Abstract
Time dependent inhibition (TDI) may confound drug interaction predictions. Recently, models were generated for an array of TDI kinetic schemes using numerical analysis of microsomal assays. Additionally, a distinct terminal inactivation step was identified for certain mechanism based inhibitors (MBI) following reversible metabolite intermediate complex (MIC) formation. Longer hepatocyte incubations potentially allow analysis of slow TDI and terminal inactivation. In the experiments presented here, we compared the quality of TDI parameterization by numerical analysis between hepatocyte and microsomal data. Rat liver microsomes (RLM), suspended rat hepatocytes (SRH), and sandwich-cultured rat hepatocytes (SCRH) were incubated with the prototypical CYP3A MBI troleandomycin and the substrate midazolam. Data from RLM provided a better model fit as compared to SRH. Increased CYP3A expression after dexamethasone (DEX) induction improved the fit for RLM and SRH. A novel sequential kinetic scheme, defining inhibitor metabolite production prior to MIC formation, improved the fit compared to direct MIC formation. Furthermore, terminal inactivation rate constants were parameterized for RLM and SRH samples with DEX induced CYP3A. The low expression of CYP3A and experimental error in SCRH resulted in poor data for model fitting. Overall, RLM generated data better suited for elucidation of TDI mechanisms by numerical analysis.
Keywords: Cytochrome P450, Time Dependent Inhibition, Mechanism Based Inhibition, Microsomes, Hepatocytes
Introduction
Time dependent inhibition (TDI) or mechanism based inhibition is a significant contributor to cytochrome P450 (CYP) clinical drug-drug interactions (DDIs) (Grimm et al., 2009). Clinical coadministration of TDI perpetrators may lead to increased victim substrate exposure (Pessayre et al., 1982, Olkkola et al., 1993, Arayne et al., 2005, Pinto et al., 2005a, Pinto et al., 2005b). The decrease in CYP activity is a result of enzyme inactivation through formation of covalent bonds with the CYP apoprotein or heme (López-Garcia et al., 1994, Yukinaga et al., 2007, Kang et al., 2008, Teng et al., 2010, Sridar et al., 2012), or quasi-irreversible inhibition due to metabolite intermediate complexes (MIC) with the heme (Pessayre et al., 1981b, Yamazaki et al., 1996, Bertelsen et al., 2003, Wang et al., 2005, Hutzler et al., 2006, Polasek and Miners, 2008, Salminen et al., 2011). Despite the generally understood mechanisms of TDI, in vitro predictions of clinical outcomes are still inaccurate (Grimm et al., 2009).
Preclinical evaluation of potential time-dependent inhibitors of CYPs involves in vitro kinetic experiments measuring enzyme activity with inhibitor preincubation. Liver microsomes are typically used for this assessment due to ease of use and enrichment of CYP enzymes. Using the traditional replot method to determine the TDI parameters kinact and KI, the slopes of percent remaining enzyme activity (PRA) over preincubation times (kobs) are plotted against inhibitor concentrations (Mohutsky and Hall, 2014). The replot method assumes typical Michaelis-Menten (MM) kinetics with irreversible inhibition, and it may not generate correct parameters for non-MM kinetics or complex TDI schemes with non-linear PRA plots (Korzekwa et al., 2014). A numerical method was developed to model several TDI kinetic schemes e.g. MM, quasi-irreversible or partial inactivation, non-MM enzyme-inhibitor-inhibitor (EII) (Nagar et al., 2014) with subsequent parameterization of rate constants for CYP2B6, CYP2C8 and CYP3A4 inactivation from human microsomal experimental data (Korzekwa et al., 2014). Additionally, a slow terminal inactivation step was recently identified for the quasi-irreversible inhibitor podophyllotoxin following the slowly reversible MIC with CYP3A4 (Barnaba et al., 2016). Time dependent inhibitors with a slow rate of terminal inactivation may require longer primary incubations for accurate preclinical parameter calculations. Accurate parameterization of the terminal inactivation rate constant is critical for in vivo DDI prediction.
Intact hepatocytes provide a holistic system for longer TDI incubations. Duration of viability and enzyme activity vary with culture method. Hepatocytes in suspension culture maintain uptake transporter and CYP expression after isolation with viability for approximately 4 h (Skett, 1994). Plated hepatocyte monolayers in sandwich configuration may be cultured for days and exhibit appropriate transporter polarity(LeCluyse et al., 1994), but CYP expression requires supplementation with dexamethasone (DEX) to maintain detectable activity (Swift et al., 2010). In addition to comparing microsomes and hepatocytes, an additional objective of this study is to determine if hepatocytes can provide a more stable system for TDI studies. This could allow for better experimental design leading to improved characterization of the terminal inactivation rate constant for the biphasic inactivation by MIC TDIs.
Various studies have compared TDI in human liver microsomes (HLM) to hepatocytes towards DDI predictions. Some groups have reported consistent kinact and KI values generated between both systems (McGinnity et al., 2006, Albaugh et al., 2012), while others observed an overprediction of TDI when using HLM as opposed to hepatocytes (Zhao et al., 2005, Van et al., 2007, Xu et al., 2009, Chen et al., 2011, Mao et al., 2013). In the latter cases, hepatocytes predicted a higher KI (2–10 fold) than HLM while kinact was generally in agreement between both systems with a few instances of much lower estimates in hepatocytes (Mao et al., 2013). The discrepancies between laboratories may be due to differences in experimental design and analysis. For quasi-irreversible inhibitors which exhibit concave PRA plots, consideration of early preincubation time points alone may overestimate inactivation, while single, late preincubation time points may underestimate inactivation (Korzekwa et al., 2014).
The research presented here investigated the utility of primary rat hepatocytes for modeling of TDI by numerical analysis. Comparing data generated from rat liver microsomes (RLM), primary rat hepatocytes in suspension (SRH) and in sandwich culture (SCRH), we modeled TDI of rat CYP3A23 using the quasi-irreversible inhibitor troleandomycin (TAO) and the substrate midazolam (MDZ). N-dealkylation and oxidation of TAO by CYP3A results in a nitroso function and subsequent MIC formation with the heme (Pessayre et al., 1981a) causing quasi-irreversible inactivation. A MM, quasi-irreversible kinetic scheme was used as the base model for parameterization of rate constants. We calculated KI for each data set and kinact as a net rate constant of MIC formation, reversion to free enzyme, and irreversible terminal inactivation. In addition, each system was compared with and without CYP3A23 induction by DEX to examine the effect of increased expression on model fitting. We also considered a novel sequential scheme to more accurately characterize the inhibitor metabolite formation and heme complexation steps. The resultant parameterization and implications for each experimental system will be discussed.
Materials and Methods
Materials
All buffers, media, and supplements were purchased from Life Technologies (Carlsbad, CA, USA) except for BSA (Thermofisher, Waltham, MA, USA), ascorbic acid (Sigma, St. Louis, MO, USA), collagenase (Sigma, St. Louis, MO, USA), Percoll™ (GE, Pittsburgh, PA, USA), FBS (Hyclone, Logan, UT, USA), Matrigel™ (BD Corning, Tewksbury, MA, USA), BCA protein assay kit (Pierce Thermofisher, Waltham, MA, USA), midazolam (Cambridge Isotope Laboratories, Andover, MA, USA), 4OH-midazolam (Sigma, St. Louis, MO, USA), troleandomycin (Enzo Life Sciences, Farmingdale, NY, USA), dexamethasone (Cayman Chemical Company, Ann Arbor, MI, USA), and diltiazem (Sigma, St. Louis, MO, USA). Multi-well cell culture plates were purchased from BD Corning (Tewksbury, MA, USA).
