Abstract
Background
Gestational diabetes mellitus (GDM) is associated with increased risk for diabetes mellitus, metabolic syndrome, and cardiovascular disease. We evaluated whether GDM is associated with incident chronic kidney disease (CKD), controlling for pre-pregnancy risk factors for both conditions.
Study Design
Prospective cohort
Setting & Participants
Of 2,747 women (aged 18–30 years) enrolled in the Coronary Artery Risk Development in Young Adults (CARDIA) Study in 1985–86, we studied 820 who were nulliparous at enrollment, delivered at least one pregnancy > 20 weeks’ gestation and had kidney function measures during 25 years of follow-up.
Predictor
GDM was self-reported by women for each pregnancy.
Outcomes
CKD was defined as development of estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2 or urine albumin-creatinine ratio ≥ 25 mg/g at any one CARDIA examination in years 10, 15, 20 or 25.
Measurements
HRs for developing CKD were estimated for women who developed GDM versus women without GDM using complementary log-log models, adjusting for pre-pregnancy age, systolic blood pressure, dyslipidemia, body mass index, smoking, education, eGFR, fasting glucose, physical activity level (all measured at the CARDIA examination prior to first pregnancy), race, and family history of diabetes. We explored for an interaction between race and GDM.
Results
Over a mean follow-up of 20.8 years, 105 of 820 (12.8%) women developed CKD, predominantly increased urine albumin excretion (98 albuminuria only, 4 decreased eGFR only, 3 both). There was evidence of a GDM-race interaction on CKD risk (p=0.06). Among black women, the adjusted HR for CKD was 1.96 (95% CI, 1.04–3.67) in GDM compared to those without GDM. Among white women, the HR was 0.65 (95% CI, 0.23–1.83).
Limitations
Albuminuria was assessed by single untimed measurements of urine albumin and creatinine.
Conclusions
GDM is associated with subsequent development of albuminuria among black women in CARDIA.
Index words: gestational diabetes mellitus (GDM), chronic kidney disease (CKD), incident CKD, albuminuria, pregnancy, diabetes mellitus, race/ethnicity, African American, CKD risk factor
Introduction
Gestational diabetes mellitus (GDM), i.e., glucose intolerance with onset or first recognition during pregnancy, affects about 6% of pregnancies in the United States, where the prevalence of GDM has been increasing over time.1,2 GDM is known to be associated with subsequent development of cardiovascular risk factors including type 2 diabetes mellitus3–6 and metabolic syndrome7,8 as well as subclinical atherosclerosis8,9 and manifest cardiovascular disease.10–12 Chronic kidney disease (CKD), defined by increased urine albumin excretion (albuminuria) and/or reduced estimated glomerular filtration rate (eGFR), affects approximately 13.6% of US adults (2007–2012).13 Reduced eGFR and albuminuria are independent risk factors for all-cause mortality, cardiovascular mortality and end-stage renal disease in the general population.14,15
The relationship between GDM and subsequent CKD is unclear. Studies have reported an association between GDM and early stage CKD16, increased albuminuria among women with a history of GDM compared to those without GDM history,17,18 and a higher prevalence of albuminuria among women with GDM history who later developed diabetes compared to normoglycemic women.19 Other studies have not found differences in albuminuria between women with and without GDM history,20,21 although the cross-sectional nature, lack of pre-pregnancy measurement of shared risk factors for GDM and CKD, or short duration of follow-up time since pregnancy constrained several studies. The aim of this study was to estimate the association between GDM and CKD in a longitudinal, prospective population-based study of young adults that includes pre-pregnancy assessments of kidney function and shared risk factors for GDM and CKD.
Methods
Study Design
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a prospective, population-based cohort study that enrolled 5,115 black and white participants aged 18–30 years in 1985–1986.22 Follow-up examinations occurred at 2, 5, 7, 10, 15, 20 and 25 years (2010–2011) after the initial examination. Participants were recruited from Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. All participants gave informed consent, and the appropriate institutional review boards approved this study.
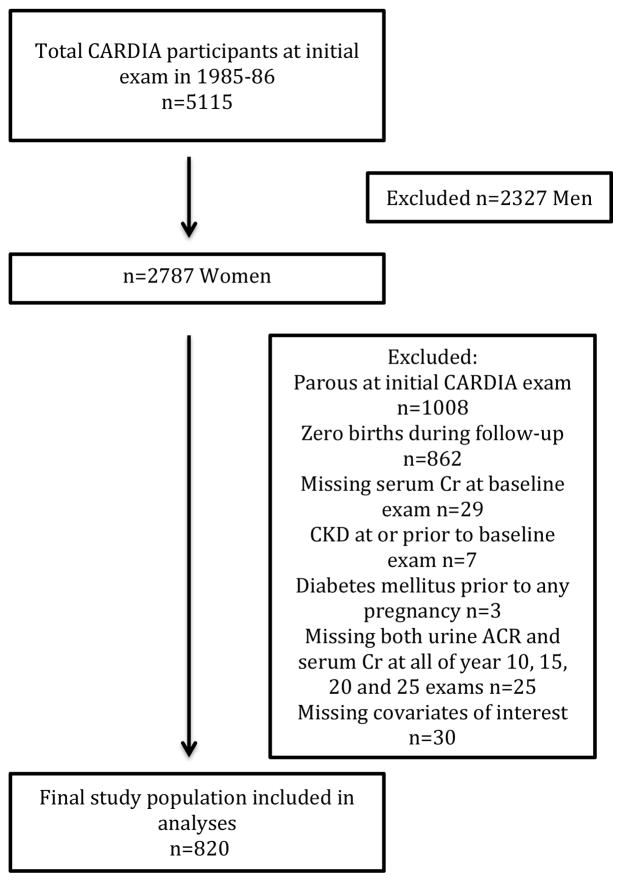
Sample Selection Criteria
Women were asked at each cohort examination whether they were currently pregnant or breastfeeding, and about the number of pregnancies, abortions, miscarriages, stillbirths and live births since the last exam. The first reported pregnancy > 20 weeks’ gestation was treated as the “index” pregnancy. Women were included in our analytic cohort at the examination prior to the index pregnancy, and all covariates were selected from that examination. For example, for a woman who reported her first birth at CARDIA examination year 15, pre-pregnancy covariates were selected from the prior examination in year 10. Urine specimens used to define our outcome were collected at CARDIA years 10, 15, 20 and 25. Women who reported a first birth prior to the 10-year follow-up examination had their covariates assessed at the first CARDIA examination. As shown in Figure 1, we excluded women who were parous at the initial CARDIA examination (n=1008), women with zero births at the end of CARDIA follow-up (examination year 25, n=862), women with CKD prior to or at the baseline examination (n=7), women with diabetes mellitus prior to any pregnancy (n=3), women missing measurement of baseline CKD or missing measures of both albuminuria and eGFR at all four examinations where the outcome was measured (n=25), and women missing data for covariates of interest (n=30). Baseline CKD was defined as GFR < 60 ml/min/1.73m2 or self-reported kidney disease other than nephrolithiasis or pyelonephritis or a urine albumin-creatinine ratio (ACR) ≥ 25 mg/g adjusted for race and gender (urine ACR was not measured at the year 0 examination). Compared to the women included in these analyses, women excluded from this study were more likely to be older, black, with higher systolic blood pressure, body mass index (BMI), LDL (low density lipoprotein)-cholesterol levels, eGFR, and fasting plasma glucose at enrollment into CARDIA (Table S1, available as online supplementary material). The women excluded also were more likely to be smokers and have less education, while being more likely to have metabolic syndrome and family history of CKD compared with the included study population.
