Abstract
Background & Aims
Chronic, excessive alcohol consumption leads to alcoholic liver disease (ALD) characterized by steatosis, inflammation, and eventually cirrhosis. The hepatocyte specific microRNA 122 (MIR122) regulates hepatocyte differentiation and metabolism. We investigated whether an alcohol-induced decrease in level of MIR122 contributes to development of ALD.
Methods
We obtained liver samples from 12 patients with ALD and cirrhosis and 9 healthy individuals (controls) and analyzed them by histology and immunohistochemistry. C57Bl/6 mice were placed on a Lieber-DeCarli liquid diet, in which they were fed ethanol for 8 weeks, as a model of ALD, or a control diet. These mice were also given injections of CCl4, to increase liver fibrosis, for 8 weeks. On day 28, mice with ethanol-induced liver disease and advanced fibrosis, and controls, were given injections of recombinant adeno-associated virus 8 vector that expressed the primary miR-122 transcript (pri-MIR122, to overexpress MIR122 in hepatocytes) or vector (control). Two weeks before ethanol feeding, some mice were given injections of a vector that expressed an anti-MIR122, to knock down its expression. Serum and liver tissues were collected; hepatocytes and liver mononuclear cells were analyzed by histology, immunoblots, and confocal microscopy. We performed in silico analyses to identify targets of MIR122 and chromatin immunoprecipitation quantitative PCR analyses in Huh-7 cells.
Results
Levels of MIR122 were decreased in liver samples from patients with ALD and mice on the Lieber-DeCarli diet, compared with controls. Transgenic expression of MIR122 in hepatocytes of mice with ethanol-induced liver disease and advanced fibrosis significantly reduced serum levels of alanine aminotransferase (ALT) and liver steatosis and fibrosis, compared to mice given injections of the control vector. Ethanol feeding reduced expression of pri-MIR122 by increasing expression of the spliced form of the transcription factor grainyhead like transcription factor 2 (GRHL2) in livers tissues from mice. Levels of GRHL2 were also increased in liver tissues from patients with ALD, compared with controls; increases correlated with decreases in levels of MIR122 in human liver. Mice given injections of the anti-MIR122 before ethanol feeding had increased steatosis, inflammation, and serum levels of ALT compared to mice given a control vector. Levels of hypoxia inducible factor 1 alpha (HIF1A) mRNA, a target of MIR122, were increased in liver tissues from patients and mice with ALD, compared with controls. Mice with hepatocyte-specific disruption of Hif1a developed less-severe liver injury following administration of ethanol, injection of anti-MIR122, or both.
Conclusions
Levels of MIR122 decrease in livers from patients with ALD and mice with ethanol-induced liver disease, compared with controls. Transcription of MIR122 is inhibited by GRHL2, which is increased in livers of mice and patients with ALD. Expression of an anti-MIR122 worsened the severity of liver damage following ethanol feeding in mice. MIR122 appears to protect the liver from ethanol-induced damage by reducing levels of HIF1A. These processes might be manipulated to reduce the severity of ALD in patients.
Keywords: miR-122, AAV, mouse model, ethanol, gene expression
Introduction
Chronic alcohol consumption accounts for nearly 50% of liver-related deaths in the United States, however, no effective therapies exist for patients. While early steatosis in alcoholic liver disease (ALD) is reversible, chronic, excessive alcohol consumption leads to steatohepatitis and fibrosis. Acute alcoholic hepatitis has a 30–50% 30-day mortality and the standard of care with steroids has limited benefits and significant side effects. Alcoholic cirrhosis (Liennec cirrhosis) is the single greatest cause of hepatocellular cancer (HCC)1,2. Thus, identification of novel therapeutic targets is a major clinical need in ALD2.
ALD is characterized by liver steatosis, inflammation, and progressive fibrosis2–4. Alcohol-triggered hepatocyte steatosis and cell death in combination with bacterial lipopolysaccharide (LPS) due to alcohol-induced “leaky gut” results in the activation and infiltration of immune cells. The subsequent release of inflammatory cytokines causes further hepatocyte cell death and results in perpetuation of liver injury5.
Hepatic microRNAs (miRNAs) have crucial roles in maintaining liver homeostasis, mitochondrial function, and regulating oncogenesis6,7. MIR122 constitutes 70% of all miRNAs in mature hepatocytes, or approximately 130,000 copies per cell, with negligible expression in other cells and tissues8. Mice with germline and liver-specific deletion of MIR122 display steatosis at birth, spontaneous progression to fibrosis, and HCC9. In humans, liver MIR122 expression inversely correlates with HCC survival and metastasis, while MIR122 inhibition reduces hepatitis C virus (HCV) viremia, serum triglycerides, and cholesterol10–12. These observations suggest that MIR122 has diverse and pleiotropic effects on hepatocytes and liver diseases and prompted us to explore the role of MIR122 in ALD.
Encoded on chromosome 18, MIR122 is transcribed as a ~4.7 kb noncoding pri-miRNA transcript by RNA polymerase II which is then rapidly processed into a 66-nucleotide (nt) pre-MIR122 by Drosha. Subsequently, the pre-miRNA is shuttled into the cytoplasm where it is processed into its mature 23-nt form. While factors that maintain the high level of expression of MIR122 in the healthy liver have been well studied, little is known about the regulation of MIR122 expression in disease states.
In this study, we explored the hypothesis that alcohol modulates MIR122 expression in the liver and contributes to pathogenic features of ALD. We demonstrate a significant reduction of MIR122 expression in hepatocytes and show restoration of MIR122 levels had therapeutic benefits in ALD. Further, we discovered that the decrease in MIR122 was due to inhibition of MIR122 transcription by chronic alcohol-induced increases in the grainyhead-like 2 transcriptional regulator. We found that alcohol-induced MIR122 reduction is hepatocyte-specific and it mediates steatosis and inflammation through its primary target, hypoxia-inducible factor-1 alpha (HIF1α).
Materials and Methods
Human Liver Samples
Human liver samples were provided by the NIH-funded Liver Tissue Procurement and Cell Distribution System (N01-DK-7-0004/HHSN26700700004C) Cell Distribution System (N01-DK-7-0004/HHSN26700700004C). Demographics can be found in Supplementary Table 1. Healthy liver samples were provided as age-matched controls.
Animal use
All animals received care in compliance with protocols approved by the Institutional Animal Use and Care Committee of the University of Massachusetts Medical School. As previously described13 mice were acclimated to a Lieber-DeCarli liquid diet of 5% ethanol (vol/vol) over a period of 1 week, then maintained on the 5% diet for 4 weeks. Wild-type (WT) mice (C57/BL6), Alb-Cre, and HIF1flox/flox mice were purchased from Jackson Laboratories (Bar Harbor, ME)14. Mice were treated by tail vein injection with AAV vectors at 6×1011 genome copies/mouse or approximately 3×1013 genome copies/kg15.
Murine model of advanced alcoholic fibrosis
Model of advanced fibrosis was adapted from previously published work16. Mice were gradually started on a Lieber-DeCarli liquid diet with 2% ethanol (vol/vol) for a period of 2 week, which was increased to 4% and then 5% each two weeks. During this time 0.5ul/kg carbon tetrachloride (CCl4) or corn oil was given every 3rd day for 8 weeks. On day 28, CCl4 injections were held for 1 week and 6×1011 viral particles of AAV8 containing either pri-MIR122 or Scrambled vector were administered intravenously. CCl4 injections resumed on day 35 and continued every 3 days until day 56. Mice were sacrificed 48 hours following the last dose of CCl4.
Statistical Analysis
Statistical significance was determined using two-tailed t-test; two-way ANOVA with Dunnett’s multiple comparison post-test were used to compare the means of multiple groups. Outliers were determined using the ROUT method and a q of 1%. Data are shown as mean ± SEM and were considered statistically significant at *P < 0.05, **P<0.005, and ***P<0.0005. GraphPad Prism 7.02 (GraphPad Software Inc.) was used for analysis.
