Abstract
This study examined whether marijuana use was associated with clinically problematic outcomes for patients with depression and alcohol use disorder (AUD). The sample consisted of 307 psychiatry outpatients with mild to severe depression and past 30-day hazardous drinking/drug use, who participated in a trial of substance use treatment. Participants were assessed for AUD based on DSM-IV criteria. Measures of marijuana use, depression symptoms, and functional status related to mental health were collected at baseline, 3, and 6 months. Differences in these outcomes were analyzed among patients with and without AUD using growth models, adjusting for treatment effects. Marijuana was examined as both an outcome (patterns of use) and a predictor (impact on depression and functioning). Forty percent used marijuana and about half the sample met AUD criteria. Fewer patients with AUD used marijuana than those without AUD at baseline. Over 6 months, the proportion of patients with AUD using marijuana increased compared to those without AUD. Patients with AUD using marijuana had greater depressive symptoms and worse functioning than those without AUD. These findings indicate that marijuana use is clinically problematic for psychiatry outpatients with depression and AUD. Addressing marijuana in the context of psychiatry treatment may help improve outcomes.
Keywords: depression, alcohol use disorder, marijuana, marijuana use
1. Introduction
Prior research examining the comorbidity of psychiatric conditions and substance use disorders (SUDs) suggests that alcohol use disorders (AUDs) are significantly comorbid with depression (Grant et al., 2004; Schuckit, 2009; Conner et al., 2009; Pacek et al., 2013). In the United States, 7% (about 16 million) of adults aged 18 or older had major depressive episodes in 2014–2015, and the prevalence of AUDs (DSM-IV abuse or dependence) among adults with major depressive episodes was 14%, twice as prevalent with depression as any other SUD (Substance Abuse and Mental Health Services Administration [SAMHSA], 2015). Given the substantial comorbidity of depression and AUD, the characteristics and subsequent outcomes of persons with these disorders have become high priorities for prevention and treatment research.
The significant comorbidity of depression and AUD found in the general population is more striking in clinical populations. Specifically, studies conducted with either psychiatry or addiction treatment seeking samples have found 50%–70% of patients with depression had AUDs (Sullivan et al., 2005; Conner et al., 2009). In addition, patients with depression and AUDs who present for psychiatry treatment have higher rates of drug use, more severe depressive symptoms, and functional impairment than patients with depression but without AUDs (Conner et al., 2009; Sher et al., 2008). A general population-based study found that individuals with co-occurring alcohol and marijuana use disorders were more likely to have major depressive episodes relative to those with either alcohol or marijuana use disorder alone (Pacek et al., 2012). Longitudinal studies indicate that patients with both depression and AUDs continue to demonstrate greater depressive symptoms and functional impairment over time than patients with depression alone (Conner et al., 2009). Less is known, however, about the extent of drug use over time, and whether it has differential effects on clinical outcomes for those with depression and AUDs. Although a review by Conner et. al., (2009) concluded that drug use was associated with greater depression severity and functional impairment in treatment seeking patients with depression and AUD, this review is almost 10 years old and was limited to cross-sectional studies. To our knowledge, there has been no recent longitudinal examination of symptom and functional outcomes in terms of marijuana use among psychiatry outpatients with depression and AUD.
Marijuana is the most commonly used drug in the U.S. (SAMHSA, 2015), with 8.3% of adults reporting past month use. General population-based research among individuals with depression has found no association between marijuana use and depression pathology over time (Feingold et al., 2017). Yet, research in clinical samples has shown that marijuana use is associated with worse overall psychopathology and poorer functioning among psychiatry patients with depression, and that these adverse clinical outcomes persist over time (Bahorik et al., 2013). Psychiatry patients with comorbid depression and AUD (Conner et al., 2009; Sullivan et al., 2005) may have additional problems related to marijuana use, owning to its association with poor clinical outcomes among clinical samples (Bahorik et al., 2017; Trull et al., 2016; Bahorik et al., 2013). A study focused on marijuana use in psychiatry patients with depression and AUD may characterize an important subgroup at risk of poor clinical outcomes and contribute information to future prevention and intervention strategies.
We explored whether marijuana use was associated with clinically problematic outcomes for patients with depression and AUD by analyzing 6 month follow up data in a secondary analysis of 307 individuals who participated in a randomized trial for substance use treatment, delivered in a psychiatry outpatient setting. This larger question was addressed through carrying out three study aims. First, we examined whether differences in marijuana use existed at baseline between patients with and without AUD. Second, we examined whether differences in marijuana use existed over 6 months between patients with and without AUD. Finally, we investigated whether differences existed between patients with and without AUD in terms of marijuana use, depressive symptom and functional outcomes over the follow-up. Building on our prior work showing that marijuana use has adverse effects on depression (Bahorik et al., 2017), findings will provide important information about the differential impact of marijuana use on those with comorbid depression and AUD, and inform drug use prevention and intervention efforts.
2. Methods
2.1. Participants and procedures
Data for this secondary analysis were drawn from individuals who had participated in a randomized controlled trial of motivational interviewing (MI) for substance use treatment for patients with depression, delivered in an outpatient psychiatry setting. Patients were recruited from Kaiser Permanente Southern Almeda Medical Center Department of Psychiatry in Union City and Fremont, California. These psychiatry clinics provide evaluation, psychotherapy, and medication management for patients with a range of mental health conditions. These psychiatry clinics do not provide specialized services for individuals who present for treatment with serious substance use problems. At these psychiatry clinics, individuals are screened by telephone prior to intake, and those reporting serious alcohol or drug problems are referred to the Kaiser Chemical Dependency Recovery Program (CDRP), located in the Union City medical center in a separate building from the psychiatry clinic. The parent MI trial sought to provide substance use services to psychiatry patients who used drugs or alcohol but who are not referred to CDRP for treatment. The results and methodological details of the parent MI trial are reported elsewhere (Satre et al., 2016).