In Vivo Dosing with Dexamethasone
Compliance with federal regulations regarding the proper care and humane treatment of animals was overseen by the Temple University Institutional Animal Care and Use Committee in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals by the U.S. Department of Health and Human Services and National Institutes of Health, Office of Laboratory Animal Welfare. Male Sprague Dawley rats (Charles River Laboratories, Malvern, PA, USA) weighing between 225 and 250 g were used for hepatocyte and microsomal preparations. Rats were housed in a 12 h light/dark cycle room and allowed food and water ad libitum. Forty-eight and 24 h before liver perfusion, 15 mg/mL dexamethasone (DEX) in corn oil at 100 mg/kg or corn oil alone were administered intraperitoneally.
Microsomal and hepatocyte preparations upon DEX treatment were subjected to quantitative PCR and western blot analysis in order to check the level of induction of CYP3A23 upon DEX treatment. These data are provided in Supplementary Materials.
Hepatocyte Isolation
Rats were anesthetized and the portal vein cannulated. Pre-warmed perfusion buffer (HBSS without Ca2+ and Mg2+ supplemented with 0.5 μM EDTA, 4 μg/mL insulin, ascorbic acid, and 5% BSA) was pumped through the cannula at 25 mL/min and the inferior vena cava immediately severed. The liver was perfused for 10 min. Digestion buffer (HBSS supplemented with 4 μg/mL insulin, 50 μg/mL ascorbic acid, 5% BSA, and 70 units/mL collagenase type IV) was subsequently pumped through the cannula at 25 mL/min for approximately 8 min until the liver was adequately digested. The liver was removed, placed in a sterile vessel containing isolation William’s E media (supplemented with 4 μg/mL insulin, 292 μg/mL L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 10% FBS, and 1 μM DEX), and separated with forceps to release hepatocytes. The cells were centrifuged and washed with isolation William’s E media. The cells were resuspended in isolation William’s E media supplemented with 37% Percoll™ solution (1X DPBS in Percoll™), centrifuged, washed, then resuspended in plating William’s E media (supplemented with 4 μg/mL insulin, 292 μg/mL L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 5% FBS, and 1 μM DEX). Cell number and viability (>80%) were calculated using trypan blue exclusion.
Microsomal Preparation and Incubations
A pellet from the hepatocyte isolation was resuspended in cold homogenizing buffer (10 mM EDTA, 0.154 M KCl, 0.25 M sucrose, 0.05 M phosphate buffer pH 7.5) and homogenized in a Teflon dounce. The homogenate was centrifuged at 12,500 g for 15 min at 4°C. The resulting supernatant was ultracentrifuged at 105,000 g for 70 min at 4°C. The pellet was resuspended in cold microsome buffer (20% glycerol (v/v), 80% 0.1 M phosphate buffer pH 7.5, 10 mM EDTA) and homogenized again in a Teflon dounce. Protein concentrations were determined by BCA protein assay according to the manufacturer’s protocol.
Incubations to assess time dependent inhibition were completed in two parts. The primary incubation contained the CYP3A inhibitor troleandomycin (TAO) at a final concentration of 0, 0.5, 1, 2, 4, or 8 μM in 0.1% ACN, 0.5 mg/mL microsomes, and NADPH regenerating system in 0.1 M phosphate buffer. Following incubation in microcentrifuge tubes for 0, 5, 15, 30, and 45 min in a 37°C water bath with shaking at 40 RPM, an aliquot was diluted 20X into the secondary incubation with a final concentration of CYP3A substrate 100 μM midazolam (MDZ) in 0.1% ACN, and NADPH regenerating system in 0.1 M phosphate buffer. After 3 min at 37°C with shaking at 40 RPM, the reactions were stopped with an equal volume of cold stop solution (50 ng/mL diltiazem internal standard (IS) in ACN).
Hepatocyte Suspension Incubations
Following isolation, hepatocytes were diluted to 7 × 105 cells/mL in HBSS and preincubated in uncoated 24-well plates for 10 min at 37°C and 5% CO2 on an orbital shaker. TAO was added at final concentrations of 0, 0.5, 1, 2, 4, or 8 μM in 0.1% ACN and incubated for 0, 5, 15, 30, and 45 min at 37°C and 5% CO2 on an orbital shaker at 100 RPM. MDZ was added at a final concentration of 100 μM and 1% ACN. After 10 min at 37°C and 5% CO2 on an orbital shaker at 100 RPM, the reactions were stopped with an equal volume of cold stop solution.
Hepatocyte Monolayer Incubations
Following isolation, cells were seeded onto collagen-coated 24-well plates at 7 × 105 cells/mL and 0.5 mL/well and incubated at 37°C and 5% CO2. Three hours after seeding, media was aspirated, replaced with fresh media, and returned to the incubator overnight. Cells were overlaid with 0.25 mg/mL Matrigel™ in William’s E media (without phenol red, supplemented with 4 μg/mL insulin, 292 μg/mL L-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, and 100 nM DEX). Media was changed after 24 and 72 h with 5 μM DEX for induced samples.
At 96 h, cells were washed with prewarmed HBSS. TAO was added at final concentrations of 0, 0.5, 1, 2, 4, or 8 μM in 0.1% ACN and incubated for 0, 5, 15, 30, and 45 min at 37°C and 5% CO2 on an orbital shaker at 100 RPM. MDZ was added at a final concentration of 300 μM MDZ and 1% ACN. After 60 min at 37°C and 5% CO2 on an orbital shaker at 100 RPM, the reactions were stopped with an equal volume of cold stop solution. The hepatocyte layer was scraped and transferred for analysis.
LC-MS/MS Analysis
All samples were centrifuged at 10,000g for 10 min at 4°C, and supernatant removed for analysis. A Luna 3 μm C18(2) 100 Å 30 × 2 mm LC column (Phenomenex, Torrance, CA, USA) was used for the analysis. The gradient chromatography was conducted using 0.1% (v/v) formic acid in filtered deionized water (mobile phase A) and 0.1% (v/v) formic acid in 100% ACN (mobile phase B) at a flow rate of 0.300 mL/min. The linear gradient was 10% mobile phase B to 0.5 min, 95% to 2 min, and then 10% to 8 min. The injection volume was 5 μL.
Positive ion electrospray tandem MS was conducted with an AB Sciex API 4000 triple quadrupole MS using multiple reaction monitoring detection mode controlled by Analyst™ (AB Sciex, Framingham, MA, USA) operating software. The respective parameters for 4OH-MDZ and diltiazem were Q1: 342.1 and 415.5, Q3: 324.9 and 178.4, DP: 56 and 41, CE: 31 and 49, CXP: 10 and 34.
Analyst™ was used for integration of the chromatographic peak areas for the analytes and IS. The software calculated the 1/x weighted least-squares linear regression relating peak area ratios (relative to IS) to reference sample concentrations for standard curve generation. Concentrations of unknown samples were interpolated from the standard curve using the peak area ratios relative to the IS.