Additional Methods found in Supplementary Materials
Results
Chronic alcohol decreases hepatic MIR122 expression in humans and mice
Because the role of MIR122 in ALD is unknown, first, we hypothesized that alcohol may regulate MIR122 levels in hepatocytes. Investigation of human diseased livers revealed that MIR122 expression was significantly reduced (by 2-fold) in patients with alcoholic cirrhosis when compared to healthy controls (Fig. 1A, Supplementary Table 1), raising the question of whether chronic alcohol could promote liver injury by inhibiting MIR122. To further dissect the role of MIR122, we used the Lieber-DeCarli chronic alcohol diet in mice that results in early features of ALD including liver injury, steatosis and inflammation17. We found that MIR122 expression was significantly reduced in the livers of alcohol-fed mice compared to pair-fed controls (Fig. 1B) and the extent of MIR122 reduction was equivalent to that seen in the livers of patients with alcoholic cirrhosis.
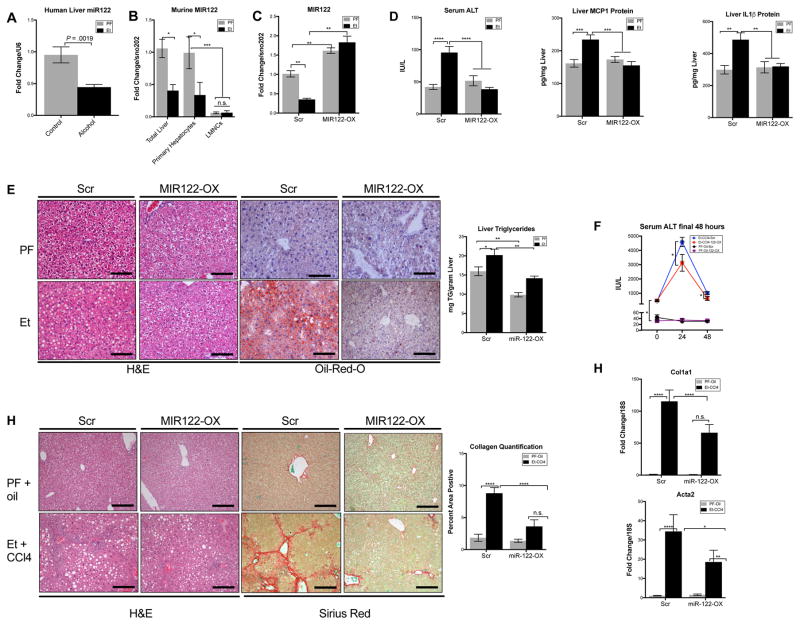
Fig. 1. Treatment with rAAV8-MIR122-OX protects from ALD by restoring the alcohol-induced reduction of MIR122 expression.
Expression of MIR122 in (A)human livers (n=9–12/group), (B)murine livers (n=8–14/group), murine hepatocytes and liver mononuclear cells (LMNCs) of pair-fed and alcohol-fed mice (n=5/group). Hepatic (C)MIR122 expression, from livers of either Et-fed WT mice treated with rAAV8-Scr or rAAV8-MIR122-OX vectors (n=8–12/group). Liver injury, inflammation, and steatosis was assessed by (D) serum ALT, liver MCP-1 and IL-1β protein levels, and (E) H&E, ORO staining, hepatic triglycerides concentrations, respectively. (F)ALT at 0-, 24-, and 48-hours following final dose of CCl4(n=6–10). Hepatic fibrosis was evaluated by measuring (G)Sirius-red staining and the percent-positive area stained was quantified using ImageJ and (H)Col1a1 and Acta2 expression from total liver RNA (n=6–10). Scale bars; 100 μm. Student’s t-test or two-way ANOVA.
To determine the cell specificity of the MIR122 reduction, we isolated primary hepatocytes and liver mononuclear cells (LMNCs) from mice. MIR122 was selectively decreased in hepatocytes and not in LMNCs from alcohol-fed mice (Fig. 1B) compared to pair-fed controls. This supported our hypothesis that alcohol-induced changes in MIR122 in the total liver are hepatocyte-specific.
Restoration of MIR122 in hepatocytes rescues mice from alcohol-induced liver injury in vivo
Given the essential role of MIR122 in hepatocyte homeostasis, we hypothesized that if the reduction of MIR122 by alcohol is a causal factor in ALD, then restoration of mature MIR122 in the livers of alcohol-fed mice will reverse disease severity. To assess the potential of MIR122 restoration to reverse alcohol-injury, we developed a recombinant adeno-associated virus 8 (rAAV8) vector expressing pri-MIR122 (MIR122-OX) and a scrambled control vector (scr). rAAV8 vectors have been shown to have tropism for hepatocytes and can safely maintain sustained transgene expression for years in the liver18,19. In a preliminary experiment, we established that wild-type, alcohol-fed mice developed significant liver injury by week 2 of the 5-week alcohol feeding (Supplementary Fig. 1A). We also determined that our rAAV8 MIR122-OX construct requires 3 weeks for full expression in the liver (Supplementary Fig. 1B). Therefore, we treated pair-fed and alcohol-fed mice with 6×1011 viral particles containing Scr or MIR122-OX construct via tail-vein injection on week 2 of a 5-week alcohol feeding (Supplementary Fig. 1C).
Treatment with the rAAV8-MIR122-OX effectively increased mature MIR122 levels in the livers of pair-fed and alcohol-treated mice (Fig. 1C). Overexpression of MIR122 in hepatocytes attenuated alcohol-induced increases in serum ALT (Fig. 1D), and steatosis indicated by histology and liver triglyceride levels (Fig. 1E). Furthermore, MIR122-OX treatment reduced key inflammatory cytokines, MCP-1 and IL-1β, (Fig. 1D) in alcohol-fed mice.
Therapeutic restoration of MIR122 reduces liver injury and fibrosis in an alcohol+CCl4 model of advanced fibrosis
The Lieber DeCarli alcohol mouse model results in a modest increase in early markers of fibrosis and represents early alcoholic liver disease. Therefore, to examine the therapeutic potential of MIR122 models more akin to clinical liver disease we sought a more aggressive model of ALD, by combining alcohol and carbon tetrachloride (CCl4) to induce fibrosis20,21,22. Briefly, mice were fed with increasing alcohol concentrations of LDC diet over 8 weeks and either CCl4 or Corn Oil i.p. every 72 hours. On day 28, mice were treated with either pri-MIR122 or scrambled vector (Supplementary Fig. 1E). AAV8 vectors have previously been shown to be equally effective in transducing both healthy and cirrhotic livers23. Indeed, we noted equivalent activity of co-expressed Gaussia Luciferase (GLuc) in the serum CCl4 treated mice 2 weeks following injection in both Oil and CCl4-treated mice (Supplementary Fig. 1F).
Following 8 weeks of alcohol and CCl4, scrambled vector-treated mice demonstrated a robust increase in liver injury as measured by serum ALT (Fig. 1F–0 hours) and fibrosis (Fig. 1G–H) with respect to PF+Oil treated controls. Importantly, MIR122-OX-treated mice exhibited reduced ALT at 24 hours and 48 hours following final CCl4 dose compared to scr-treated controls (Fig. 1F). Furthermore, MIR122-OX-treated mice revealed significantly reduced fibrosis as measured by Sirius red staining (Fig. 1G), and by significantly lower expression of Acta2 and Col1a1 (Fig. 1H), encoding α-SMA and Type-I collagen, in Et+CCl4 mice when compared to scrambled controls.
