Abstract
Researchers have long debated whether salient stimuli can involuntarily “capture” visual attention. Here we review evidence for a recently discovered inhibitory mechanism that may help resolve this debate. This evidence suggests that salient stimuli naturally attempt to capture attention, but capture can be avoided if the salient stimulus is suppressed before it captures attention. Importantly, the suppression process can be more or less effective as a result of changing task demands or lapses in cognitive control. Converging evidence for the existence of this suppression mechanism comes from multiple sources, including psychophysics, eye-tracking, and event-related potentials. We conclude that the evidence for suppression is strong, but future research will need to explore the nature and limits of this mechanism.
Keywords: attention capture, suppression, inhibition, visual attention
A New Role for Inhibition in the Guidance of Visual Attention
In daily life, certain types of visual stimuli seem to automatically attract our attention. For example, Figure 1a shows a red cardinal in a homogeneous background of green leaves. Phenomenologically, the red cardinal “pops out” from the scene, generating the impression that it automatically captures attention. This leads to a conundrum: If salient objects automatically attract visual attention, then our attention would constantly be captured by irrelevant information, making it difficult for us to achieve our goals. However, if these stimuli do not automatically capture attention, then why does the red cardinal in Figure 1a seem to pop out? And why are brightly colored stimuli used as visual warning signals for traffic signs, slippery floors, and building exits (Figure 1b)?
Figure 1.

Examples of attention capture. Salient stimuli, such as the uniquely colored bird (A), seem to automatically attract visual attention. For this reason, salient stimuli are often used as warning signals (B). However, researchers disagree about whether such stimuli attract attention in a truly automatic manner.
The purpose of the current paper is to review the burgeoning research suggesting that inhibitory processes play a key role in the avoidance of visual distraction. We review several recent advances in the understanding of this suppressive process using psychophysical, eye tracking, and electrophysiological techniques. We also note some limitations of the existing research and provide suggested directions for future research.
The Attention Capture Debate
Researchers studying attention capture (see Glossary) aim to determine if, when, and how certain types of stimuli involuntarily attract visual attention (see Box 1 for explanations of key terms). Knowing the answer to this question would allow us to design more effective visual warning signals in applied settings and develop more accurate basic science models of visual search. However, solving the riddle of attention capture has been a devilishly difficult undertaking – one fraught with perplexing empirical discrepancies and thorny theoretical issues.
Box 1: Defining Attention, Capture, Automatic, Involuntary, and Salience.
The term “attention” may refer to several different cognitive phenomena [90], but it is typically used in the attention capture literature as a shorthand for selective attention, a set of processes by which some stimuli receive greater processing resources or greater weight in decisions at the expense of others [91]. In the context of vision, selective attention may involve overt shifts of gaze to objects of interest or covert changes in the allocation of internal processing resources without eye movements [92,93].
Classically, shifts of both covert and overt attention are described as being driven either by top-down goals (e.g., the desire to find a red apple) or bottom-up sensory features (e.g., bright or moving objects). However, attention may also be driven by a variety of unconscious factors such as priming or reward associations that are internal (i.e., not solely a result of the current sensory input) but may work in opposition to the observer’s conscious goals. Together, these unconscious factors are termed reward and selection history [29,94]. The present review focuses mainly on goal-driven and sensory-driven allocation of attention because little research has examined suppression on the basis of reward and selection history [41].
Attention is said to be captured when it is directed to an object even though the observer has no goal of attending that object. Capture is said to be fully automatic when it is solely a result of stimulus properties and is not influenced by goals or experience (see also [95,96]). However, capture can be involuntary even if it is not fully automatic: Looking for a red apple may cause attention to be captured by a red shirt. In this case, the capture interferes with the observer’s goals (making it involuntary), but it is a consequence of the goals rather than being solely a result of the sensory properties of the red apple [11].
Stimuli that produce automatic attention capture are often described as “salient.” However, the term salience is also sometimes applied to stimuli that attract attention because of associations or previous experience (e.g., food, money). We use the phrase physical salience to denote salience that arises solely from the physical properties of the stimuli. Relevance, on the other hand, refers to how well an item matches a participant’s attentional set.
Theories of attention capture have traditionally been divided into two competing classes. According to stimulus-driven theories, physically salient objects will capture attention, regardless of the observer’s intentions [1–3]. For example, imagine a pedestrian searching for a blue storefront sign. According to stimulus-driven theories, a bright yellow door would automatically capture the pedestrian’s attention, even though it is definitely not what the pedestrian is looking for. Several types of visual features have been proposed to capture attention. Here we focus on uniquely colored objects in relatively homogenous backgrounds (called color singletons, like the cardinal and stop sign in Figure 1) [4–6].
Stimulus-driven theories of attention capture have been supported by studies demonstrating that color singletons interfere with visual search even when participants know they are task-irrelevant [4,7,8]. For example, in the additional singleton paradigm, participants search for a circle target amongst diamonds and make a speeded response regarding the orientation of a line inside the target (Figure 2a). On some trials, one of the items is a color singleton. The target is never the color singleton, and the participants are told they can ignore the singleton, but its presence slows task performance under certain conditions. That is, response times (RTs) may be slower for stimulus arrays containing the singleton compared to singleton-absent arrays (called a singleton presence cost). This is taken as evidence that the color singleton captured visual attention, temporarily disrupting visual search (but see [9,10]).
Figure 2.
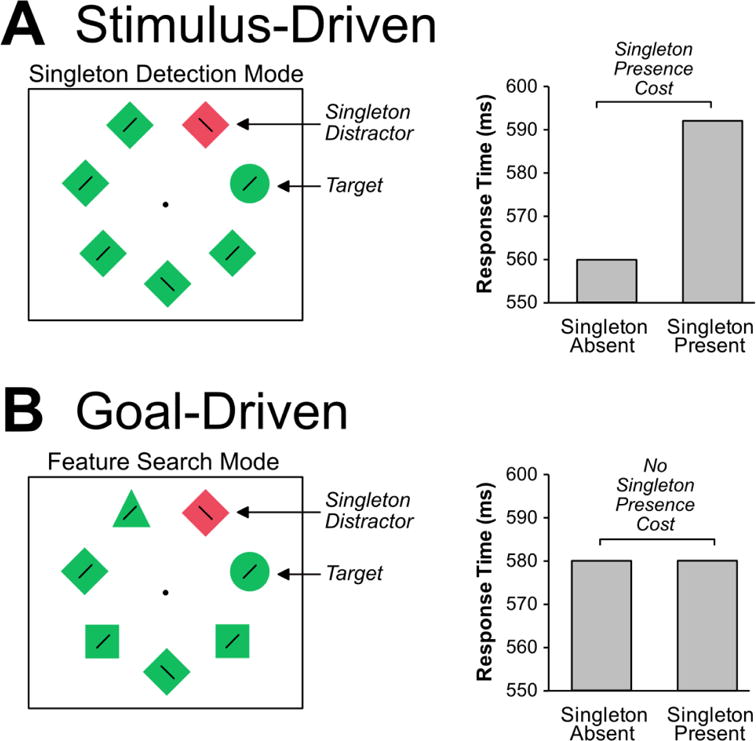
Conflicting results in a typical attention capture task. In both versions, participants search for the green circle and make a speeded response indicating the tilt of a line inside (left or right). On half of trials, a red singleton appears at a nontarget location. (A) Stimulus-driven theorists frequently use a version of the task where the target circle appears amongst homogenous distractors (i.e., all diamonds). This leads a large singleton presence cost, indicating capture. (B) Goal-driven theorists use a version of the task where the circle target appears amongst heterogeneous distractors. This leads to no singleton presence cost, indicating no capture. These stimuli and data are illustrative, based on a combination of several studies.
