Abstract
Background
Computerized tomography (CT) imaging is routine in oncologic care and can be used to measure muscle quantity and composition that may improve prognostic assessment of older patients. This study examines the association of single-slice CT-assessed muscle measurements with a frailty index in older adults with cancer.
Methods
Using the Carolina Senior Registry, we identified patients with CT imaging within 60 days +/– of geriatric assessment (GA). A 36-item Carolina Frailty Index was calculated. Cross-sectional skeletal muscle area (SMA) and skeletal muscle density (SMD) were analyzed from CT scan L3 lumbar segments. SMA and patient height (m2) were used to calculate skeletal muscle index (SMI). Skeletal Muscle Gauge (SMG) was calculated by multiplying SMI × SMD.
Results
Of the 162 patients, mean age 73, 53% were robust, 27% pre-frail, and 21% frail. Significant differences were found between robust and frail patients for SMD (29.4 vs 24.1 HU, p<0.001) and SMG (1188 vs 922 AU, p=0.003), but not SMI (41.9 vs 39.5 cm2/m2, p=0.29). After controlling for age and gender, for every 5 unit decrease in SMD, the prevalence ratio of frailty increased by 20% (PR = 1.20 [1.09, 1.32]) while the prevalence of frailty did not differ based on SMI.
Conclusions
Muscle mass (measured as SMI) was poorly associated with a GA-based frailty index. Muscle density, which reflects muscle lipid content, was more associated with frailty. Although frailty and loss of muscle mass are both age-related conditions that are predictive of adverse outcomes, our results suggest they are separate entities.
Keywords: frailty, sarcopenia, skeletal muscle index, muscle attenuation, skeletal muscle gauge, cancer, geriatric oncology
Introduction
Cancer is primarily a disease of older adults, with the majority of new cancer diagnoses and cancer deaths occurring in patients over the age of 65.1 By 2030, 70% of all new cancer diagnoses will occur in adults over 65 years of age.2 The treatment of older adults with cancer is complicated by the heterogeneous aging process and is associated with a wide range in treatment tolerability. Frailty is a medical condition? status? with multiple causes and contributors that ultimately increase an individual’s vulnerability for adverse outcomes such as increased dependency and/or death.3,4 Many frail older adults lack the physiologic reserve to recover from surgical operations or from the acute toxicities of chemotherapy or radiation.5,6 Several methods exist for defining frailty, yet frailty assessment is rarely employed in routine clinical practice and decision-making. Recent publications have highlighted the benefits of using a frailty index in older adults with cancer that categorizes patients as robust (or non-frail), pre-frail, and frail, and have shown it to be predictive of all-cause mortality, as well as increased severe chemotherapy toxicity and hospitalizations.7,8 Developing more precise and easier methods to identify frail, at-risk older adults with cancer is a focus of ongoing research.9,10
Low muscle mass, commonly termed sarcopenia within oncology, is highly prevalent in older adults, and has been associated with increased chemotherapy toxicity, surgical complications, and mortality.11–14 Computerized tomography (CT) imaging, utilized in routine oncologic care for staging, disease monitoring, and surveillance, can also be used to assess muscle mass without significant resource allocation. Consistent methods for utilizing CT imaging to achieve practical and precise measurements of body composition have been developed.15,16 CT-based body composition measures represent a nascent opportunity to improve the assessment of older patients and provide additional prognostic information, including the identification of frail, at-risk patients.
This study examines associations between single-slice, CT-assessed skeletal muscle measurements and a frailty index derived from a brief geriatric assessment. Our specific focus is on older adults diagnosed with cancer, in which treatment decisions require careful attention to prognosis and the potential for treatment toxicities. As both frailty and low muscle mass are highly prevalent age-related conditions that are associated with adverse outcomes, we sought to better understand their interrelationship.