We noted that GLuc activity, indicating transgene expression, decreased following repeated administration of CCl4 in both scr and MIR122-treated mice (Supplementary Fig. 1F). This loss of GLuc expression was also associated with a reduction of MIR122 in both MIR122-OX and Scr-treated fibrotic animals while PF-oil treated MIR122-OX mice maintained a robust level of MIR122 overexpression (Supplementary Fig. 1G). Together, this indicates that MIR122 over-expression was effective in reducing fibrosis and liver injury, while the reduction of the transgene occurred due to the repeated CCl4 injections23.
Alcohol inhibits MIR122 transcription via alternate splicing of grainyhead-like 2 transcription factor
The question remained as to the mechanism by which MIR122 expression is reduced by alcohol. Surprisingly, both PF and alcohol-fed WT mice treated with rAAV8-MIR122-OX achieved similar expression levels of mature MIR122 (Fig. 1C). Given that our U6-promoter MIR122-OX construct required processing by canonical miRNA maturation mechanisms, we hypothesized that chronic ethanol did not inhibit the maturation or stabilization of MIR122 but rather its transcription.
miRNAs are encoded by their own genes and regulated transcriptionally24. Using TaqMan probes specific for the endogenous MIR122 primary transcript (pri-MIR122), we found that alcohol reduces pri-MIR122 expression in livers of human alcoholic cirrhosis patients approximately 2-fold (Fig. 2A). Livers of alcohol-fed mice and, more specifically, isolated hepatocytes also showed significant reductions in pri-MIR122 expression (Fig. 2B) similar to the reduction of mature MIR122 (Fig. 1A, B) suggesting transcriptional regulation. Of note, we found reduced pri-MIR122 expression in hepatocytes of alcohol-fed mice treated with MIR122-OX (Supplementary Fig. 1D) indicating that the effect of chronic alcohol on MIR122 transcription is independent of liver injury, or mature MIR122 levels.
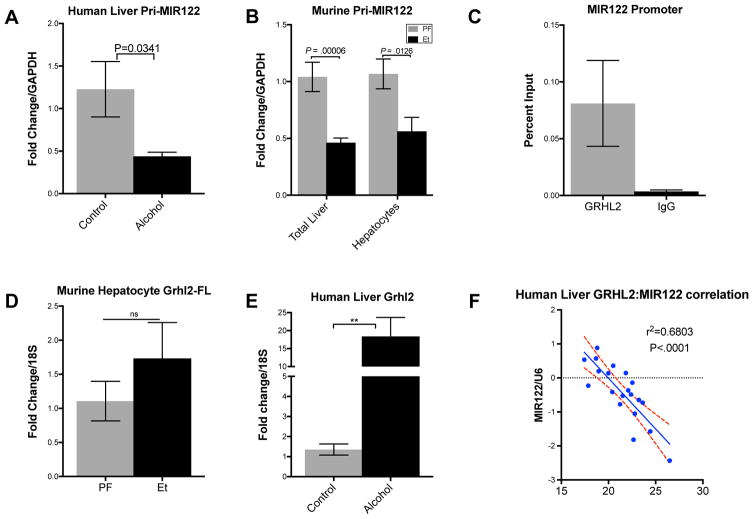
Fig. 2. Grainyhead-like 2 inversely correlates with MIR122 expression in human livers.
Pri-MIR122 expression in (A)human livers (n=10–12/group) and (B)alcohol-fed WT murine livers (n=7–8/group) and hepatocytes (n=5/group). ChIP-qPCR of the GRHL binding results presented as % input of (C)GRHL2 and respective IgG controls. (D)GRHL2 mRNA expression in murine livers. (n=3/group) (E)GRHL2 mRNA expression and correlation with MIR122 expression in human livers (n=10–12/group). Student’s t-test, two-way ANOVA, and Pearson’s correlation coefficient.
These observations turned our attention to grainyhead like-2 (GRHL2), a homolog of the Drosophila grainyhead transcriptional regulator, which was recently suggested as a potential repressor of MIR122 expression in progenitor cells during hepatic differentiation 25. However, the role of grainyhead proteins in hepatic pathophysiology has yet to be described.
Using in silico analysis, we identified a conserved grainyhead dimer binding site approximately 300 bp upstream of the MIR122 transcription start site (TSS), (Supplementary Fig. 2A). First, we performed chromatin immunoprecipitation-qPCR in Huh-7 cells which confirmed the putative GRHL binding site in the MIR122 promoter (Fig. 2C). Total liver extracts from alcohol-fed mice showed a modest, but not statistically significant increase in GRHL2 mRNA expression (Fig. 2D). However, we found robust increases in GRHL2 expression in the livers of alcoholic cirrhosis patients (Fig. 2E) when compared to healthy controls. Furthermore, this 18-fold increase in GRHL2 demonstrated a significant inverse correlation with MIR122 expression (Fig. 2F) in human alcoholic cirrhotic livers.
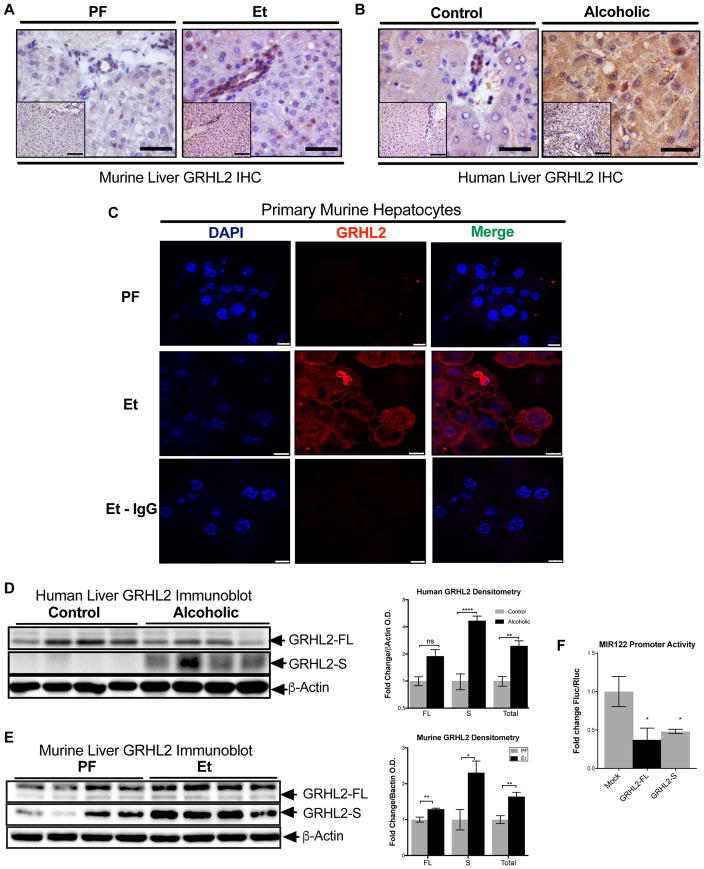
In normal, healthy human livers and mouse livers immunohistochemistry evaluation revealed that GRHL2 was primarily localized to the biliary epithelium, a finding consistent with previous reports (Fig. 3A–C) 26,27. In alcohol-fed mice and in livers of alcoholic patients, we detected increased GRHL2 staining that appeared to localize both to the cytoplasm and nucleus of hepatocytes (Fig. 3A–C). While western blot analysis from total livers revealed only a moderate increase in the expression of the 70 kDa GRHL2 (GRHL2-FL) (Fig. 3D, E), the levels of the 49 kDa GRHL2 splice variant (GRHL2-S), previously described as a “dominant negative” variant, were significantly increased in both human and murine livers (Fig. 3D, E) 28. To dissect the functional relevance of this finding, we co-transfected a luciferase reporter containing the human MIR122 promoter with either the GRHL2-FL or the GRHL2-S isoforms into Huh-7 cells. Our results showed that both the full-length (FL) and spliced variant forms of GRHL2 potently inhibited MIR122 expression (Fig. 3F). Taken together, our data suggest that alcohol regulates MIR122 expression by selectively increasing the spliced form of GRHL2 in hepatocytes.