Model Fitting
Data used in model fitting were generated from the average of at least two experiments with samples in triplicate. Each experiment used saturating concentrations of MDZ as determined in preliminary assays (data not shown). TAO was assumed to exhibit Michaelis Menten, quasi-irreversible inhibition (Fig 1A). Initial values for rate constants were estimated as follows. The association constant for both MDZ and TAO (k1, k4) was set at 270 μM−1 min−1 according to published measurements for the association rate of CYP3A substrates (Barnaba et al., 2016). The dissociation constant of MDZ (k2) was set based on a Km of 10 μM from experiments in rat liver microsomes (data not shown) with a value of 2700 min−1. Km was approximated by k2/k1 since MDZ kcat (k3, approx. 10 min−1) determined from experimental samples without inhibitor was significantly lower than k2 (2700 min−1). KI was approximated by k5/k4 and determined from the inhibitor concentration resulting in 50% activity at the longest preincubation time, approximately 1 μM, for a k5 of 270 min−1. An estimate of k7 (MIC intermediate formation) was determined from the initial slope of inactivation vs time at the highest TAO concentration. Inherent experimental enzyme loss (k10) was estimated from the decrease in activity over preincubation time without TAO. Additional models used in fitting included a sequential model (Fig 1B) that incorporated a TAO metabolism event and subsequent enzyme-inhibitor metabolite complex formation for inactivation. Here the inhibitor’s metabolite formation rate constant k6 was fixed at 50 min−1, in order to provide a lag for inactivation. The model was tested by varying k6 over a range of 25 – 100 min−1 to evaluate variability in kinact estimates. ESI schemes considering separate binding sites for TAO and MDZ were also examined (Fig 1C, D), but did not improve model fitting.
Fig. 1.
Quasi-irreversible kinetic schemes. E, enzyme; ES, enzyme substrate; EI, enzyme inhibitor; EM, enzyme inhibitor metabolite; ESI, enzyme substrate inhibitor; ESM, enzyme substrate/inhibitor metabolite; E*, enzyme metabolite intermediate complex; E**, inactivated enzyme; S, substrate; P, product; I, inhibitor; M, inhibitor metabolite. (A) Michelis-Menten (MM) quasi-irreversible kinetic scheme including rates for loss of all enzyme species. (B) Sequential scheme adding inhibitor metabolite formation, M, and enzyme inhibitor metabolite binding, EM, prior to metabolite intermediate complexation, E*. (C) ESI scheme describing simultaneous binding of substrate and inhibitor to enzyme. (D) Sequential ESI scheme adding enzyme substrate/inhibitor metabolite binding, ESM.
When fitting to the hepatocyte data, k5 could not be accurately estimated, presumably due to high experimental error. The k5 value estimated from the microsomal data could not be used, because the hepatocyte experiments showed minimal competitive inhibition (observed at preincubation time = 0 min). Therefore, the KI value for hepatocytes was fixed at 10 μM (k5 = 2700 min−1). The model was tested by varying k5 over a range of 1350 – 5400 min−1 and k6 over a range of 25 – 100 min−1 to evaluate variability in kinact estimates.
Differential equations for the kinetic schemes were parameterized using the NonlinearModelFit function in Mathematica 10.4 (Wolfram Research, Champagne, IL). The NDSolve function was used to solve the differential equations with MaxSteps -> 100,000 and PrecisionGoal -> ∞. Comparison of the corrected Akaike information criteria (AICc) value was used when evaluating different models for the same dataset.
kinact was calculated as a net rate constant (Cleland, 1975) with the following formula:
Where k7 is the rate of MIC formation (E*), k8 is the rate of reversion to free enzyme (E), and k9 the rate of terminal inactivation (E**).
The replot method (Silverman, 1995) was used for estimation of KI and kinact using the following equation:
Where [I] is the inhibitor concentration, kobs is the inactivation rate constant which represents the slope of each [I] in the PRA plot.
In Vitro-In Vivo Extrapolation (IVIVE)
DDI predictions were performed using the static model described within the FDA guidance (https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf). The following equation was used:
Where AUC’po and AUCpo are the areas under the curve in the presence and absence of inhibitor, respectively, and the other terms are described in Table 2. Enzyme synthesis rates (kdeg,h and kdeg,g), inhibitor related parameters (fup, Ig, Ih and Isys) and substrate related parameters (Fg and fm) were obtained from the literature as shown in Table 2. TDI parameters generated using replot and numerical methods were used for DDI predictions. Since no rat studies with TAO have been reported, human inhibitor concentrations were used for all calculations. Systemic concentrations (Isys and Isys,u) were used when MDZ was dosed via oral route whereas hepatic portal vein concentrations (Ih and Ih,u) and gut concentration [I]g were used when MDZ was dosed via oral route.
Table 2.
Literature values used for DDI prediction.
| Parameter | Values | Reference |
|---|---|---|
| Fg (fraction escaping gut) | 0.75 | (Kotegawa et al., 2002, Matsuda et al., 2012) |
| fm (fraction metabolized by CYP3A) | 0.93 | (Kirby et al., 2011) |
| kdeg,h (Natural degradation rate constant for hepatic CYPs) | 0.00051 min−1 | (Correia, 1991, Mao et al., 2011) |
| kdeg,g (Natural degradation rate constant for gut CYPs) | 0.000481 min−1 | |
| fup (fraction unbound in plasma) | 0.038 | (Fahmi et al., 2009) |
| Ig (Concentration in gut) | 149 μM | |
| Ih (Concentration in portal vein) | 0.15 μM | |
| Isys (Average systemic concentration) | 1.76 μM | (Fahmi et al., 2008) |
Results
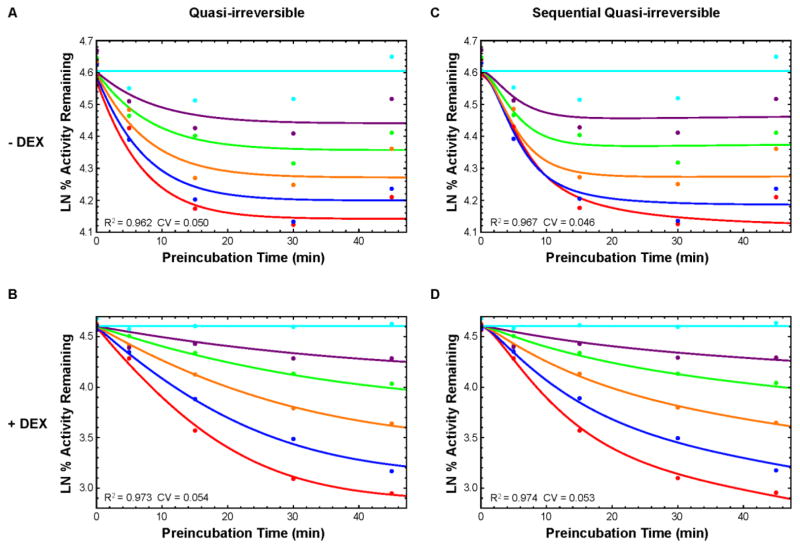
TDI experiments and subsequent modeling utilized a two-step incubation method: a primary preincubation with the inhibitor TAO at various concentrations and time points, followed by a secondary incubation with the substrate MDZ. Percent remaining activity (PRA) for each TAO concentration was plotted versus preincubation time. All experimental PRA plots showed a concave upward shape indicative of quasi-irreversible inhibition (Fig. 2, 3, and 4) (Korzekwa et al., 2014). Using the RLM data to parameterize the quasi-irreversible kinetic scheme (Fig. 1A), the model provided a better fit to the induced set compared to the uninduced set (R2: 0.973 and 0.962 respectively, Fig. 2A and B, Table 1). However, the model poorly fit the 5 min preincubation data points (Fig. 2A). The plot suggested a slight lag before inactivation. We hypothesized the lag was due to TAO n-dealkylation and oxidation before nitroso/heme MIC formation. Therefore, we applied a sequential (Seq) kinetic scheme adding inhibitor metabolite formation (M) prior to the metabolic intermediate complexation (E*, Fig. 1B). The sequential model generated a better fit for the early preincubation times in RLM compared to the direct MIC formation model (Fig. 2C & D), and a slightly better fit overall based on AICc (− DEX Direct: −166.1, Seq: −170.3; + DEX Direct: −116.5, Seq: −118.6).
