Abstract
Objectives
This study aimed to examine the previously unknown long-term spatio-temporal patterns in diarrheal morbidity and mortality across age groups and geography in Brazil under the light of evolving socioeconomic factors and interventions.
Methods
Nationwide mortality (1979–2014) and hospitalization (1998–2014) data were obtained from the Brazilian Ministry of Health. Analyses of long-term secular trends and seasonality of diarrheal morbidity and mortality were performed in EPIPOI (www.epipoi.info).
Results
For most states, the primary peak in mortality risk among children under 5 years occurred from December-April (summer/early autumn) from 1979–1988. From 2000–2005 (before the 2006 implementation of rotavirus vaccination), the pattern switched to June–October (winter/early spring). By 2007–2014, the peak in mortality shifted back towards summer/early autumn. A similar pattern was observed for hospitalizations. These patterns were particularly apparent in non-equatorial regions of the country. In contrast, the risk of diarrhea-related death among older children (5–19 years) did not demonstrate well-defined seasonality or spatial patterns.
Conclusions
Rotavirus vaccination policies were associated with a shift in the timing of seasonal peaks in children under 5, reminiscent of the summer diarrhea period common decades prior. Additionally, young children were shown to have distinct disease patterns compared to other age groups, suggesting different etiologies.
Keywords: diarrhea, interrupted time-series analysis, Brazil, spatio-temporal analysis, seasonal variation, hospitalization, mortality
INTRODUCTION
Considerable progress has been made in understanding diarrheal disease, yet it remains a serious public health challenge globally, responsible for up to 1.5 million deaths in 2012.[1] While research in the field commonly focuses on children under 5 years of age due to their particularly high burden of disease, diarrheal illness is an important cause of morbidity and mortality throughout the lifespan.[2] Global estimates suggest that 4.5 billion episodes of diarrhea may occur annually with more than 1.7 billion episodes among children less than 5 years of age and 2.8 billion episodes among older children and adults.[3], [4] Although moderate and severe diarrhea comprise a relatively small proportion of episodes, such illness represents nearly 620 million cases each year.[5] Diarrheal morbidity and mortality remain a significant global health issue, and progress in reducing diarrheal disease morbidity among older children and adults has been relatively stagnant over the last three decades, highlighting the need for further research and interventions.[4], [6]
Distinct seasonal patterns of diarrheal disease have helped to elucidate different etiologies (such as bacterial vs. viral) in certain populations and in specific environments, however, inconsistencies across regions and age groups have left unanswered questions about trends seen in a broader variety of settings.[6],[7], [8] Rotavirus is the leading cause of severe diarrhea and diarrhea-related deaths in young children globally.[3], [9] The virus is typically considered a winter disease in non-equatorial, temperate climates although it often demonstrates less pronounced seasonality in tropical climates and among adults.[6], [7] Conversely, bacterial diarrhea occurs more often during warm, rainy months.[10],[11],[12], [13] Among older children and adults in low- and middle-income countries, the most common causes of diarrhea-related hospitalization are often bacterial, chiefly E. coli, while in high-income countries, Campylobactor spp. and Salmonella spp. are of primary concern.[6],[4],[14],[15], [16] Additional factors such as region, level of development, temperature, rainfall and humidity have also been implicated with patterns of infectious diarrhea.[8,15,17,18]
Diarrheal disease continues to afflict the Brazilian population. Brazil provides a unique context in which to examine the spatial and seasonal patterns in diarrheal disease due to its large size, socioeconomically diverse population and heterogeneous climate. Existing literature on diarrheal disease trends in the country are typically short-term,[18] limited to young children,[18,19] or limited to certain regions of Brazil.[19,20] In this paper we examined the evolution of diarrheal morbidity and mortality risks across age groups and geography in Brazil, discussing the previously unknown long-term spatio-temporal patterns under the light of evolving socioeconomic factors and interventions.
MATERIALS AND METHODS
Study area
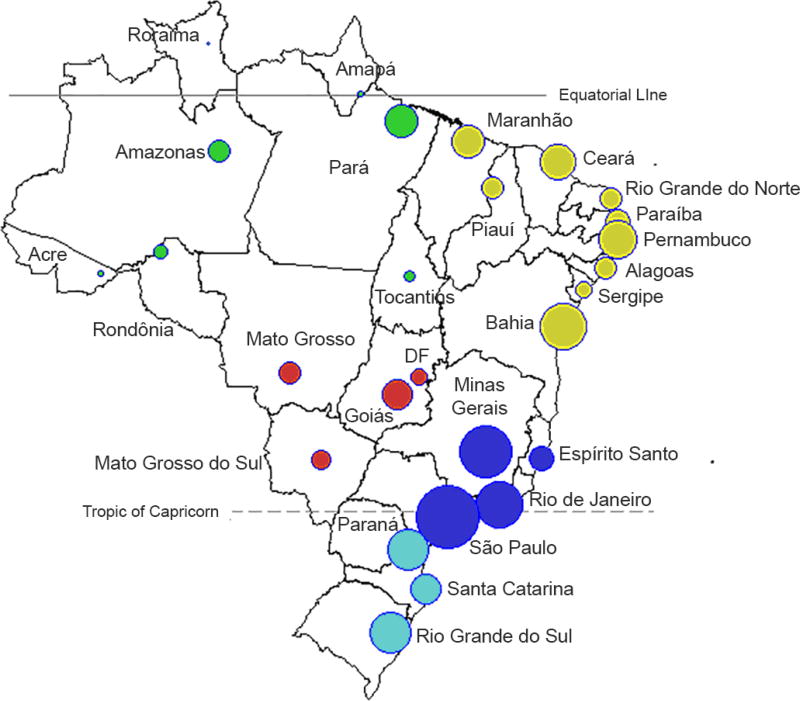
Brazil occupies a large area (8.5 million square kilometers) representing almost half of the South American continent. It has a population of 206.1 million (in 2014), concentrated mostly in the Southeast and Northeast regions (Figure 1). The country encompasses varied landscapes from the Amazon rainforest and arid regions of the equatorial region, to the savannas, rainforests and temperate climates of the non-equatorial regions. Brazil is a middle-income country with an average GDP per capita of $11,172.52 in 2015 (69th in the world).[21] It consists of 26 states and the Federal District. For spatial analyses, states are represented by the latitudes and longitudes of their respective capitals.