Fig. 3. Alcohol inhibits MIR122 in hepatocytes via alternatively spliced GRHL2.
GRHL2 immunihistochemistry of (A)murine and (B)human livers. Scale bars; full-size=100 μm, inset=200 μm. (C)Immunostaining of isolated primary hepatocytes from PF- and Et-fed mice. Scale bars; PF and Et=10 μm, Et+IgG=7.5 μm. Representative immunoblots for (D)murine (n=8/group) and (E)human (n=10/group) GRHL2 from total liver lysate. (F)The effect of GRHL2-FL or GRHL2-S on MIR122 promoter activity (n=4/group) in nuclear lysates from huh-7 cell lines. Student’s t-test.
MIR122 inhibition in hepatocytes recapitulates and augments the hepatocellular damage and steatosis induced by chronic alcohol
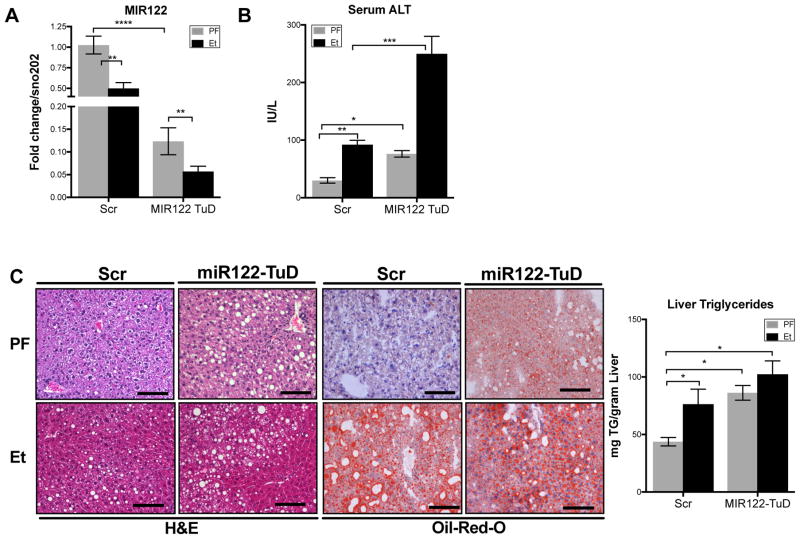
Given the alcohol-related reduction of MIR122 in murine hepatocytes and the ameliorating effect of MIR122 restoration on the pathogenic features of ALD, we hypothesized that direct inhibition of MIR122 in vivo will mimic features of alcoholic-induced liver pathology. To inhibit MIR122 in vivo, we developed a rAAV8 vector system expressing an anti-MIR122 Tough Decoy (TuD). We have previously reported that AAV-delivered anti-MIR122 TuDs are able to maintain sustained inhibition of MIR122 in murine livers15. Wild type (WT) mice were treated with rAAV8-scrambled (Scr) or rAAV8-anti-MIR122-TuD (MIR122 TuD) 2-weeks prior to initiation of the chronic alcohol feeding to permit full vector expression (Supplementary Fig. 3A).
In WT mice, rAAV8 MIR122 TuD achieved a robust and sustained knockdown of MIR122 in both pair-fed and alcohol-fed mice (Fig. 4A). Interestingly, in addition to the rAAV8 MIR122 TuD-induced reduction, liver MIR122 levels were further decreased by ethanol feeding compared to pair-fed, TuD-treated mice (Fig. 4A) suggesting that alcohol may have a direct effect on MIR122. TuD-mediated inhibition of MIR122 alone resulted in a significant increase in serum ALT (Fig. 4B) and hepatic steatosis (Fig. 4C) that was equivalent to that induced by the chronic alcohol diet. Remarkably, alcohol feeding in the MIR122-TuD treated mice resulted in even higher serum ALT (Fig. 4B) without a combinatorial increase in hepatic lipid accumulation (Fig. 4C). Together, these data suggest that MIR122 inhibition drives triglyceride accumulation predisposing hepatocytes to injury upon exposure to alcohol.
Fig. 4. The reduction of MIR122 recapitulates ALD pathogenesis.
(A)Hepatic MIR122 expression (B)serum ALT, (C)H&E and Oil-Red-O (ORO) staining and triglyceride concentrations from the total livers of scrambled or MIR122-TuD treated WT following 5-week LDC (n=8–14/group). Student’s t-test or two-way ANOVA.
MIR122 inversely correlates with HIF1α in ALD
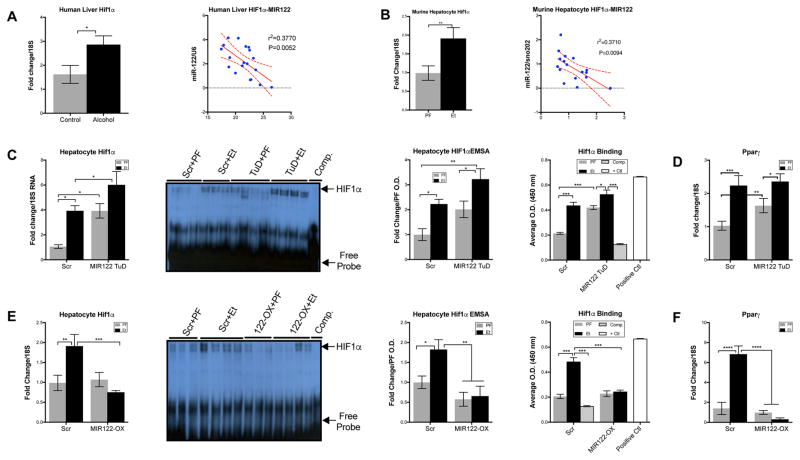
To assess potential mechanisms by which decreased levels of MIR122 could mediate liver injury and steatosis, we searched for MIR122 targets that may contribute to the development of ALD. Our lab has previously identified that HIF1α as a target of MIR122 in vitro 29. While the role of HIF1α activation in ALD has been studied, a direct relationship between MIR122 and HIF1α in vivo has yet to be explored14. Our present analysis of the livers of alcoholic cirrhosis patients and hepatocytes of alcohol-fed mice revealed an increase of HIF1α mRNA that showed a significant inverse correlation with MIR122 expression (Fig. 5A, B respectively). This correlation suggested that HIF1α regulation by MIR122 in hepatocytes may be a key element in the pathogenesis of ALD.
Fig. 5. MIR122 loss mediates hepatic steatosis and inflammation through HIF1α.
Expression of HIF1α and correlation to MIR122 expression in (A)human livers (n=9–12/group) and (B)murine hepatocytes (n=8–14/group). HIF1α expression and activity measured by mRNA, EMSA and HIF1α binding assay from isolated primary hepatocyte nuclei, and PPARγ mRNA in total livers isolated after 5 weeks of PF or Et diet from; (C–D)Scr or MIR122-TuD treated WT mice and (E–F)MIR122-OX treated mice. Student’s t-test or two-way ANOVA (n=8–14/group).
MIR122 regulates HIF1α in vivo
Alcohol and knockdown of MIR122 in the livers of WT mice increased HIF1α mRNA individually and additively (Fig. 5C). To confirm that the increase in HIF1α mRNA represents increased DNA binding capacity, we performed both EMSAs and antibody-mediated transcription factor binding assays using a HIF1α consensus binding oligonucleotides on hepatocyte nuclear extracts. Our results showed that TuD-mediated inhibition of MIR122 resulted in increased HIF1α DNA binding at baseline, equivalent to alcohol-feeding alone (Fig. 5C). Furthermore, the combination of alcohol and MIR122-TuD-inhibition yielded an additive increase in HIF1α mRNA and DNA-binding activity suggesting that alcohol regulates HIF1α via MIR122 (Fig. 5C). Indeed, we found that the increase in HIF1α activity correlated with an increase in PPARγ mRNA expression (Fig. 5C–D), a direct target of HIF1α activation and a key factor in driving steatosis30–33.