Figure 1.
Study population selection. Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; Cr, creatinine; CKD, chronic kidney disease; ACR, albumin-creatinine ratio.
Parity and Gestational Diabetes Mellitus
At each examination, women were asked if they had diabetes and whether they had diabetes only during pregnancy. Self-report of GDM was validated for 200 births between baseline and year 10 in 165 CARDIA women by medical record abstraction of laboratory data. The sensitivity of reports of ever having GDM was 100% (20 of 20) and specificity was 92% (134 of 145).3
Women were included if nulliparous (no live births of > 20 weeks’ gestation) at baseline, and transitioned across follow-up time intervals (0–10, >10–15, >15–20, and >20–25) in which the number of births (parity) and GDM status were updated. The number of births was cumulative to the end of follow up (i.e. examination year of development of CKD or year 25). Once women developed GDM, they were classified as having GDM for all subsequent follow-up time (which may have included additional pregnancies).
Chronic Kidney Disease
Single, untimed urine specimens were collected for measurement of urine albumin and creatinine at the years 10, 15, 20 and 25 examinations. Urine albumin was measured by nephelometry with a specific anti-albumin monoclonal antibody. In years 10, 15, and 20, urine creatinine was measured by the Jaffe method. In year 25, urine creatinine was measured by the Roche enzymatic method. Based on creatinine excretion across 3 days of 24-hour urine collections obtained in a CARDIA subsample (n=839),23 calibration constants were used in our study to adjust for gender and race-specific differences in urinary creatinine excretion as in Murtaugh et al24 using the formula albumin/(k*creatinine) where k=0.88 in black women (no adjustment needed for female gender).
Blood samples were drawn from seated participants after at least 8 hours of fasting. Serum creatinine was measured at years 0, 10, 15, and 20 by the modified-rate Jaffe method. In year 25, creatinine was measured by the Roche enzymatic method. Samples from years 10, 15, 20 and 25 have been calibrated to National Institute of Standards and Technology Standards as recommended by the National Kidney Disease Education Program Laboratory Working Group.25 The eGFR was calculated using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.26 The accuracy of the CKD-EPI equation over a range of kidney function, clinical characteristics and racial backgrounds has been documented. 26, 27
Incident CKD was defined in this study as the development of eGFR < 60 ml/min/1.73 m2, or urine ACR ≥ 25 mg/g after one or more births during follow up, at any one CARDIA examination in years 10, 15, 20 and 25. In examination years where only one CKD measure was available for an individual, either eGFR or ACR was used to define the outcome.
Other Covariates
Information on race, sex, and age was self-reported, and trained interviewers assessed sociodemographic information including highest level of education completed, medical history, family history and medication use. Education level, measured at each examination, was used as a measure of socioeconomic status, and categorized as high school or less or more than high school. Physical activity was assessed using the interviewer-administered CARDIA Physical Activity History assessment at each examination.28 Family history of diabetes mellitus was defined by a report of one or more first-degree relatives having diabetes mellitus at one or more examinations, and family history of CKD was defined as report of one or more first-degree relatives with kidney disease.
Sitting blood pressure was measured by trained technicians who chose the cuff size appropriate to the arm circumference. After an initial 5-minute rest, blood pressure was measured three times at 1-minute intervals using the Hawksley (Lancing, Sussex, UK) random-zero sphygmomanometer through year 15 and the oscillometric Omron (Omron Corp., Schaumberg, IL) HEM907XL in years 20 and 25. Calibration equations were established to calibrate the Omron measurements to the random-zero device.29 The average of the second and third measurements were used. Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg or use of antihypertensive medications.
Weight and height were measured at each examination, to the nearest 0.5 cm and nearest 0.2 kg, respectively.30 BMI was computed as weight in kilograms divided by height in meters squared.
Glucose was measured using the hexokinase ultraviolet method at years 0, 10, 15 and 20 using hexokinase coupled to glucose-6-phosphate dehydrogenase.31 At year 25 fasting glucose was measured by the Roche Modular P hexokinase method. Blood lipids and lipoproteins were measured at each examination.32 Total cholesterol and triglycerides (TG) were measured enzymatically, high-density lipoprotein (HDL) cholesterol was determined by precipitation with dextran sulfate-magnesium chloride, and low-density lipoprotein cholesterol was calculated using the Friedewald equation.32 Diabetes mellitus was defined as a fasting glucose ≥ 126 mg/dl, hemoglobin A1c ≥ 6.5%, 2 hour oral glucose tolerance test (OGTT) ≥ 200 mg/dl and/or use of diabetes medications. Glucose intolerance was defined as fasting plasma glucose ≥ 100 mg/dl, 2 hour OGTT ≥ 140 mg/dl or use of diabetes medications. Insulin resistance was defined as TG-HDL cholesterol ratio ≥ 1.7 for black women and TG-HDL cholesterol ratio ≥ 2.2 for white women33 or by the Homeostatic Model Assessment of Insulin Resistance (HOMA-IR).34,35 Metabolic syndrome was defined as any 3 of waist girth > 88 cm, TG ≥ 150 mg/dl, HDL cholesterol < 50 mg/dl, SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg or use of antihypertensive medications, and fasting glucose ≥ 100 mg/dl.