Figure 1.
Map of 26 administrative states plus the Capital District ("DF"). The location of each capital is represented by circles, where the size of the symbol is proportionate to the population size of each state (log10 scale). The colors refer to the five Brazilian administrative regions: North (green), Northeast (yellow), Central West (red), Southeast (dark blue), and South (light blue)
Data
Mortality data for the period between 1979 and 2014 was obtained from the System on Mortality Information of the Brazilian Ministry of Health (SIM/MS). These datasets are uniformly and systematically collected throughout the year and cover approximately 90% of the population. Regional differences in coverage ranged from 83.1% in the North region to 96.7% in the Southeast region (data available for 2008).[22] The only state in which coverage was 100% was the Federal District, located in the Central West region of Brazil.
We used hospitalization data from 1998 to 2014 from the nationwide administrative database of the Unified Health System (Sistema Único de Saúde, SUS), which records all hospitalizations paid by the public sector. Data are publicly available, from which we used primary and secondary level record information on cause of hospitalization (ICD-9 and ICD-10 coded), patient age, state of residence and admission dates. These data include hospitalizations occurring in public hospitals at the federal, state and municipal levels and those occurring in private and non-profit hospitals under contract to the SUS. Visits and treatments occurring at outpatient settings (e.g., health clinics, medical offices and emergency wards) are not included in this database. See supplemental information for more details.
The records of deaths/hospitalizations coded as diarrhea were filtered using codes ICD-9 codes of 001–009 (1979 – 1994) and ICD-10 codes of A00–A09 (1995 – 2014). The count of records which fulfilled the criteria above were aggregated and organized in a 3-D matrix, containing the following units in each one of the "dimensions" of the matrix: Time: monthly counts; Geography: 27 geographic units (the 26 states plus the Federal District); Age: aggregated into groups as shown in Figure 2 and Figure 3. Only records which contained information for all variables were considered; only 0.1% and 0.06% mortality and hospitalization records, respectively, were found to be missing data and thus excluded from the analysis
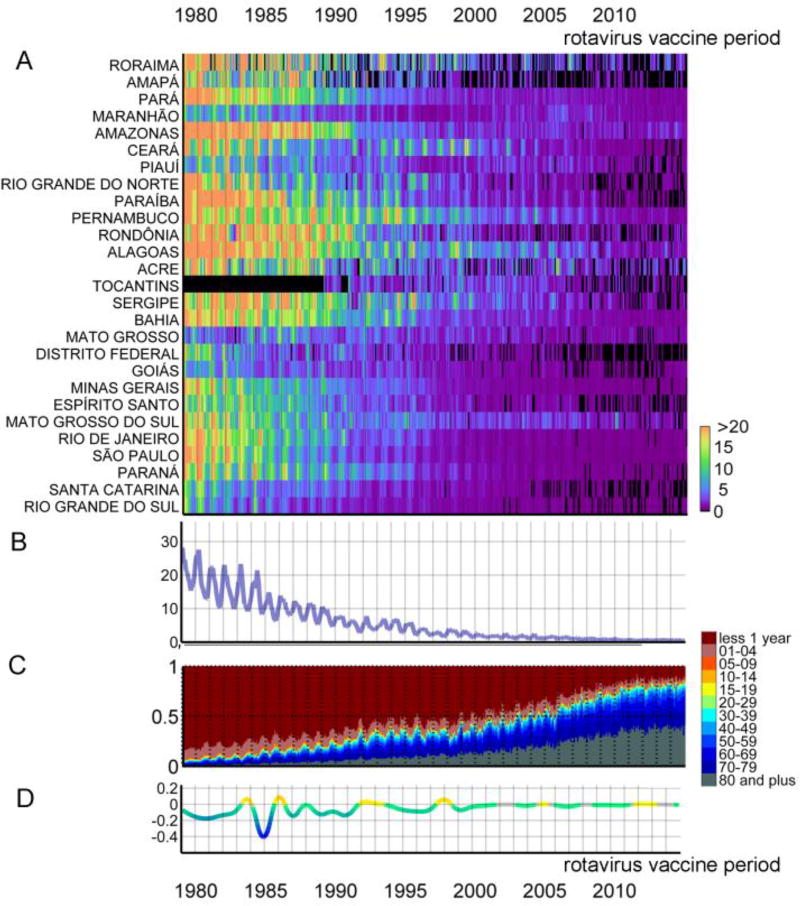
Figure 2.