Methods
Participants
The sample for this cross-sectional study was derived from the Carolina Senior Registry (CSR) (NCT01137825). The CSR was developed in 2009 as a clinic-based registry to collect geriatric assessment (GA) data on older adults (65+) with cancer. The CSR utilizes a well-validated GA tool designed specifically for use with older adults with cancer.17–19 For a detailed description of the CSR, including recruiting procedures, sampling methods, and the performance of assessments, please see Williams et al.19 Using electronic medical records, we identified CSR patients recruited through outpatient clinics of the North Carolina Cancer Hospital that had CT abdominal imaging within 60 days of completing the GA. This study was approved by the Institutional Review Board of the University of North Carolina (IRB #15-1524).
Frailty Index
The primary outcome in our study is frailty, as determined by the 36-item Carolina Frailty Index (CFI). This cancer-specific frailty index was created based on the principles of deficit accumulation and methodology reported by Searle et al and uses 36 items from the GA.8,20,21 The CFI includes multiple items pertaining to deficits in basic and instrumental activities of daily living, comorbidities, cognition, depression, and nutrition. Guerard et al showed that the CFI was predictive of all-cause and cancer-specific mortality in older adults with cancer independent of cancer stage, cancer type, age, sex, and number of comorbidities.8 A similarly constructed frailty index was also associated with increased grade 3/4 chemotherapy toxicity and hospitalizations in older adults undergoing chemotherapy.7 A list of CFI variables is provided in Supplement 1. Each variable/deficit is rated between 0 and 1, where a higher score indicates greater frailty. A score is achieved by calculating the total number of deficits divided by the total number of variables assessed. For instance, if 8 deficits are identified in a patient of 36 possible deficits, then that person’s frailty index is 8/36= 0.22. The CFI is a continuous variable with a range of 0–1 and categorized patients as robust (<0.2), pre-frail (0.2–0.35), or frail (>0.35) based on cumulative deficit counts. Patients were included in our final sample if they answered at least half (18) of the 36 CFI variables.
Body composition analysis
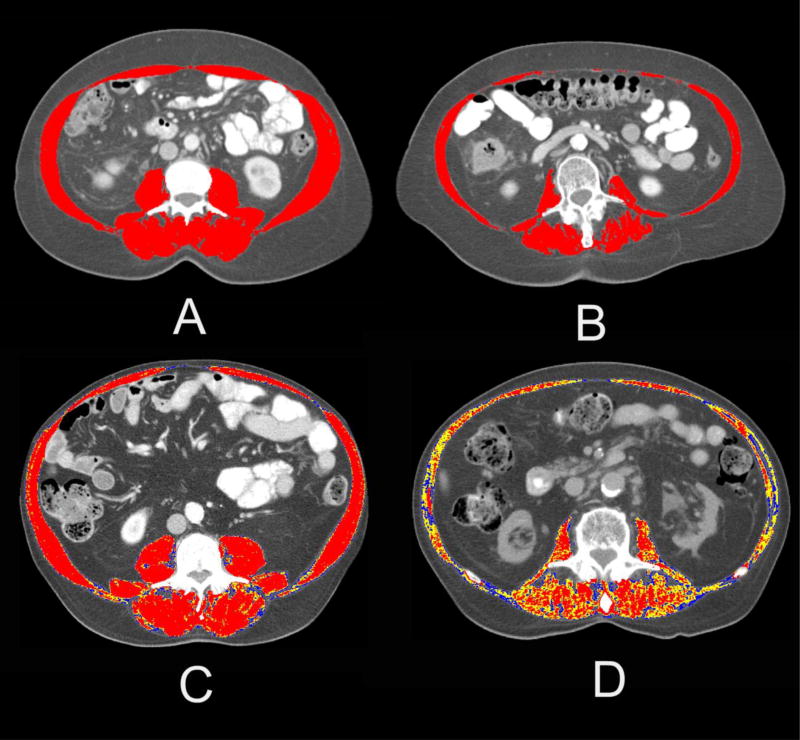
Abdominal CT images were acquired from the UNC Picture Archiving and Communication office. Using AGFA-Impax (version 6) radiological software (Mortsel, Belgium), transverse sections at the L3 vertebral level were identified and extracted for external analysis. Automated Body composition Analyzer using Computed tomography image Segmentation (ABACS) software was then used to analyze the L3 lumbar segments.15,22 The software recognizes muscle tissue based on density thresholds between −29 and +150 Hounsfield Units (HU) and provides an unbiased estimation of the cross-sectional skeletal muscle area. The software also uses a priori information about the L3 muscle shape to avoid mislabeling parts of the neighboring organs that have HU values similar to those of muscle tissue. Images were then reviewed and corrected for accuracy and verified by two authors (GRW, SSS). The measured skeletal muscle area (SMA) was then normalized for height (in meters) to calculate a skeletal muscle index (SMI) (cm2/m2). Skeletal muscle density (SMD) was derived by