Abstract
Cholangiocytes, epithelial cells that line the biliary epithelium, are the primary target cells for cholangiopathies including primary sclerosing cholangitis and primary biliary cholangitis. Quiescent cholangiocytes respond to biliary damage and acquire an activated neuroendocrine phenotype to maintain the homeostasis of the liver. The typical response of cholangiocytes is proliferation leading to bile duct hyperplasia, which is a characteristic of cholestatic liver diseases. Current studies have identified various signaling pathways that are associated with cholangiocyte proliferation/loss and liver fibrosis in cholangiopathies using human samples and rodent models. Although recent studies have demonstrated that extracellular vesicles and microRNAs could be mediators that regulate these messenger/receptor axes, further studies are required to confirm their roles. This review summarizes current studies of biliary response and cholangiocyte proliferation during cholestatic liver injury with particular emphasis on the secretin/secretin receptor axis.
Keywords: Biliary damage, cholangiocytes, bile ducts, ductular reactions
1. Introduction
The intrahepatic and extrahepatic biliary epithelium is lined with bile duct epithelial cells, i.e., cholangiocytes [1]. Cholangiocytes are active secretory cells that modify the composition of canalicular bile by secreting water, bicarbonate, and chloride ions [2, 3]. Although cholangiocytes are normally quiescent, these cells can also respond to liver or biliary damage and show various specific reactions in response to experimental triggers including partial hepatectomy, bile duct ligation (BDL), and bile acid feeding [4, 5]. Various genetically modified mouse models, such as MDR2−/− and Tgfbr2−/− mice, are also used for experimental cholestatic liver injury [6]. Cholangiocytes are the main target of cholangiopathies, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC). Characteristic responses of cholangiocytes during cholestatic liver injury include enhanced cholangiocyte proliferation, related changes in biliary apoptosis and liver fibrosis, and upregulation of the cytokine expression such as interleukin (IL)-6 [7–9]. In addition, recent studies have shown that cholangiocyte senescence is a characteristic of PSC and that it is associated with other cholangiopathies [10–12]. Cholangiocytes are functionally heterogeneous cells and respond differently to specific pathological triggers. The gastrointestinal peptide hormone, secretin (SCT), binds to secretin receptor (SR), and the SCT/SR axis is critically involved in functions of cholangiocytes and pathophysiology of cholestatic liver injury [13]. Although various extracellular messengers and their receptors have been reported to regulate cholangiocyte proliferation, detailed mechanisms of regulation of cholangiocyte responses are not fully understood [14, 15]. This review summarizes current understandings of mechanisms of cholangiocyte responses and their regulations with particular emphasis on the SCT/SR axis that is only expressed in cholangiocytes in the liver [16, 17].
2. Cholangiocyte heterogeneity
Intrahepatic bile ducts are heterogeneous in external diameter (5–200 μm) and individual cholangiocytes are also heterogeneous in diameter [17]. In rats, bile ducts with a diameter greater than 15 μm are termed large bile ducts and cholangiocytes lining those ducts are termed large cholangiocytes. Small bile ducts have a diameter less than 15 μm and consist of small cholangiocytes [18]. Small and large cholangiocytes have differences not only in diameter but also in protein expression and functions [17, 19]. Large but not small cholangiocytes express SR, cystic fibrosis transmembrane conductance regulator (CFTR), Cl−/HCO3− anion exchanger 2 (AE2), and somatostatin receptor (SSTR2) [16, 20–22]. Large but not small cholangiocytes perform ductular secretion induced by SCT [16]. In addition, previous studies have shown that small and large cholangiocytes respond differently to various experimental biliary damage.
BDL is a surgical obstruction of the common bile duct and is widely used in rodents as an animal model of cholestasis and cholestatic liver injury in humans [23]. During BDL, cholangiocytes extensively proliferate and biliary hyperplasia is observed in rodent livers [24–26]. However, only large cholangiocytes proliferate during BDL. After one week of BDL, large bile duct mass increases but small bile duct mass remains pre-BDL levels [20]. Carbon tetrachloride (CCl4) administration by oral gavage is also commonly used to induce chronic liver damage, fibrosis, and hepatocellular carcinoma in rodents [27]. During CCl4 administration, large but not small cholangiocytes are damaged by enhanced apoptosis, and small cholangiocytes de novo proliferate to compensate for the functional loss of large cholangiocytes [28, 29]. Interestingly, these studies have also shown that small cholangiocytes, which do not express endogenous SR at normal conditions, de novo express SR when large cholangiocytes are damaged. Later studies also demonstrated that γ-aminobutyric acid damages large cholangiocytes but induces differentiation of small into large cholangiocytes in a Ca2+-dependent pathway [30, 31]. These studies suggest that small cholangiocytes have characteristics of progenitor cells and differentiate into large cholangiocytes when large bile ducts are damaged and need to be repaired.