Monthly diarrhea deaths. (A) Risk by state per 100,000 children under 5, sorted by the latitude of their capitals (The state of Tocantins is included beginning in 1988, the year of its secession from the state of Goiás) (B) Risk per 100,000 children under 5 (C) Proportions of deaths per age group (D) Trend of risk of death per 100,000 children under 5.
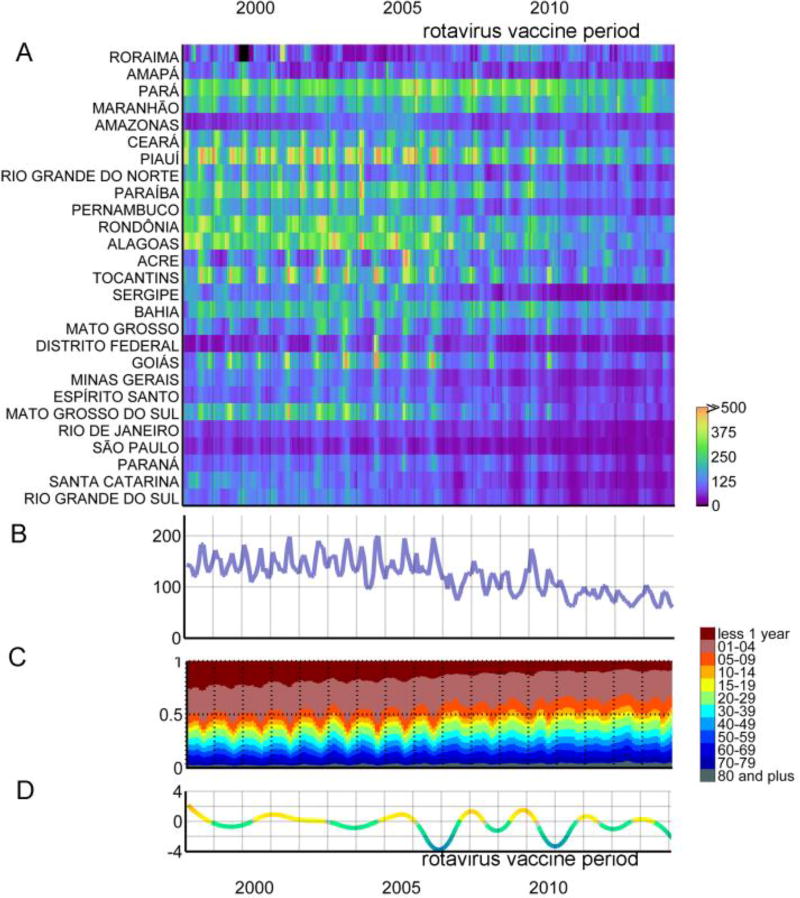
Figure 3.
Monthly diarrhea hospitalizations. (A) Risk by state per 100,000 children under 5, sorted by the latitude of their capitals (B) Risk per 100,000 children under 5 (C) Proportions of hospitalizations per age group (D) Trend of risk of hospitalization per 100,000 children under 5.
In order to obtain risk estimates, we divided the age-, state-, and month-specific case data by relevant population sizes and calculated disease rates per 100,000. The population dataset was structurally identical to the death/hospitalization-related counterparts and was obtained through temporal interpolation of data for each age group and state of the Brazilian decadal censuses (Alonso et al, in press). Since the age- and state-specific populations in each month were used, risk (or incidence proportion) was calculated and is referenced throughout the paper.[23] The reference period for calculating the mortality and hospitization risks was the entire study time period used for each at a monthly resolution (and for each state and age group).
Data Analyses
Visual inspection of cases coded as diarrhea by month and state (sorted by latitude) was performed using the heat-grid graphic,[24] displayed for the under 5 population in Figure 2A (for deaths) and Figure 3A (for hospitalizations) and the 5 and older population in eFigure1A (for deaths) and eFigure2A (for hospitalizations). Visual inspection of the relative contribution of each age group to deaths/hospitalizations per month was likewise performed (Figures 2C and 3C).
Subsequently, in the mortality dataset we identified different periods that were relatively homogenous (stationary): "Transition period" (1979 to 1988), "Modern pre-rotavirus vaccine period" (2000 to 2005) and "Modern post-rotavirus vaccine period" (2007 to 2014). The years between these periods were transitional and were avoided to minimize noise in the comparison of stationary periods enabling us to better characterize the amplitude and timing of peaks. For each stationary period and for each dataset (mortality and hospitalization), we built a periodic annual function from which timing and amplitude of the primary and secondary peaks were extracted.[24] To detect the relevant periods of increases or decreases in cases, a spline model was built for each mortality risk and hospitalization risk time series and the monthly gradient of those models were inspected.
Software
Excel (Microsoft©, Redmont, WA) was used to archive data once they were aggregated from the original source. Data processing was conducted using scripts written in Matlab R2007 (MathWorks©, Natick, WA). Finally, data analyses were performed and figures generated using the freely available software Epipoi (www.epipoi.info).[24]
RESULTS
The analyzed dataset contained nearly 519,000 deaths (1979 to 2014) and over 8.5 million hospitalizations (1998 to 2014) due to diarrhea. Of the deaths, two thirds (67.0%) occurred among children under 1 year of age and 9.5% among children aged 1 to 4 years. Hospitalizations were similarly distributed with a majority of hospitalizations among the young, however, children aged 1 to 4 years made up the largest proportion with 28.0%.