Statistical Analysis
Baseline characteristics were compared between women with and without reported GDM pregnancies. Pre-pregnancy characteristics correspond to the examination at the beginning of the interval during which a woman first reports a birth, where the intervals are CARDIA examination years 0–10, 10–15, 15–20, 20–25. The probability of not having CKD at each time interval was calculated by product limit estimation, and cumulative incidence of CKD within each time interval was calculated as 1 minus survival, for parous women with and without exposure to GDM. To account for interval censored data, complementary log-log models were used to estimate hazard ratios (HRs) of CKD by the method of Prentice and Gloecker,36 comparing women with and without a history of GDM. Confounders were chosen a priori based on a directed acyclic graph.37 DAGgitty38 was used to determine the minimally sufficient set, which included baseline (from the CARDIA examination prior to first pregnancy) age, race, smoking, dyslipidemia, SBP, BMI, physical activity score, and education level (for socioeconomic status) as well as any report of family history of diabetes. Additionally, baseline fasting glucose and eGFR were included in the adjusted model. Effect measure modification between race and the exposure was calculated by the addition of an interaction term to each model and considered significant for likelihood ratio tests with p-values less than 0.1. To analyze change in eGFR over time, percent annualized decline in eGFR was calculated for each interval and used as the outcome measure in linear mixed models. Linear mixed models were chosen to account for within-subject correlation of repeated measure using the autoregressive (1) correlation structure based on corrected Akaike Information Criterion. The same minimally sufficient set of baseline confounders was included in the adjusted model, as well as baseline fasting glucose and eGFR. Analyses were conducted using SAS version 9.4 (Cary, NC) and type 1 error was set at 5% level of significance for two-sided tests.
Results
Study Participants
A total of 101/820 (12.3%) of the women in the analytic sample reported a GDM pregnancy. Women with GDM were more likely to have family history of diabetes, they more frequently had a family history of CKD, and they had higher pre-pregnancy BMI (Table 1). There was no difference between those with and without GDM for pre-pregnancy age, race, education, eGFR, smoking status, fasting glucose, waist circumference, HDL-C levels, systolic blood pressure, diastolic blood pressure and total physical activity score.
Table 1.
Baseline characteristics by GDM status
| Variable | Parous, no GDM N=719 |
GDM N=101 |
p-value |
|---|---|---|---|
| Agea (years) | 25.8 ± 5.1 | 25.3 ± 5.1 | 0.3 |
| Race | 0.8 | ||
| Black | 289 (40.2) | 42 (41.6) | |
| White | 430 (59.8) | 59 (58.4) | |
| Education | 0.5 | ||
| ≤High school | 179 (24.9) | 21 (20.8) | |
| >High school | 540 (75.1) | 80 (79.2) | |
| eGFR (ml/min/1.73 m2) | 119.2 ± 17.8) | 118.4 ± 16.7 | 0.7 |
| Smoking status | 0.3 | ||
| Never | 474 (65.9) | 62 (61.4) | |
| Former | 101 (14.1) | 12 (11.9) | |
| Current | 144 (20.0) | 27 (26.7) | |
| BMI (kg/m2) | 23.5 ± 4.9 | 24.7 ± 5.6 | 0.03 |
| Waist circumference (cm) | 71.9 ± 9.9 | 74.3 ± 11.5 | 0.05 |
| Fasting glucose (mg/100 ml) | 80.5 ± 7.3 | 81.6 ± 9.2 | 0.3 |
| HDL cholesterol (mg/dl) | 57.5 ± 12.8 | 55.5 ± 13.0 | 0.1 |
| SBP (mmHg) | 105.4 ± 9.1 | 106.2 ± 9.4 | 0.4 |
| DBP (mmHg) | 66.2 ± 8.9 | 67.6 ± 8.6 | 0.2 |
| Family history of diabetes | 0.002 | ||
| Yes | 291 (40.5) | 58 (57.4) | |
| No | 428 (59.5) | 43 (42.6) | |
| Family history of CKD | 0.02 | ||
| Yes | 54 (9.0) | 15 (17.7) | |
| No | 548 (91.0) | 70 (82.4) | |
| Total physical activity scoreb | 316 [174–504] | 258 [182–476] | 0.3 |
Note: Characteristics are for the sample of 820 CARDIA women who were nulliparous at baseline examination and delivered ≥1 births during follow-up (mean, 20.8 years) and had measures of CKD. Values for categorical variables are given as count (percentage); for continuous variables, as median [interquartile range]. P-values were calculated using t-test for continuous variables and by Fisher’s Exact Test or chi-square where appropriate for categorical variables except where noted.
Baseline characteristics were taken from the examination at the beginning of interval during which participants reported their first pregnancy, except family history of diabetes which could be reported at any examination, and family history of CKD which was only collected at examination year 25. Conversion factors for units: glucose in mg/dl to mmol/L, x0.05551, cholesterol in mg/dl to mmol/L, x0.02586.
Abbreviations: BP, blood pressure; BMI, body mass index; CARDIA, Coronary Artery Risk Development in Young Adults; CKD, chronic kidney disease; DBP, diastolic blood pressure; GDM, gestational diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SBP, systolic blood pressure
Age at CARDIA examination prior to first reported pregnancy
p-value was calculated using Kruskal-Wallis test.