However, not all researchers agree that physically salient stimuli automatically capture visual attention. According to goal-driven theories, only stimuli that match the features of the search target will capture attention [11–15]. Put another way, a salient stimulus has no intrinsic ability to attract attention and will capture attention only if it matches what the observer is “looking for” (the observer’s attentional set). Consider again the pedestrian searching for a blue storefront sign. According to goal-driven theories, other blue objects in the scene might capture attention – such as a blue sports car or a blue umbrella on a street-side café table. This is sometimes called “contingent capture” because capture is a contingent on the pedestrian’s goals and yet runs counter what the pedestrian is actually trying to accomplish (and is therefore involuntary; [11]). It is important to note that, according to these models, physically salient items that fall outside of the pedestrian’s attentional set, such as a bright yellow door, will not capture attention.
Goal-driven theories are supported by studies demonstrating that the observer’s attentional set impacts whether salient stimuli capture attention [11–15]. For example, in the previously mentioned additional singleton paradigm, the target was itself a shape singleton because it appeared amongst homogenously shaped distractors (Figure 2a). Thus, it is plausible that participants established an attentional set for singletons more generally. In turn, this singleton detection mode causes the irrelevant color singleton to capture attention [6,12]. Consistent with this proposal, singleton presence costs can be eliminated by modifying the stimuli so that participants cannot find the target by looking for a singleton and must instead search for a specific feature value (called feature search mode) [12,16].
At face value, stimulus-driven and goal-driven theories make opposite predictions about when to expect attention capture, but these competing theoretical perspectives have flourished for over two decades. Problematically, each side has developed several “escape hatches” that allow them to explain away seemingly incompatible results (e.g., [1,8,9,17]). This has made the theories difficult to falsify, leading to an empirical stalemate. It is clear that top-down goals have an impact on attention capture, but there are many circumstances where irrelevant salient items seem to capture attention. The field desperately needs to move toward a coherent resolution of this debate.
Suppression as a Step Forward in the Attention Capture Debate
One potential resolution is to propose that physically salient stimuli do have an intrinsic ability to attract attention, but that inhibitory processes can suppress these stimuli if participants exert cognitive control. Initial evidence that salient stimuli may sometimes be suppressed came from a study of macaque monkeys which indicated that gaze was actually inhibited from moving to salient items ([18]; described in more detail below). This was followed by an event-related potential (ERP) study that led to the development of the signal suppression hypothesis [19], which states that attention capture can be prevented by a top-down inhibitory mechanism. If unsuppressed, salient stimuli will automatically capture visual attention, which is consistent with stimulus-driven theories. This is inconsistent with goal-driven theories, which predict that capture occurs only when an item matches the attentional set and is not triggered by physical salience alone. However, the signal suppression model also predicts that singletons will fail to capture attention when these stimuli are suppressed, which is consistent with goal-driven theories (and many experiments in which no capture is observed [11–14]). This is inconsistent with stimulus-driven theories, which propose that the initial shift of visual attention is guided entirely by salience.
To better understand how suppression and capture might interact, it might first help to review some basic assumptions about how visual attention is guided (Figure 3). Researchers generally assume that the visual system first parses search displays into feature maps, which represent the locations of specific feature values. Separate feature maps are constructed for different feature dimensions, such as color and shape [20–24]. From these feature maps, an attentional priority map is constructed, where items with a higher priority are more likely to be attended first. Stimulus-driven theories predict that color singletons will have a high priority, thus attracting the initial shift of attention. Goal-driven theories predict that items matching the attentional set will have a high priority, thus attracting attention. The signal suppression hypothesis agrees with stimulus-driven theories that color singletons will ordinarily have a high priority and therefore capture attention. However, it also proposes that a top-down inhibitory mechanism can suppress this item (assuming that the participants are in a state of good attentional control), preventing capture of attention by the singleton. Moreover, if the singleton is sufficiently suppressed, processing at the location of the singleton should be reduced below baseline levels of processing.
Figure 3.
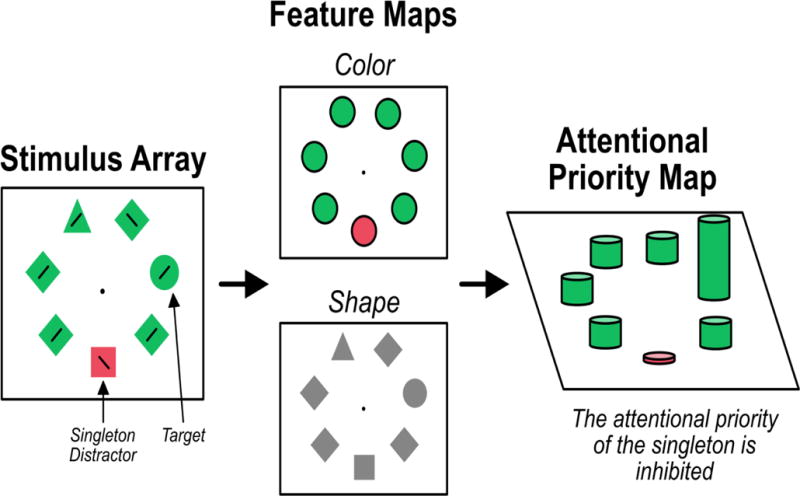
Mechanisms underlying the guidance of visual attention. The stimulus is first represented by a series of feature maps, which denote the location of a simple feature in the visual field. These feature maps are used to construct an attentional priority map. When singletons fail to capture attention, the signal suppression hypothesis predicts that the attentional priority at the singleton will be lower than baseline levels. This inhibition will result in relatively poor processing at the singleton location.
A crucial feature of the signal suppression hypothesis is that inhibition of the salient singleton is enacted before the initial shift of visual attention (see Box 2). One might suppose that a salient item cannot be suppressed without first being selected by some kind of attentional mechanism. However, we propose that suppression can guided by preattentive feature information and therefore occurs prior to the transmission of information to the priority map [25]. This notion of attentional guidance is not new or particularly controversial – it is a key feature of many models of visual search [21,24].
Box 2: The Time Course of Attentional Suppression.
The signal suppression hypothesis proposes that suppression of the salient item occurs before an initial shift of attention. This runs counter to an alternative account commonly invoked by stimulus-driven theorists: the rapid disengagement account [7,97,98]. According to this account, visual attention initially moves to the most salient item in a display, but the salient item can be rapidly rejected so that the target is attended with little delay. Thus, unlike the signal suppression hypothesis, this account predicts that suppression is only possible after an initial shift of visual attention to the most salient object. Relatedly, some studies have suggested that search items cannot be ignored unless they are first attended (the ignoring paradox; [77,99]).