averaging the HU of skeletal muscle of the cross-sectional image. The attenuation of skeletal muscle is a non-invasive radiological technique to indirectly assess muscle fat content, known as myosteatosis. The density of skeletal muscle is inversely related to muscle fat content.23 Figure 1 illustrates the visual differences of these various muscle measures. Images A and B show two patients with identical BMI but varying skeletal muscle quantity, with image B representing a patient with low muscle mass (low SMI). Images C and D represent two patients with similar BMI that primarily differ in terms of muscle density, as shown by the color variation.
Figure 1. Examples of representative abdominal Computerized Tomography (CT) images.
Images A and B illustrate differences in skeletal muscle mass in patients with the same BMI (BMI=25). For the top portion of the figure, the red area highlights the skeletal muscle area between −29 and +150 Hounsfield Units (HU). Images C and D represent two patients with contrasting values of muscle radiodensity. For the lower portion of the figure, the red area represents skeletal muscle within the normal range of radiodensity (+30 to +150 HU), the yellow represents +1 to +29 HU, and the blue represents 0 to −29 HU.
To integrate both the quantity (SMI) and attenuation/quality (SMD), we calculated the skeletal muscle gauge (SMG) by multiplying SMI × SMD. The actual units for SMG are (cm2 tissue area × average HU)/(m2 height); for simplicity we chose to represent them as arbitrary units (AU). This method was first introduced by Weinberg et al and showed better correlation of SMG with aging than either SMD or SMI alone.24 SMG has also been associated with toxicity and survival in patients with metastatic breast cancer receiving first-line taxane therapy.25
Statistical analysis
Descriptive statistics were used to characterize the baseline characteristics of the sample. Kruskall-Wallis and Wilcoxon non-parametric tests were used to compare distributions of skeletal muscle measures between frailty groups and gender. Pearson correlation coefficients were used to evaluate associations of continuous CFI with skeletal muscle measures. Prevalence Ratios (PR) and 95% confidence interval (CI) for the prevalence of frailty were calculated using Poisson regression,26 with both unadjusted and adjusted (age, gender, BMI) PR estimated. SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC) was used for all analyses.
Results
Study population
Of the 771 patients available within the CSR, 207 had available CT imaging within 60 days of completion of the GA. Of those with available imaging, 185 were adequate for body composition evaluation, of which 162 had sufficient GA variables (answered at least 18 of the 36 variables) to calculate a frailty index. For the 162 patients included in this study, the median age was 71 (interquartile range [IQR] 68–77), 57% were female, and 83% were white (see Table 1). The most common cancer types were breast cancer (29%), lung cancer (18%), and gastrointestinal malignancies (14%). The majority of patients had the GA performed during treatment (50%). Using the CFI, 85 patients (53%) were identified as robust, 43 patients (27%) as pre-frail, and 34 patients (21%) as frail. The median time between GA performance and receipt of CT imaging was 2 days (IQR −16 to 0), and the majority of patients (83%) had the GA and imaging performed within 30 days of each other.