In vivo restoration of MIR122 in hepatocytes prevented the alcohol-induced increase in HIF1α mRNA, DNA binding activity, and PPARγ mRNA expression, a HIF1α target gene involved in lipogenesis (Fig. 5E–F)34. Overall, these data demonstrate that restoration of MIR122 in hepatocytes may suppress the pathogenic features of ALD via inhibition of HIF1α in vivo and indicates that hepatocyte-specific MIR122 delivery could be a therapeutic consideration in ALD.
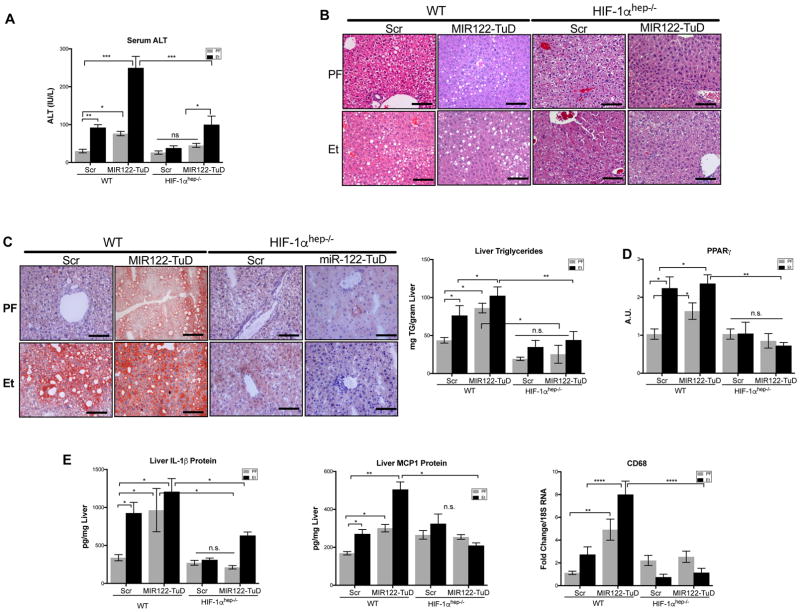
Hepatocyte-specific knockout of HIF1α protects from liver injury, steatosis, inflammation, and fibrosis induced by alcohol or MIR122 reduction
Based on the discovery that MIR122 is decreased by alcohol and that MIR122 inhibits HIF1α in hepatocytes, we postulated that if MIR122 regulates HIF1α in vivo, then mice with a hepatocyte-specific knockout of HIF1α (HIF1αhep−/−) will be protected from the MIR122-TuD and/or alcohol-induced liver injury (Supplementary Fig. 3A, B).
In support of this hypothesis, we found that in contrast to WT mice, HIF1αhep−/− mice were protected from liver injury (Fig. 6A) and steatosis (Fig. 6B, C) whether induced by alcohol or MIR122 inhibition alone, or in combination. Furthermore, HIF1αhep−/− also displayed no increase in PPARγ (Fig. 6D) associated with the knockdown of MIR122 and alcohol in WT mice. These data suggested first, that the loss of MIR122 in hepatocytes directly triggers an increase of HIF1α, resulting in steatosis and hepatocyte injury in the liver. Second, alcohol and exogenous MIR122 inhibition additively decrease MIR122, inducing HIF1α, and subsequently, PPARγ.
Fig. 6. Hepatocyte specific HIF1αKO mice are protected from loss of MIR122 and chronic alcohol.
Liver Injury assessed by (A)serum ALT and (B)H&E staining of WT and HIF1αhep−/− following 5-weeks of LDC or PF diet treated with either AAV8-Scr or MIR122-TuD vectors. Hepatic triglycerides assessed by (C)ORO staining and triglyceride quantification. Downstream HIF1α function was measured by total liver (D)PPARγ mRNA. Hepatic inflammation was assessed by (E)IL-1β and MCP1 protein, and CD68 mRNA. Student’s t-test or two-way ANOVA (n=8–14/group).
Analysis of H&E sections and qPCR evaluation of immune cell markers revealed increased immune cell infiltration, CD68 (Fig. 6B, E) in anti-MIR122 TuD-treated, or alcohol-fed mice with an increase in mice treated with both TuD and alcohol. We also found increased levels of IL-1β and MCP-1 protein (Fig. 6E), in the livers of alcohol and MIR122 TuD-treated WT mice. In contrast, HIF1αhep−/− mice treated with either MIR122 inhibition or chronic alcohol showed a reduction in inflammatory cell infiltration and activation compared to WT mice (Fig. 6E), suggesting that, in addition to steatosis, liver inflammation induced by MIR122 decrease is also attenuated by hepatocyte-specific HIF1α deficiency.
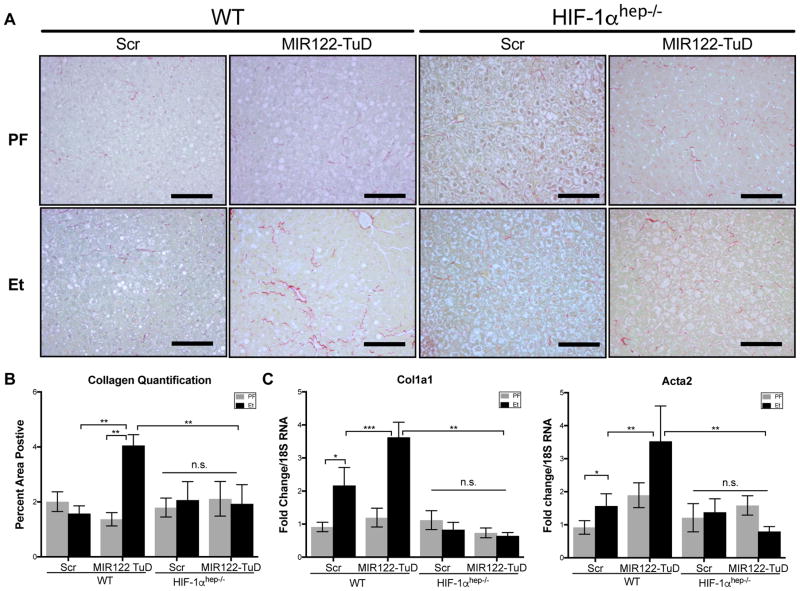
The development of fibrosis indicates progression of ALD as a result of sustained hepatocyte injury, inflammation, and stellate cell activation. As the 5-week LDC alcohol feeding model represents early features of ALD, prominent fibrosis was not seen by Sirius Red staining (Fig. 7A, B) however, there were significant increases in pro-collagen-1α and Acta2 mRNA levels (Fig. 7C) in alcohol-fed WT mice. The combination MIR122 TuD and alcohol feeding significantly increased liver expression of fibrosis markers compared to alcohol alone or pair-fed WT controls (Fig. 7A–C). Importantly, the increase in fibrosis in the MIR122 TuD+Et group was abrogated in the HIF1αhep−/− mice (Fig. 7A–C). Together, these data strongly suggest that the increased pro-inflammatory and pro-fibrotic state are secondary to HIF1α-mediated hepatocyte injury due to the reduction of MIR122.
Fig. 7. MIR122 loss mediates hepatic fibrosis through HIF1α.
Hepatic fibrosis was evaluated by (A)Sirius Red staining and the (B)percent-positive area stained was quantified using ImageJ (n=6–14). Additional markers of fibrosis were further assessed by expression of (C)col1a1 and Acta2 from total liver RNA (n=6–14). Two-way ANOVA. Scale bars: 100 μm.