However, there is now good reason to believe that salient objects can be suppressed without being initially attended. First, several ERP studies suggests that when salient items elicit the suppression-related PD component, there is no evidence of a preceding attentional shift to that location (i.e., no N2pc) [19,38,63]. Second, in the capture-probe paradigm, even when the probe and search array onset simultaneously and last for only 100 ms, there is still a robust probe suppression effect [69]. This short probe duration leaves little time for attention to move to and reject the singleton item. Finally, in the oculomotor capture paradigm, even the fastest eye movements are guided away from the singleton [68]. If the salient object was attended before suppression, one might predict that the fastest eye movements should be guided toward the singleton, because the eye movement is initiated while the item is still covertly attended (see [98]).
How is it possible to suppress a salient object without first selecting it? As discussed in the main text, feature-based attention mechanisms may be used to decrease the priority of items that contain a particular feature value (e.g., all green items). Substantial research shows that feature-based attention can operate in parallel on the entire visual input, without requiring prior selection of specific objects [100–102].
It is not yet known whether suppression is the result of an intentional strategy or occurs involuntarily as a result of previous encounters with salient distractors. A large line of research suggests that factors such as scene context [26] or implicit knowledge about the previous trial [27,28] can play a surprisingly large role in the size of observed capture effects (called selection history; for a review, see [29]). The signal suppression hypothesis is currently agnostic about whether suppression arises automatically from selection history, and this will be an important question for future research (see the Outstanding Questions box).
Evidence for Suppression of Salient Singletons
The signal suppression hypothesis postulates a new inhibitory mechanism, and this section reviews the converging evidence for this mechanism from electrophysiological, eye tracking and behavioral studies.
The PD Component
Early support for the signal suppression hypothesis came from ERP studies use of the N2pc and PD components. The N2pc component is an electrophysiological index of the covert deployment of visual attention [30–34]. It is a negative-going deflection observed over the visual cortex contralateral to an attended object, typically beginning 150–200 ms after stimulus onset [35,36]. The PD component is much like the opposite of the N2pc component. Whereas N2pc is a negative potential contralateral to a to-be-attended stimulus, the PD is a positive potential contralateral to a to-be-ignored stimulus. An early study postulated that this lateralized positivity reflects suppression of distracting stimuli during visual search [37].
Later studies showed that the PD component is observed when a salient color singleton fails to capture attention. For example, in one study, participants reported whether a specific target letter (e.g., a large A) was present or absent within an array of several letters ([19]; Figure 4a). The target letter elicited an N2pc, indicating that it attracted visual attention. A salient color singleton was present on some trials, which one might expect to capture attention. However, the singleton elicited no N2pc and instead elicited a PD component. This was interpreted as evidence that the singleton was suppressed, preventing attention capture. Many studies have corroborated this basic pattern of results: under conditions where singletons elicit a PD component, it typically is not followed by an N2pc component [38–44].
Figure 4.
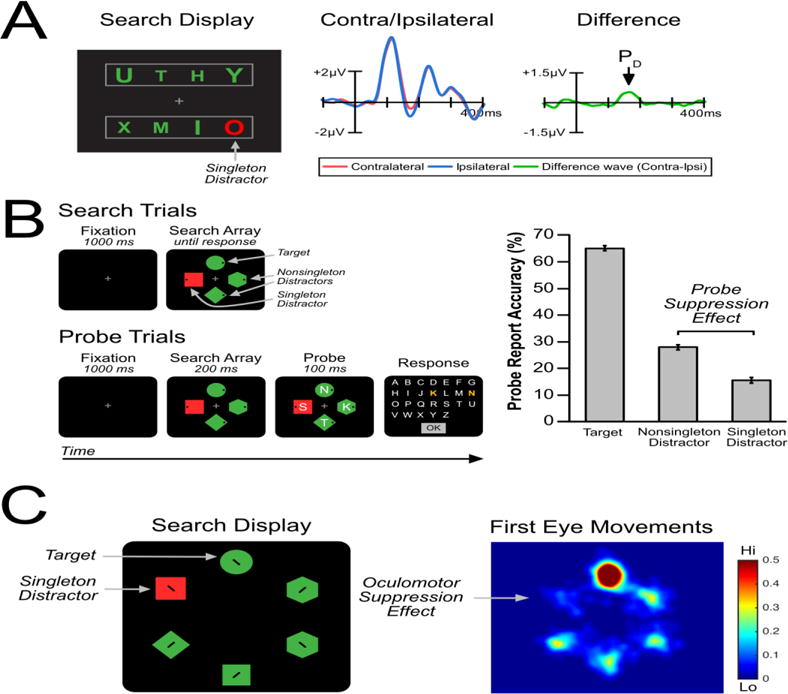
Converging evidence for the suppression of salient-but-irrelevant singletons. (A) In an ERP capture task, participants searched for a specific target (e.g., large green A) and attempted to ignore a salient color singleton (red O). Salient singletons elicited an electrophysiological index of suppression called the PD component [19]. (B) In the capture-probe paradigm, participants search for a green circle and report the location of a dot. On a random subset of probe trials, letters briefly appear in each search location and participant tried to recall as many letters as possible. Probe recall accuracy was impaired at the singleton location compared to other nontarget locations [69]. (C) In an oculomotor capture task, participants searched for a green circle. As shown in the heat map, first eye movements were biased away from the singleton distractor, suggesting that it was inhibited [68].
Associating a neural signal with an underlying cognitive operation can be difficult [45,46]. To illustrate, consider this puzzling fact: some studies find that salient singletons elicit both a PD and an N2pc in the same ERP waveform [40,47–53]. At face value, this pattern of results seems paradoxical because, on the one hand, the singleton seems to capture attention (indexed by the N2pc) and yet, on the other hand, the singleton seems to be suppressed (indexed by the PD). If suppression prevents capture, how could such a result occur? One might argue that the PD indexes some other cognitive process than suppression, such as a salience signal from the singleton [54–56]. But other accounts could explain these results while still maintaining that the PD reflects suppression. For example, perhaps the singleton was suppressed, but not enough to prevent capture. Or perhaps capture and suppression happen on different trials [57] or in different participants [58,59], producing both an N2pc and a PD in the grand average even though they never occurred on the same trial. Finally, in many cases the N2pc is followed by a PD component [40,51–53,60], which may reflect a suppressive process that returns attention back to a neutral state after an episode of attentional focusing is complete [61] (but see [62]). As a result, multiple sources of converging evidence will be needed to determine what specific process is reflected by the PD component.
There is some preliminary evidence suggesting that the PD component specifically indexes suppression. First, in visual search tasks, the magnitude of the PD component is larger on trials with faster RTs [38,39], consistent with the idea that effective suppression of the singleton allows participants to quickly locate the target. Second, in a study that concurrently measured ERPs and eye movements, the PD component was elicited only on trials where gaze shifted directly to the target, but not on trials where gaze was initially captured by the singleton [57]. Third, groups of individuals with good attentional control demonstrate a large PD component to salient items, whereas groups of individuals with poor control exhibit an N2pc ([63,64]). All of these lines of evidence are consistent with the hypothesis that the PD component reflects a suppressive process. However, it cannot be assumed that all contralateral positivities reflect the same underlying PD component [46,65], and future research is needed to definitively link the PD component with processes of attentional suppression.