Brazil experienced tremendous declines in the risk of diarrhea-related death during the 36 year period, as shown in Figure 2A and especially apparent among children under age 5 in Figure 2B (see Supplemental Digital Content eFigure 5 for examples of state-specific time series). A majority of the decline in death from 1979 through 2014 can be attributed to the reduced risk among children less than 1 year of age (Figure 2C). Children under 1 year of age accounted for 84.4% of diarrheal deaths in 1979 and only 12.3% by 2014. Consequently, the age group responsible for the largest proportion of diarrheal deaths shifted from young children to those 70 years old and older (Figure 2C) though this age group simultaneously saw a gradual decline in risk of diarrhea-related death over the time period. As shown by the gradients of change in the spline model (Figure 2D), the most dramatic decline in risk of death for children less than 5 years of age occurred until 1992 (except the years of 1983 and 1985–6, separated by a particularly strong brief period of reduction of risk). This was followed by a more gradual decline in risk through the remainder of the 1990s before it leveled off and remained relatively stable throughout subsequent years. The nineties were punctuated by interruptions of this decrease in the years of 1992 and 1997–8.
Risk of hospitalization demonstrated a similar pattern to that seen in the risk of mortality for the corresponding timeframe, including a mild decline from the late 1990s onward. Even though the period of data available for hospitalization spans only the last 17 years of the mortality series, it also showed that the decline in hospitalization risk was mainly among children less than 1 year of age, though this shift was more subtle (Figure 3C).
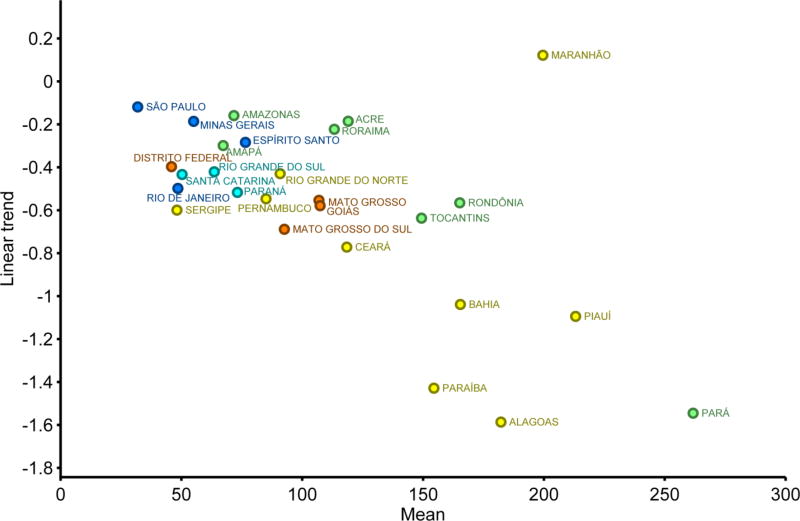
The risk of death and hospitalization caused by diarrhea, as well as the seasonality of such events, differed by region. The highest risks for both death and hospitalization occurred in the North and Northeast regions whereas the southern regions faced the lowest risk - not only historically, but also in recent years (Figures 2A). This can be observed in the scatter plot of Figure 4, which compares average risk with the linear trend of hospitalizations for the period 2007–2014. This graphic shows, for most states, a direct relation of progress toward less diarrhea in places where hospitalization incidence was higher. Nevertheless, in one state (Maranhão) the situation remains less optimistic, as the higher average risk of diarrhea hospitalization was not matched by strong progress in its reduction.
Figure 4.
Average risk per 100,000 and the linear trend of hospitalizations in children under 5 caused by diarrhea in Brazil per state for the period 2007–2014. Colors represent the different regions of Brazil (see map in Figure 1).
Seasonality
When analyzing the aggregated mortality risk data for the whole country, the first two decades were characterized by single annual high amplitude summer peaks that typically occurred early in the year, corresponding to summer and early autumn months, with no visually noticeable change in timing of the peak. In contrast, beginning in 2000, more subtle annual peaks appeared. This was generally true by region as well, though the North and Central West regions had less pronounced peaks.
Nationally, among children less than 5 years old, single annual peaks in mortality risk (Figure 2B) and bimodal peaks in morbidity (Figure 3B) were apparent. Children and adolescents ages 5 to 19 as well as adults ages 20 and older had less discernable seasonality for diarrheal mortality throughout the same period, often with bimodal peaks at the beginning and middle of the year (eFigure 1). Older children ages 5 to 19 presented, as expected, a transitional pattern between the younger children (with a more dramatic decline in risk) and adults but with no clear change in pattern. Bimodal peaks in morbidity were generally observed for older children and adults throughout the period (eFigure 2). Nevertheless, such generalizations at the national level miss the socioeconomic and climatologic diversity across the Brazilian geography. Therefore, nuanced analyses at a finer resolution (state level) were performed, as below.