As noted in the MIR122-OX study, the inhibition of MIR122 expression due to alcohol was independent of mature MIR122 levels. We also find reduced pri-MIR122 expression in the hepatocytes of alcohol-fed mice treated with either MIR122-TuD, as well as in HIF1αhep−/− (Supplementary Fig. 3C) further corroborating our hypothesis that the inhibition of MIR122 due to alcohol is independent of liver injury.
Discussion
The pathomechanism of ALD involves several key factors including hepatic fat deposition and activation of inflammatory pathways which can all be regulated by microRNAs5,7,35,36. In this report, we show that chronic alcohol reduces MIR122 levels in human livers and murine hepatocytes and that this MIR122 reduction contributes to liver injury, steatosis, and inflammation in ALD. Our experiments in chronic alcohol reveal a novel mechanism of alcohol-induced inhibition of MIR122 via upregulation of GRHL2. We also discovered that in vivo inhibition of MIR122 with a rAAV8–delivered anti-MIR122 TuD induces steatosis and inflammation similar to alcohol in the liver and in combination with alcohol augments the features of ALD. We show for the first time that features of ALD induced by direct MIR122 inhibition can be prevented in hepatocyte-specific HIF1α deficient mice thereby mechanistically linking MIR122 reduction to increased HIF1α activation in ALD14. We show that therapeutic AAV8-mediated restoration of MIR122 reverses hepatic injury via inhibiting HIF1α after alcohol administration and thereby decreasing liver injury, steatosis, inflammation, and fibrosis (Supplementary Fig. 4). We further showed that MIR122 restoration was also effective in reducing liver fibrosis in an aggressive model of liver fibrosis induced by a combination of chronic alcohol and CCL4.
While this is the first report to mechanistically describe the alcohol-induced reduction in MIR122 in human livers and mice, other studies found decreased liver MIR122 levels in patients and in murine models of nonalcoholic steatohepatitis (NASH), bile duct ligation, and hepatocellular cancer (HCC)1,29,37,38. In those studies, the loss of MIR122 was associated with increased steatosis, disease severity, as well as sensitivity to HCC development, metastasis, and mortality1,29,37,38. MIR122 has been found to regulate many pathways including those governing hepatic metabolism, cellular differentiation, and proliferation – all of which may contribute to ALD pathogenesis6,39,40. Our data indicates that the increase of HIF1α in hepatocytes, a direct MIR122 target, is a key mediator of ALD pathogenesis. Furthermore, we show that over-expression of MIR122 in murine hepatocytes can inhibit this pathway and reverse alcohol-induced steatosis and liver injury.
Our results show that the inhibition of MIR122 by alcohol or an rAAV-mediated MIR122 inhibitor results in a baseline increase in HIF1α, steatosis, and hepatocyte injury. This observation is similar to the phenotype previously described in mice with in vivo overexpression of HIF1α in hepatocytes (HIF1dPA)14,41. Here we found that reduction of MIR122 in hepatocytes with chronic alcohol (MIR122-TuD+Et), resulted in a dramatic increase in hepatic injury, greater than alcohol or MIR122 reduction alone (Fig. 4B) without an increase in steatosis (Fig. 4C). This phenomenon may be due to the further reduction in MIR122 seen in TuD+Et mice when compared to TuD+PF mice. Previous studies showed that mice that had higher HIF1α expression in hepatocytes developed steatosis and liver injury even in the absence of alcohol14,41. When given alcohol, HIF1dPA mice exhibited a combinatorial phenotype similar to that seen in MIR122-TuD+Et, having greater liver injury than either treatment alone, and further increasing HIF1α mRNA expression. Alternately, we cannot rule out that alcohol may increase HIF1α mRNA expression by pathway(s) other than MIR122.
Our studies with MIR122 restoration via a gene therapy approach using a rAAV8 MIR122-OX indicate that targeting MIR122 balance in hepatocytes can ameliorate steatosis, liver injury, and fibrosis in chronic early and late-stage alcohol-induced liver pathology. These experiments provide a mechanistic role for reduced MIR122 in the pathogenesis of ALD and highlight the therapeutic potential of MIR122 restoration in hepatocytes.
Two previous studies have explored the therapeutic potential of MIR122 modulation with contradictory results9,42. Esau, et al. used an anti-MIR122 anti-sense-oligonucleotide (ASO) to knockdown MIR122 in the liver in a high fat diet (HFD) model42. They demonstrated that short-term inhibition of MIR122 in the liver reduced steatosis in HFD-fed mice. These findings, however, were in sharp contrast to work by Hsu, et al. who showed that liver-specific-knockouts (122−/−LKO) had increased susceptibility to myc-induced HCC. Our work provides the first evidence to indicate that restoration of MIR122 in hepatocytes ameliorates the features of ALD, defining a novel treatment indication of normalizing liver MIR122 levels in ALD9.
It has been shown that MIR122 is an essential host factor for HCV replication and represents a therapeutic target in HCV infection43–46. Therapeutic inhibition of MIR122 using Miravirsen or SPC3649, a Locked Nucleic Acid (LNA) anti-MIR122 oligo, was developed to treat HCV infection44,47,48. Recently completed phase 2a trials using Miravirsen to treat HCV infection yielded reduced HCV viral load at low therapeutic concentrations, with no adverse events45,47. Additionally, these groups and others have demonstrated a cardio-protective role for MIR122 inhibition. AAV, ASO, and LNA inhibition of MIR122 have all demonstrated a decrease in serum cholesterol and triglycerides without hepatotoxicity15,45,47. Our findings, however, raise the concern that sustained inhibition of MIR122 could result in progressive liver injury as a potential complication of chronic MIR122 inhibitor therapies47,49.
Our present observation in livers with ALD appears to be contrary to previous in vitro findings where we have shown an increase in MIR122 during acute exposure to EtOH in hepatoma cell lines43,50. However, exposure in vitro represents an acute exposure model rather than chronic alcohol consumption. Further, most hepatoma cell lines exhibit a decreased baseline expression of MIR122 due to mutations and dysregulated transcription factor networks when compared to primary cells making findings with respect MIR122 modulation difficult to generalize8,43.
While much attention has been placed on an understanding of the mature form of MIR122 in liver physiology and its characterization as an oncomir, few studies have explored the upstream mediators that regulate pri-MIR122 expression51. Recent publications have demonstrated the essential functions of GRHL2 in development and differentiation with conflicting evidence on its role in EMT, and cancer progression25,52,26. A strong correlation of GRHL2 expression with various epithelial cells, undifferentiated progenitor cells, and chronic disease states such as atopic dermatitis and psoriasis were described53,54. Uniquely, hepatocytes are a rare subset of epithelial cells that do not normally express GRHL226. We too find GRHL2 expression in the biliary epithelium in normal liver while GRHL2 was expressed in hepatocytes in ALD both in human and mouse livers. Our results show that overexpression of the full-length (FL) GRHL2 variant inhibits MIR122 in humans and mice. We also discovered that the effect of alcohol is to specifically increase the alternatively spliced form of GRHL2, which inhibits MIR122 expression equivalent to the FL isoform. This spliced isoform is the result of alternative splicing in exon 1 which results in a protein that retains the conserved C-terminal DNA binding domain, but lacks the N-terminal transactivation domain, thereby inhibiting transcriptional activity (Supplementary Fig. 2B).