Behavioral and Oculomotor Evidence for Suppression
Although the PD experiments have suggested that salient items are suppressed, these studies are not entirely conclusive because they lacked direct evidence that processing at the singleton location was actually inhibited below baseline levels of processing. Recent studies have addressed this shortcoming by developing behavioral and eye tracking paradigms that assess the processing of each item in the stimulus array.
The capture-probe paradigm is used to assess suppression of covert attention by intermixing two types of trials (see Figure 4b). On search trials, participants perform a typical attention capture task: they search for a target item (e.g., a green diamond) and make a speeded response regarding the location of a dot inside this shape (left or right). On some trials, a color singleton appears at a nontarget location. The key to this paradigm is that probe trials are randomly intermixed with the search trials. On probe trials, letters briefly appear inside each search object, followed by a mask. On these trials, participants do not have to locate the target and instead report as many letters as possible. The probability that the letter at a given location is reported is used as a measure of the processing at that location. That is, participants should be more likely to report letters that appeared at attended objects and less likely to report letters at suppressed objects (as previously validated by [66,67]). Note that because probe trials are rare and randomly intermixed with search trials, participants shift attention in the same manner on both search and probe trials.
This paradigm has been used under conditions that promote capture (by encouraging singleton detection mode) and under conditions that discourage capture (by encouraging feature search mode). Under conditions that promote capture (as in Figure 2a), probe report accuracy was higher for the letter at the location of the color singleton than for letters at nonsingleton distractor locations. This provides an important validation of the probe method. However, under conditions that discouraged capture (as in Figure 2b), probe report accuracy was lower for the letter at the location of the color singleton than for letters at the nonsingleton distractor locations (a probe suppression effect). This clearly demonstrates that processing at the singleton location is reduced relative to baseline levels of processing.
A second paradigm uses eye tracking to assess the suppression of overt attention (see Figure 4c). This paradigm is much like the capture-probe paradigm, except that no probe trials are included and the search displays are designed to encourage eye movements (e.g., search items appear at a greater eccentricity, and participants report the orientation of a small tilted line inside the target). The landing position of the first eye movement is used to assess the level of processing at each location. If the singleton is suppressed, gaze should be less likely to be directed to the singleton than to the nonsingleton distractor items (an oculomotor suppression effect).
This paradigm has been used in both humans and macaque monkeys [18,25,68]. In both species, the first eye movement was less likely to go to the color singleton than to a given nonsingleton distractor (under conditions that discouraged capture, as in Figure 2b). In other words, gaze selectively avoided the singleton, consistent with an interpretation that this location was suppressed. This oculomotor suppression effect occurred even for the fastest subset of eye movements, suggesting that suppression was initiated rapidly (see Box 2 for a discussion of the time course of suppression).
Another line of evidence suggesting that salient items are suppressed comes from response times in the additional singleton paradigm (e.g., Figure 2b). Mean response times are sometimes faster on singleton-present trials than on singleton-absent trials [25,68–70]. This singleton presence benefit presumably indicates that the suppressed singleton was excluded from visual search, effectively decreasing number of items to be searched. Other capture paradigms show analogous effects on mean response time when salient items might be inhibited. For example, in the spatial cuing paradigm, participants are sometimes faster to respond on trials where a salient cue appears at a nontarget location than when it appears at a target location [27,71,72].
Mechanisms of Suppression
Now that we have established the converging evidence for suppression, we will review some potential mechanisms by which the visual system might determine which items in a given stimulus array should be suppressed. As illustrated in Figure 3, we distinguish between three classes of models. According to first-order feature suppression models, the visual system inhibits items on the basis of their individual feature values. For example, if observers are repeatedly exposed to a green singleton that is never the target, they may learn to suppress green items. According to second-order feature models, however, the visual system requires no information about the specific feature value of to-be-suppressed salient item. Instead, the salient item is suppressed on the basis of its status as a feature discontinuity (which is a second-order stimulus feature; [73–75]). For example, if observers are repeatedly exposed to a green singleton that is never the target, they may learn to suppress any feature discontinuity on the color feature map. Finally, according to global salience models, participants may simply suppress items with a high weighting in the attentional priority map.
A key difference is that first-order feature suppression models posit the observer requires foreknowledge about the specific feature value of the to-be-ignored item, whereas the other models predict that singletons can be suppressed even if the particular color of the singleton is not known in advance. Most prior studies of suppression have held the feature value of the to-be-ignored item constant across the experimental session (e.g., [38,63,64,68,69,76]), making it impossible to determine whether featural foreknowledge is necessary for suppression.
A simple way to distinguish between these models is to randomly swap the singleton and nonsingleton colors across trials, making it impossible for observers to know which color to suppress (e.g., the arrays could sometimes contain a green singleton amongst red nonsingleton items and sometimes contain a red singleton amongst green nonsingleton items). For both the probe-suppression and eye tracking paradigms shown in Figure 4, suppression was eliminated when the singleton and nonsingleton colors swapped randomly trial-by-trial. Indeed, significant capture of attention by the singleton was obtained under these conditions, even though the tasks were designed to discourage capture via feature search mode. These results are consistent with first-order feature suppression models but inconsistent with the second-order feature suppression model and the global salience suppression models. In other words, suppression appears to be possible only when the observer knows in advance the features of the to-be-suppressed item.
Other evidence supporting first-order feature suppression models comes from studies demonstrating that the ability to avoid color singletons develops as gradually as participants gain experience with the specific feature value of the singleton [25,70,77,78]. For example, in one study, participants performed a behavioral capture paradigm where the color of the singleton remained constant for a block of 48 trials and then changed to a new color for the next block (with the nonsingleton color remaining constant across the entire session)[70]. A singleton presence cost was observed in the first half of each block with a given singleton color, indicating that the singleton captured attention. However, the singleton presence cost was eliminated in the second half of each block, suggesting that participants learned to suppress the singleton. The capture returned when the singleton color changed in the next block, indicating that the specific color of the singleton was being used in the prior block to determine which item should be suppressed. Even stronger evidence was obtained in an eye tracking version of this paradigm [25], which made it possible to determine whether the location of the singleton was suppressed below baseline levels. For the first few trials of a block of trials with a particular color (i.e., immediately after the color of the singleton changed), eye movements were biased toward the singleton (Figure 5; oculomotor capture). However, after many trials with a given singleton color, eye movements became biased away from the color singleton (oculomotor suppression). This is again consistent with the hypothesis that singletons are suppressed on the basis of their color and not on the basis of their status as singletons or their high levels of salience.
Figure 5.