Children under 5 years of age
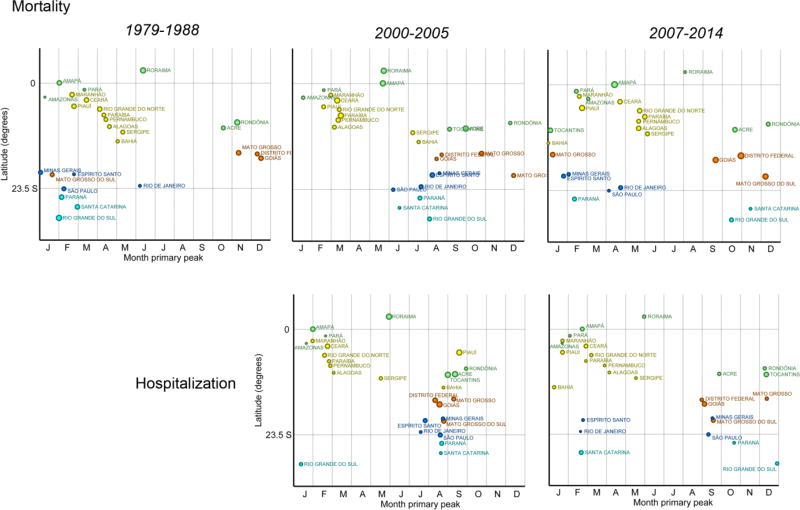
Mortality among children less than 5 years of age exhibited the most dramatic changes in seasonality as well as mortality and hospitalization frequency over the study period. Details on the seasonal patterns of risk of diarrheal deaths among children under 5 years of age are highlighted in the scatterplots shown in Figure 5 comparing three specific time periods. During the first decade for which data were available, 1979 – 1988, a time of economic transition and development for the country (the “transition period”), the primary peak in the risk of mortality most frequently occurred from December through April (summer and early autumn), regardless of latitude, with few states showing peaks elsewhere during the year. By 2000 – 2005, a “modern pre-rotavirus vaccine period” just prior to the 2006 implementation of the rotavirus vaccine for children under 1 year of age, a very different pattern was observed. During this time, the highest risk of mortality for most states within the non-equatorial (South, Southeast, and Central West) regions had shifted towards the winter season, clustering between June and October. In contrast, the primary peaks for several states in the equatorial (North and Northeast) regions of the country, remained similar, occurring throughout the year, with many remaining early autumn peaks. The strong tendency for winter peaks for the non-equatorial states shown in the previous period had dissipated by 2007 – 2014 (a “modern post-rotavirus period”), when nearly no states experienced their highest risk of diarrheal deaths within the winter season.
Figure 5.
Primary peak in risk of diarrheal mortality (first row) and hospitalizations (second row) per 100,000 children under 5 years in Brazil by state for three periods: "Transition period" (1979 to 1988), "Modern pre-rotavirus vaccine period" (2000 to 2005) and "Modern post-rotavirus vaccine period" (2007 to 2014). The size of circles are proportional to the amplitude of the seasonality. Colors represent the different regions of Brazil (see map in Figure 1).
A similar relationship between time period and highest risk was seen for hospitalizations among this age group (Figure 5). From 2000 – 2005, the highest risk of hospitalization for non-equatorial states occurred most commonly between July and September (winter) while the equatorial states generally demonstrated peaks between January and early march (summer). As with mortality, the highest risk of hospitalization from 2007 – 2014 occurred outside the winter season for a majority of non-equatorial states.
Older children, adolescents, and adults
The risk of diarrhea-related death among children and adolescents ages 5 to 19 years displayed nearly no pattern in regard to timing of peak by geographic location (see Supplemental Digital Content eFigure 3). Throughout a majority of the study period, the highest risk of death for individual states ranged throughout the year. Diarrhea-related hospitalization for this age group tended to have the highest incidence clustered in February (for all regions except the Central West) and September (for the Central West region), which spread slightly over time. Similar patterns in deaths and hospitalizations were observed among adults aged 20 years and older (see Supplemental Digital Content eFigure 4).
DISCUSSION
Like many other developing countries,[5,25] substantial changes in health and diarrheal disease have occurred in Brazil in the past few decades[26] (additional information available from the recent Global Burden of Disease analysis at http://ihmeuw.org/3z6r [27]). Primarily a killer of Brazilian children under 1 year of age in the late 1970s and 1980s, the populations with the highest burden of diarrhea deaths today are those 70 years of age and older – and even so with lower levels of risk than before – showing the success of many public health interventions and technological advances in recent decades. Our study not only confirmed those uplifting trends, but also uncovered two very interesting and novel phenomena, which are scientifically and operationally valuable.
The first is that we can observe the evolution of diarrheal disease patterns and identify the success of rotavirus vaccination policies by looking at seasonal patterns. This is detected not only quantitatively by the change in risk of death over the 36-year period but, because some pathogens have defined seasonal patterns, also qualitatively, with important changes in the seasonal components. We show that Brazil, although with significant deficiencies in basic sanitation,[28] has not only surpassed the pre-sanitation era (which, as previously shown, is characterized by strong summer seasonality), but also the pre-rotavirus vaccination era (which featured winter peaks) when examining trends in diarrheal morbidity and mortality among children under 5. This can be detected in non-equatorial Brazil, where the colder winter season demonstrates a viral diarrhea signature when the predominantly summer bacterial diarrhea signal is removed in the pre-rotavirus vaccination era. In the current post-sanitation and post-rotavirus vaccination era, the seasonality of diarrhea in non-equatorial regions still resembles the pre-sanitation era qualitatively (summer peaks), but not quantitatively, with the risk in 2014 being only a fraction of that of 1979. This analytical approach can be applicable to similar epidemiological inquiries in other settings.