Fig. 2.
Liver microsome data and model fitting. Natural log of percent remaining activity vs preincubation time (PRA) plots of TAO TDI quasi-irreversible model (solid lines) fit to data (points). The TAO concentrations are 0 (cyan), 0.5 (purple), 1 (green), 2 (orange), 4 (blue), or 8 (red) μM. Left (A,B): Direct enzyme-inhibitor quasi-irreversible inactivation. Right (C,D): Sequential enzyme-inhibitor metabolite quasi-irreversible inactivation. Top (A,C): Without DEX treatment. Bottom (B,D): With DEX treatment.
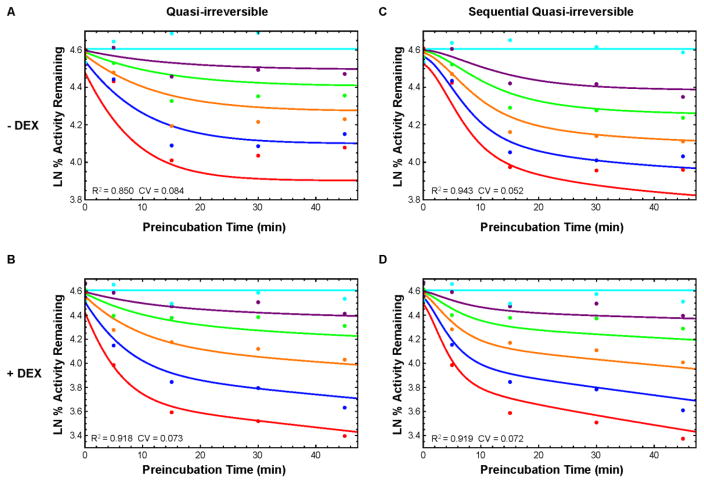
Fig. 3.
Hepatocyte suspension data and model fitting. Natural log of percent remaining activity vs preincubation time (PRA) plots of TAO TDI quasi-irreversible model (solid lines) fit to data (points). The TAO concentrations are 0 (cyan), 0.5 (purple), 1 (green), 2 (orange), 4 (blue), or 8 (red) μM. Left (A,B): Direct enzyme-inhibitor quasi-irreversible inactivation. Right (C,D): Sequential enzyme-inhibitor metabolite quasi-irreversible inactivation. Top (A,C): Without DEX treatment. Bottom (B,D): With DEX treatment.
Fig. 4.
Hepatocyte sandwich data and model fitting. Natural log of percent remaining activity vs preincubation time (PRA) plots of TAO TDI quasi-irreversible model (solid lines) fit to data (points) with DEX treatment. The TAO concentrations are 0 (cyan), 0.5 (purple), 1 (green), 2 (orange), 4 (blue), or 8 (red) μM. (A): Direct enzyme-inhibitor quasi-irreversible inactivation. (B): Sequential enzyme-inhibitor metabolite quasi-irreversible inactivation.
Table 1.
Kinetic parameters determined numerically for direct and sequential (Seq) TDI models with data from rat liver microsomes (RLM), rat hepatocyte suspensions (SRH) and rat hepatocyte sandwich cultures (SCRH) with and without dexamethasone (DEX) treatment. The following parameters were fixed: k1 = 270 μM−1 min−1, k2 = 2700 min−1, k4 = 270 μM−1 min−1. Estimated parameters are listed as parameter estimate ± SE.
| RLM | SRH | SCRH | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Direct | Seq | Direct | Seq | Direct | Seq | ||
| − DEX | k3 (min−1) | 19.3±0.35 | 19.2±0.31 | 19.4±0.66 | 19.0±0.37 | ||
| k5 (min−1) | 364±153 | 639±287 | 27002 | 27002 | |||
| k6 (min−1) | NA1 | 502 | NA1 | 502 | |||
| k7 (min−1) | 0.079±0.024 | 0.326±0.020 | 0.190±0.075 | 1.39±0.44 | |||
| k8 (min−1) | 0.099±0.028 | 0.236±0.077 | 0.059±0.023 | 0.129±0.025 | |||
| k9 (min−1) | 02 | 02 | 02 | 02 | |||
| k10 (min−1) | 0.009±0.001 | 0.009±0.001 | 0.006±0.001 | 0.003±0.001 | |||
| kinact (min−1) | ND3 | ND3 | ND3 | ND3 | |||
| KI (μM) | 1.35±0.57 | 2.37±1.06 | 10* | 10* | |||
| kinact/KI (μM−1 min−1) | ND3 | ND3 | ND3 | ND3 | |||
| AICc | −166.1 | −170.3 | −22.7 | −52.3 | |||
| R2 | 0.962 | 0.967 | 0.850 | 0.943 | |||
| CV | 0.050 | 0.046 | 0.084 | 0.052 | |||
|
| |||||||
| + DEX | k3 (min−1) | 17.3±0.27 | 17.3±0.23 | 18.8±0.53 | 18.6±0.52 | 17.7±1.13 | 16.1±0.28 |
| k5 (min−1) | 1192±235 | 1459±297 | 27002 | 27002 | 27002 | 27002 | |
| k6 (min−1) | NA1 | 502 | NA1 | 502 | NA1 | 502 | |
| k7 (min−1) | 0.123±0.016 | 0.177±0.037 | 0.367±0.103 | 0.881±0.314 | 0.587±0.202 | 30.9±7.98 | |
| k8 (min−1) | 0.015±0.002 | 0.030±0.014 | 0.060±0.024 | 0.116±0.038 | 0.035±0.008 | 0.060±0.007 | |
| k9 (min−1) | 02 | 0.0150±0.0145 | 0.00771±0.00767 | 0.0103±0.0053 | 02 | 02 | |
| k10 (min−1) | 0.003±0.001 | 0.0032 | 0.004±0.001 | 0.004±0.001 | 0.003±0.001 | 02 | |
| kinact (min−1) | ND3 | 0.012±0.010 | 0.007±0.006 | 0.009±0.005 | ND3 | ND3 | |
| KI (μM) | 4.41±0.87 | 5.40±1.10 | 102 | 102 | 102 | 102 | |
| kinact/KI (μM−1 min−1) | ND3 | 0.002±0.002 | 0.0007±0.0006 | 0.0009±0.0005 | ND3 | ND3 | |
| AICc | −116.5 | −118.6 | 16.6 | 16.7 | −29.3 | −54.8 | |
| R2 | 0.973 | 0.974 | 0.918 | 0.919 | 0.795 | 0.907 | |
| CV | 0.054 | 0.053 | 0.073 | 0.072 | 0.080 | 0.054 | |
Not Applicable.