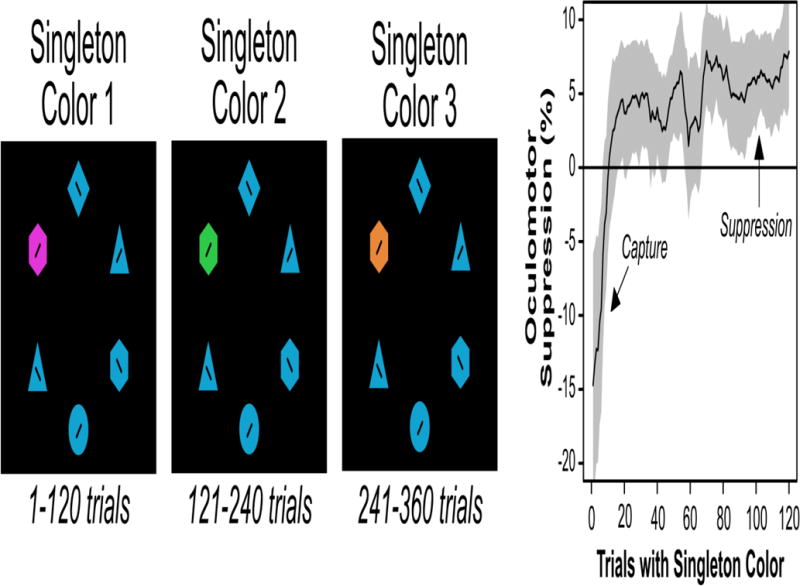
An eye-tracking task in which the singleton color is blocked. The left panel shows the target color and the singleton colors, each of which was used for one block of 120 trials. The right panel shows the amount of oculomotor suppression at the singleton location over the course of a block of trials (running average across sets of 10 trials). In the first few trials with a new singleton color, the eyes are captured by the singleton. As participants gain experience with the singleton color value, the eyes are biased away from the singleton [25].
Despite clear evidence that suppression was based on first-order features in these experiments, other experiments suggest that, given enough practice, people may learn to suppress singletons even without foreknowledge of singleton color. First, in an experiment with over 2000 trials per participant, color singletons elicited a PD component even though the singleton and nonsingleton colors swapped randomly from trial to trial [19] (see also [43]). Second, similarly another study found that participants who were given experience with several different singleton colors did not exhibit capture by a singleton of a novel color [79]. However, neither of these studies provided direct evidence that the singleton location was suppressed below background levels, so additional research is needed to determine whether people can learn to suppress singletons without foreknowledge of the singleton’s color.
Relation to Traditional Models of Visual Search
Although the evidence for suppression of salient singletons is quite new, this idea is remarkably consistent with traditional models of visual search in which attention is assumed to be guided by simple features [21,23,24,80,81]. For example, Wolfe’s guided search theory [21] explicitly proposes that first-order featural information can be used to guide attention toward items containing task-relevant feature values (which would in turn bias attention away from color singletons). We call the prioritization of task-relevant features target-feature upweighting [82] to highlight the distinction between this mechanism and mechanisms that involve directly suppressing the distractor features.
It is surprisingly difficult to empirically distinguish between target-feature upweighting and distractor-feature suppression. This is especially the case if one assumes that upweighting is not necessarily applied to the exact feature values of the target but can be flexibly adjusted depending on the expected nontarget feature values. For example, the visual system may strategically boost values shifted away from the target value to increase discriminability between the target and the likely distractors [83–85]. Additionally, the visual system may boost large swaths of feature space rather than a narrow feature value [85]. With such modifications, target-feature upweighting can explain virtually any instance of reduced processing of a salient distractor. Consider, for example, the aforementioned eye-tracking task in which the singleton color was blocked (see Figure 5). When the singleton is pink, participants may adopt an attentional set for the target color that is shifted away from that value (e.g., bluish-green). If the singleton then changes to green, participants may be boosting a region of color space that includes the singleton color. After a few trials, they may adjust their attentional set to values shifted away from green (e.g., purplish-blue).
At first glance, this may seem like depressing news for the signal suppression model. But there is a hidden silver lining: Previous evidence for guidance of attention toward relevant feature values could instead be explained by the suppression of irrelevant feature values. This point was originally made by Anne Treisman during the debate between Guided Search and Feature Integration models [86]. The potential role of inhibition in visual search has also come to light in recent research on “templates for rejection” [87,88]. Note that suppression-based accounts of selective attention could be especially successful if one assumes the same flexibility that we gave to upweighting models – that the visual system may inhibit features shifted away from the actual distractor value or may inhibit large swaths of the feature space.
Finally, it is worth mentioning that suppression and upweighting models are not mutually exclusive, and there is some evidence that both may operate concurrently to guide visual attention [84,89]. In short, the inhibition/upweighting problem will be an important issue, not only on future research on attention capture, but for all researchers who are interested in the guidance of visual attention (see Outstanding Questions). As far as we know, no widely accepted method exists for separating the contributions of suppression versus upweighting – developing such a method will be a crucial step for future research.
Concluding Remarks
The attention capture debate has been a challenging undertaking for visual attention researchers – one abound with perplexing empirical discrepancies and theoretical contradictions. Resolving this debate has abundant practical as well as theoretical implications, and for this reason, it has occupied many researchers for decades. There is now converging evidence from psychophysical, eye-tracking, and ERP studies suggesting that, when salient items fail to capture attention, performance at the singleton location is inhibited. This inhibitory mechanism is a new lead in the longstanding attention capture debate, but additional research is needed to determine the boundary conditions for this inhibitory mechanism and to determine its broader role in the guidance of visual selective attention.
Trends Box.
-
-
Researchers have long debated whether salient stimuli involuntarily attract visual attention. But much of this research has failed to consider the potentially crucial role of inhibitory processes in preventing distraction.
-
-
ERP studies demonstrate that when salient items fail to capture attention, they elicit a distractor positivity (PD) that reflects a suppressive process.
-
-
Psychophysical and eye-tracking studies demonstrate that processing at the location of a salient distractor can be inhibited below baseline levels. This suppression manifests as a reduced probability of reporting stimuli presented at that location or a reduced probability of directing gaze to that location.
-
-
Current research is exploring exactly how the visual system determines which items should be suppressed.
Outstanding Questions.
-
-
Do behavioral, eye tracking, and electrophysiological indexes of suppression all reflect the same underlying cognitive mechanism? More evidence is needed to definitively link the PD component to suppression and to determine whether multiple different processes can produce PD-like contralateral positivities.
-
-
Do separate mechanisms exist for suppressing irrelevant features and upweighting relevant features? If so, how are they coordinated to guide visual attention? One might imagine that different task demands or constraints may encourage subjects to use one strategy over the other.
-
-
Is suppression a direct result of voluntary goals or an automatic consequence of having encountered salient distractors on previous trials? It is natural to assume that the suppression of salient distractors arises from a top-down strategy, but it may instead reflect unconscious priming from previous trials (selection history).
-
-
How does the visual system determine which items should be suppressed? Some evidence suggests that the visual system selects items for suppression on the basis of simple features (e.g., the color green). However, other evidence suggests the visual system can learn to suppress items defined by second-order feature discontinuities or items defined by high salience.
-
-
Can suppression be applied to other types of salient stimuli in addition to color singletons? It is unclear if other well-studied classes of salient stimuli, such as abrupt onsets or motion singletons, can be actively suppressed. In addition, little research has examined whether and how suppression is used to avoid capture of attention by items that would otherwise attract attention on the basis of reward.
-
-
What factors cause lapses in inhibition that leave the visual system vulnerable to capture? For example, it seems reasonable to suspect that high cognitive load, mental fatigue, and poor task strategy could reduce inhibition. Such research could have profound implications for attention capture in applied settings.