Table 1.
Patient characteristics
| Total Sample (n=162) |
|
|---|---|
|
| |
| Age, median (IQR) | 71 (68–77) |
|
| |
| Gender, n (%) | |
| Male | 69 (43) |
| Female | 93 (57) |
|
| |
| Race, n (%) | |
| White | 134 (83) |
| Black/Other | 28 (17) |
|
| |
| Education, n (%) | |
| Some high school | 23 (14) |
| High school degree | 75 (47) |
| Associates/Bachelor’s degree | 35 (22) |
| Advanced degree | 29 (18) |
|
| |
| Cancer type, n (%) | |
| Breast cancer | 46 (29) |
| Lung cancer | 29 (18) |
| GI malignancy | 22 (14) |
| GU malignancy | 17 (11) |
| Hematological malignancy | 16 (10) |
| Other | 28 (18) |
|
| |
| Treatment Phase, n (%) | |
| Before treatment | 49 (31) |
| During treatment | 78 (50) |
| After treatment | 30 (19) |
|
| |
| Carolina Frailty Index, n (%) | |
| Robust (0–0.2) | 85 (53) |
| Pre-frail (0.2–0.35) | 43 (27) |
| Frail (>0.35) | 34 (21) |
Abbreviations: IQR, interquartile range; GI, gastrointestinal; GU, genitourinary
Body composition metrics
The median BMI was 26.5 (IQR 23.2–29.1), SMI 40.7 cm2/m2 (IQR 35.5–47.3), SMD 26.0 HU (IQR 26.1–31.3), and SMG 1095 AU (IQR 826–1362). Significant differences were found between men and women for SMI and SMG (44.8 versus 37.7 cm2/m2, p<0.001, and 1220 versus 1020 AU, p=0. 003, respectively). There were no differences in SMD or BMI by sex. The patient sample exhibited a wide variation in body composition.
Frailty and skeletal muscle measures
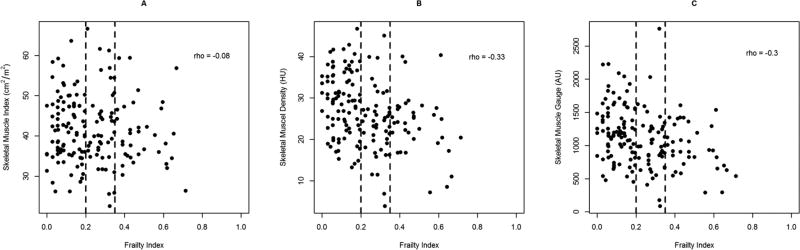
Overall, no significant differences were found between robust, pre-frail, and frail patients for SMI (41.9, 40.0, 39.5 cm2/m2, p=0.60) (see Table 2). Significant differences were found between robust and frail patients for SMD (29.4 vs 24.1 HU, p=<0.001) and SMG (1188 vs 922 AU, p=0.003). Using CFI as a continuous variable, SMI had weak negative correlation with the frailty index (rho= −0.08) (see Figure 2). Both SMD and SMG had moderate negative correlation with CFI (rho= −0.33 and −0.30, respectively).
Table 2.
Median skeletal muscle measures and Prevalence Ratios† for frailty (frail & pre-frail combined).
| Median | Unadjusted Prevalence Ratio (95% CI) |
Adjusted (for age & gender) Prevalence Ratio (CI) |
Adjusted (for age, gender, & BMI) Prevalence Ratio (CI) |
|||
|---|---|---|---|---|---|---|
| Robust (n=85) |
Pre-frail (n=43) |
Frail (n=34) |
||||
| Skeletal Muscle Index (cm2/m2) | 41.9 | 40.0 | 39.5 | 1.02 (0.93, 1.13) | 0.95 (0.84, 1.07) | 1.01 (0.89, 1.14) |
| Skeletal Muscle Density (HU) | 29.4 | 24.4** | 24.1** | 1.23 (1.13,1.35)** | 1.20 (1.09, 1.32)** | 1.14 (1.00,1.31) |
| Skeletal Muscle Gauge (AU) | 1188 | 987** | 922** | 1.07 (1.03, 1.12)* | 1.06 (1.01, 1.11)* | 1.04 (0.98, 1.10) |
p=<0.05
p=<0.01 as compared to Robust
Prevalence Ratios for pre-frail and frail vs robust are for 5 cm2/m2 decrease in skeletal muscle index, 5 HU decrease in skeletal muscle density, and 100 AU decrease in skeletal muscle gauge.