Fixed value.
Not Defined.
The hepatocyte experimental data showed little competitive inhibition (Fig 3 and 4, 0 min. preincubation). However, the model fitting was unsuccessful with parameterization of KI of approximately 10 μM or higher with significant standard error. In order to parameterize the remaining rates, the KI was fixed to 10 μM for all hepatocyte sets. For the SRH data sets, a better fit was modeled with DEX induced experiments compared to uninduced (R2: 0.918 and 0.850 respectively, Fig. 3A & B, Table 1). The sequential model generated a better fit against the earlier preincubation time points, with marked improvement for the uninduced SRH data (AICc Direct: −22.7, Seq: −52.3) and similar fit for the induced data (AICc Direct: 16.6, Seq: 16.7). No inhibitor response was observed in uninduced SCRH experiments (data not shown). Although the sequential scheme again provided a better fit for induced SCRH (AICc Direct: −29.3, Seq: −54.8), the model did not capture the experimental data at higher TAO concentrations and time points (Fig. 4C & D, Table 1).
A terminal inactivation step was recently identified and modeled for MIC forming quasi-irreversible inhibitors (Barnaba et al., 2016). This rate constant is described in our kinetic scheme by k9 from the MIC E* to the irreversibly inactivated E** (Fig. 1). We were able to parameterize this rate constant for induced RLM (Seq k9: 0.0150±0.0145 min−1), and induced SRH (Direct k9: 0.00771±0.00767 min−1, Seq k9: 0.0103±0.0053 min−1). kinact was calculated as a net rate constant of MIC formation (k7), reversion to free enzyme (k8), and terminal inactivation (k9). kinact was similar for RLM (Seq kinact: 0.012±0.010 min−1) and induced SRH (Direct kinact: 0.007±0.006 min−1, Seq kinact: 0.009±0.005 min−1).
Due to the high uncertainty in k9 and resultant kinact estimates, sensitivity analysis was conducted over a range of fixed k5 and k6 values. For induced RLM Seq, a range of k6 between 25 – 50 min−1 provided rational solutions with R2 > 0.99, and kinact varying between 0.012 ±0.01 and 0.02±0.007 min−1. A range of k5 between 2700 – 5400 min−1 provided rational solutions (R2 > 0.99) for induced SRH Direct, with kinact varying between 0.007±0.006 and 0.008±0.005 min−1. Finally, a 3 × 3 matrix of k5 (25 – 100 min−1) and k6 (1350 – 5400 min−1) values resulted in rational solutions (R2 > 0.99) for induced SRH Seq, with kinact varying between 0.008±0.004 and 0.013±0.004 min−1.
Comparison of parameter estimates with the numerical versus the replot methods is shown in Table 3. Since the PRA plots showed concave upward curvature, data were analyzed either using all the data points or using only the log linear points on the PRA plots. In DEX induced systems, replot estimates of KI were higher using linear data as compared to all the data. However in the uninduced systems the estimates of KI were similar across enzyme preparations with the replot method. The estimates of kinact with the replot method were 2 to 4 fold higher using linear data as compared to using all data irrespective of the different DEX induced and uninduced systems. Compared with the numerical method, estimates of KI were lower and kinact were higher with the replot method. DDI predictions using KI and kinact with the replot method range from 1.4 to 11.3, and from 1.1 to 4.8 with the numerical method (Table 4).
Table 3.
Comparison of the replot and numerical methods.
| System | Method | KI (μM) | kinact (min−1) | kinact/KI (μM−1, min−1) | R2 | ||
|---|---|---|---|---|---|---|---|
| −DEX | RLM | Replot | All data points | 1.13 ± 0.45 | 0.012 ± 0.001 | 0.011 ± 0.004 | 0.984 |
| Linear | 1.45 ± 0.46 | 0.027 ± 0.003 | 0.019 ± 0.006 | 0.9996 | |||
| Numerical | Direct | 1.35 ± 0.57 | ND | ND | 0.962 | ||
| Seq | 2.37 ± 1.06 | ND | ND | 0.967 | |||
|
| |||||||
| SRH | Replot | All data points | 1.15 ± 0.05 | 0.016 ± 0.0002 | 0.014 ± 0.0006 | 0.9998 | |
| Linear | 1.35 ± 0.12 | 0.058 ± 0.002 | 0.043 ± 0.004 | 0.999 | |||
| Numerical | Direct | 10 | ND | ND | 0.850 | ||
| Seq | 10 | ND | ND | 0.943 | |||
|
| |||||||
| + Dex | RLM | Replot | All data points | 2.79 ± 0.47 | 0.050 ± 0.004 | 0.018 ± 0.003 | 0.998 |
| Linear | 5.99 ± 1.72 | 0.11 ± 0.017 | 0.018 ± 0.006 | 0.996 | |||
| Numerical | Direct | 4.41 ± 0.87 | ND | ND | 0.973 | ||
| Seq | 5.4 ± 1.1 | 0.012 ± 0.010 | 0.002 ± 0.002 | 0.974 | |||
|
| |||||||
| SCRH | Replot | All data points | 0.19 ± 0.14 | 0.011 ± 0.001 | 0.058 ± 0.043 | 0.985 | |
| Linear | 1.73 ± 0.39 | 0.041 ± 0.003 | 0.024 ± 0.006 | 0.995 | |||
| Numerical | Direct | 10 | ND | ND | 0.795 | ||
| Seq | 10 | ND | ND | 0.907 | |||
|
| |||||||
| SRH | Replot | All data points | 6.35 ± 1.9 | 0.040 ± 0.007 | 0.0063 ± | 0.994 | |
| Linear | 12.7 ± 4.6 | 0.16 ± 0.04 | 0.012 ± 0.005 | 0.995 | |||
| Numerical | Direct | 10 | 0.007 ± 0.006 | 0.0007 ± 0.0006 | 0.918 | ||
| Seq | 10 | 0.009 ± 0.005 | 0.0009 ± 0.0005 | 0.919 | |||
Table 4.