In brief, a total of 307 participants were recruited from the previously mentioned Kaiser psychiatry clinics. Participants were identified via provider referrals and self-referral in response to flyers in clinic waiting areas. Study clinicians followed up by phone with patients who were interested in the study and determined eligibility based on inclusion criteria, which required patients to be ≥ 18 years old, have Patient Health Questionnaire (PHQ-9: Kroneke et al., 2001) score ≥ 5 indicating at least mild depression, and endorse hazardous drinking (≥ 3/≥ 4 drinks/day for women/men) or illicit drug use (illicit/non-prescribed prescription drugs) within the past 30 days. The parent trial used a hazardous drinking standard slightly more conservative than that recommended for the general population because psychiatry patients are frequently prescribed antidepressants and other psychotropic medications that can have adverse interactions with alcohol and other drugs (Satre et al., 2016). Similarly, the depression score cutoff for enrollment was relatively low to capture a range of severity levels in the sample who might benefit from substance use reduction, and to include those in the maintenance phase of depression treatment as well as higher acuity patients starting care in psychiatry. Patients with mania or psychosis were excluded as such patients would likely require more intensive substance use services than the brief MI intervention model was designed to provide. The current analytic sample consisted of all 307 patients with depression (PHQ-9 ≥ 5) who enrolled in the parent trial: 149 with AUD (DSM-IV abuse/dependence), and 158 without AUD.
Participants who enrolled in the parent trial used laptop computers to complete the baseline measures (response rate: baseline 100%), including self-report assessments of past 30 day illicit drug and alcohol use, the PHQ-9, and the Mental Health Subscale (MCS-12) of the Short Form Health Survey (SF-12). Then, participants were re-assessed using the same self-report substance use, symptom, and functional assessments every 3 months via telephone interviews (response rate: 3 months 96%; 6 months 98%) by trained raters and study clinicians during the 6 month study. Patients were offered $50 gift cards for completing each interview.
After completing the baseline interviews, participants in the parent trial were randomized to one of two study arms, either MI or usual care. The MI intervention consisted of one 45-minute session followed by two 15-minute telephone “booster” sessions (Satre et al., 2013), about two weeks apart. MI sessions were delivered within 6 weeks of enrollment, based on MI counseling approach principles by Miller and Rollnick (Miller and Rollnick, 2012). Participants in the control group were given a 2-page brochure, produced by the National Institute of Health National Office of Drug Control Policy as part of their Fast Fact Series (United States Department of Justice, 2003), on use risks specific to the substances reported at baseline (Satre et al., 2013; Dunn et al., 2001). Patients also continued to receive usual depression care based on current best practices for medication management and evidence-based psychological treatment (Kaiser Permanente Care Management Institute, 2006). All patients provided written informed consent at an in-person appointment in the same psychiatry clinic where they received usual care. Procedures were approved by the University of California, San Francisco (UCSF) and Kaiser Permanente Institutional Review Boards.
2.2. Measures
2.2.1. Demographic characteristics
Patient demographic characteristics were identified via self-report questionnaire at baseline. Responses were coded for descriptive analyses for age (continuous), gender (=1 if female, male), race ethnicity (= 1 if white, = 2 if black, = 3 if Hispanic, = 4 if Asian, = 5 if other/unknown), income (= 1 if ≥50K, else), marital status (= 1 if married, else), employment status (= 1 if employed, else). These demographic patient characteristics also served as covariates in longitudinal analyses, except for income owing to its correlation with employment and the outcomes under study. Responses were recoded for longitudinal analyses for age (18–29 – reference; 30–39; 40–49; 50+) and race/ethnicity (= 1 if white, otherwise).
2.2.2. Treatment characteristics
Treatment characteristics consisted of the intervention assignment from the parent study, as well as psychiatry service use measures from each interview (baseline, 3, 6 months), which served as covariates in longitudinal analyses. We include a treatment variable consisting of two categories (= 1 if MI, usual care) as a longitudinal covariate because MI was found to be more effective at reducing marijuana use than the usual care control in the parent trial (Satre et al., 2016). Psychiatry service use data were derived from electronic health records, with the measure reflecting the number of psychiatry visits in the 30 days prior to each interview (range 0 to 20, M = 1.71; SD = 2.87), providing a continuous covariate.
2.2.3. Alcohol use disorders
Alcohol abuse and dependence at baseline were assessed via a self-report questions derived from the Structured Clinical Interview, Non-Patient Version (SCID-I-N/P; First et al., 1995) for DSM-IV (American Psychological Association [APA], DSM-IV, 4th ed., 1994). Alcohol abuse and dependence diagnoses were collapsed into a single AUD variable (=1 if AUD, no AUD), providing a dichotomous measure of AUD.
2.2.4. Alcohol and drug use
Past 30-day alcohol and drug use were assessed during study interviews (baseline, 3, and 6 months) via patient self-report. Specifically, patients were asked: (1) “How many days in the past 30 days have you used alcohol” and (2) “How many days in the past 30 have you used drugs (e.g., marijuana, cocaine, amphetamine, stimulants, sedatives other than as prescribed, opioids other than as prescribed, heroin and ecstasy)”. Patients were coded as using if they endorsed any use (all coded, any use = 1, no use), providing dichotomous measures. All alcohol and drug use measures were examined for descriptive purposes at baseline. Marijuana use was a predictor/outcome under study in longitudinal analyses, and alcohol use was a covariate.
2.2.5. Depressive symptomatology
The PHQ-9 (Kroneke et al., 2009) was used to index depressive symptoms and to derive a measure of baseline major depression. The PHQ-9 is a 9-item self-report questionnaire, based on the DSM-IV criteria for depression, used to assess depression symptoms in the prior 2 weeks. Patients rate how often they experience different indicators of depressive symptoms on a 4-point Likert type scale (0 = “not present” to 3 = “nearly every day”). Ratings were summed to generate a total PHQ-9 score, providing a continuous measure of depression severity, with higher scores indicating more severe depression (total score range: 0–27; cutoff scores: 5–9 = mild, 10–14 = moderate, 15–19 = moderately severe, and 20–17 = severe). As used in prior work (Manea et al., 2012), patients were coded as likely meeting the DSM-IV criteria for major depression if they had a total PHQ-9 score ≥ 10 (1 = ≥ 10 PHQ-9 major depression, else), providing a dichotomous measure for baseline analyses. Depressive symptoms, measured by total PHQ-9 score, served as a covariate/outcome in longitudinal analyses.