Acknowledgments
This review was made possible by National Research Service Award F32EY024834 to N.G. from the National Eye Institute and by NIH Grants R01MH076226 and R01MH065034 to S.J.L.
Glossary Box
- Abrupt onset
An object that appears suddenly in an otherwise static image
- Attention capture
The allocation of attention to an object involuntarily, whether or not this is directly consistent with the observer’s goals
- Attentional set
A template for search that contains the target features (also called an attentional control setting). In layman’s terms, “what the participant was looking for.”
- Color singleton
A uniquely colored object on a homogenously colored background
- ERP
Event-related potential a neural response to a sensory or cognitive event that is extracted from the EEG (electroencephalogram).
- Feature search mode
When an observer adopts an attentional set for a specific target-defining property (see also “singleton detection mode”)
- Goal-driven theories
Theories proposing that the allocation of attention is driven solely by the match between a stimulus and the attentional set, with no special allocation of attention to physically salient stimuli (also called top-down theories)
- N2pc
An ERP component that is a negative-going deflection in electrodes over visual cortex contralateral to the to-be-attended region of space. It is widely considered to be an index of attentional allocation. “N2” means that it’s part of the second major negative ERP response, and “pc” indicates that it is posterior and contralateral
- Oculomotor capture
When eye movements are more likely to be directed to a task-irrelevant salient stimulus compared to some baseline measure of attentional allocation. In other words, when a physically salient item attracts gaze above chance levels
- Oculomotor suppression
When first eye movements are less likely to be directed to a task-irrelevant salient stimulus compared to some baseline measure of attentional allocation
- PD (Distractor Positivity)
An ERP component that is a positive-going deflection observed at electrodes over visual cortex contralateral to a to-be-ignored object. This ERP component is a putative index of suppression
- Probe suppression effect
In the capture-probe paradigm, when participants are less likely to report probe letters at the location of the singleton than at the nonsingleton distractors (i.e., performance at the singleton location is below baseline)
- Relevance
How well a search item matches the viewer’s attentional set
- Salience
The extent to which a given item is noticeable. This term is often restricted to cases in which the item is noticeable solely by virtue of its physical properties (termed physical salience), but it is sometimes used to for cases in which a stimulus is noticeable as a result of the observer’s prior experience (see Box 1 for more details)
- Signal suppression hypothesis
A model of attention capture which proposes that salient stimuli generate a strong bottom-up salience signal, but that this salience signal can be suppressed by a top-down mechanism
- Singleton detection mode
When an observer adopts an attentional set for any unique salient item (i.e., a broad attentional set for any type of singleton or “pop out” stimulus)
- Singleton presence benefit
Faster RTs in a visual search task when a task-irrelevant singleton is present than when it is absent (which suggests that the singleton was suppressed, reducing the number of items to be searched)
- Singleton presence cost
Slower RTs in a visual search task when a task-irrelevant singleton is present than when it is absent (which suggests that the singleton captured attention)
- Stimulus-driven theories
Theories proposing that physically salient stimuli automatically capture attention in a manner that is not influenced by an observer’s goals (also called bottom-up theories)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Theeuwes J. Top–down and bottom–up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Franconeri SL, Simons DJ. Moving and looming stimuli capture attention. Percept Psychophys. 2003;65:999–1010. doi: 10.3758/bf03194829. [DOI] [PubMed] [Google Scholar]
- 3.Yantis S, Jonides J. Abrupt visual onsets and selective attention: Evidence from visual search. J Exp Psychol Hum Percept Perform. 1984;10:601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
- 4.Theeuwes J. Perceptual selectivity for color and form. Percept Psychophys. 1992;51:599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- 5.Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Percept Psychophys. 1988;43:346–354. doi: 10.3758/bf03208805. [DOI] [PubMed] [Google Scholar]
- 6.Pashler HE. Cross-dimensional interaction and texture segregation. Percept Psychophys. 1988;43:307–318. doi: 10.3758/bf03208800. [DOI] [PubMed] [Google Scholar]
- 7.Theeuwes J. Top-down search strategies cannot override attentional capture. Psychon Bull Rev. 2004;11:65–70. doi: 10.3758/bf03206462. [DOI] [PubMed] [Google Scholar]
- 8.Belopolsky AV, et al. The size of an attentional window modulates attentional capture by color singletons. Psychon Bull Rev. 2007;14:934–938. doi: 10.3758/bf03194124. [DOI] [PubMed] [Google Scholar]
- 9.Folk CL, Remington RW. Selectivity in distraction by irrelevant featural singletons: Evidence for two forms of attentional capture. J Exp Psychol Hum Percept Perform. 1998;24:847–858. doi: 10.1037//0096-1523.24.3.847. [DOI] [PubMed] [Google Scholar]
- 10.Becker SI. Irrelevant singletons in pop-out search: Attentional capture or filtering costs? J Exp Psychol Hum Percept Perform. 2007;33:764–787. doi: 10.1037/0096-1523.33.4.764. [DOI] [PubMed] [Google Scholar]
- 11.Folk CL, et al. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- 12.Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Percept Psychophys. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- 13.Lien MC, et al. Contingent attentional capture by top-down control settings: Converging evidence from event-related potentials. J Exp Psychol Hum Percept Perform. 2008;34:509–530. doi: 10.1037/0096-1523.34.3.509. [DOI] [PubMed] [Google Scholar]
- 14.Lien MC, et al. Attentional capture by singletons is contingent on top-down control settings: Evidence from electrophysiological measures. Vis cogn. 2010;18:682–727. [Google Scholar]
- 15.Lien MC, et al. Attentional capture with rapidly changing attentional control settings. J Exp Psychol Hum Percept Perform. 2010;36:1–16. doi: 10.1037/a0015875. [DOI] [PubMed] [Google Scholar]
- 16.Leber AB, Egeth HE. It’s under control: Top-down search strategies can override attentional capture. Psychon Bull Rev. 2006;13:132–138. doi: 10.3758/bf03193824. [DOI] [PubMed] [Google Scholar]
- 17.Burnham BR, Bulletin P. Displaywide visual features associated with a search display’s appearance can mediate attentional capture. Psychon Bull Rev. 2007;14:392–422. doi: 10.3758/bf03194082. [DOI] [PubMed] [Google Scholar]
- 18.Ipata AE, et al. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat Neurosci. 2006;9:1071–1076. doi: 10.1038/nn1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawaki R, Luck SJ. Capture versus suppression of attention by salient singletons: Electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception, Psychophys. 2010;72:1455–1470. doi: 10.3758/APP.72.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe JM. Guided Search 4.0: Current progress with a model of visual search. In: Gray WD, editor. Integrated models of cognitive systems. Oxford University Press; 2007. pp. 99–119. [Google Scholar]