Although small and large cholangiocytes respond to BDL and CCl4 differently, both cells respond similarly to bile acids. Small and large cholangiocytes isolated from rats show increased proliferation and expression of Na+-dependent apical bile acid transporter against taurocholate and taurolithocholate in vitro [32, 33]. Taurocholate feeding also attenuates large cholangiocyte damage caused by CCl4 by inducing proliferation and inhibiting apoptosis in rats [34]. Both small and large cholangiocytes also respond similarly to α-naphthylisothiocyanate (ANIT). ANIT feeding damages bile ducts and increases apoptosis as well as proliferation in both small and large cholangiocytes in rats [35]. Histamine is a mediator for the local immune system inducing inflammation. Studies have shown that histamine induces cell proliferation in both small and large cholangiocytes [36, 37]. These findings suggest that while small and large cholangiocytes have different functions and mechanisms for proliferation and injury response, these two subsets of cholangiocytes share the same response (or similar response) to specific triggering agents.
3. Mechanisms of cholangiocyte proliferation and functions
Adenosine 3′,5′-cyclic monophosphate (cAMP) is a key messenger in cholangiocytes for their proliferation and function. The neuropeptide hormone SCT binds to SR that is expressed only in the basolateral membrane of cholangiocytes, and this SCT binding and SR activation elevate intracellular cAMP levels leading to enhanced cell proliferation, exocytosis and ductular secretion in cholangiocytes [38, 39]. Elevated intracellular cAMP levels increase bicarbonate secretion from cholangiocytes in bile through CFTR-dependent ATP release [40]. Triggering agents that induce cAMP outputs, such as follicle stimulating hormone or forskolin, increase intracellular cAMP levels in cholangiocytes leading to proliferation via cAMP-dependent PKA/MEK/ERK1/2/Elk-1 signaling [41, 42]. As mentioned above, large but not small cholangiocytes express CFTR and SR, and therefore only large cholangiocytes perform CFTR- or SR-dependent proliferation and bicarbonate secretion in the liver. Another study has also shown that large but not small cholangiocytes are involved in SCT-induced ductular secretion [16].
In small cholangiocytes, cAMP levels are involved in proliferation, but Ca2+ signaling is also important. During histamine-induced small cholangiocyte proliferation, inositol 1,4,5-trisphosphate (IP3) levels are increased [37]. IP3 binds to IP3 receptor (IP3R), which is a calcium channel located on the endoplasmic reticulum (ER), a calcium storage organelle in the cell. Activated IP3R by IP3 binding releases Ca2+ from the ER into the cytosol. This Ca2+ release in the cytosol activates calcineurin (CN) and calmodulin (CaM). CN phosphorylates nuclear factor of activated T-cells (NFAT) proteins, and CaM leads to activation of CaM-dependent kinase (CaMK) [43, 44]. Studies have shown that small cholangiocytes proliferate through this IP3/Ca2+/CaMK signaling [36, 37]. Another study has shown that activation and nuclear translocation of NFAT2 (also known as NFATc1) are increased in proliferating small cholangiocytes [45]. This Ca2+/CN/NFATc1 pathway is also associated with cell proliferation in hepatocellular carcinoma [46]. In large cholangiocytes, however, Ca2+ signaling may not be critical for cell proliferation during cholestatic liver injury. A study has shown that expression levels of IP3R are decreased in large cholangiocytes during BDL [47]. Although cAMP pathways may be more important for large cholangiocyte proliferation than IP3/Ca2+ pathways, Ca2+ signaling may be more important than cAMP pathways for ductular secretion in large cholangiocytes. Large but not small cholangiocytes perform ductular secretion induced by triggers [16], and a previous study has demonstrated that trigger-induced Cl− secretion is Ca2+-dependent [48]. This study has also shown that the impact of Ca2+-induced ductular secretion is significantly greater than cAMP-induced secretion mediated by Ca2+-activated K+ channel in cholangiocytes. These findings suggest that cAMP as well as Ca2+ signaling pathways are involved in cholangiocyte proliferation and secretion depending on its heterogeneity. Figure 1 summarizes the mechanisms of cell proliferation in small and large cholangiocytes.
Figure 1. Mechanisms of cholangiocyte proliferation.