2.2.6. Functioning
Functional outcomes were measured with the PHQ-9 and the MCS-12 subscale of the SF-12, (Ware et al., 1998). PHQ-9 item 10, which is not part of the total depression severity score, assessed functional impairment related to depression in the prior 2 weeks. Patients were asked: “How difficult has it been to do work, take care of things at home, or get along with other people”, rated on a 4-point Likert-type scale (1 = “not difficult at all” to 4 = “very difficult”). Patients were coded as having impaired functioning if they endorsed any impairment (1 = ≥ 2 impaired functioning, else), providing a dichotomous measure. The MCS-12, a 12-item subscale of the SF-12, indexed mental health status. The MCS-12 asks patients to self-report functional impairment related to their mental health status in the past 4 weeks. Responses were summed to generate a total MCS-12 score (12-items, range 0 to 100, M = 50; SD = 10), with lower scores indicating worse mental health functioning (Ware et al., 1998), providing a continuous score.
2.3. Data analysis
Statistical analyses were carried out in R version 3.3.1 (R Development Core Team, 2016) and significance was defined at p < .05. The first analytic aim was focused on describing the baseline sample and included an examination of the extent of marijuana and other drug use by those with AUD. Longitudinal analyses identified overall patterns of marijuana use and differences in these rates by those with AUD. In the final analyses, we investigated the longitudinal effect of marijuana use on symptom and functional outcomes in the sample and whether marijuana use had a differential impact on these outcomes for those with AUD.
2.3.1. Participant characteristics analyses at baseline
Baseline analyses began with using means and frequencies to describe patient characteristics and identify those who met AUD criteria. We used χ2 tests (categorical) and independent t tests (continuous) to identify differences between patients with AUD and those without AUD. Frequencies were then used to examine marijuana and other drug use and χ2 tests were employed to identify differences in marijuana and drug use for those with AUD and those without AUD.
2.3.2. Longitudinal analyses investigating overall marijuana use patterns and differences in rates of use among those with AUD
To examine patterns of marijuana use, and differences in the proportion of patients with and without AUD using marijuana over time, a series of mixed-effects growth models were computed. This approach is a form of hierarchical linear/non-linear modeling where repeated measurement occasions are nested within individuals, which allows for the estimation of inter-individual (between-persons) variability in intra-individual (within-persons) patterns of change over time (Raudenbush and Bryk, 2009). Generalized growth models using penalized quasi likelihood estimation were fit to the data, to compute the parameter estimates of binary marijuana use outcomes. These analyses began with unconditional growth models predicting marijuana use from time (coded: 0 = baseline; 1 = 3 months; 2 = 6 months) to examine patterns of marijuana (= 1 if any marijuana use, = 0 if no marijuana use) use over time. Conditional growth models were constructed predicting marijuana use from AUD (reference = non-AUD), and a time × AUD interaction, to examine differences between patents with and without AUD using marijuana over the study. Conditional growth models adjusted for age, gender, race, employment status, marital status, treatment assignment, time-varying psychiatry visits, time-varying depressive symptoms, as well as the initial levels marijuana use. Rather than discard partial study completers (about 4.0% of the sample) and bias the final sample analyzed, the expectation maximization method was used to handle missing data during maximum likelihood estimation at the time of analysis.
2.3.3. Longitudinal analyses examining effects of marijuana on symptom and functional outcomes and differential associations of use with these outcomes among those with AUD
These analyses began with conditional growth models predicting symptom (PHQ-9 score) and functional outcomes (PHQ-9 item-10 and MCS-12) from time and time-varying marijuana use (= 1 if marijuana use, else), to investigate the effect of marijuana use on these clinical outcomes. We continued to build upon the previous conditional models by predicting symptom and functional measures from time and a time × AUD interaction, to investigate whether these clinical outcomes varied by patients with and without AUD over the study. Finally, moderated analyses were conducted, predicting clinical outcomes from time and a time × AUD × marijuana use interaction, to determine whether continued marijuana use had different effects on the clinical outcomes of those with and without AUD. Conditional growth models (including the marijuana moderated models) adjusted for age, gender, race, employment status, marital status, treatment assignment, time-varying psychiatry visits, time-varying alcohol use, as well as the initial levels of the outcome variable under study (e.g., PHQ-9 or MCS-12). As with the marijuana use outcome models, the expectation maximization method was used to handle missing data during maximum likelihood estimation at the time of analysis.
3. Results
3.1. Participant characteristics at baseline
As shown in Table 1, the sample was 70.3% women, 38.1% white, 21.1% Hispanic, 14.0% Asian, 21.8% black, and 4.2% other race/ethnicity. Participants were 37 years old on average (SD = 13.10), and 53.0% had a household income ≥ $50K. Fifty-six percent of the sample had PHQ-9 scores (score ≥ 10) that suggested the presence of DSM-IV major depressive disorder. Most patients were employed (64.6%), and less than half were married (42.0%). A considerable number of participants had AUD, with 149 (48.5%) of the 307 meeting the DSM-IV criteria. Few significant differences existed between participants who had AUD compared to those without AUD at baseline. Both groups were equally likely to have a psychiatry visit within 30 days of baseline, and there were no significant differences with respect to age, race/ethnicity, employment or marital status, income, major depression, depression severity, or functioning (Table 1).
Table 1.
Patient characteristics at baseline.