DDI prediction using the static model
| Substrate | [I] | DDI Prediction | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| RLM | SRH | |||||||
|
| ||||||||
| Replot | Numerical | Replot | Numerical | |||||
|
| ||||||||
| All data | Linear data | Seq | All data | Linear data | Direct | Seq | ||
| MDZ (IV) | Isys | 10.7 | 11.3 | 4.8 | 8.2 | 10.6 | 2.7 | 3.1 |
| Isys,u | 2.8 | 2.9 | 1.3 | 1.7 | 2.3 | 1.1 | 1.1 | |
|
| ||||||||
| MDZ (oral) | Ih | 5.9 | 6.1 | 2.1 | 3.3 | 4.9 | 1.5 | 1.6 |
| Ih,u | 1.6 | 1.6 | 1.3 | 1.4 | 1.5 | 1.3 | 1.3 | |
Discussion
The experiments presented here summarize our efforts to evaluate the utility of data generated from microsomes and hepatocytes in numerical modeling of time dependent inhibition. The numerical method has been used previously to solve a variety of TDI kinetic schemes from microsomal experimental data (Korzekwa et al., 2014, Nagar et al., 2014). Hepatocytes represent a holistic system to examine longer incubation times especially critical for slow time dependent inhibitors as well as more complex schemes inherent to a cellular system. TAO mediated TDI of MDZ metabolism by CYP3A23 served as the representative pathway. A comparison of human microsomes and hepatocytes would be ideal. However, rat preparations were used in the present study due to the prohibitive cost of obtaining human liver hepatocytes from a reasonably large pool of donors. The difference in kinetic parameters for TAO mediated TDI of CYP3A in this work compared to previously published results (Nagar et al., 2014) is likely due to species differences.
Since TDI experiments measure decrease in enzyme activity over multiple preincubation times and inhibitor concentrations to assess the magnitude of inactivation, we hypothesized that increased enzyme expression would result in improved experimental data. Our initial studies examined expression of CYP3A23 in freshly isolated SRH as well as SCRH after treatment with CYP3A23 inducer DEX. Consistent with previous reports, RNA, protein, and activity were much lower in untreated SCRH than SRH (Supplementary Material). Treatment with 0.1μM DEX, typically used in SCRH to maintain expression (Swift et al., 2010), yielded low RNA and protein compared to SRH. A concentration of 5μM DEX gave maximum induction over the time tested (data not shown) but still considerably lower than SRH. The mechanism for reduced basal and induced CYP3A23 expression in SCRH compared to SRH remains unclear. The induced change in CYP3A23 protein corresponded with RNA, but not activity. This result may indicate a certain fraction of expressed CYP3A23 is unavailable for enzymatic activity.
The quasi-irreversible MM kinetic scheme provided an adequate model fit for the data generated by RLM and SRH. However, a new sequential metabolism model improved the fit for uninduced RLM (AICc Direct: −166.1, Seq: −170.3) and uninduced SRH (AICc Direct: −22.7, Seq: −52.3). This scheme (Fig. 1B) includes an additional metabolic step that represents the sequential oxidations required for nitroso formation (Barnaba et al., 2016), prior to inactivation. Sequential metabolism results in a lag due to the formation of mandatory intermediates. For the induced RLM and SRH, the non-sequential and sequential models fit equally well (AICc Direct: −116.5, Seq: −118.6; Direct: 16.6, Seq: 16.7, respectively). Nevertheless, sequential metabolism must be involved for all systems, since TAO is a known MIC-forming TDI. The higher catalytic activity in the induced systems may reduce the lag time for nitroso intermediate formation and reduce the need for a sequential model. In these experiments, low levels of CYP3A23 in sandwich cultures do not provide enough resolution for TDI parameterization as the amount of inactivation was very low in the DEX treated cells (Supplementary Material and Fig. 4). Sandwich cultures without DEX treatment showed no response to TAO treatment (data not shown).
Since no competitive inhibition was observed with hepatocytes, a higher apparent KI value was required. Other laboratories have reported higher KI values for TDI in hepatocytes when compared to microsomes (Zhao et al., 2005, Van et al., 2007, Xu et al., 2009, Chen et al., 2011, Mao et al., 2013). One possible explanation is that substrate and inhibitor can bind simultaneously, requiring an ESI, quasi-irreversible model (Fig 1C, D). However, optimization of an ESI model did not result in a better fit to the data (data not shown). A low fraction unbound (fu) of TAO in hepatocytes as compared to RLM would result in an apparent higher KI. However reported TAO fu values in human microsomes are similar or lower than in hepatocytes (fum: 0.154–0.93, fuh: 0.567–0.99 (Zhao et al., 2005, Xu et al., 2009)). Permeability and possibly transporter activity could affect the early preincubation time points in hepatocytes since TAO must cross the cell membrane into the cytosol before interacting with CYP3A23. The macrolide antibiotic erythromycin, similar in structure to TAO, was shown to have much lower permeability than MDZ in Caco-2 cells (ERY Papp: 4.4 nm/s, MDZ Papp: 324 nm/s) (Gertz et al., 2013). This may result faster MDZ accessibility to CYP3A23 at the zero time point in hepatocytes, thereby increasing the apparent KI. Additionally, it was reported that inhibition of efflux by P-gp in human cryopreserved hepatocytes treated with TAO decreased CYP3A activity suggesting that TAO is a P-gp substrate (Zhao et al., 2005). Efflux of TAO would decrease the effective cellular concentration in hepatocytes and result in a higher apparent KI.
A recent report describes an important modification to the mechanism of inactivation by MIC-forming TDIs (Barnaba et al., 2016). The initial decrease in enzyme activity observed with a PRA plot is not due to enzyme inactivation, but due to the formation of a reversible intermediate. This intermediate must be further reduced to inactivate the enzyme, resulting in a biphasic inactivation curve. It is the second phase that is primarily responsible for the kinact. Unfortunately, the second phase is difficult to characterize experimentally due to the need for long preincubation times. In the present study, we were able to characterize kinact values as a net rate constant of MIC formation, reversion, and terminal inactivation for induced RLM (Seq kinact: 0.012±0.010 min−1) and induced SRH (Direct kinact: 0.007±0.006 min−1, Seq kinact: 0.009±0.005 min−1). The conventional replot method or use of the initial linear inactivation phase does not consider the terminal inactivation step, which may be the cause of previously reported overpredictions of DDI (Zhao et al., 2005, Van et al., 2007, Xu et al., 2009, Chen et al., 2011, Albaugh et al., 2012, Mao et al., 2013). This can be seen in Table 3, where kinact values, when characterized were much lower with the numerical method than with the replot method. Although in vivo rat inhibition data is not available, parameters obtained with the numerical method always predict a lower DDI potential than the replot method (Table 4).
Others have compared the use of microsomes to hepatocytes (Mao et al., 2011, Mao et al., 2016) and concluded that use of hepatocytes resulted in better DDI predictions. However, many factors are not well defined in the IVIVE approach to predict TDI mediated DDIs. For example, the value used for kdeg, incubation conditions (e.g. +/− plasma, secondary incubation time, and enzyme source), and inhibitor concentrations used can significantly impact the predicted DDIs. If the observed over prediction of DDIs is primarily due to using the initial, reversible phase of the inactivation curves, any modification that decreases the rate of inactivation will result in better IVIVEs. For truly accurate DDI predictions, it will be necessary to determine the appropriate values of kdeg, use relevant inhibitor concentrations, and determine kinact/KI using an appropriate kinetic model.
In summary, we have evaluated the use of microsomes, suspension hepatocytes, and sandwich-cultured hepatocytes, to characterize TDI. In this study, microsomes provided the best data for the numerical method. Intact cellular membranes, transport, as well as data error in our hepatocyte experiments may have contributed to the poor model fits. It is important to note that time dependent inhibitors often exhibit complex, non-Michaelis-Menten kinetics. Here we report a novel scheme to model sequential metabolism and TDI. As described previously, the numerical approach can model these complexities whereas the standard replot method cannot (Korzekwa et al., 2014, Nagar et al., 2014). Perhaps with improvements to in vitro systems and experimental design, the numerical method can be used to better characterize the kinetic complexities of TDI.