Small and large cholangiocytes have different mechanisms for proliferation. Proliferation for small cholangiocytes, which are smaller in size and have a larger nucleus to cytosol ratio compared to large cholangiocytes, is dependent on intracellular Ca2+ release induced by IP3 while large cholangiocyte proliferation is cAMP-dependent. Binding of messengers such as histamine for small cholangiocytes and SCT for large cholangiocytes to their corresponding receptors triggers these pathways leading to cholangiocyte proliferation. When large cholangiocytes are damaged by triggers such as γ–aminobutyric acid or CCl4, small cholangiocytes begin to express SR and differentiate into large cholangiocytes in order to maintain bile duct homeostasis and function. Signaling mechanisms on how damaged large cholangiocytes initiate differentiation in small cholangiocytes are still unknown.
4. Pathways associated with cholangiocyte responses and their regulations
Cholangiocytes are responsive cells and various messengers and regulators for cholangiocyte proliferation have been identified to date [15]. Recent studies have shown that some messengers and their receptors are critically involved in cholangiopathies and cholangiocyte responses to injury. This section summarizes specific pathways that are associated with cholangiocyte responses in cholestatic liver injury.
4.1. The secretin/secretin receptor axis
As described above, large cholangiocytes express various membrane proteins and channels, which small cholangiocytes do not express endogenously, such as SR, CFTR, SSTR2, and AE2 [19]. The signaling pathway of SCT and its receptor SR is one of the most studied messengers/receptors in cholestatic liver injury. It has been suggested that the SCT/SR axis is associated with cholestatic liver injury because rats show elevated expression of SR after BDL [49]. SCT administration into rats increases expression of SR and CFTR as well as intracellular cAMP levels and cholangiocyte proliferation in vivo [50]. SCT is expressed by cholangiocytes and S cells which are located primarily in the mucosa of the duodenum [51]. During BDL, both large cholangiocytes and S cells show elevated SCT expression [52]. SCT−/− mice show attenuated bile duct hyperplasia after BDL compared to wild-type mice suggesting that SCT is a key messenger in regulating large cholangiocyte proliferation in an autocrine/paracrine manner [52]. SR−/− mice also show reduced intracellular cAMP levels, ERK1/2 phosphorylation and cholangiocyte proliferation during BDL [53]. In human PSC, bile duct hyperplasia as well as liver fibrosis are often observed as common characteristics [54]. A recent study has shown that PSC patients show higher expression levels of SCT and SR in the liver compared to healthy individuals [55]. Mdr2−/− mice are widely used as a mouse model of human PSC [56, 57]. This study has also demonstrated that administration of SR antagonist into BDL or Mdr2−/− mice attenuated both bile duct hyperplasia and liver fibrosis compared to respective controls [55]. These findings suggest that the SCT/SR axis is an important signaling pathway for cholangiocyte responses in cholangiopathies. In addition, we have demonstrated that SR−/− mice show less liver steatosis with high fat diet compared to wild-type mice suggesting that the SCT/SR axis may play a key role not only in cholangiopathies but also in non-alcoholic steatohepatitis (NASH) [58]. Further studies will reveal functional roles of cholangiocytes in lipid metabolisms and liver steatosis during the development of non-alcoholic fatty liver disease (NAFLD) and NASH. Figure 2 summarizes functions of the SCT/SR axis in liver diseases.
Figure 2. The function of the SCT/SR axis in liver diseases.
SCT binds to SR that is expressed only in large cholangiocytes in the liver, and SCT binding and SR activation lead to elevated exocytosis and ductular secretion. Recent studies have shown that human patients with cholangiopathies such as PSC show elevated expression of SCT and SR indicating an association between the SCT/SR axis and cholestatic liver injury. Studies using SCT−/− and SR−/− mice have demonstrated that bile duct hyperplasia and liver fibrosis are attenuated in those knockout mice during BDL suggesting the functional contribution of the SCT/SR axis to the pathogenesis of cholangiopathies. A recent study has also demonstrated that the SCT/SR axis may be responsible for liver steatosis leading to NAFLD and NASH.
4.2 The substance P/neurokinin-1 receptor axis
Substance P (SP) is a neuropeptide that binds to neurokinin-1 receptor (NK-1R). It is known that SP induces inflammatory responses by elevating cytokine expression [59]. In cholestatic liver injury, elevated serum levels of SP are observed in patients with chronic liver disease as well as in cholestatic rat models compared to respective controls suggesting an association between SP production and cholestatic liver damage [60]. Elevated SP secretion is also identified in cholangiocarcinoma (CCA) cell lines and administration of NK-1R antagonist L-733, 060 inhibits proliferation of CCA cells [61]. A study has demonstrated that BDL mice show elevated NK-1R expression in large cholangiocytes, and NK-1R−/− mice show attenuated large cholangiocyte proliferation and bile duct hyperplasia [62]. This study has also demonstrated that SP administration elevates intracellular cAMP levels followed by cell proliferation in large cholangiocytes suggesting that the SP/NK-1R axis is associated with large cholangiocyte proliferation. A recent study has shown that serum levels of SP and NK-1R expression in the liver are elevated in human PSC patients compared to healthy individuals [63]. In addition, SP administration induces bile duct hyperplasia and liver fibrosis in wild-type mice, and administration of NK-1R antagonist attenuates liver fibrosis in Mdr2−/− mice and BDL mice [63]. These findings suggest that the SP/NK-1R axis plays a key role in bile duct proliferation and fibrosis in cholangiopathies.