| Overall Sample N = 307 |
Patients without AUD n = 158 |
Patients with AUD n = 149 |
||
|---|---|---|---|---|
|
|
|
|||
| Variable | M (SD) | M (SD) | M (SD) | Pa |
| Race/ethnicity | ||||
| —n (% White) | 117 (38.1) | 61 (40.9) | 56 (35.4) | 0.382 |
| —n (% Black) | 67 (21.8) | 31 (20.8) | 34 (21.5) | 0.989 |
| —n (% Hispanic) | 65 (21.1) | 36 (24.1) | 31 (19.6) | 0.409 |
| —n (% Asian) | 43 (14.0) | 18 (12.0) | 25 (15.8) | 0.435 |
| —n (% other/unknown) | 15 (4.2) | 3 (2.0) | 12 (7.5) | 0.056 |
| Age | 37.00 (13.10) | 36.49 (13.71) | 37.95 (12.61) | 0.330 |
| Gender—n (% female) | 216 (70.3) | 106 (67.0) | 110 (73.8) | 0.243 |
| Marital Status—n (% married) | 129 (42.0) | 71 (44.9) | 58 (38.9) | 0.341 |
| Employment—n (% employed) | 114 (64.6) | 102 (64.5) | 102 (68.4) | 0.547 |
| Income—n (% > 50k) | 163 (53.0) | 88 (55.6) | 75 (50.3) | 0.989 |
| PHQ-9 Depressionb score | 10.26 (6.00) | 13.49 (5.55) | 14.36 (5.61) | 0.170 |
| PHQ-9 Major Depression — n (% score > 10) | 172 (56.0) | 83 (52.5) | 89 (59.7) | 0.248 |
| PHQ-9 Functioning—n (% impaired) | 159 (51.7) | 75 (47.4) | 84 (56.3) | 0.148 |
| MCS-12 Mental Healthc Functioning score | 26.17 (11.73) | 30.10 (9.98) | 28.98 (9.08) | 0.282 |
| Alcohol use—n (% alcohol use) | 283 (92.1) | 134 (84.8) | 149 (100.0) | <0.001 |
| Marijuana use— n (% marijuana use) | 124 (40.7) | 76 (48.1) | 49 (32.8) | <0.001 |
| Opioid use—n (% opioid use) | 20 (6.5) | 8 (5.0) | 12 (8.0) | 0.408 |
| Sedative use—n (% sedative use) | 16 (5.2) | 8 (5.0) | 8 (5.3) | 0.891 |
| Amphetamine use—n (% amphetamine use) | 7 (2.2) | 5 (3.1) | 2 (1.3) | 0.492 |
| Stimulant use—n (% stimulant use) | 7 (2.2) | 5 (3.1) | 2 (1.3) | 0.492 |
| Cocaine use—n (% cocaine use) | 5 (1.6) | 2 (1.2) | 3 (2.0) | 0.947 |
| Ecstasy use—n (% ecstasy use) | 9 (2.9) | 4 (2.5) | 5 (3.3) | 0.928 |
| Psychiatry visits (average visitsd) | 1.71 (2.87) | 1.68 (2.46) | 1.70 (3.21) | 0.933 |
| MI treatment condition—n (% MI) | 154 (50.1) | 89 (56.3) | 65 (43.6) | 0.034 |
Note. N = 307. AUD = alcohol use disorder; MI = motivational interviewing.
Bivariate analyses were computed using χ2 (categorical) or independent sample t-tests (continuous) for differences between patients with and without AUD.
PHQ-9 = Patient Health Questionnaire; Higher mean scores indicate worse depression severity.
Mental Health Subscale (MCS-12) of the Short Form Health Survey (SF-12); Lower mean scores indicate worse mental health functioning.
Average number of psychiatry department visits within 30-days of baseline.
Marijuana was the most commonly used drug, with 124 (40.7%) participants reporting use at baseline. Prescription opioids were used by a small proportion (n = 20, 6.5%) of participants, and the use of other drugs was minimal. Few differences existed between participants with and without AUD regarding drug use at baseline, however, patients without AUD were significantly more likely to use marijuana (48.1%) than those with AUD (32.8%), (all p’s < 0.001), (Table 1).
3.2. Longitudinal patterns of marijuana use and differences in rates of use among participants with AUD
An examination of the proportion of patients who used marijuana at each assessment period revealed that marijuana use was the highest at baseline (40.7%), and then decreased at 3 (32.5%) and 6 months (32.2%), (Figure 1). Results of the unconditional model revealed a similar pattern (B = −1.20 [95% CI = −1.924, −0.492], p < 0.001), indicating that the proportion of patients using marijuana significantly declined over the study (Table 2).
Figure 1.
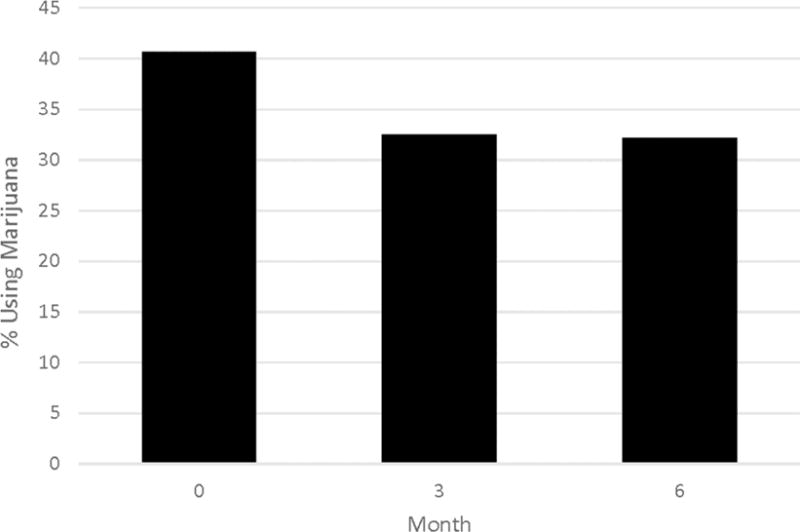
Marijuana use among patients with depression at baseline, 3, and 6 months.
Table 2.
Longitudinal growth models of marijuana use outcomes among patients with depression with and without AUD.