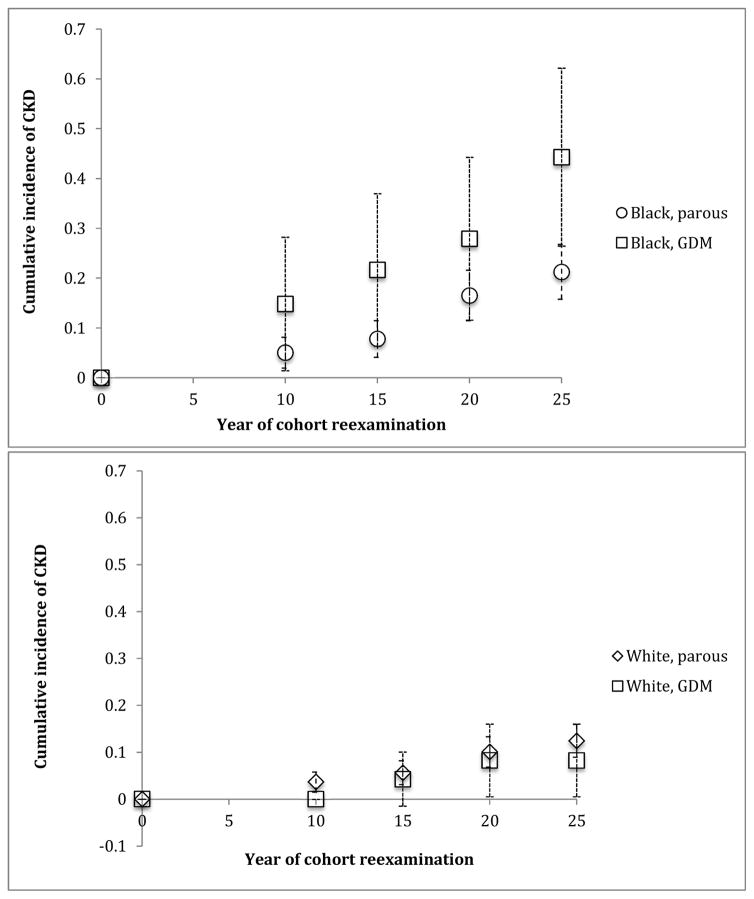
A total of N=105 incident cases of CKD accrued over mean follow-up time of 20.8 years. Of those, 98 had albuminuria only, 4 had decreased eGFR only, and 3 met both CKD criteria. Overall, among women with GDM, 17/101 (16.8%) developed CKD compared to 88/719 (12.2%) without GDM (p=0.2). Among black women with GDM, 13/42 (31.0%) developed CKD compared to 45/289 (15.6%) without GDM (p=0.03). Among white women who reported GDM, 4/59 (6.8%) developed CKD compared to 43/430 (10.0%) without GDM (p=0.6). The cumulative incidence of CKD at each examination interval was greater among those with GDM compared to those without although this result was not statistically significant (Table 2). The relative hazard of incident CKD was higher among women with GDM compared to parous women without a history of GDM, although not statistically significant in the crude model (Table 3). Adjustment for confounders did not significantly change the result. There was evidence of interaction between GDM and race (likelihood ratio test interaction p=0.06). Among black women, the HR for CKD was 1.96 (95% CI, 1.04–3.67) for those with GDM versus parous women without GDM. Among white women, the HR was 0.65 (95% CI, 0.23–1.83). The incidence curves by race and GDM status are presented in Figure 2.
Table 2.
Cumulative incidence of CKD at examination years 0 to 25 by GDM status among parous CARDIA women with follow-up births
| Exam year | No. of events | No. at risk | Cumulative Incidence (95% CI) |
|---|---|---|---|
| GDM | |||
| 0 | 0 | 66 | 0 (0–0) |
| 10 | 4 | 62 | 0.065 (0.003–0.126) at year 10 |
| 15 | 4 | 72 | 0.116 (0.040–0.193) at year 15 |
| 20 | 4 | 73 | 0.165 (0.079–0.250) at year 20 |
| 25 | 5 | 69 | 0.225 (0.131–0.320) at year 25 |
| Parous, no GDM | |||
| 0 | 0 | 520 | 0 (0–0) |
| 10 | 21 | 499 | 0.042 (0.024,–0.060) at year 10 |
| 15 | 13 | 547 | 0.065 (0.044–0.086) at year 15 |
| 20 | 34 | 531 | 0.125 (0.097–0.152) at year 20 |
| 25 | 20 | 534 | 0.158 (0.127–0.188) at year 25 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; CKD, chronic kidney disease; GDM, gestational diabetes mellitus
Table 3.
Hazard ratios for CKD by GDM status among CARDIA women nulliparous at baseline who self-reported interim births during mean 20.8 years of CARDIA follow-up
| HR (95% CI) | |||
|---|---|---|---|
| Model | Parous, no GDM | GDM | p for interaction |
| Crude model | 1.00 (reference) | 1.46 (0.87–2.45) | |
| Model 1a | 1.00 (reference) | 1.33 (0.78–2.26) | |
| Model 2b | 0.06 | ||
| Black women | 1.00 (reference) | 1.96 (1.04–2.67) | |
| White women | 1.00 (reference) | 0.65 (0.23–1.83) | |
Note: 820 subjects contributed 2407 observations to each model. Each subject can contribute one observation for each CARDIA examination year she has data available for the outcome.
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; CKD, chronic kidney disease; GDM, gestational diabetes mellitus; HR, hazard ratio.
Model 1 includes variables for age, race, body mass index, smoking, family history of diabetes, fasting glucose, baseline estimated glomerular filtration rate, education, high-density lipoprotein cholesterol, systolic blood pressure and physical activity score.
Model 2 is model 1 + interaction term for race and GDM.
Figure 2.
Incident CKD in parous women with and without GDM, by race. Abbreviations: CKD, chronic kidney disease, GDM gestational diabetes mellitus.
We examined whether the development of glucose intolerance, insulin resistance, diabetes mellitus, metabolic syndrome, or hypertension contributed to explain the association between GDM and CKD. Black women were more likely than white women to develop insulin resistance as defined by HOMA-IR, metabolic syndrome, hypertension and diabetes mellitus (Table 4). However, there was no evidence of effect measure modification between interval development of those factors and the association between CKD and GDM in parous black women (Table 5).
Table 4.
Cumulative incidence of metabolic dysfunction in parous CARDIA women by race
| Variable | Black n=331 |
White n=489 |
p-value |
|---|---|---|---|
| Glucose intolerancea | 0.08 | ||
| Yes | 121 (36.6) | 150 (30.7) | |
| No | 210 (63.4) | 339 (69.33) | |
| Insulin resistanceb | 0.6 | ||
| Yes | 141 (42.6) | 198 (40.5) | |
| No | 190 (57.4) | 291 (59.5) | |
| HOMA-IR | <0.001 | ||
| >4 | 114 (34.4) | 63 (12.9) | |
| ≤4 | 217 (65.6) | 426 (87.1) | |
| HOMA-IR | <0.001 | ||
| ≥ 2.73 | 192 (58.0) | 145 (29.7) | |
| <2.73 | 139 (42.0) | 344 (70.4) | |
| Metabolic syndromec | <0.001 | ||
| Yes | 72 (21.8) | 60 (12.3) | |
| No | 259 (78.3) | 429 (87.7) | |
| Diabetes mellitus | <0.001 | ||
| Yes | 41 (12.4) | 22 (4.5) | |
| No | 290 (87.6) | 467 (95.5) | |
| Hypertension | <0.001 | ||
| Yes | 115 (34.7) | 69 (14.1) | |
| No | 216 (65.3) | 420 (85.9) | |
| GDM | 0.8 | ||
| Yes | 42 (12.7) | 59 (12.1) | |
| No | 289 (87.3) | 430 (87.9) | |
| CKD | 0.001 | ||
| Yes | 58 (17.5) | 47 (9.6) | |
| No | 273 (82.5) | 442 (90.4) |
Notes: Values presented are number (percentage). P-values obtained from Fisher’s exact test.