Abbreviations: HU, Hounsfield Units; AU, Arbitrary Units; CI, Confidence Intervals; BMI, Body Mass Index.
Figure 2.
Scatter plots of skeletal muscle measures plotted against frailty index. Abbreviations: HU, Hounsfield Units; AU, Arbitrary Units.
Both unadjusted and after controlling for age and gender, similar relationships between body composition measures and CFI were seen. After adjusting for age and gender, for every 5 unit decrease in SMD, the prevalence ratio of frailty (frail and pre-frail combined, versus robust) increased by 20% (PR = 1.20 [1.09, 1.32]) and for every 100 AU unit decrease in SMG, the prevalence ratio of frailty (frail and pre-frail) increased by 6% (PR = 1.06 [1.01, 1.11]). The prevalence of frailty did not differ based on SMI (see table 2). After additionally adjusting for BMI, only SMD showed a suggestion of adding additional information with PR=1.14 (1.00, 1.31), p = 0.0575.
Discussion
Although low skeletal muscle mass, commonly known as sarcopenia within oncology, has been associated with adverse outcomes in oncology, we found only a weak association between muscle mass and frailty in our sample of older adults with cancer. The prevalence of frailty did not differ based on SMI in either unadjusted or adjusted analyses. Instead, skeletal muscle density, which indirectly represents the composition and presence of muscle lipid content, was more associated with frailty than muscle quantity. Decreased muscle density was significantly related to an increased prevalence of frailty even after controlling for age and gender; however, this finding was only borderline significant after additionally controlling for BMI. This suggests that the quality of muscle, rather than quantity, may be more important in identifying and understanding frail older patients.
Over the past decade, there has been an increased interest in the role of muscle mass in oncology, with numerous articles highlighting the negative impact of low muscle mass on adverse events.12 However, this increased risk of adverse outcomes does not appear to be directly related to underlying frailty. Although sarcopenia and frailty have some commonalities and are often used interchangeably, they appear to represent separate entities with different constructs.27,28 The term “sarcopenia” specifically connotes a loss of skeletal muscle and function, while frailty is a broader term used to indicate reduced homeostatic reserves.3,29 The most apparent overlap of sarcopenia and frailty is impaired physical function and disability.27 As the evidence for a frailty phenotype was conceptualized by Fried et al, loss of muscle mass was hypothesized as a core component of the frailty cycle.4 Understanding the causal relationship between sarcopenia and frailty is complicated and controversial and limited by our lack of understanding of the aging process.27,30 There is growing consensus that although sarcopenia may be a component of frailty, frailty is more multifaceted than sarcopenia alone.3,29,31,32 The general concept of frailty goes beyond physical factors and encompasses social and psychological dimensions as well, including social support and cognitive function.33 Furthermore, therapeutic approaches to the two age-related conditions may also vary. For example, while the treatment of sarcopenia is focused predominantly on increasing strength and muscle mass, frailty requires a broader focus of the diverse underlying pathophysiology.28
Discordance between frailty and low muscle mass has been found by other investigators, as well. Preliminary work from the ONCOSARCO project in Europe has come to a similar conclusion of variation? Divergence? between these conditions. Using either the Fried criteria or the Balducci criteria, frailty was not associated with sarcopenia (using the European Working Group on Sarcopenia in Older Persons [EWGSOP] definition).34 Other studies outside oncology have shown similar results.28,35,36 Using three different definitions of sarcopenia and two different frailty definitions, Reijnierse et al showed little uniformity? in prevalence rates of the two conditions in older adults without cancer.28 Comparing studies is difficult, as neither sarcopenia nor frailty has a consensus definition and a variety of definitions are employed. Studies using a physical frailty definition tend to show more overlap with sarcopenia because this approach uses low handgrip strength and weight loss as part of its criteria.28,35 Similarly, sarcopenia definitions that include reduced handgrip strength and low gait speed, such as EWGSOP, have more concordance with frailty. While there is some overlap between sarcopenia and frailty, the consensus is that they are distinct.3,29,31,32