- 21.Wolfe JM. Guided search 2.0: A revised model of visual search. Psychon Bull Rev. 1994;1:202–238. doi: 10.3758/BF03200774. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe JM, et al. Guided Search : An Alternative to the Feature Integration Model for Visual Search Guided Search : An Alternative to the Feature Integration Model for Visual Search. 2016;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- 23.Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- 24.Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2:194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- 25.Gaspelin N, Luck SJ. Distinguishing Among Potential Mechanisms of Singleton Suppression. J Exp Psychol Hum Percept Perform. doi: 10.1037/xhp0000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosman JD, Vecera SP. Context-dependent control over attentional capture. J Exp Psychol Hum Percept Perform. 2013;39:836–848. doi: 10.1037/a0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belopolsky AV, et al. What is top-down about contingent capture ? Attention, Perception, Psychophys. 2010;72:326–341. doi: 10.3758/APP.72.2.326. [DOI] [PubMed] [Google Scholar]
- 28.Maljkovic V, Nakayama K. Priming of pop-out: I. Role of features. Mem Cognit. 1994;22:657–672. doi: 10.3758/bf03209251. [DOI] [PubMed] [Google Scholar]
- 29.Awh E, et al. Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends Cogn Sci. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 31.Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 1994;20:1000–14. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- 32.Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalogr Clin Neurophysiol. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- 33.Woodman GF, Luck SJ. Serial deployment of attention during visual search. J Exp Psychol Hum Percept Perform. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- 34.Luck SJ. In: Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. Luck SJ, Kappenman ES, editors. Oxford University Press; 2012. pp. 329–360. [Google Scholar]
- 35.Hopf JM, et al. Neural sources of focused attention in visual search. Cereb cortex (New York, NY 1991) 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- 36.Hopf JM, et al. The Neural Site of Attention Matches the Spatial Scale of Perception. J Neurosci. 2006;26:3532–3540. doi: 10.1523/JNEUROSCI.4510-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey C, et al. Electrophysiological indices of target and distractor processing in visual search. J Cogn Neurosci. 2009;21:760–775. doi: 10.1162/jocn.2009.21039. [DOI] [PubMed] [Google Scholar]
- 38.Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. J Neurosci. 2014;34:5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jannati A, et al. Tracking target and distractor processing in fixed-feature visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 2013;39:1713–30. doi: 10.1037/a0032251. [DOI] [PubMed] [Google Scholar]
- 40.Kiss M, et al. Attentional Capture by Salient Distractors during Visual Search Is Determined by Temporal Task Demands. J Cogn Neurosci. 2012;24:749–759. doi: 10.1162/jocn_a_00127. [DOI] [PubMed] [Google Scholar]
- 41.Sawaki R, Luck SJ. Active suppression of distractors that match the contents of visual working memory. Vis cogn. 2011;19:956–972. doi: 10.1080/13506285.2011.603709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspelin N, Luck SJ. Electrophysiological and Behavioral Evidence of Suppression of Salient-But-Irrelevant Stimuli. J Cogn Neurosci. doi: 10.1162/jocn_a_01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burra N, Kerzel D. Attentional capture during visual search is attenuated by target predictability: Evidence from the N2pc, Pd, and topographic segmentation. Psychophysiology. 2013;50:422–430. doi: 10.1111/psyp.12019. [DOI] [PubMed] [Google Scholar]
- 44.Burra N, Kerzel D. The distractor positivity (Pd) signals lowering of attentional priority: Evidence from event-related potentials and individual differences. Psychophysiology. 2014;51:685–696. doi: 10.1111/psyp.12215. [DOI] [PubMed] [Google Scholar]
- 45.Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Kappenman ES, Luck SJ. ERP components: The ups and downs of brainwave recordings. In: Kappenman ES, Luck SJ, editors. The Oxford Handbook of ERP Components. Oxford University Press; 2012. pp. 3–30. [Google Scholar]
- 47.Feldmann-Wüstefeld T, Schubö A. Intertrial priming due to distractor repetition is eliminated in homogeneous contexts. Attention, Perception, Psychophys. 2016;78:1935–1947. doi: 10.3758/s13414-016-1115-6. [DOI] [PubMed] [Google Scholar]
- 48.Feldmann-Wüstefeld T, et al. You see what you have learned. Evidence for an interrelation of associative learning and visual selective attention. Psychophysiology. 2015;52:1483–1497. doi: 10.1111/psyp.12514. [DOI] [PubMed] [Google Scholar]
- 49.Feldmann-Wüstefeld T, et al. Rewarded visual items capture attention only in heterogeneous contexts. Psychophysiology. 2016;53:1063–1073. doi: 10.1111/psyp.12641. [DOI] [PubMed] [Google Scholar]
- 50.Feldmann-Wüstefeld T, Schubö A. Context homogeneity facilitates both distractor inhibition and target enhancement. J Vis. 2013;13:1–12. doi: 10.1167/13.3.11. [DOI] [PubMed] [Google Scholar]
- 51.Sawaki R, et al. A common neural mechanism for preventing and terminating the allocation of attention. J Neurosci. 2012;32:10725–10736. doi: 10.1523/JNEUROSCI.1864-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawaki R, Luck SJ. How the brain prevents and terminates shifts of attention. In: Mangun GR, editor. Cognitive Electrophysiology of Attention. Elsevier; 2014. pp. 16–29. [Google Scholar]
- 53.Hilimire MR, Corballis PM. Event-related potentials reveal the effect of prior knowledge on competition for representation and attentional capture. Psychophysiology. 2014;51:22–35. doi: 10.1111/psyp.12154. [DOI] [PubMed] [Google Scholar]
- 54.Pomerleau VJ, et al. The attentional blink freezes spatial attention allocation to targets, not distractors: Evidence from human electrophysiology. Brain Res. 2014;1559:33–45. doi: 10.1016/j.brainres.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 55.Corriveau I, et al. Electrophysiological evidence of multitasking impairment of attentional deployment reflects target-specific processing, not distractor inhibition. Int J Psychophysiol. 2012;86:152–159. doi: 10.1016/j.ijpsycho.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Barras C, Kerzel D. Active suppression of salient-but-irrelevant stimuli does not underlie resistance to visual interference. Biol Psychol. 2016;121:74–83. doi: 10.1016/j.biopsycho.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Weaver MD, et al. A temporal dependency account of attentional inhibition in oculomotor control. Neuroimage. 2017;147:880–894. doi: 10.1016/j.neuroimage.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Fukuda K, Vogel EK. Individual differences in recovery time from attentional capture. Psychol Sci. 2011;22:361–368. doi: 10.1177/0956797611398493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukuda K, Vogel EK. Human variation in overriding attentional capture. J Neurosci. 2009;29:8726–8733. doi: 10.1523/JNEUROSCI.2145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawaki R, Luck SJ. Active suppression after involuntary capture of attention. Psychon Bull Rev. 2013;20:296–301. doi: 10.3758/s13423-012-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dukewich KR, Klein RM. Inhibition of return: A phenomenon in search of a definition and a theoretical framework. Attention, Perception, Psychophys. 2015;77:1647–1658. doi: 10.3758/s13414-015-0835-3. [DOI] [PubMed] [Google Scholar]