| Variable | Marijuana Use
|
|||
|---|---|---|---|---|
| B | 95%CI | SE | p | |
| Unconditional Growth Model | ||||
| Time | −1.20 | −1.924, −0.492 | 0.36 | <0.001 |
| Conditional Growth Modela | ||||
| Ageb | ||||
| 30–39 | −0.90 | −1.286, −0.217 | 0.26 | <0.001 |
| 40–49 | −0.92 | 1.389, −0.158 | 0.30 | <0.001 |
| 50+ | −1.30 | −1.773, −0.676 | 0.27 | <0.001 |
| Female | −0.38 | −0.782, 0.103 | 0.22 | 0.091 |
| Employed | −0.16 | −0.647, 0.212 | 0.21 | 0.444 |
| White | −0.33 | −0.823, 0.162 | 0.25 | 0.193 |
| Married | −0.35 | −0.784, 0.070 | 0.22 | 0.105 |
| MI Treatment | −0.10 | −0.469, 0.333 | 0.20 | 0.609 |
| AUD | −0.64 | −1.080, −0.276 | 0.24 | <0.001 |
| Time | −0.67 | −1.139, −0.413 | 0.18 | <0.001 |
| Time × Ageb | ||||
| Time × 30–39 | 0.11 | −0.205, 0.437 | 0.16 | 0.484 |
| Time × 30–39 | 0.12 | −0.245, 0.491 | 0.19 | 0.517 |
| Time × 50+ | 0.39 | 0.070, 0.728 | 0.16 | 0.018 |
| Time × Female | 0.14 | −0.123, 0.414 | 0.13 | 0.294 |
| Time × Employed | 0.27 | 0.017, 0.539 | 0.13 | 0.038 |
| Time × White | 0.21 | −0.087, 0.513 | 0.15 | 0.168 |
| Time × Married | 0.25 | −0.002, 0.510 | 0.13 | 0.055 |
| Time × MI Treatment | 0.03 | −0.209, 0.276 | 0.13 | 0.789 |
| Psychiatry Visitsc | −0.01 | −0.057, 0.018 | 0.01 | 0.329 |
| Depression Symptomsd | 0.04 | 0.015, 0.060 | 0.01 | <0.001 |
| Time × AUD | 0.25 | 0.012, 0.497 | 0.12 | 0.042 |
Note. N = 307. B = beta coefficient; SE = standard error; 95% CI = confidence intervals; p = p- values < .05 are presented in boldface; MI Treatment = Motivation interviewing treatment condition; AUD = alcohol use disorder.
Conditional growth models were fit using penalized quasi likelihood estimation.
reference = ages 18 – 29.
Psychiatry visits = Time-varying covariate estimating the average number of psychiatry visits prior to each interview.
Depression symptoms = Time-varying covariate estimating the average depression severity prior to each interview.
Differences between patients using marijuana with and without AUD over 6 months were then investigated. Results of the conditional model showed that the proportion of participants using marijuana with AUD increased significantly (B = 0.25 [95% CI = 0.012, 0.497], p = 0.042) compared to those without AUD using marijuana over 6 months (Table 2; Figure 2).
Figure 2.
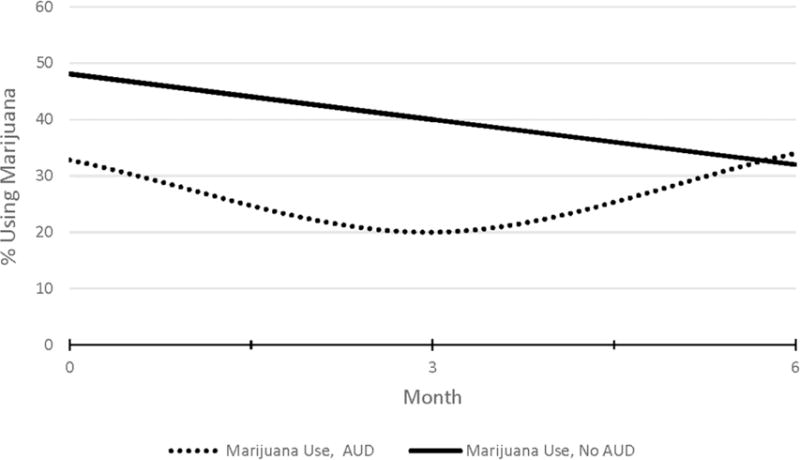
Six-month patterns of marijuana use among patients with depression with and without alcohol use disorder.
3.3. Longitudinal effect of marijuana use on symptom and functional outcomes and the differential association of marijuana use on these clinical outcomes among participants with AUD
As shown in Table 3, marijuana use was associated with worse functioning over time (PHQ-9, item-10: B = 0.57 [95% CI = −1.000, −0.001], p <.001, MCS-12: B = −2.34 [95% CI = −3.932, −0.751], p < 0.001). Marijuana use was also associated with worse depression symptoms over time (B =1.28 [95% CI = 0.507, 2.060], p <0.001). Although no significant differences were found between patients with and without AUD in terms of these outcomes over time in terms of the conditional models (p’s > 0.254), results of the moderated models revealed significant interactions between these groups and marijuana use on both PHQ-9 depression and functional outcomes. Specifically, participants with AUD using marijuana had higher depression symptoms compared with those without AUD using marijuana (B = 1.64 [95% CI = 0.089, 3.200], p = 0.038), and had worse PHQ-9, item-10 functional outcome scores (B = 1.00 [95% CI = 0.262, 1.748], p = 0.009), (Table 3).
Table 3.
Longitudinal growth models of clinical outcomes among patients with depression who use marijuana with and without AUD.