The relationship of muscle composition with clinical outcomes has been elucidated over the last decade, facilitated by the use of advanced imaging approaches, including CT and magnetic resonance (MR) imaging.23 These modalities enable the quantification not only of muscle size, but also muscle attenuation. Goodpaster et al demonstrated that the attenuation of thigh muscle obtained via CT imaging correlates well with muscle triglyceride content.37 The importance of skeletal muscle radiodensity with clinical outcomes was first described outside of oncology. The Health, Aging and Body Composition (Health ABC) study was designed to prospectively evaluate changes in body composition in older community-dwelling adults. Health ABC investigators were among the first to show that muscle attenuation was independently associated with muscle strength, lower extremity performance, and mobility loss in older adults.38–40 Within oncology, reduced muscle attenuation has been shown to be prognostic of mortality and outcomes in multiple cancers.11,25,41–43 We have recently shown that lower muscle attenuation is also associated with physical function impairments and poorer performance on the Timed Up and Go test in older adults with cancer.44 Although it remains unclear why muscle fat infiltration is more related to frailty than muscle size, this is likely related to the impact of intramuscular fat on strength and physical performance.39 As the characterization of muscle attenuation is relatively new, the relationship between reduced attenuation of muscle to clinical outcomes remains unknown; however, our data suggest that this may in part be related to underlying frailty. Given the multitude of proposed sarcopenia definitions in the literature and the lack of a consensus definition,45 we chose to examine skeletal muscle metrics as continuous variables without dichotomization to better understand the association with frailty. This approach enables a more thorough understanding of the relationship of skeletal muscle measures without making erroneous assumptions regarding cut-points. There are several barriers to creating a unified definition of sarcopenia, including marked differences in rates of age-related muscle loss observed among different ethnic groups.46 More research is needed to develop a consensus on diagnostic cut-points for sarcopenia in oncology and elsewhere. The Sarcopenia 2 Project seeks to establish evidence-based cut-points for low muscle mass and strength, and although the project does not incorporate any oncologic study populations, it is the largest planned-pooled analysis and promises to provide the most evidence-based definition of sarcopenia to date (http://www.fnih.org/what-we-do/current-research-programs/biomarkers-consortium-sarcopenia-2-project).
Our study has some limitations. This was a single center, retrospective cross-sectional review of an institutional registry. As such, no causal relationship between frailty and skeletal muscle measures can be inferred. There are also several different definitions of frailty available in the literature. We chose to use the deficit accumulation approach as described originally by Rockwood, because this approach has been specifically validated in older adults with cancer.7,8,47 Regardless of the definition of frailty that was used, findings similar to ours have been found in other studies.28,34 The CSR contains limited treatment information; therefore, we are not able to draw any conclusions regarding the impact of cancer treatments on frailty or skeletal muscle measures. Some patients (n=23) were excluded from our analyses because they did not answer a sufficient number of GA variables (≥18 of the 36 variables) to calculate the CFI. Although we do not know the reason for these patients’ incomplete, this could resulted in some selection bias in our final sample. Our study also has important strengths. We purposefully chose not to use a specific definition of sarcopenia due to the lack of consensus, and instead chose to examine skeletal muscle measures as continuous variables to examine the relationship between skeletal muscle and frailty. We also incorporated a novel integrated measure of skeletal muscle that incorporates both quantity and quality into a combined metric. Although this integrated muscle measure was not more correlated with the CFI than SMD alone in our study, we have recently shown SMG to be more associated with adverse outcomes than either SMI or SMD in adults with early stage breast cancer, and encourage further incorporation in future studies.48
In conclusion, skeletal muscle mass alone poorly correlates with a frailty index in a population of older adults with cancer. Muscle composition – as represented by skeletal muscle density – appears more related to frailty than just skeletal muscle quantity. Although frailty and sarcopenia are both age-related conditions that are predictive of increased risk for adverse outcomes, mounting evidence suggests they are separate entities. Further research is necessary to decide which condition is most predictive and clinically useful in assessing older adults with cancer and assisting in treatment decisions.