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CKD, chronic kidney disease; GDM, gestational diabetes mellitus; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance.
Table 5.
Association between CKD and GDM by presence of glucose intolerance, insulin resistance, metabolic syndrome, diabetes, and hypertension in parous black CARDIA women
| HR (95% CI) | |||
|---|---|---|---|
| Variable | no GDM | GDM | LRT p for interaction of GDM & variable |
| Glucose intolerancea | 0.4 | ||
| Yes | 1.00 (reference) | 1.70 (0.71–4.07) | |
| No | 1.00 (reference) | 2.98 (1.23–7.26) | |
| Insulin resistanceb | 0.1 | ||
| Yes | 1.00 (reference) | 1.23 (0.47–3.23) | |
| No | 1.00 (reference) | 3.80 (1.69–8.55) | |
| HOMA-IR | 0.6 | ||
| >4 | 1.00 (reference) | 1.83 (0.75–4.44) | |
| ≤4 | 1.00 (reference) | 2.60 (1.08–6.28) | |
| HOMA-IR | 0.3 | ||
| ≥2.73 | 1.00 (reference) | 1.77 (0.89–3.91) | |
| <2.73 | 1.00 (reference) | 3.77 (1.41–10.13) | |
| Metabolic syndromec | 0.5 | ||
| Yes | 1.00 (reference) | 1.55 (0.55–4.35 | |
| No | 1.00 (reference) | 2.50 (1.15–5.43) | |
| Diabetes mellitus | 0.9 | ||
| Yes | 1.00 (reference) | 2.05 (0.66–6.36) | |
| No | 1.00 (reference) | 1.95 (0.87–4.37) | |
| Hypertension | 0.3 | ||
| Yes | 1.00 (reference) | 1.39 (0.47–4.10) | |
| No | 1.00 (reference) | 2.94 (1.38–6.26) | |
Note: 331 subjects contributed 948 observations to each model. Each subject can contribute one observation for each CARDIA exam year she has data available for the outcome.
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; CKD, chronic kidney disease; GDM, gestational diabetes mellitus; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; HR, hazard ratio; LRT, likelihood ratio test
No differences were seen in percent-annualized eGFR change between parous women with and without GDM (Table 6), nor was there evidence of an interaction between race and GDM (Wald p for interaction ≥ 0.2).
Table 6.
Differences in percent annualized decline in eGFR for parous CARDIA women by GDM history
| Model | no GDM | GDMa |
|---|---|---|
| Crude | 1.00 (reference) | 0.064 (−0.16 to 0.29) |
| Model 1b | 1.00 (reference) | 0.013 (−0.21to 0.24) |
Note: 820 subjects contributed 2407 observations to each model. Each subject can contribute an observation for each CARDIA.
examination year she has data available for the outcome measurement.
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; eGFR, estimated glomerular filtration rate; GDM, gestational diabetes mellitus.
Result may be interpreted as the difference in percent annual decline in eGFR (% ml/min/1.73 m2); values in parenthese are 95% confidence interval.
Model 1 is adjusted for race, age, body mass index, smoking, family history of diabetes, fasting glucose, baseline eGFR, education, high-density lipoprotein cholesterol, systolic blood pressure, and physical activity score.
Sensitivity Analysis
Normal pregnancy affects renal physiology, resulting in an increase in eGFR and a small increase in albuminuria.39 As a sensitivity analysis, measures of CKD were set to missing for any examination year when a woman was pregnant, and complementary log-log models were again used to estimate HRs of CKD. Results did not differ from the initial analysis. Among black women, the HR was 1.96 (95% CI, 1.04–3.67) for those with GDM compared to those without GDM, while among white women the HR was 0.64 (95% CI, 0.23–1.81).
Women with pregnancies before the initial CARDIA examination who also reported interim births during follow-up (n=387) were included in a sensitivity analysis with the main study sample (total N=1207 for this analysis). Only births occurring after the initial CARDIA examination were considered, and covariates were taken from the CARDIA examination at the beginning of the interval during which women reported their first interim birth. Overall, results did not differ significantly from the main analysis (Tables S2 and S3). In addition, the analysis was repeated considering only women with albuminuria as the outcome. Results were not different from the main analysis.
Discussion
GDM was associated with development of CKD, predominantly albuminuria, among black women, but not white women in this study of parous women. The association was similar after adjusting for pre-pregnancy risk factors for GDM and CKD, including age, SBP, dyslipidemia, BMI, smoking, socioeconomic status, eGFR, fasting glucose, and physical activity level as well as race and family history of diabetes.
Biomarkers of endothelial dysfunction are elevated in women with recent GDM compared to controls40 and have been shown to predict the development of diabetes independent of other known risk factors.41,42 Elevated levels of these biomarkers have also been associated with onset and progression of albuminuria as well as increased risk of death among patients with type 2 diabetes.43 Given the increased cardiovascular and mortality risk associated with albuminuria even in the general population,14,44 several authors have proposed that endothelial dysfunction, at least in part, may explain the link between albuminuria, cardiovascular disease and mortality.45,46
Only a small number of women in this cohort developed eGFR < 60 ml/min/1.73 m2, and no association was seen between GDM and eGFR decline. Glomerular hyperfiltration, or abnormally high eGFR, is seen in early diabetic kidney disease along with albuminuria, and may predict progressive diabetic nephropathy.47–49 Although we used the CKD-EPI equation in our study, as it was developed to be more accurate at higher eGFRs,26 among diabetics this equation and other estimation formulas may fail to detect hyperfiltration and underestimate eGFR decline when compared to measured GFR.50
A prior, although cross-sectional, analysis reported results similar to ours. Women in the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP) with self-reported GDM, but without subsequent diabetes, had higher odds of microalbuminuria compared to women without any diabetes history.16 An interaction with race and GDM was also seen in the KEEP population, with the association between GDM and albuminuria found in black women, but not white women. Another cross-sectional analysis of women with GDM but not subsequent diabetes in the NHANES (National Health and Nutrition Examination Survey) was equivocal in relating moderately increased albuminuria to GDM.18 The divergent results in the latter two studies may reflect differences in age of the women with GDM history (mean ages, 32.2 years in NHANES and 51.5 years in KEEP), and the population-based design of NHANES and the kidney disease risk setting in KEEP.