| Predictor | Depression Symptoms | Depression Functioning | Mental Health Functioning | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| B | 95% CI | p | B | 95% CI | p | B | 95% CI | p | |
| Unconditional Growth Model | |||||||||
| Time | −2.83 | −3.201, −2.525 | <0.001 | −0.80 | −0.956, −0.659 | <0.001 | 4.56 | 3.802, 5.334 | <0.001 |
|
| |||||||||
| Conditional Growth Modela | |||||||||
| Ageb | |||||||||
| 30–39 | −0.16 | −1.762, 1.422 | 0.833 | 0.92 | 0.352, 1.722 | <0.001 | 1.38 | −1.575, 4.354 | 0.357 |
| 40–49 | 0.70 | −1.11, 2.526 | 0.448 | 0.66 | −0.047, 1.496 | 0.063 | 0.07 | −3.320, 3.469 | 0.965 |
| 50+ | 0.74 | −0.909, 2.395 | 0.376 | 1.13 | 0.545, 1.959 | <0.001 | 1.59 | −1.490, 4.676 | 0.310 |
| Female | 2.63 | 1.331, 3.944 | <0.001 | 0.57 | 0.909, 1.208 | 0.020 | 4.19 | −6.631, −1.768 | <0.001 |
| Employed | −1.29 | −2.576, −0.020 | 0.046 | 0.40 | −0.960, 0.122 | 0.107 | 0.87 | −3.257, 1.500 | 0.468 |
| White | 0.45 | −1.011, 1.926 | 0.540 | 0.52 | −0.024, 1.232 | 0.071 | 1.74 | −0.900, 4.473 | 0.210 |
| Married | −0.42 | −1.697, 0.844 | 0.509 | 0.16 | −0.340, 0.737 | 0.509 | 034 | −2.020, 2.711 | 0.774 |
| MI Treatment | 0.19 | −0.995, 1.390 | 0.744 | 0.48 | 0.056, 1.066 | 0.093 | 0.80 | −1.408, 3.026 | 0.473 |
| AUD | 0.69 | −0.506, 1.904 | 0.254 | 0.32 | −0.060, 1.190 | 0.172 | 1.05 | −3.301, 1.193 | 0.356 |
| Time | −1.43 | −2.475, −0.404 | <0.001 | 0.02 | −0.536, 0.655 | 0.918 | 1.44 | −0.903, 3.788 | 0.227 |
| Time × Ageb | |||||||||
| Time × 30–39 | 0.31 | −0.588, 1.226 | 0.490 | 0.45 | −0.536, 0.655 | 0.029 | 0.54 | −2.606, 1.513 | 0.602 |
| Time × 30-39 | −0.31 | −1.358, 0.720 | 0.547 | 0.16 | −0.988, −0.001 | 0.472 | 0.53 | −1.822, 2.892 | 0.656 |
| Time × 50+ | −0.08 | −1.021, 0.848 | 0.855 | 0.17 | −0.755, 0.221 | 0.112 | 0.55 | −2.679, 1.564 | 0.605 |
| Time × Female | 1.15 | −1.910, −0.397 | <0.001 | 0.17 | −0.541, 0.265 | 0.318 | 1.99 | 0.275, 3.708 | 0.023 |
| Time × Employed | −0.46 | −1.209, 0.269 | 0.212 | 0.26 | −0.786, −0.025 | 0.112 | 2.18 | 0.511, 3.866 | 0.010 |
| Time × White | 0.38 | −1.238, 0.459 | 0.368 | 0.31 | −0.829, 0.086 | 0.103 | 0.69 | −2.618, 1.238 | 0.482 |
| Time × Married | 0.15 | −0.569, 0.881 | 0.637 | 0.41 | −0.600, 0.160 | 0.385 | 0.92 | −0.723, 2.567 | 0.271 |
| Time × MI Treatment | −0.35 | −1.042, 0.327 | 0.306 | 0.34 | −0.767, −0.044 | 0.027 | 0.25 | −1.303, 1.808 | 0.749 |
| Psychiatry Visitsc | −0.07 | −0.184, 0.093 | 0.203 | −0.01 | −0.073, 0.227 | 0.437 | 0.04 | −0.192, 0.272 | 0.735 |
| Marijuana | 1.28 | 0.507, 2.060 | <0.001 | 0.57 | 0.210, 1.479 | <0.001 | −2.34 | 3.932, −0.7511 | <0.001 |
| Alcohol | 0.36 | −1.305, 0.583 | 0.453 | 0.42 | −1.000, −0.001 | 0.035 | 0.11 | −1.868, 2.909 | 0.911 |
| Time × AUD | 0.69 | −0.506, 1.904 | 0.254 | 0.32 | −0.918, −0.009 | 0.176 | −0.09 | −1.656, 1.461 | 0.902 |
| Marijuana Moderated Growth Modeld | |||||||||
| Time × AUD × Marijuana | 1.64 | 0.089, 3.200 | 0.038 | 1.00 | 0.262, 1.748 | 0.009 | −0.07 | −3.451, 3.305 | 0.966 |
Note. N = 307. B = beta coefficient; SE = standard error; 95% CI = confidence intervals; p = p- values < .05 are presented in boldface; MI Treatment = Motivation interviewing treatment condition; AUD = alcohol use disorder.
Conditional growth models with depression symptoms and mental health functioning were fit using restricted maximum likelihood estimation. Conditional growth models with depression functioning were fit using penalized quasi likelihood estimation.
reference = ages 18 – 29.
Psychiatry visits = Time-varying covariate estimating the average number of psychiatry visits prior to each interview.
Only a priori moderator effects of interest are presented.
4. Discussion
Drug use is often comorbid with both depression and AUD, and may negatively affect the outcomes of patients receiving psychiatric services. We conducted secondary analyses of 307 patients with depression and AUD from a trial of substance use treatment for depression, and examined marijuana use, depressive symptoms, and functional outcomes over 6 months.
Consistent with prior research on alcohol and drug use among psychiatry patients with depression (Sullivan et al., 2005; Conner et al., 2009; Bahorik et al., 2013; Trull et al., 2016), approximately half the sample met DSM-IV criteria for AUD at baseline, with a relatively high proportion of patients reporting marijuana use (~40%). Overall, this proportion was slightly higher than documented (~37%) in prior studies among psychiatry treatment samples (Trull et al., 2016) and may reflect normalizing views about marijuana within California (Hasin et al., 2017).
Over 6 months, the proportion of patients in our overall sample who used marijuana significantly declined. Yet the proportion of patients with AUD who used marijuana significantly increased over the follow-up compared to patients without AUD. These findings extend prior work showing a decreasing trend in psychiatry patients using marijuana post-treatment (Bahorik et al., 2017; Bahorik et al., 2013), as well as work showing variability in marijuana use by psychiatric diagnosis over time (Bahorik et al., 2013). As expected from prior work with this sample (Bahorik et al., 2017), patients who used marijuana had worse symptoms and poorer functioning. Patients with AUD who used marijuana had worse symptoms and functional impairment than those without AUD who used marijuana. Our findings reinforce prior work with clinical samples showing poor clinical outcomes in patients with AUD and depression (vs. no AUD) (Conner et al., 2009), as well as work showing adverse effects of marijuana use among patients with depression (Bahorik et al., 2017).