Acknowledgments
Funding
Supported in part by the UNC Oncology Clinical Translational Research Training Program (NCI 5K12CA120780-07), the Breast Cancer Research Foundation (New York, NY), the University Cancer Research Fund at UNC, and the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences, National Institutes of Health (1UL1TR001111).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previously presented as an oral presentation at the 5th Annual International Conference on Frailty and Sarcopenia Research (ICFSR), Philadelphia, PA, April 28th, 2016.
Disclosures and Conflict of Interest Statements
The authors had no conflicts of interests to disclose.
Author Contributions
Study Concepts: GR Williams, AM Deal, SS Shachar
Study Design: GR Williams, AM Deal, SS Shachar
Data Acquisition: GR Williams, SS Shachar, MS Weinberg
Quality Control of Data and Algorithms: GR Williams, SS Shachar, MS Weinberg, AM Deal
Data Analysis and Interpretation: GR Williams, AM Deal, HB Muss, MS Weinberg, HK Sanoff, EJ Guerard MD, KA Nyrop, M Pergolotti, SS Shachar
Statistical Analysis: AM Deal
Manuscript Preparation: GR Williams
Manuscript Editing: GR Williams, AM Deal, HB Muss, MS Weinberg, HK Sanoff, EJ Guerard MD, KA Nyrop, M Pergolotti, SS Shachar
Manuscript Review: GR Williams, AM Deal, HB Muss, MS Weinberg, HK Sanoff, EJ Guerard MD, KA Nyrop, M Pergolotti, SS Shachar
References
- 1.Surveillance Epidemiology and End Results (SEER) program. http://seer.cancer.gov/faststats/index.php.
- 2.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 5.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Critical reviews in oncology/hematology. 2011;78(2):138–149. doi: 10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. American journal of surgery. 2012;204(2):139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Cohen HJ, Smith D, Sun CL, et al. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016 doi: 10.1002/cncr.30269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerard E, Deal A, Chang Y, et al. Frailty index developed from a cancer-specific geriatric assessment and the association with all-cause mortality among older adults with cancer. J Natl Compr Canc Ne. 2017 doi: 10.6004/jnccn.2017.0122. in press. [DOI] [PubMed] [Google Scholar]
- 9.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2015;26(6):1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 10.Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(24):2587–2594. doi: 10.1200/JCO.2013.55.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(12):1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 12.Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. doi: 10.1016/j.ejca.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Broughman JR, Williams GR, Deal AM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. Journal of geriatric oncology. 2015;6(6):442–445. doi: 10.1016/j.jgo.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. British journal of cancer. 2012;107(6):931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popuri K, Cobzas D, Esfandiari N, Baracos V, Jagersand M. Body Composition Assessment in Axial CT Images using FEM-based Automatic Segmentation of Skeletal Muscle. IEEE transactions on medical imaging. 2015 doi: 10.1109/TMI.2015.2479252. [DOI] [PubMed] [Google Scholar]
- 16.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Me. 2008;33(5):997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 17.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. Journal of Clinical Oncology. 2011;29(10):1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 19.Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. Journal of geriatric oncology. 2014;5(3):245–251. doi: 10.1016/j.jgo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Guerard E, Deal A, Williams G, Jolly T, Wood W, Muss H. Construction of a frailty index for older adults with cancer using a geriatric assessment. J Clin Oncol 33, 2015 (suppl; abstr 9535) 2015;33(supplement) abstr 9535. [Google Scholar]
- 21.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC geriatrics. 2008;8:24. doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung H, Cobzas D, Birdsell L, Lieffers J, Baracos V. Automated segmentation of muscle and adipose tissue on CT images for human body composition analysis. Paper presented at: SPIE Medical Imaging. 2009 [Google Scholar]