Several studies have shown greater risk for incident type 2 diabetes among black women with GDM history compared to white women with GDM history.5,6 Diabetes is a well-established risk factor for CKD,51,52 and development of type 2 diabetes mellitus after a GDM pregnancy could explain the association between GDM and incident CKD. Go et al19 evaluated a cohort of 289 black women with GDM a median of 11 years after delivery and reported a prevalence of moderately increased albuminuria of 11% in normoglycemic women versus 36% in women who had overt DM after GDM. Kew et al21 tested women who were 3 years postpartum, and found an association between current glucose intolerance and elevated urine ACR, but not GDM and moderaltely increased albuminuria, although glucose intolerance was treated as a confounder of the association between GDM and moderately increased albuminuria. We consider post-partum development of glucose intolerance and type 2 diabetes mellitus to be an intermediate on the causal pathway between GDM and CKD, and did not adjust for it to avoid introducing bias.53,54 The presence of glucose intolerance, insulin resistance, diabetes mellitus, and metabolic syndrome prior to albuminuria onset did not explain the association between incident albuminuria and GDM seen among black women in our study. Other potential explanations for the association between GDM and CKD in black women include genetic variation, blood pressure elevation following GDM, differences in lactation practices, or differences in the severity of GDM. At least one study reported that genes flanking the major histocompatibility complex are associated with diabetes mellitus, insulin resistance, hypertension and moderately increased albuminuria in black women with a history of GDM.55 Black women have been shown to develop hypertension, a risk factor for CKD, more often and more rapidly following a GDM pregnancy compared to white women.56 In our cohort, black women were more likely than white women to develop hypertension prior to onset of albuminuria, however the presence of hypertension did not seem to account for the association between incident albuminuria and GDM seen among black women. Higher lactation intensity and longer duration are associated with lower incidence of type 2 diabetes 2 years after a GDM pregnancy,57 and longer lactation duration was associated with lower metabolic syndrome incidence years after a GDM delivery.31 Failure to initiate breastfeeding was reported to be higher among black women,58 including a failure to initiate breastfeeding after GDM pregnancy.59 The severity of GDM may be greater in black than in white women, as they may be more likely to require incident insulin treatment for GDM60 and may have more severe complications of GDM.61
Among the limitations of this study is that GDM was self-reported, although the validity of self-reported GDM in CARDIA was high versus medical record review3, similar to other validation studies of self-reported GDM.62,63 Urine specimens were not obtained at the initial CARDIA examination, so urine ACR ≥ 25 mg/g at year 0 could not be used as exclusion criteria for women who entered the cohort during the interval from year 0 to year 10. Although some women with underlying kidney disease may have been included in the cohort, a high prevalence of albuminuria is unlikely in women aged 18–30 years old. Albuminuria was assessed by untimed measurements of urinary albumin and creatinine and thus associated with significant within-person variability,64 although this practice is supported by strong associations of albuminuria using single ACR measurements with cardiovascular and kidney outcomes documented in the literature. 15,65,66 Few women developed reduced eGFR, but results were similar when reduced eGFR was removed from the outcome definition. The strengths of this study include the racially diverse study population, long duration of follow-up and the availability of longitudinal measures of CKD and pre-pregnancy risk factors for both CKD and GDM.
We conclude that black women in CARDIA with a history of GDM are at risk for subsequent albuminuria, independent of traditional risk factors for CKD. Early detection of albuminuria allows for both lifestyle and medical interventions, which slow progression of disease and reduce cardiovascular risk, yet early stages of CKD are asymptomatic.67 Other pregnancy complications, including preeclampsia and gestational hypertension, have been associated with incident maternal CKD.68–70 Pregnancy may present a window of opportunity to identify women at risk of CKD and implement prevention strategies. Replication of our results would suggest that following a GDM pregnancy, black women may benefit from counseling about the need for screening for albuminuria and risk modification strategies.
Supplementary Material
Table S1: Exam year 0 characteristics compared for CARDIA women included and excluded from this analysis.
Table S2: Cumulative incidence of CKD by history of GDM among all CARDIA women reporting interim births at exam years 0, 10, 15, 20, and 25.
Table S3: HRs for CKD by history of exposure to GDM among all CARDIA women reporting interim births during mean 20.8 y of follow-up.
Acknowledgments
Support: The CARDIA Study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI); R01 DK090047 (Dr Gunderson, PI), K01 DK059944 (Dr Gunderson, PI) and 5K24DK103992 (Dr Bibbins-Domingo, PI) from the National Institute of Diabetes, Digestive, and Kidney Diseases; the Intramural Research Program of the National Institute on Aging (NIA), and an intra-agency agreement between NIA and NHLBI (AG0005). The funders of the study had no role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
This manuscript has been reviewed by CARDIA for scientific content and consistency of data interpretation with previous CARDIA publications.
Footnotes
Authors’ Contributions: Research idea and study design: EWD, EPG, CEL, SME, MJF, AVK, GH; data acquisition: EWD, EPG, CEL, KB-D, HK; data analysis/interpretation: EWD, MAP, SME, MJF, AVK, GH; statistical analysis: EWD, MAP; supervision or mentorship: GH, EPG, CEL. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Peer Review: Evaluated by two external peer reviewers, with editorial input from a Statistics/Methods Editor, an Associate Editor, and the Editor-in-Chief.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Exam year 0 characteristics compared for CARDIA women included and excluded from this analysis.
Supplementary Table S2 (PDF). Cumulative incidence of CKD by history of GDM among all CARDIA women reporting interim births at exam years 0, 10, 15, 20, and 25.
Supplementary Table S3 (PDF). HRs for CKD by history of exposure to GDM among all CARDIA women reporting interim births during mean 20.8 y of follow-up.