Supplementary Material
Acknowledgments
We would like to thank Mr. Jaydeep Yadav for his technical assistance with the replot method analyses.
Funding details
This work was supported by the NIH/NIGMS
Footnotes
Disclosure Statement
The authors report no conflicts of interest.
References
- Albaugh DR, Fullenwider CL, Fisher MB, Hutzler JM. Time-dependent inhibition and estimation of CYP3A clinical pharmacokinetic drug-drug interactions using plated human cell systems. Drug Metab Dispos. 2012;40:1336–44. doi: 10.1124/dmd.112.044644. [DOI] [PubMed] [Google Scholar]
- Arayne MS, Sultana N, Bibi Z. Grape fruit juice-drug interactions. Pak J Pharm Sci. 2005;18:45–57. [PubMed] [Google Scholar]
- Barnaba C, Yadav J, Nagar S, Korzekwa K, Jones JP. Mechanism-Based Inhibition of CYP3A4 by Podophyllotoxin: Aging of an Intermediate Is Important for in Vitro/in Vivo Correlations. Mol Pharm. 2016;13:2833–43. doi: 10.1021/acs.molpharmaceut.6b00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen KM, Venkatakrishnan K, Von Moltke LL, Obach RS, Greenblatt DJ. Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab Dispos. 2003;31:289–93. doi: 10.1124/dmd.31.3.289. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu L, Monshouwer M, Fretland AJ. Determination of time-dependent inactivation of CYP3A4 in cryopreserved human hepatocytes and assessment of human drug-drug interactions. Drug Metab Dispos. 2011;39:2085–92. doi: 10.1124/dmd.111.040634. [DOI] [PubMed] [Google Scholar]
- Cleland WW. Partition analysis and the concept of net rate constants as tools in enzyme kinetics. Biochemistry. 1975;14:3220–4. doi: 10.1021/bi00685a029. [DOI] [PubMed] [Google Scholar]
- Correia MA. Cytochrome P450 turnover. Methods Enzymol. 1991;206:315–25. doi: 10.1016/0076-6879(91)06101-8. [DOI] [PubMed] [Google Scholar]
- Fahmi OA, Hurst S, Plowchalk D, Cook J, Guo F, Youdim K, Dickins M, Phipps A, Darekar A, Hyland R. Comparison of different algorithms for predicting clinical drug-drug interactions, based on the use of CYP3A4 in vitro data: predictions of compounds as precipitants of interaction. Drug Metabolism and Disposition. 2009;37:1658–1666. doi: 10.1124/dmd.108.026252. [DOI] [PubMed] [Google Scholar]
- Fahmi OA, Maurer TS, Kish M, Cardenas E, Boldt S, Nettleton D. A combined model for predicting CYP3A4 clinical net drug-drug interaction based on CYP3A4 inhibition, inactivation, and induction determined in vitro. Drug Metabolism and Disposition. 2008;36:1698–1708. doi: 10.1124/dmd.107.018663. [DOI] [PubMed] [Google Scholar]
- Gertz M, Cartwright CM, Hobbs MJ, Kenworthy KE, Rowland M, Houston JB, Galetin A. Cyclosporine inhibition of hepatic and intestinal CYP3A4, uptake and efflux transporters: application of PBPK modeling in the assessment of drug-drug interaction potential. Pharm Res. 2013;30:761–80. doi: 10.1007/s11095-012-0918-y. [DOI] [PubMed] [Google Scholar]
- Grimm SW, Einolf HJ, Hall SD, He K, Lim HK, Ling KH, Lu C, Nomeir AA, Seibert E, Skordos KW, Tonn GR, Van Horn R, Wang RW, Wong YN, Yang TJ, Obach RS. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug Metab Dispos. 2009;37:1355–70. doi: 10.1124/dmd.109.026716. [DOI] [PubMed] [Google Scholar]
- Hutzler JM, Melton RJ, Rumsey JM, Schnute ME, Locuson CW, Wienkers LC. Inhibition of cytochrome P450 3A4 by a pyrimidineimidazole: Evidence for complex heme interactions. Chem Res Toxicol. 2006;19:1650–9. doi: 10.1021/tx060198m. [DOI] [PubMed] [Google Scholar]
- Kang P, Liao M, Wester MR, Leeder JS, Pearce RE, Correia MA. CYP3A4-Mediated carbamazepine (CBZ) metabolism: formation of a covalent CBZ-CYP3A4 adduct and alteration of the enzyme kinetic profile. Drug Metab Dispos. 2008;36:490–9. doi: 10.1124/dmd.107.016501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. Complex Drug Interactions of HIV Protease Inhibitors 1: Inactivation, Induction and Inhibition of Cytochrome P450 3A by Ritonavir or Nelfinavir. Drug Metabolism and Disposition. 2011 doi: 10.1124/dmd.110.037523. dmd. 110.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzekwa K, Tweedie D, Argikar UA, Whitcher-Johnstone A, Bell L, Bickford S, Nagar S. A numerical method for analysis of in vitro time-dependent inhibition data. Part 2. Application to experimental data. Drug Metab Dispos. 2014;42:1587–95. doi: 10.1124/dmd.114.058297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotegawa T, Laurijssens BE, Von Moltke LL, Cotreau MM, Perloff MD, Venkatakrishnan K, Warrington JS, Granda BW, Harmatz JS, Greenblatt DJ. In vitro, pharmacokinetic, and pharmacodynamic interactions of ketoconazole and midazolam in the rat. Journal of Pharmacology and Experimental Therapeutics. 2002;302:1228–1237. doi: 10.1124/jpet.102.035972. [DOI] [PubMed] [Google Scholar]
- Lecluyse EL, Audus KL, Hochman JH. Formation of extensive canalicular networks by rat hepatocytes cultured in collagen-sandwich configuration. Am J Physiol. 1994;266:C1764–74. doi: 10.1152/ajpcell.1994.266.6.C1764. [DOI] [PubMed] [Google Scholar]
- López-Garcia MP, Dansette PM, Mansuy D. Thiophene derivatives as new mechanism-based inhibitors of cytochromes P-450: inactivation of yeast-expressed human liver cytochrome P-450 2C9 by tienilic acid. Biochemistry. 1994;33:166–75. doi: 10.1021/bi00167a022. [DOI] [PubMed] [Google Scholar]
- Mao J, Johnson TR, Shen Z, Yamazaki S. Prediction of crizotinib-midazolam interaction using the Simcyp population-based simulator: comparison of CYP3A time-dependent inhibition between human liver microsomes versus hepatocytes. Drug Metab Dispos. 2013;41:343–52. doi: 10.1124/dmd.112.049114. [DOI] [PubMed] [Google Scholar]
- Mao J, Mohutsky MA, Harrelson JP, Wrighton SA, Hall SD. Prediction of CYP3A mediated drug-drug interactions using human hepatocytes suspended in human plasma. Drug Metabolism and Disposition. 2011 doi: 10.1124/dmd.110.036400. dmd. 110.036400. [DOI] [PubMed] [Google Scholar]
- Mao J, Tay S, Khojasteh CS, Chen Y, Hop CE, Kenny JR. Evaluation of Time Dependent Inhibition Assays for Marketed Oncology Drugs: Comparison of Human Hepatocytes and Liver Microsomes in the Presence and Absence of Human Plasma. Pharm Res. 2016;33:1204–19. doi: 10.1007/s11095-016-1865-9. [DOI] [PubMed] [Google Scholar]