The second notable finding is the seasonality of diarrheal deaths and hospitalizations among the different age groups, showing that young children have distinct annual patterns compared to other age groups, probably due to different pathogens to which, somehow, older ages are less sensitive. Interestingly, for both older children and adults, a seasonal pattern in hospitalizations but not mortality was observed. These findings ma y suggest that the pathogens responsible for severe diarrheal disease among these age groups may cause seasonal peaks in hospitalizations on top of an underlying burden of especially severe diease (causing mortality) that remains relatively constant throughout the year.
The sustained transition among the under 5 year-old population away from primarily winter peaks, a potential signature of rotavirus-dominated diarrheal disease, is suggestive of a shift in pathogen-specific disease burden and supported by etiologic data. Prior to vaccination, rotavirus among hospitalized children was seen most commonly during the winter months.[29] In contrast, after the nationwide initiation of the vaccine, the prevalence of rotavirus among hospital-treated children with diarrhea was substantially reduced[19] and, in some areas, was not the predominant virus during the winter season.[9,20] Vaccine coverage increased rapidly after its introduction in the country with some models suggesting that coverage was as high as 70% by the end of 2007 and nearly 90% by 2014.[30] This shift in seasonality among the young Brazilian population supports existing evidence of the vaccine’s success in various settings.[9,31]
Evaluation of long-term spatial and temporal trends across age groups hints at differential etiology and potential effects of vaccination across the age groups. In the immediate pre-vaccination era, peak risk in diarrheal disease mortality among Brazilians ages 5 to 19 and 20 and older demonstrated little seasonal pattern. This can be distinguished from the more well-defined pattern observed in the 20 and older populations after the 2006 initiation of rotavirus vaccination among infants when most states experience peak mortality risk from January through June (mid-summer through early winter). Both populations experienced a shift in peak hospitalization from the summer months towards autumn months, mimicking, though to a lesser extent, the exclusion of winter peaks seen among the vaccinated population. Specifically examining hospitalizations, the immediate post-vaccination period for children under 5 included a substantial decline in the rate of hospitalizations (Figure 3B and Figure 3D; regression slope: 0.50; 95% C.I.: −0.64 to −0.36; see eTable 4 for state level data). A similar decline was not apparent among the 5 and older population (eFigure 2B and eFigure 2D; regression slope: −0.06; 95% C.I.: −0.07 to −0.05; eTable 4 for state level data), potentially suggesting limited indirect effects of the rotavirus vaccine among the older population shortly after its introduction. This contrast adds to existing evidence of the limited effects of vaccination among non-vaccinated populations in the county.[18] In-depth questions regarding indirect effects, the role of accumulation of immunity or waning effectiveness of vaccination require further investigation and microbiological analysis.[32], [33]
It is important to note that the analyses of the impact of a specific intervention are blurred by the set of complementary, but statistically competing, factors. This is especially important for trends, which reflect not only effectiveness of vaccinations, but improvements in living conditions and medical services.[34] Recent studies aiming to ascertain more about the existing burden and mechanisms of diarrheal illness in Brazil have identified several determinants of disease, including socioeconomic status, sanitation conditions, and prenatal care, among other factors.[35] Specifically for Brazil, a nationwide program called ‘Pact for Health’ was introduced in 2006, with one of its goals being to reduce diarrhea and pneumonia deaths among young children. The program created local committees to monitor child mortality and trained medical staff in proper treatment and eduation about these diseases.[34] These measures likely added to the effect of immunization programs, including the introduction of the rotavirus vaccine in the same year, potentially by reducing the severity of disease and alterning transmission patterns..
The analysis described here is not without limitations. Mortality data captured cause of death for a vast majority of the population around the country; however, coverage differed slightly by region and may have potentially varied by year if collection methods were modified over time. As mentioned previously, the hospitalization data included in the analysis is limited to illness diagnosed in hospital discharge records. These data exclude individuals with less severe disease that may have sought treatment at outpatient clinics or private offices, and those for which no medical treatment was sought. Further constraints result from the geographic analysis which was limited to state-level data, linked with latitudinal values for each state’s capital. This level of detail allows for identification of high-level geographic associations but does not enable detailed analysis within states and may not represent within state patterns related to population density or distribution. Over time, shifts in proportion of people seeking care in the private versus public sector likely occurred and potentially occurred differentially by state. Fortunately, such differences are not expected to impact the results as neither seasonality nor diarrheal admission trends are expected to be sensitive to the type of hospital. Finally, it is important to note limitations related to the ICD coding in this analysis. Several issues should be considered, including possible changes in how healthcare providers coded rotavirus and other gastroenteritis diseases with the shift in ICD coding, changes in rotavirus testing patterns that may have accompanied the introduction of the vaccine, and unknown validity of the ICD coding for gastroenteritis in Brazil. This analysis attempted to minimize these challenges by including all ICD-9 codes specific to infectious causes of gastroenteritis (excluding gastrointestinal illness presumed to be non-infectious) and the corresponding ICD-10 codes.