Financial Disclosure: Dr Lewis has received research funding from Novo Nordisk and the National Institutes of Health. The other authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: temporal trends 1989 through 2004. American journal of obstetrics and gynecology. 2008 May;198(5):525.e521–525. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG: an international journal of obstetrics and gynaecology. 2017 Apr;124(5):804–813. doi: 10.1111/1471-0528.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderson EP, Lewis CE, Tsai AL, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007 Dec;56(12):2990–2996. doi: 10.2337/db07-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirkola J, Pouta A, Bloigu A, et al. Prepregnancy overweight and gestational diabetes as determinants of subsequent diabetes and hypertension after 20-year follow-up. The Journal of clinical endocrinology and metabolism. 2010 Feb;95(2):772–778. doi: 10.1210/jc.2009-1075. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Chen L, Horswell R, et al. Racial differences in the association between gestational diabetes mellitus and risk of type 2 diabetes. Journal of women’s health (2002) 2012 Jun;21(6):628–633. doi: 10.1089/jwh.2011.3318. [DOI] [PubMed] [Google Scholar]
- 6.Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011 Dec;54(12):3016–3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Childbearing is associated with higher incidence of the metabolic syndrome among women of reproductive age controlling for measurements before pregnancy: the CARDIA study. American journal of obstetrics and gynecology. 2009 Aug;201(2):177e171–179. doi: 10.1016/j.ajog.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karoli R, Siddiqi Z, Fatima J, Shukla V, Mishra PP, Khan FA. Assessment of noninvasive risk markers of subclinical atherosclerosis in premenopausal women with previous history of gestational diabetes mellitus. Heart views: the official journal of the Gulf Heart Association. 2015 Jan-Mar;16(1):13–18. doi: 10.4103/1995-705X.152995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunderson EP, Chiang V, Pletcher MJ, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. Journal of the American Heart Association. 2014;3(2):e000490. doi: 10.1161/JAHA.113.000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG: an international journal of obstetrics and gynaecology. 2014 Nov;121(12):1530–1536. doi: 10.1111/1471-0528.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaul P, Savu A, Nerenberg KA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: a population-level analysis. Diabetic medicine: a journal of the British Diabetic Association. 2015 Feb;32(2):164–173. doi: 10.1111/dme.12635. [DOI] [PubMed] [Google Scholar]
- 12.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2009 Sep 15;181(6–7):371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.System USRD. 2015 USRDS annual data report: Epidemiology of Kidney Disease in the United States. Bethesda, Maryland: National Institues of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 14.Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney international. 2011 Jul;80(1):93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomback AS, Rekhtman Y, Whaley-Connell AT, et al. Gestational diabetes mellitus alone in the absence of subsequent diabetes is associated with microalbuminuria: results from the Kidney Early Evaluation Program (KEEP) Diabetes care. 2010 Dec;33(12):2586–2591. doi: 10.2337/dc10-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman S, Rabinerson D, Bar J, et al. Microalbuminuria following gestational diabetes. Acta obstetricia et gynecologica Scandinavica. 1995 May;74(5):356–360. doi: 10.3109/00016349509024428. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Cheng YJ, Beckles GL. Cardiovascular disease risk profiles in women with histories of gestational diabetes but without current diabetes. Obstetrics and gynecology. 2008 Oct;112(4):875–883. doi: 10.1097/AOG.0b013e31818638b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go RC, Desmond R, Roseman JM, Bell DS, Vanichanan C, Acton RT. Prevalence and risk factors of microalbuminuria in a cohort of African-American women with gestational diabetes. Diabetes care. 2001 Oct;24(10):1764–1769. doi: 10.2337/diacare.24.10.1764. [DOI] [PubMed] [Google Scholar]
- 20.Friedman AN, Marrero D, Ma Y, et al. Value of urinary albumin-to-creatinine ratio as a predictor of type 2 diabetes in pre-diabetic individuals. Diabetes care. 2008 Dec;31(12):2344–2348. doi: 10.2337/dc08-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kew S, Swaminathan B, Hanley AJ, et al. Postpartum microalbuminuria after gestational diabetes: the impact of current glucose tolerance status. The Journal of clinical endocrinology and metabolism. 2015 Mar;100(3):1130–1136. doi: 10.1210/jc.2014-3814. [DOI] [PubMed] [Google Scholar]
- 22.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. Journal of clinical epidemiology. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs DR, Jr, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender- and race-specific determination of albumin excretion rate using albumin-to-creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. American journal of epidemiology. 2002 Jun 15;155(12):1114–1119. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 24.Murtaugh MA, Jacobs DR, Jr, Yu X, Gross MD, Steffes M. Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. American journal of epidemiology. 2003 Oct 1;158(7):676–686. doi: 10.1093/aje/kwg208. [DOI] [PubMed] [Google Scholar]
- 25.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clinical chemistry. 2006 Jan;52(1):5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padala S, Tighiouart H, Inker LA, et al. Accuracy of a GFR estimating equation over time in people with a wide range of kidney function. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2012 Aug;60(2):217–224. doi: 10.1053/j.ajkd.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderssen N, Jacobs DR, Jr, Sidney S, et al. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) American journal of epidemiology. 1996 Feb 15;143(4):351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 29.Gunderson EP, Chiang V, Lewis CE, et al. Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstetrics and gynecology. 2008 Dec;112(6):1294–1302. doi: 10.1097/AOG.0b013e31818da09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR., Jr Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. American journal of public health. 1997 Apr;87(4):635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunderson EP, Jacobs DR, Jr, Chiang V, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults) Diabetes. 2010 Feb;59(2):495–504. doi: 10.2337/db09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Annals of epidemiology. 1996 May;6(3):235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 33.Kats D, Knowles JW, Reaven GM, Sharrett AR, Nambi V, Heiss G. Abstract MP37: The Triglyceride to High-density Lipoprotein Cholesterol Ratio, an Estimate of Insulin Resistance, is Associated with Incident Coronary Heart Disease. The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2016 Mar 1;133(Suppl 1):AMP37. [Google Scholar]
- 34.Gunderson EP, Quesenberry CP, Jr, Jacobs DR, Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: The CARDIA study. American journal of epidemiology. 2010 Nov 15;172(10):1131–1143. doi: 10.1093/aje/kwq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008 Feb;196(2):696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978 Mar;34(1):57–67. [PubMed] [Google Scholar]