- Matsuda Y, Konno Y, Satsukawa M, Kobayashi T, Takimoto Y, Morisaki K, Yamashita S. Assessment of intestinal availability of various drugs in the oral absorption process using portal vein-cannulated rats. Drug Metabolism and Disposition. 2012;40:2231–2238. doi: 10.1124/dmd.112.048223. [DOI] [PubMed] [Google Scholar]
- Mcginnity DF, Berry AJ, Kenny JR, Grime K, Riley RJ. Evaluation of time-dependent cytochrome P450 inhibition using cultured human hepatocytes. Drug Metab Dispos. 2006;34:1291–300. doi: 10.1124/dmd.106.009969. [DOI] [PubMed] [Google Scholar]
- Mohutsky M, Hall SD. Irreversible enzyme inhibition kinetics and drug-drug interactions. Methods Mol Biol. 2014;1113:57–91. doi: 10.1007/978-1-62703-758-7_5. [DOI] [PubMed] [Google Scholar]
- Nagar S, Jones JP, Korzekwa K. A numerical method for analysis of in vitro time-dependent inhibition data. Part 1. Theoretical considerations. Drug Metab Dispos. 2014;42:1575–86. doi: 10.1124/dmd.114.058289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola KT, Aranko K, Luurila H, Hiller A, Saarnivaara L, Himberg JJ, Neuvonen PJ. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Descatoire V, Konstantinova-Mitcheva M, Wandscheer JC, Cobert B, Level R, Benhamou PJ, Jaouen M, Mansuy D. Self-induction by triacetyloleandomycin of its own transformation into a metabolite forming a stable 456 nm-absorbing complex with cytochrome P-450. Biochem Pharmacol. 1981a;30:553–8. doi: 10.1016/0006-2952(81)90125-8. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Konstantinova-Mitcheva M, Descatoire V, Cobert B, Wandscheer JC, Level R, Feldmann G, Mansuy D, Benhamou JP. Hypoactivity of cytochrome P-450 after triacetyloleandomycin administration. Biochem Pharmacol. 1981b;30:559–64. doi: 10.1016/0006-2952(81)90126-x. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Larrey D, Vitaux J, Breil P, Belghiti J, Benhamou JP. Formation of an inactive cytochrome P-450 Fe(II)-metabolite complex after administration of troleandomycin in humans. Biochem Pharmacol. 1982;31:1699–704. doi: 10.1016/0006-2952(82)90671-2. [DOI] [PubMed] [Google Scholar]
- Pinto AG, Horlander J, Chalasani N, Hamman M, Asghar A, Kolwankar D, Hall SD. Diltiazem inhibits human intestinal cytochrome P450 3A (CYP3A) activity in vivo without altering the expression of intestinal mRNA or protein. Br J Clin Pharmacol. 2005a;59:440–6. doi: 10.1111/j.1365-2125.2005.02343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AG, Wang YH, Chalasani N, Skaar T, Kolwankar D, Gorski JC, Liangpunsakul S, Hamman MA, Arefayene M, Hall SD. Inhibition of human intestinal wall metabolism by macrolide antibiotics: effect of clarithromycin on cytochrome P450 3A4/5 activity and expression. Clin Pharmacol Ther. 2005b;77:178–88. doi: 10.1016/j.clpt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Polasek TM, Miners JO. Time-dependent inhibition of human drug metabolizing cytochromes P450 by tricyclic antidepressants. Br J Clin Pharmacol. 2008;65:87–97. doi: 10.1111/j.1365-2125.2007.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen KA, Meyer A, Imming P, Raunio H. CYP2C19 progress curve analysis and mechanism-based inactivation by three methylenedioxyphenyl compounds. Drug Metab Dispos. 2011;39:2283–9. doi: 10.1124/dmd.111.041319. [DOI] [PubMed] [Google Scholar]
- Silverman RB. [10] Mechanism-based enzyme inactivators. Methods in enzymology. 1995;249:240–283. doi: 10.1016/0076-6879(95)49038-8. [DOI] [PubMed] [Google Scholar]
- Skett P. Problems in using isolated and cultured hepatocytes for xenobiotic metabolism/metabolism-based toxicity testing-Solutions? Toxicol In Vitro. 1994;8:491–504. doi: 10.1016/0887-2333(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Sridar C, D’agostino J, Hollenberg PF. Bioactivation of the cancer chemopreventive agent tamoxifen to quinone methides by cytochrome P4502B6 and identification of the modified residue on the apoprotein. Drug Metab Dispos. 2012;40:2280–8. doi: 10.1124/dmd.112.047266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift B, Pfeifer ND, Brouwer KL. Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev. 2010;42:446–71. doi: 10.3109/03602530903491881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng WC, Oh JW, New LS, Wahlin MD, Nelson SD, Ho HK, Chan EC. Mechanism-based inactivation of cytochrome P450 3A4 by lapatinib. Mol Pharmacol. 2010;78:693–703. doi: 10.1124/mol.110.065839. [DOI] [PubMed] [Google Scholar]
- Van LM, Swales J, Hammond C, Wilson C, Hargreaves JA, Rostami-Hodjegan A. Kinetics of the time-dependent inactivation of CYP2D6 in cryopreserved human hepatocytes by methylenedioxymethamphetamine (MDMA) Eur J Pharm Sci. 2007;31:53–61. doi: 10.1016/j.ejps.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Wang YH, Jones DR, Hall SD. Differential mechanism-based inhibition of CYP3A4 and CYP3A5 by verapamil. Drug Metab Dispos. 2005;33:664–71. doi: 10.1124/dmd.104.001834. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen Y, Pan Y, Skiles GL, Shou M. Prediction of human drug-drug interactions from time-dependent inactivation of CYP3A4 in primary hepatocytes using a population-based simulator. Drug Metab Dispos. 2009;37:2330–9. doi: 10.1124/dmd.108.025494. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Urano T, Hiroki S, Shimada T. Effects of erythromycin and roxithromycin on oxidation of testosterone and nifedipine catalyzed by CYP3A4 in human liver microsomes. J Toxicol Sci. 1996;21:215–26. doi: 10.2131/jts.21.4_215. [DOI] [PubMed] [Google Scholar]
- Yukinaga H, Takami T, Shioyama SH, Tozuka Z, Masumoto H, Okazaki O, Sudo K. Identification of cytochrome P450 3A4 modification site with reactive metabolite using linear ion trap-Fourier transform mass spectrometry. Chem Res Toxicol. 2007;20:1373–8. doi: 10.1021/tx700165q. [DOI] [PubMed] [Google Scholar]
- Zhao P, Kunze KL, Lee CA. Evaluation of time-dependent inactivation of CYP3A in cryopreserved human hepatocytes. Drug Metab Dispos. 2005;33:853–61. doi: 10.1124/dmd.104.002832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.