IHC and proteomic screens have detected GRHL2 in a subset of HCCs suggesting that gain of GRHL2 expression may play a role in promoting hepatic neoplasia. Tanaka et al. found an association between a genome copy number gain of GRHL2 in tumor tissues of patients with recurrent HCC52. However, it is important to note that these findings conveyed increases of the GRHL2 genomic locus on chromosome 8, a frequent occurrence in HCC, and that increased copy number of GRHL2 has been shown not to translate into increased protein or mRNA. Furthermore, their work demonstrated that siRNA knockdown of GRHL2 inhibits HCC tumor growth was performed in Huh-6 cell lines which lack GRHL2 mRNA while harboring copy number gains of chromosome 826,52. Work done by Tanimizu and colleagues found a loss of GRHL2 expression in differentiating murine hepatocyte progenitors and that increasing GRHL2 was associated with a decrease in MIR122 expression25. Little is known about the full-length or spliced isoforms of GRHL2, and their biological significance in hepatic pathology. Our data suggest that the alcohol-induced increase in GRHL2 in mature hepatocytes inhibits MIR122 and drives ALD pathogenesis. We speculate that increase in GRHL2 expression in hepatocytes in ALD may also represent an early alteration of hepatocyte de-differentiation that can promote preneoplastic changes in the alcoholic liver.
Of note, we found that alcohol combined with rAAV8-MIR122-TuD resulted in an additive effect, further reducing MIR122 levels and increasing liver injury when compared to MIR122-TuD or alcohol treatment alone. We suggest the combined effect is explained by the combination of alcohol-induced transcriptional inhibition by GRHL2 and MIR122-TuD-mediated reduction in mature MIR122.
The treatment of ALD is a complex process involving reversal of parenchymal cell injury and suppression of inflammation55. After 40 years of clinical trials, steroids remain the controversial standard of care, with no new FDA-approved treatments available2,56–58. Based on our results, we speculate that GRHL2 may serve as a prognostic marker of hepatocyte differentiation or disease progression and that in vivo therapeutic correction of GRHL2 splicing or expression changes in ALD may be beneficial to correct MIR122 dysregulation. However, until the role of GRHL2 in liver disease is explored further, we propose that downstream intervention with MIR122 restoration to treat ALD may constitute a safer, simpler, and more effective approach.
While the use of viral vector gene therapy is gaining increasing acceptance, they have both advantages and disadvantages and their use is not without limitations with respect to the treatment of chronic liver disease. First, viral vectors deliver their transgenes as non-integrating episomes which are lost following repeated regenerative cycles51,59. Immediately following AAV treatment, we noted equivalent serum GLuc activity in both CCl4 and oil-treated controls. However, GLuc activity rapidly declined in subsequent weeks in only CCl4-treated mice (Supplementary Fig. 1F) indicating transgene was expelled during replicative cycles. In accordance with this, we noted no difference in MIR122 RNA between MIR122-OX and Scrambled in following 8 weeks of CCl4 compared scrambled treated controls, while PF-Oil mice maintained a robust level of MIR122 overexpression (Supplementary Fig. 1G). Secondly, while AAVs have markedly reduced immunogenicity when compared to other viral delivery systems, neutralizing antibodies to the virus do develop following their use, requiring the use of other AAV serotypes dosing should repeated treatment be required60. Given our data that vector expression was well maintained following 3 weeks of chronic alcohol alone (Fig. 1C), the use of AAV-mediated delivery still remains a viable long-term therapeutic in ALD where cell turnover is less than that seen with repeated CCl4 administration.
Recent clinical trials in the UK using rAAV8 vectors to treat Hemophilia B deficiency has demonstrated that a single peripheral-vein dose can safely and effectively achieve sustained transgene expression for 16 months after treatment18. Furthermore, previous work has also demonstrated that AAV8 are equally effective in transducing both healthy and cirrhotic livers when administered intravenously23. This single dose approach provides an added benefit in treating patients with ALD who are frequently lost to follow-up compared to other miRNA therapies that require monthly dosing59. Of note, in WT-PF mice treated with rAAV8 MIR122-OX, the degree to which we restored MIR122 was greater than anticipated (Fig. 1C); however, there was no hepatotoxicity as a result. Given that 130,000 copies of MIR122 are in each hepatocyte, overexpression amounts to nearly 70,000 extra copies per cell, therefore, treatments that over-express MIR122 may have a large therapeutic window8.
MIR122 restoration has been considered as an HCC therapy, where its loss has been reported10,11,40,61. However, treatment at such a late stage of ALD is difficult and gene therapy is often precluded due to tumor size9. Our data supports a novel treatment indication for MIR122 restoration, as a therapy in both early and late stage ALD to prevent and reverse hepatic injury and cirrhosis that warrants further exploration in the clinical setting.
Supplementary Material
Acknowledgments
Funding: This work was supported by grant funding from the NIAAA, R01-AA020744 to GS and F30AA022283 to AS.
The authors would like to thank members of the Szabo lab for their stimulating intellectual discussions and scientific input. The hGRHL2-FL and hGRHL2-DN plasmids were a kind gift by Dr. Volker Assmann of the Institute of Tumor Biology at University Medical Center Hamburg-Eppendorf. We thank Dr. David Garlick of the Cancer Biology Department at UMass Medical School (UMMS) for histopathological examination of liver tissue sections. The authors are grateful to services provided by the UMMS DERC Morphology Core including: immunohistochemistry, vector (AAV) production, and CFAR (Primer Synthesis). Lastly, the authors thank Dr. Jeffrey Nickerson, Dr. Jean Underwood, and the Cell & Development Biology Confocal Core at UMMS for their training and support in the use of the Leica TCS SP5 II Laser Scanning Confocal Microscope.
Footnotes
Competing interests: The authors have nothing to disclose.
Author contributions: AS, AVA, YH, PL, BG, AI-V, JX, SB, GG, & GS designed the experiments. DT, BS, AS, AVA, SR, NM, PL, BG, AI-V, DC, JL, JX, LZ, & KK performed the animal experiments. AA, AS, AVA, SR, & YH acquired, analyzed, and interpreted the data. AS, JL, JX, LZ, & GG developed and administered AAV vectors. AS & GS interpreted the data and wrote the manuscript. AS & GS obtained the funding and provided overall study concept and supervision.