The present study has implications for future drug use prevention and treatment efforts initiated in psychiatry settings. Patterns of marijuana use are rapidly changing as legislation to legalize use for recreational and medical purposes spreads (Volkow et al., 2014; Volkow et al., 2016; Hasin et al., 2017). This has considerable implications for health systems, as evidenced by the recent increases in emergency department services observed for patients using marijuana with psychiatric conditions (Campbell et al., 2017). The observation of high emergency department utilization among this population may be explained by associations of marijuana use with adverse health effects, such as addiction to other drugs, high prevalence of AUD, poor functioning, and worse depression severity (Volkow et al., 2014; Volkow et al., 2016; Bahorik et al., 2017; Conner et al., 2009). Although medical marijuana proponents often suggest that the drug may be used to effectively treat depression (Colorado PotGuide.com, 2015; Gregorie, 2015), its safety and efficacy in depression treatment has not been thoroughly examined or established (Volkow et al., 2014; Belenduik et al., 2015). In addition, research with medical dispensary clients has found depressive symptoms to be associated with marijuana use problems, with only about 10% of clients reporting a reduction of symptoms as a primary benefit of use (Bonn-Miller et al., 2014). Future studies among psychiatry samples could examine the degree to which marijuana may potentially alleviate symptom distress relative to its intrinsic risk to this population. However, results suggest that marijuana is more likely to have adverse effects on the health of psychiatry patients who have AUD and depression, based on the unfavorable outcomes observed. The parent MI trial found the substance use intervention to be effective in reducing marijuana use (Satre et al., 2016), and this strategy may be especially helpful to patients with depression who also have AUD.
Recent reports indicate that marijuana can interfere with the assessment and treatment of patients with AUD and depression (Moss et al., 2015; Brecht et al., 2008). For example, clinicians often identify and initiate treatment for the substance for which help is sought (Brecht et al., 2008), and this may result in under-detected comorbid drug or alcohol use problems, and unmet treatment needs. In addition, research with dispensary clients has suggested that the DSM-5 criteria for cannabis withdrawal overlap with depressive symptoms (e.g., sleep difficulty, decreased appetite, depressed mood, etc.) (APA, DSM-5, 5th ed., 2013). Thus, clients reporting marijuana use to medicate depression may not suffer from depression, but from cannabis withdrawal (Bonn-Miller et al., 2014). The potential for cannabis withdrawal to mirror depressive symptoms may further contribute to under-detected drug use problems and unmet treatment needs.
Regardless of cause, patients in depression treatment samples often have AUDs or use marijuana (Satre et al., 2016; Bahorik et al., 2017; Conner et al., 2009; Sullivan et al., 2005), and there is a need to initiate efforts in psychiatry treatment contexts that focus on marijuana use. This will be important as psychiatry providers often do not advise patients to reduce drug use in the context of depression treatment (Satre et al., 2014), and patients who use drugs and have depression often receive services in psychiatry contexts rather than specialty addiction treatment (Edlund et al., 2012). Future work should address marijuana use, in addition to alcohol and depression symptoms, among patients with depression and AUD in psychiatry treatment settings.
Limitations should be noted. Patients were recruited from an outpatient psychiatry setting, which may limit generalizability. Our enrollment criteria required participants to have mild depression based on having a PHQ-9 score ≥ 5. Yet, a PHQ-9 score of 10 only indicates the presence of major depression based on the DSM-IV criteria, after which thorough diagnostic assessments are required before patients can be assigned a formal diagnosis of major depressive disorder based on the DSM-IV or DSM-5 criteria. As only the PHQ-9 was available to measure depression in this study, and a relatively low cutoff score was used for enrollment, many of our participants would not have met criteria for major depressive disorder. Our findings should be considered within the context of these caveats. We know from the parent study that 12.0% had cannabis dependence (Satre et al., 2016), and it is possible that some participants were reporting symptoms consistent with cannabis withdrawal syndrome rather than depression. Our measure for AUD is limited because of its focus on the DSM-IV criteria and its reliance on self-report information. Due to changes in the DSM-5 criteria for AUD, our estimates based on the DSM-IV criteria may underestimate AUD compared to studies using the DSM-5. Our finding of worse functioning for AUD patients using marijuana was limited to PHQ-9 functional impairment, which was assessed by one item and limited to depression-related functioning. Our use of the MCS-12 to measure mental health functioning is limited because of its global focus and its incorporation of depression symptomatology into the measurement (Ware et al., 1998). Future work would benefit from examining indicators of functional impairment potentially less confounded with symptoms. Statistical tests were computed without adjustment for multiple inference testing. Marijuana use was dichotomized, which reduces statistical power and our understanding of patterns over time. We could not examine drug use other than marijuana over time due to low base rates. Because data on patterns of use and the primary compounds of marijuana were not available (e.g., delta-9-tetrahydrocannabionl and cannabidiol), we are precluded from commenting on the contribution of these factors to the outcomes studied. All measures were based on self-report, and future work may benefit from confirmatory structured assessments as well as laboratory tests to provide a more accurate assessment of psychiatric symptoms and drug use, respectively.
While more research is required to replicate these results, findings indicate that whether patients with depression and AUD experience clinically problematic outcomes may be influenced by marijuana use. It would be valuable for future treatment and prevention efforts to assess and address marijuana in the context of outpatient psychiatry treatment, and such efforts should focus on patients with depression and AUD, in order to improve patient outcomes.
Highlights.
Patients with depression frequently used marijuana and nearly half met the AUD criteria.
Fewer patients with AUD were using marijuana at baseline compared to patients without AUD.
Patients with AUD using marijuana increased over 6 months compared to those without AUD.
Patients with AUD using marijuana had worse clinical outcomes than patients without AUD.
Addressing marijuana use in outpatient psychiatry treatment may help improve outcomes.
Acknowledgments
We thank Georgina Berrios for assistance in conducting the study.
Role of funding source
This study was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA: R01 AA020463) and the National Institute on Drug Abuse (NIDA: T32DA007250). The funding sources provided no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Author; Washington, DC: 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Author; Washington, DC: 2013. [Google Scholar]
- Bahorik AL, Leibowitz AS, Sterling SA, Travis A, Weisner CM, Satre DD. Patterns of marijuana use among psychiatry patients with depression and its impact on recovery. J Affect Disord. 2017;213:168–171. doi: 10.1016/j.jad.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahorik AL, Newhill CE, Eack SM. Characterizing the longitudinal patterns of substance use among individuals diagnosed with serious mental illness after psychiatric hospitalization. Addiction. 2013;108:1259–1269. doi: 10.1111/add.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belendiuk KA, Baldini L, Bonn-Miller MO. Narrative review of the safety and efficacy of marijuana for the treatment of commonly state-approved medical and psychiatric disorders. Addict Sci Clin Pract. 2015;10:10. doi: 10.1186/s13722-015-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht ML, Huang D, Evans E, Hser YI. Polydruguse and implications for longitudinal research: The-year trajectories for heroin, cocaine, and methamphetamine users. Drug Alc Depend. 2008;96:193–201. doi: 10.1016/j.drugalcdep.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Boden MT, Bucossi MM, Babson KA. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am J Drug Alc Abuse. 2014;40:23–30. doi: 10.3109/00952990.2013.821477. [DOI] [PubMed] [Google Scholar]
- Campbell CI, Bahorik AL, Kline-Simon AH, Satre DD. The role of marijuana use disorder in predicting emergency department and inpatient encounters: A retrospective cohort study. Drug Alc Depend. 2017;178:170–175. doi: 10.1016/j.drugalcdep.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado PotGuide.com. Cannabis as a treatment for depression. 2015 [Colorado PotGuide.com Web site] March 15, 2015. Available at: https://www.coloradopotguide.com/colorado-marijuana-blog/2015/march/16/cannabis-as-a-treatment-for-depression/. Accessed September 6, 2016.