The analysis presented here analyzes data from three and half decades during which Brazil experienced substantial changes. In fact, the country was still a military dictatorship when the series began (the transition to democracy occurred in 1985) and the country later went through a severe economic crisis.[36] It is encouraging to realize that, despite these changes, the trends in infant diarrhea (an important indicator of social progress) had been declining almost without interruption. This study is limited in its ability to detangle the weight of a multitude of factors that contributed to this trend. Nevertheless, we believe that, with an original approach, we were able to demonstrate the role of one of those factors (rotavirus vaccination).
Analysis of the long-term, monthly mortality and hospitalization data across all ages from each state in Brazil provided an opportunity to discern spatial and seasonal patterns in diarrheal illness throughout the country. Such analysis contributes to the growing body of literature and a better conceptual understanding of the burden of diarrheal disease, its drivers, and value of certain interventions across age groups. Substantial reductions in diarrheal disease have occurred over time and following the nationwide introduction of a rotavirus vaccine to children under 1 year of age. Along with these changes were shifts in the distribution of disease burden by age group and geography.
Supplementary Material
HIGHLIGHTS.
Diarrheal pathogens have distinct regional seasonal signatures in Brazil.
Infant seasonal diarrheal disease signature shows rotavirus vaccination success.
Diarrheal disease burden in Brazil shifted by age group and geography.
Acknowledgments
The authors are grateful to the Department of Vital Statistics, a branch of the Brazilian Ministry of Health, for providing the mortality and hospitalization data. We also thank Cynthia Schuck-Paim for the background information relating to the hospitalization data. Thanks to Roger Glass, Peter Kilmarx, Cecile Viboud, Rodolfo Acuña-Soto and the anonymous referees for their valuable suggestions to our manuscript.
Funding: This work was performed in the context of the MISMS study, a multinational research program focused on the epidemiology and evolutionary dynamics of influenza led by the Fogarty International Center (WJA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016 Oct;388(10053):1459–544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO; [cited 2015 Aug 8]. Global Health Observatory Data Repository [Internet] Available from: http://apps.who.int/gho/data/node.main.CODWORLD?lang=en. [Google Scholar]
- 3.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013 Apr 20;381(9875):1405–16. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker CLF, Black RE. Diarrhoea morbidity and mortality in older children, adolescents, and adults. Epidemiol Infect. 2010 Sep;138(9):1215–26. doi: 10.1017/S0950268810000592. [DOI] [PubMed] [Google Scholar]
- 5.Lamberti LM, Fischer Walker CL, Black RE. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health. 2012 Apr 6;12:276. doi: 10.1186/1471-2458-12-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer Walker CL, Sack D, Black RE. Etiology of Diarrhea in Older Children, Adolescents and Adults: A Systematic Review. [cited 2015 Aug 13];PLoS Negl Trop Dis [Internet] 2010 Aug 3;4(8) doi: 10.1371/journal.pntd.0000768. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2914743/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68(2):171–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Patel MM, Pitzer V, Alonso WJ, Vera D, Lopman B, Tate J, et al. Global Seasonality of Rotavirus Disease. Pediatr Infect Dis J. 2013 Apr;32(4):e134–47. doi: 10.1097/INF.0b013e31827d3b68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) The Lancet Global Health. 2015 Sep;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaper JB, Morris JG, Levine MM. Cholera. Clin Microbiol Rev. 1995 Jan;8(1):48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed SF, Farheen A, Muzaffar A, Mattoo GM. Prevalence of Diarrhoeal Disease, its Seasonal and Age Variation in under- fives in Kashmir, India. Int J Health Sci (Qassim) 2008 Jul;2(2):126–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes TAT, Rassi V, MacDonald KL, Ramos SRTS, Trabulsi LR, Vieira MAM, et al. Enteropathogens Associated with Acute Diarrheal Disease in Urban Infants in São Paulo, Brazil. J Infect Dis. 1991 Aug 1;164(2):331–7. doi: 10.1093/infdis/164.2.331. [DOI] [PubMed] [Google Scholar]
- 13.Cockburn TA, Cassanos JG. Epidemiology of endemic cholera. Public Health Rep. 1960 Sep;75:791–803. [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TF, McMillian MB, Scallan E, Frenzen PD, Cronquist AB, Thomas S, et al. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996–2003. Epidemiology and Infection. 2007 Feb;135(02):293. doi: 10.1017/S0950268806006765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Z, Hu W, Zhang Y, Wang X, Zhou M, Su H, et al. Exploration of diarrhoea seasonality and its drivers in China. [cited 2015 Aug 8];Sci Rep [Internet] 2015 Feb 4;:5. doi: 10.1038/srep08241. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4316158/ [DOI] [PMC free article] [PubMed]
- 16.Etard J-F. Childhood mortality and probable causes of death using verbal autopsy in Niakhar, Senegal, 1989–2000. International Journal of Epidemiology. 2004 Dec 1;33(6):1286–92. doi: 10.1093/ije/dyh259. [DOI] [PubMed] [Google Scholar]
- 17.Levy K, Hubbard AE, Eisenberg JN. Seasonality of rotavirus disease in the tropics: a systematic review and meta-analysis. Int J Epidemiol. 2009 Dec;38(6):1487–96. doi: 10.1093/ije/dyn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.do Carmo GMI, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in Diarrhea Mortality and Admissions after Routine Childhood Rotavirus Immunization in Brazil: A Time-Series Analysis. PLoS Med. 2011 Apr 19;8(4):e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurgel RG, Bohland AK, Vieira SCF, Oliveira DMP, Fontes PB, Barros VF, et al. Incidence of Rotavirus and All-Cause Diarrhea in Northeast Brazil Following the Introduction of a National Vaccination Program. Gastroenterology. 2009 Dec;137(6):1970–5. doi: 10.1053/j.gastro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 20.Raboni SM, Damasio GAC, Ferreira CEO, Pereira LA, Nogueira MB, Vidal LR, et al. Acute gastroenteritis and enteric viruses in hospitalised children in southern Brazil: aetiology, seasonality and clinical outcomes. Mem Inst Oswaldo Cruz. 2014 Jul;109(4):428–35. doi: 10.1590/0074-0276140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IMF. Gross domestic product per capita, current prices. [cited 2015 Oct 27];Report for Selected Countries and Subjects [Internet] 2015 Available from: http://www.imf.org/
- 22.DATASUS. Vital Statistics Brazil. Brazilian ministry of health [Internet] 2016 Available from: http://www.datasus.gov.br.