References
- 1.Ambade A, Satishchandran A, Szabo G. Alcoholic hepatitis accelerates early hepatobiliary cancer by increasing stemness and miR-122-mediated HIF-1α activation. Sci Rep. 2016;6:21340. doi: 10.1038/srep21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalasani N, Szabo G. Alcoholic and Non-Alcoholic Fatty Liver Disease. Springer; 2015. [Google Scholar]
- 3.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. doi: 10.1038/nrgastro.2015.35. [DOI] [PubMed] [Google Scholar]
- 4.Iracheta-Vellve A, Petrasek J, Satishchandran A, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol. 2015;63:1147–1155. doi: 10.1016/j.jhep.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 6.Szabo G, Satishchandran A. MicroRNAs in alcoholic liver disease. Semin Liver Dis. 2015;35:36–42. doi: 10.1055/s-0034-1397347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo G, Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 9.Hsu S-H, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulouarn C, Factor VM, Andersen JB, et al. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spaniel C, Honda M, Selitsky SR, et al. microRNA-122 abundance in hepatocellular carcinoma and non-tumor liver tissue from Japanese patients with persistent HCV versus HBV infection. PLoS ONE. 2013;8:e76867. doi: 10.1371/journal.pone.0076867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang J, Guo JT, Jiang D, et al. Liver-Specific MicroRNA miR-122 Enhances the Replication of Hepatitis C Virus in Nonhepatic Cells. 2008;82:8215–8223. doi: 10.1128/JVI.02575-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrasek J, Iracheta-Vellve A, Saha B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249–256. doi: 10.1189/jlb.3AB1214-590R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath B, Levin I, Csak T, et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J, Ameres SL, Friedline R, et al. Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat Meth. 2012;9:403–409. doi: 10.1038/nmeth.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roychowdhury S, Chiang DJ, McMullen MR, et al. Moderate, chronic ethanol feeding exacerbates carbon tetrachloride-induced hepatic fibrosis via hepatocyte-specific hypoxia-inducible factor 1 α. Pharmacol Res Perspect. 2014;2:e00061–13. doi: 10.1002/prp2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandon-Warner E, Schrum LW, Schmidt CM, et al. Rodent Models of Alcoholic Liver Disease: Of Mice and Men. Alcohol (Fayetteville, NY) 2012;46:715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrasek J, Bala S, Csak T, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol. 2016;64:1378–1387. doi: 10.1016/j.jhep.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roychowdhury S, Chiang DJ, McMullen MR, et al. Moderate, chronic ethanol feeding exacerbates carbon tetrachloride-induced hepatic fibrosis via hepatocyte-specific hypoxia-inducible factor 1 α. Pharmacol Res Perspect. 2014;2:e00061–13. doi: 10.1002/prp2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobrevals L, Enguita M, Rodriguez C, et al. AAV vectors transduce hepatocytes in vivo as efficiently in cirrhotic as in healthy rat livers. 2011;19:411–417. doi: 10.1038/gt.2011.119. [DOI] [PubMed] [Google Scholar]
- 24.Finnegan EF, Pasquinelli AE. MicroRNA biogenesis: regulating the regulators. Critical Reviews in Biochemistry and Molecular Biology. 2013;48:51–68. doi: 10.3109/10409238.2012.738643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanimizu N, Kobayashi S, Ichinohe N, et al. Downregulation of miR122 by grainyhead-like 2 restricts the hepatocytic differentiation potential of adult liver progenitor cells. Development. 2014;141:4448–4456. doi: 10.1242/dev.113654. [DOI] [PubMed] [Google Scholar]
- 26.Riethdorf S, Frey S, Santjer S, et al. Diverse expression patterns of the EMT suppressor grainyhead-like 2 (GRHL2) in normal and tumour tissues. Int J Cancer. 2015 doi: 10.1002/ijc.29841. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 27.Ponten F, Jirström K, Uhlen M. The Human Protein Atlas--a tool for pathology. J Pathol. 2008;216:387–393. doi: 10.1002/path.2440. [DOI] [PubMed] [Google Scholar]
- 28.Werner S, Frey S, Riethdorf S, et al. Dual Roles of the Transcription Factor Grainyhead-like 2 (GRHL2) in Breast Cancer. Journal of Biological Chemistry. 2013;288:22993–23008. doi: 10.1074/jbc.M113.456293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Csak T, Bala S, Lippai D, et al. microRNA-122 regulates hypoxia-inducible factor-1 and vimentin in hepatocytes and correlates with fibrosis in diet-induced steatohepatitis. Liver Int. 2015;35:532–541. doi: 10.1111/liv.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettinelli P, Videla LA. Up-regulation of PPAR-gamma mRNA expression in the liver of obese patients: an additional reinforcing lipogenic mechanism to SREBP-1c induction. J Clin Endocrinol Metab. 2011;96:1424–1430. doi: 10.1210/jc.2010-2129. [DOI] [PubMed] [Google Scholar]
- 31.Pettinelli P, Del Pozo T, Araya J, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–1086. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Souza-Mello V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. WJH. 2015;7:1012. doi: 10.4254/wjh.v7.i8.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan J, Suter M, Windak R, et al. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metabolism. 2009;9:512–524. doi: 10.1016/j.cmet.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Belanger AJ, Luo Z, Vincent KA, et al. Hypoxia-inducible factor 1 mediates hypoxia-induced cardiomyocyte lipid accumulation by reducing the DNA binding activity of peroxisome proliferator-activated receptor alpha/retinoid X receptor. BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS. 2007;364:567–572. doi: 10.1016/j.bbrc.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 35.O’Shea RS, Dasarathy S, McCullough AJ, et al. Alcoholic liver disease. Hepatology. 2009;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 36.Bala S, Marcos M, Kodys K, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaki Y, Saito Y, Takasugi A, et al. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from non-alcoholic steatohepatitis. Cancer Sci. 2014;105:1254–1260. doi: 10.1111/cas.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu S-H, Wang B, Kutay H, et al. Hepatic Loss of miR-122 Predisposes Mice to Hepatobiliary Cyst and Hepatocellular Carcinoma upon Diethylnitrosamine Exposure. The American Journal of Pathology. 2013;183:1719–1730. doi: 10.1016/j.ajpath.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagschal A, Najafi-Shoushtari SH, Wang L, et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat Med. 2015;21:1290–1297. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandiera S, Pfeffer S, Baumert TF, et al. miR-122--a key factor and therapeutic target in liver disease. J Hepatol. 2015;62:448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Balamurugan K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int J Cancer. 2016;138:1058–1066. doi: 10.1002/ijc.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esau C, Davis S, Murray SF, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Bukong TN, Momen-Heravi F, Kodys K, et al. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. In: Luo G, editor. PLoS Pathog. Vol. 10. 2014. p. e1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sedano CD, Sarnow P. Hepatitis C Virus Subverts Liver-Specific miR-122 to Protect the Viral Genome from Exoribonuclease Xrn2. 2014;16:257–264. doi: 10.1016/j.chom.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elmen J, Lindow M, Silahtaroglu A, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Research. 2007;36:1153–1162. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.JOPLING CL, NORMAN KL, SARNOW P. Positive and negative modulation of viral and cellular mRNAs by liver-specific microRNA miR-122. Cold Spring Harb Symp Quant Biol. 2006;71:369–376. doi: 10.1101/sqb.2006.71.022. [DOI] [PubMed] [Google Scholar]
- 47.Janssen HLA, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 48.Jopling CL, Schütz S, Sarnow P. Position-Dependent Function for a Tandem MicroRNA miR-122-Binding Site Located in the Hepatitis C Virus RNA Genome. 2008;4:77–85. doi: 10.1016/j.chom.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanford RE, Hildebrandt-Eriksen ES, Petri A, et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou W, Bukong TN, Kodys K, et al. Alcohol Facilitates HCV RNA Replication Via Up-Regulation of miR-122 Expression and Inhibition of Cyclin G1 in Human Hepatoma Cells. Alcohol Clin Exp Res. 2012;37:599–608. doi: 10.1111/acer.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thakral S, Ghoshal K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Curr Gene Ther. 2015;15:142–150. doi: 10.2174/1566523214666141224095610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tanaka Y, Kanai F, Tada M, et al. Gain of GRHL2 is associated with early recurrence of hepatocellular carcinoma. J Hepatol. 2008;49:746–757. doi: 10.1016/j.jhep.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Chen W, Liu ZX, Oh J-E, et al. Grainyhead-like 2 (GRHL2) inhibits keratinocyte differentiation through epigenetic mechanism. Cell Death and Disease. 2012;3:e450–10. doi: 10.1038/cddis.2012.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menke C, Cionni M, Siggers T, et al. Grhl2 is required in nonneural tissues for neural progenitor survival and forebrain development. genesis. 2015;53:573–582. doi: 10.1002/dvg.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosato V, Abenavoli L, Federico A, et al. Pharmacotherapy of alcoholic liver disease in clinical practice. Int J Clin Pract. 2016;70:119–131. doi: 10.1111/ijcp.12764. [DOI] [PubMed] [Google Scholar]
- 56.Sanyal AJ, Gao B, Szabo G. Gaps in Knowledge and Research Priorities for Alcoholic Hepatitis. 2015;149:4–9. doi: 10.1053/j.gastro.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiNubile MJ. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N Engl J Med. 2015;373:281–282. doi: 10.1056/NEJMc1506342. [DOI] [PubMed] [Google Scholar]
- 58.Frazier TH, Stocker AM, Kershner NA, et al. Treatment of alcoholic liver disease. Therap Adv Gastroenterol. 2011;4:63–81. doi: 10.1177/1756283X10378925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Therapy. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masat E, Pavani G, Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov Med. 2013;15:379–389. [PubMed] [Google Scholar]
- 61.Bai S, Nasser MW, Wang B, et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.