- 62.Livingstone AC, et al. Signal enhancement, not active suppression, follows the contingent capture of visual attention. J Exp Psychol Hum Percept Perform. 2017;43:219–224. doi: 10.1037/xhp0000339. [DOI] [PubMed] [Google Scholar]
- 63.Gaspar JM, et al. Inability to suppress salient distractors predicts low visual working memory capacity. Proc Natl Acad Sci U S A. 2016;113:3693–3698. doi: 10.1073/pnas.1523471113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sawaki R, et al. Hyperfocusing of Attention on Goal-Related Information in Schizophrenia: Evidence From Electrophysiology. J Abnorm Psychol. 2016;126:106–116. doi: 10.1037/abn0000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luck SJ. An Introduction to the Event-Related Potential Technique. 2nd. MIT Press; 2014. [Google Scholar]
- 66.Kim MS, Cave KR. Spatial Attention in Visual Search for Features and Feature Conjunctions. Psychol Sci. 1995;6:376–380. [Google Scholar]
- 67.Sperling G. The information available in brief visual presentations. Psychol Monogr Gen Appl. 1960;74:1–29. [Google Scholar]
- 68.Gaspelin N, et al. Suppression of overt attentional capture by salient-but-irrelevant color singletons. Attention, Perception, Psychophys. 2017;79:1–18. doi: 10.3758/s13414-016-1209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaspelin N, et al. Direct Evidence for Active Suppression of Salient-but-Irrelevant Sensory Inputs. Psychol Sci. 2015;22:1740–1750. doi: 10.1177/0956797615597913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vatterott DB, Vecera SP. Experience-dependent attentional tuning of distractor rejection. Psychon Bull Rev. 2012;19:871–878. doi: 10.3758/s13423-012-0280-4. [DOI] [PubMed] [Google Scholar]
- 71.Anderson BA, Folk CL. Dissociating location-specific inhibition and attention shifts: Evidence against the disengagement account of contingent capture. Attention, Perception, Psychophys. 2012;74:1183–1198. doi: 10.3758/s13414-012-0325-9. [DOI] [PubMed] [Google Scholar]
- 72.Schönhammer JG, et al. Which kind of attention is captured by cues with the relative target colour? Vis cogn. 2017;6285:1–12. [Google Scholar]
- 73.Cavanagh P, Mather G. Motion: The long and short of it. Spat Vis. 1989;4:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- 74.Chubb C, Sperling G. Two motion perception mechanisms revealed through distance- driven reversal of apparent motion. Proc Natl Acad Sci U S A. 1989;86:2985–2989. doi: 10.1073/pnas.86.8.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Julesz B. Experiments in the visual perception of texture. Sci Am. 1975;232:34–43. doi: 10.1038/scientificamerican0475-34. [DOI] [PubMed] [Google Scholar]
- 76.Chisholm JD, et al. Reduced attentional capture in action video game players. Attention, Perception, Psychophys. 2010;72:667–671. doi: 10.3758/APP.72.3.667. [DOI] [PubMed] [Google Scholar]
- 77.Cunningham CA, Egeth HE. Taming the White Bear: Initial Costs and Eventual Benefits of Distractor Inhibition. Psychol Sci. 2016 doi: 10.1177/0956797615626564. [DOI] [PubMed] [Google Scholar]
- 78.Zehetleitner M, et al. Top-down control of attention: It’s gradual, practice-dependent, and hierarchically organized. J Exp Psychol Hum Percept Perform. 2012;38:941–957. doi: 10.1037/a0027629. [DOI] [PubMed] [Google Scholar]
- 79.Vatterott DB, et al. Rejecting salient distractors: Generalization from experience. Attention, Perception, Psychophys. doi: 10.3758/s13414-017-1465-8. [DOI] [PubMed] [Google Scholar]
- 80.Wolfe JM, et al. Guided search: An alternative to the feature integration model for visual search. J Exp Psychol Hum Percept Perform. 1989;15:419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- 81.Wolfe JM. Guided search 4.0. Integr Model Cogn Syst. 2006 doi: 10.1007/978-94-011-5698-1_30. [DOI] [Google Scholar]
- 82.Bichot NP, et al. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- 83.Scolari M, Serences JT. Adaptive Allocation of Attentional Gain. J Neurosci. 2009;29:11933–11942. doi: 10.1523/JNEUROSCI.5642-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navalpakkam V, Itti L. Search Goal Tunes Visual Features Optimally. Neuron. 2007;53:605–617. doi: 10.1016/j.neuron.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 85.Becker SI, et al. The role of relational information in contingent capture. J Exp Psychol Hum Percept Perform. 2010;36:1460–1476. doi: 10.1037/a0020370. [DOI] [PubMed] [Google Scholar]
- 86.Treisman AM, Sato S. Conjunction Search Revisited. J Exp Psychol Hum Percept Perform. 1990;75:459–478. doi: 10.1037//0096-1523.16.3.459. [DOI] [PubMed] [Google Scholar]
- 87.Arita JT, et al. Templates for rejection: Configuring attention to ignore task-irrelevant features. J Exp Psychol Hum Percept Perform. 2012;38:580–584. doi: 10.1037/a0027885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Beck VM, Hollingworth A. Evidence for Negative Feature Guidance in Visual Search Is Explained by Spatial Recoding. J Exp Psychol Hum Percept Perform. 2015;41:1190–1196. doi: 10.1037/xhp0000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersen SK, Müller MM. Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature-selective attention. Proc Natl Acad Sci U S A. 2010;107:13878–82. doi: 10.1073/pnas.1002436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pashler HE. The psychology of attention. The MIT Press; 1998. [Google Scholar]
- 91.Luck SJ, Vecera SP. Attention. In: Pashler H, Yantis S, editors. Steven’s handbook of experimental psychology (3rd ed.), Vol. 1: Sensation and perception. John Wiley & Sons Inc; 2002. pp. 235–286. [Google Scholar]
- 92.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 93.Posner MI, et al. Attended and unattended procesing modes: The role of set for spatial location. In: Pick HL, Saltzman IJ, editors. Modes of Perceiving and Processing Information. Lawrence Erlbaum Associates; 1978. [Google Scholar]
- 94.Anderson Ba, et al. Value-driven attentional capture. Proc Natl Acad Sci U S A. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychol Rev. 1977;84:1–66. [Google Scholar]
- 96.Yantis S, Johnston JC. On the locus of visual selection: Evidence from focused attention tasks. J Exp Psychol Hum Percept Perform. 1990;16:135–149. doi: 10.1037//0096-1523.16.1.135. [DOI] [PubMed] [Google Scholar]
- 97.Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 98.van Zoest W, et al. The Role of Stimulus-Driven and Goal-Driven Control in Saccadic Visual Selection. J Exp Psychol Hum Percept Perform. 2004;30:746–759. doi: 10.1037/0096-1523.30.4.749. [DOI] [PubMed] [Google Scholar]
- 99.Moher J, Egeth HE. The ignoring paradox: Cueing distractor features leads first to selection, then to inhibition of to-be-ignored items. Attention, Perception, Psychophys. 2012;74:1590–1605. doi: 10.3758/s13414-012-0358-0. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Luck SJ. Feature-based attention modulates feedforward visual processing. Nat Neurosci. 2009;12:24–25. doi: 10.1038/nn.2223. [DOI] [PubMed] [Google Scholar]
- 101.Treue S, Martínez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 102.Saenz M, et al. Global effects of feature-based attention in human visual cortex. Nat Neurosci. 2002;5:631–632. doi: 10.1038/nn876. [DOI] [PubMed] [Google Scholar]