- Conner KR, Pinquart M, Gamble SA. Meta-analysis of depression and substance use among individuals with alcohol use disorders. J Subst Abuse Treat. 2009;37:127–137. doi: 10.1016/j.jsat.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96:1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Booth BM, Han X. Who seeks care where? Mental health and substance use disorder treatment in two national samples of individuals with alcohol use disorders. J Stud Alcohol Drugs. 2012;73:635–46. doi: 10.15288/jsad.2012.73.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: A population based longitudinal study. Psychiatry Res. 2017;251:225–234. doi: 10.1016/j.psychres.2017.02.027. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis Disorders, Non-Patient Edition. New York State Psychiatric Institute; NY: 1995. [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psych. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Gregorie C. New Study Finds Marijuana to be Effective Against Depression. 2015 [Huffington Post Web site] February 6, 2015 Available at: http://www.huffingtonpost.com/2015/02/06/marijauna-depression_n_6622126.html. Accessed September 6, 2016.
- Hasin DS, Sarvet AL, Cerda M, Keyes KM, Stohl M, Galea S, Wall M. US Adult Illicit Cannabis Use, Cannabis Use Disorder, and Medical Marijuana Laws. JAMA Psychiatry. 2017;74(6):579–588. doi: 10.1001/jamapsychiatry.2017.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Permanente Care Management Institute. Diagnosis and treatment of depression in adults: 2012 clinical practice guideline. Oakland, CA: Kaiser Permanente Care Management Institute; 2006. Available at: http://www.guideline.gov/content.aspx?id=39432 Accessed January 30, 2017. [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Int Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):191–196. doi: 10.1503/cmaj.110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: helping people change. 3rd. Guilford Press; NY: 2012. [Google Scholar]
- Moss HB, Goldstein RB, Chen CM, Yi HY. Patterns of use of other drugs among those with alcohol dependence: associations with drinking behavior and psychopathology. Addict Behav. 2015;50:192–198. doi: 10.1016/j.addbeh.2015.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Malcolm RJ, Martins SS. Race/ethnicity differences between alcohol, marijuana and co-occurring alcohol and marijuana use disorders and their association with public health and social problems using a national sample. Am J Addict. 2012;21:435–444. doi: 10.1111/j.1521-0391.2012.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Martins SS, Crum RM. The bidirectional relationships between alcohol, cannabis co-occurring alcohol and cannabis use disorders with major depressive disorder: results form a national sample. J Affect Disord. 2013;148:188–195. doi: 10.1016/j.jad.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R Foundation for Statistical Computing (Version 3.3.1) Vienna, Austria: R Foundation for Statistical Computing; 2016. R: A language and environment for statistical computing. [Computer Software] [Google Scholar]
- Raudenbush DSW, Bryk DAS. Hierarchical Linear Models Second Edition: Applications and data analysis methods. Thousand Oaks, C.A.: 2009. [Google Scholar]
- Satre DD, Leibowitz A, Mertens J, Weisner C. Advising depression patients to reduce alcohol and drug use: Factors associated with provider intervention in outpatient psychiatry. Am J Addict. 2014;23:570–575. doi: 10.1111/j.1521-0391.2014.12140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Delucchi K, Lichtmacher J, Sterling SA, Weisner C. Motivational Interviewing to reduce hazardous drinking and drug use among depression patients. J Subst Abuse Treat. 2013;44:323–329. doi: 10.1016/j.jsat.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Leibowitz A, Sterling SA, Lu Y, Travis A, Weisner C. A Randomized Clinical Trial of Motivational Interviewing to Reduce Alcohol and Drug Use among Patients with Depression. J Consult Clin Psychol. 2016;84:571–579. doi: 10.1037/ccp0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M. Alcohol-use disorders. Lancet. 2009;373:493–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Sher LS, Stanley BH, Harkavy-Friedman JM, Carballo JJ, Arendt M, Brent DA, Sperling D, Lizardi D, Mann J, Quendo MA. Depressed patients with coocurring alcohol use disorders: a unique patient profile. J Clin Psychiatry. 2008;69:907–915. doi: 10.4088/jcp.v69n0604. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. (HHS Publication No. SMA 15-4927, NSDUH Series H-50).Results from the 2014 National Survey on Drug Use and Health: Summary of National Findings. 2015 Retrieved from http://www.samhsa.gov/data/
- Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am J Med. 2005;118:330–41. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Wycoff AM, Lane SP, Carpenter RW, Brown WC. Cannabis and alcohol use, affect and impulsivity I npsychiatric outpatients’ daily lives. Addiction. 2016;111:2052–2059. doi: 10.1111/add.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Justice. Questions and answers. Washington, DC: National Drug Intelligence Center; 2003. Marijuana fast facts. Available at: http://www.justice.gov/archive/ndic/pubs3/3593/3593p.pdf. Accessed January 25 2017. [Google Scholar]
- Volkow ND, Swanson JM, Evins E, DeLisi LE, Meler MH, Gonzalez R, Bloomfield MAP, Curran VH, Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse Health Effects of Marijuana Use. New Eng J Med. 2014;370:2219–2227. doi: 10.1056/NEJMra1402309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J, Kosknski M, Keller S. SF-12: How to score the SF-12 physical and mental health summary scales. 2nd. The Health Institute, New England Medical Center; Boston MA: 1998. [Google Scholar]