- 23.Centers for Disease Control and Prevention. Lession 3: Measures of Risk [Internet]. Principles of Epidemiology in Public Health Practice, Third Edition, An Introduction to Applied Epidemiology and Biostatistics. [cited 2015 Dec 30]; Available from: http://www.cdc.gov/ophss/csels/dsepd/ss1978/lesson3/section2.html.
- 24.Alonso WJ, McCormick BJ. EPIPOI: A user-friendly analytical tool for the extraction and visualization of temporal parameters from epidemiological time series. BMC Public Health. 2012 Nov 15;12(1):982. doi: 10.1186/1471-2458-12-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso WJ, AcuñA-Soto R, Giglio R, Nuckols J, Leyk S, Schuck-Paim C, et al. Spatio-temporal patterns of diarrhoeal mortality in Mexico. Epidemiology and Infection. 2012 Jan;140(01):91–9. doi: 10.1017/S0950268811000562. [DOI] [PubMed] [Google Scholar]
- 26.Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. The Lancet. 2011 May;377(9779):1778–97. doi: 10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
- 27.IHME. Seattle, WA: IHME, University of Washington, 2016; 2016. [cited 2016 Dec 7]. Causes of Death (COD) Visualization. Available from: http://vizhub.healthdata.org/cod/ [Google Scholar]
- 28.Machado AP. The Next Battle for Brazil: Public Sanitation. [cited 2016 Jan 2];Forbes [Internet] 2015 Jan 5; Available from: http://www.forbes.com/sites/arthurmachado/2015/01/05/the-next-battle-for-brazil-public-sanitation/
- 29.Bittencourt JA, Arbo E, Malysz AS, Oravec R, Dias C. Seasonal and age distribution of rotavirus infection in Porto Alegre--Brazil. Braz J Infect Dis. 2000 Dec;4(6):279–83. [PubMed] [Google Scholar]
- 30.Schuck-Paim C, Taylor R, Lindley D, Klugman KP, Simonsen L. Use of near-real-time medical claims data to generate timely vaccine coverage estimates in the US: the dynamics of PCV13 vaccine uptake. Vaccine. 2013 Dec 5;31(50):5983–8. doi: 10.1016/j.vaccine.2013.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Paternina-Caicedo A, Parashar UD, Alvis-Guzmán N, De Oliveira LH, Castaño-Zuluaga A, Cotes-Cantillo K, et al. Effect of rotavirus vaccine on childhood diarrhea mortality in five Latin American countries. Vaccine. 2015 Jul;33(32):3923–8. doi: 10.1016/j.vaccine.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luchs A, Cilli A, Morillo SG, de Cassia Compagnoli Carmona R, do Carmo Sampaio Tavares Timenetsky M. Rotavirus in adults, Brazil, 2004–2011: G2P[4] dominance and potential impact on vaccination. The Brazilian Journal of Infectious Diseases. 2014 Jan;18(1):53–9. doi: 10.1016/j.bjid.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Correia JB, Patel MM, Nakagomi O, Montenegro FMU, Germano EM, Correia NB, et al. Effectiveness of Monovalent Rotavirus Vaccine (Rotarix) against Severe Diarrhea Caused by Serotypically Unrelated G2P[4] Strains in Brazil. The Journal of Infectious Diseases. 2010 Feb;201(3):363–9. doi: 10.1086/649843. [DOI] [PubMed] [Google Scholar]
- 34.Schuck-Paim C, Taylor RJ, Simonsen L, Lustig R, Kürüm E, Bruhn CAW, et al. Challenges to estimating vaccine impact using hospitalization data. [cited 2016 Nov 28];Vaccine [Internet] 2016 doi: 10.1016/j.vaccine.2016.11.030. Available from: http://www.sciencedirect.com/science/article/pii/S0264410X16310775. [DOI] [PMC free article] [PubMed]
- 35.Genser B, Strina A, Teles CA, Prado MS, Barreto ML. Risk factors for childhood diarrhea incidence: dynamic analysis of a longitudinal study. Epidemiology. 2006 Nov;17(6):658–67. doi: 10.1097/01.ede.0000239728.75215.86. [DOI] [PubMed] [Google Scholar]
- 36.Paim J, Travassos C, Almeida C, Bahia L, Macinko J. The Brazilian health system: history, advances, and challenges. The Lancet. 2011 May;377(9779):1778–1897. doi: 10.1016/S0140-6736(11)60054-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.