- 23.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta physiologica. 2014;210(3):489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg M, Shachar S, Deal A, et al. Characterization of skeletal muscle and body mass indices in younger and older women with stage II and III breast cancer. Journal of the American Geriatrics Society. 2016;(supplement):S86. [Google Scholar]
- 25.Strulov Shachar S, Deal AM, Weinberg M, et al. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane Based Chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 27.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Frontiers in aging neuroscience. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reijnierse EM, Trappenburg MC, Blauw GJ, et al. Common Ground? The Concordance of Sarcopenia and Frailty Definitions. Journal of the American Medical Directors Association. 2016;17(4):371 e377–312. doi: 10.1016/j.jamda.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cesari M, Vellas B, Gambassi G. The stress of aging. Experimental gerontology. 2013;48(4):451–456. doi: 10.1016/j.exger.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. Journal of the American Medical Directors Association. 2011;12(6):403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina-Garrido MJ, Guillen-Ponce C, Fernandez-Felix BM. Relationship Between Sarcopenia and Frailty in a Spanish Cancer in the Elderly Unit: The ONCOSARCO Project. Journal of the American Medical Directors Association. 2016;17(8):760–761. doi: 10.1016/j.jamda.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Mijnarends DM, Schols JM, Meijers JM, et al. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: similarities and discrepancies. Journal of the American Medical Directors Association. 2015;16(4):301–308. doi: 10.1016/j.jamda.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Spira D, Buchmann N, Nikolov J, et al. Association of Low Lean Mass With Frailty and Physical Performance: A Comparison Between Two Operational Definitions of Sarcopenia-Data From the Berlin Aging Study II (BASE-II) The journals of gerontology. Series A, Biological sciences and medical sciences. 2015;70(6):779–784. doi: 10.1093/gerona/glu246. [DOI] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. Journal of applied physiology. 2000;89(1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 38.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. The journals of gerontology. Series A, Biological sciences and medical sciences. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 39.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of applied physiology. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 40.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. Journal of the American Geriatrics Society. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 41.Chu MP, Lieffers J, Ghosh S, et al. Skeletal muscle radio-density is an independent predictor of response and outcomes in follicular lymphoma treated with chemoimmunotherapy. Plos One. 2015;10(6):e0127589. doi: 10.1371/journal.pone.0127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A, Moynagh MR, Multinu F, et al. Muscle composition measured by CT scan is a measurable predictor of overall survival in advanced ovarian cancer. Gynecologic oncology. 2016;142(2):311–316. doi: 10.1016/j.ygyno.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 43.Sjoblom B, Gronberg BH, Wentzel-Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016 doi: 10.1016/j.clnu.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Williams GR, Deal AM, Muss HB, et al. Skeletal muscle measures and physical function in older adults with cancer: sarcopenia or myopenia? Oncotarget. 2017 doi: 10.18632/oncotarget.16866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bijlsma AY, Meskers CG, Ling CH, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35(3):871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva AM, Shen W, Heo M, et al. Ethnicity-related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22(1):76–82. doi: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 48.Shachar SS, Deal AM, Weinberg M, et al. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane Based Chemotherapy for Early Stage Breast Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017 doi: 10.1158/1078-0432.CCR-16-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]