- 37.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass) 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 38.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology (Cambridge, Mass) 2011 Sep;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 39.Odutayo A, Hladunewich M. Obstetric nephrology: renal hemodynamic and metabolic physiology in normal pregnancy. Clinical journal of the American Society of Nephrology: CJASN. 2012 Dec;7(12):2073–2080. doi: 10.2215/CJN.00470112. [DOI] [PubMed] [Google Scholar]
- 40.Gobl CS, Bozkurt L, Yarragudi R, et al. Biomarkers of endothelial dysfunction in relation to impaired carbohydrate metabolism following pregnancy with gestational diabetes mellitus. Cardiovascular diabetology. 2014;13:138. doi: 10.1186/s12933-014-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA: the journal of the American Medical Association. 2004 Apr 28;291(16):1978–1986. doi: 10.1001/jama.291.16.1978. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Manson JE, Tinker L, et al. Circulating levels of endothelial adhesion molecules and risk of diabetes in an ethnically diverse cohort of women. Diabetes. 2007 Jul;56(7):1898–1904. doi: 10.2337/db07-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002 Apr;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 44.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 45.Amann K, Wanner C, Ritz E. Cross-talk between the kidney and the cardiovascular system. Journal of the American Society of Nephrology: JASN. 2006 Aug;17(8):2112–2119. doi: 10.1681/ASN.2006030204. [DOI] [PubMed] [Google Scholar]
- 46.Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. Journal of the American Society of Nephrology: JASN. 2006 Aug;17(8):2106–2111. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 47.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009 Apr;52(4):691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 48.Moriya T, Tsuchiya A, Okizaki S, Hayashi A, Tanaka K, Shichiri M. Glomerular hyperfiltration and increased glomerular filtration surface are associated with renal function decline in normo- and microalbuminuric type 2 diabetes. Kidney international. 2012 Mar;81(5):486–493. doi: 10.1038/ki.2011.404. [DOI] [PubMed] [Google Scholar]
- 49.Ruggenenti P, Porrini EL, Gaspari F, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes care. 2012 Oct;35(10):2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaspari F, Ruggenenti P, Porrini E, et al. The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney international. 2013 Jul;84(1):164–173. doi: 10.1038/ki.2013.47. [DOI] [PubMed] [Google Scholar]
- 51.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012 Jan 14;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 52.McClellan WM. Epidemiology and risk factors for chronic kidney disease. The Medical clinics of North America. 2005 May;89(3):419–445. doi: 10.1016/j.mcna.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. Toward a clearer definition of confounding” revisited with directed acyclic graphs. American journal of epidemiology. 2012 Sep 15;176(6):506–511. doi: 10.1093/aje/kws127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weinberg CR. Toward a clearer definition of confounding. American journal of epidemiology. 1993 Jan 1;137(1):1–8. doi: 10.1093/oxfordjournals.aje.a116591. [DOI] [PubMed] [Google Scholar]
- 55.Acton RT, Bell DS, Collins J, et al. Genes within and flanking the major histocompatibility region are risk factors for diabetes, insulin resistance, hypertension, and microalbuminuria in African-American women. Transplantation proceedings. 1997 Dec;29(8):3710–3712. doi: 10.1016/s0041-1345(97)01079-8. [DOI] [PubMed] [Google Scholar]
- 56.Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. The American journal of cardiology. 2014 Apr 15;113(8):1364–1370. doi: 10.1016/j.amjcard.2014.01.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunderson EP, Hurston SR, Ning X, et al. Lactation and Progression to Type 2 Diabetes Mellitus After Gestational Diabetes Mellitus: A Prospective Cohort Study. Annals of internal medicine. 2015 Dec 15;163(12):889–898. doi: 10.7326/M15-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Smith MG, Dobre MA, Ferguson JE. Maternal obesity and breast-feeding practices among white and black women. Obesity (Silver Spring, Md) 2010 Jan;18(1):175–182. doi: 10.1038/oby.2009.182. [DOI] [PubMed] [Google Scholar]
- 59.Cordero L, Thung S, Landon MB, Nankervis CA. Breast-feeding initiation in women with pregestational diabetes mellitus. Clinical pediatrics. 2014 Jan;53(1):18–25. doi: 10.1177/0009922813496455. [DOI] [PubMed] [Google Scholar]
- 60.Hillier TA, Ogasawara KK, Pedula KL, Vesco KK. Markedly different rates of incident insulin treatment based on universal gestational diabetes mellitus screening in a diverse HMO population. American journal of obstetrics and gynecology. 2013 Nov;209(5):440e441–449. doi: 10.1016/j.ajog.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. American journal of obstetrics and gynecology. 2012 Oct;207(4):322e321–326. doi: 10.1016/j.ajog.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. American journal of epidemiology. 1997 Jan 1;145(1):58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- 63.Sou SC, Chen WJ, Hsieh WS, Jeng SF. Severe obstetric complications and birth characteristics in preterm or term delivery were accurately recalled by mothers. Journal of clinical epidemiology. 2006 Apr;59(4):429–435. doi: 10.1016/j.jclinepi.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 64.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2013 May;61(5):716–722. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010 Jun 12;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McQueen MJ, Gerstein HC, Pogue J, Mann JF, Yusuf S. Reevaluation by high-performance liquid chromatography: clinical significance of microalbuminuria in individuals at high risk of cardiovascular disease in the Heart Outcomes Prevention Evaluation (HOPE) Study. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2006 Dec;48(6):889–896. doi: 10.1053/j.ajkd.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Stenvinkel P. Chronic kidney disease: a public health priority and harbinger of premature cardiovascular disease. Journal of internal medicine. 2010 Nov;268(5):456–467. doi: 10.1111/j.1365-2796.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 68.Bar J, Kaplan B, Wittenberg C, et al. Microalbuminuria after pregnancy complicated by pre-eclampsia. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 1999 May;14(5):1129–1132. doi: 10.1093/ndt/14.5.1129. [DOI] [PubMed] [Google Scholar]
- 69.Mannisto T, Mendola P, Vaarasmaki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013 Feb 12;127(6):681–690. doi: 10.1161/CIRCULATIONAHA.112.128751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shahbazian N, Shahbazian H, Ehsanpour A, Aref A, Gharibzadeh S. Hypertension and microalbuminuria 5 years after pregnancies complicated by pre-eclampsia. Iranian journal of kidney diseases. 2011 Sep;5(5):324–327. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Exam year 0 characteristics compared for CARDIA women included and excluded from this analysis.
Table S2: Cumulative incidence of CKD by history of GDM among all CARDIA women reporting interim births at exam years 0, 10, 15, 20, and 25.
Table S3: HRs for CKD by history of exposure to GDM among all CARDIA women reporting interim births during mean 20.8 y of follow-up.