4.3 The gonadotropin-releasing hormone and its receptor
Gonadotropin-releasing hormone (GnRH) is a tropic peptide hormone that modulates cell proliferation. GnRH has stimulatory and inhibitory effects on cell proliferation depending on the cell type [64]. A recent study has shown that cholangiocytes express GnRH receptor 1 (GnRHR1) and its expression levels are elevated during BDL in rats [65]. This study has also demonstrated that administration of GnRH enhances bile duct mass as well as intracellular cAMP levels, expression of SR, CFTR and AE2 in cholangiocytes in vivo. GnRH Vivo-Morpholino attenuates bile duct hyperplasia and fibrosis induced by BDL suggesting that GnRH is a key messenger for cholangiocyte responses in cholestatic liver injury [65]. A recent study has shown that serum levels of GnRH and GnRHR1 expression in the liver are elevated in human PSC patients compared to healthy individuals [66]. This study has also demonstrated that GnRH and GnRHR1 expression is enhanced in cholangiocytes isolated from Mdr2−/− mice, and GnRH Vivo-Morpholino attenuates bile duct hyperplasia and fibrosis in these mice suggesting that the GnRH/GnRHR1 axis is associated with cholangiocyte proliferation and liver fibrosis in cholestatic liver injury.
4.4 Mast cell-derived histamine and histamine receptors
As described above, histamine induces small and large cholangiocyte proliferation [36, 37]. Histamine interacts with G protein-coupled histamine receptors: H1 to H4 [67]. Interestingly, agonists for H1 histamine receptor induce small cholangiocyte proliferation, and agonists for H2 induce large cholangiocyte proliferation [36, 37]. It is known that mast cells secrete histamine and the number of mast cells in the liver is increased during cholestatic liver injury such as PBC [68]. A recent study has shown that inhibition of mast cell-derived histamine secretion attenuates bile duct hyperplasia during BDL in vivo [69]. Another study has shown that serum levels of histamine are increased in Mdr2−/− mice, and inhibition of mast cell-derived histamine secretion decreases serum histamine levels as well as bile duct hyperplasia and fibrosis in these mice [70]. Mast cell-deficient mice show less liver damage, bile duct mass, and fibrosis during BDL compared to wild-type [71]. These studies suggest that histamine secreted from mast cells infiltrated during cholestatic liver injury interacts with histamine receptors and regulates cholangiocyte responses leading to bile duct hyperplasia and fibrosis.
4.5 Melatonin and melatonin 1A receptor
Melatonin is a hormone produced by the pineal gland, small intestine and liver [72]. Melatonin regulates sleep as a part of the circadian rhythm as well as cell proliferation [73]. A previous study has demonstrated that melatonin administration in rats reduces liver fibrosis and serum cytokine levels for IL-1β and IL-6 during BDL [74]. Other studies have also shown that melatonin administration protects BDL- or ANIT-induced liver damage in vivo suggesting that melatonin has therapeutic effects on cholestatic liver injury [75, 76]. A recent study has demonstrated that melatonin administration inhibits GnRH secretion and reduces bile duct hyperplasia and liver fibrosis in BDL rats (McMillin et al. in press). Melatonin interacts with G protein-coupled melatonin 1A (MT1), MT2, and MT3 receptors [77]. A study has demonstrated that melatonin administration reduces intracellular cAMP levels and large cholangiocyte proliferation in BDL rats by activation of MT1 but not MT2 melatonin receptor [78]. Melatonin is an indole produced by arylalkylamine N-acetyltransferase (AANAT) [79]. A recent study has shown that melatonin administration increases AANAT expression in cholangiocytes [80]. This study has also demonstrated that AANAT Vivo-Morpholino exacerbates BDL-induced bile duct hyperplasia, and overexpression of AANAT in cholangiocytes reduces cell proliferation in vitro suggesting an association between AANAT followed by melatonin synthesis and cholangiocyte proliferation. Melatonin secretion is induced when exposed to darkness. BDL rats housed in complete darkness for one week show elevated AANAT expression and melatonin production compared to BDL rats with 12:12-hour light-dark cycles (Wu et al. in press #1). This study has demonstrated that complete darkness reduces bile duct mass and liver fibrosis during BDL by reducing intracellular cAMP levels. These findings suggest that the AANAT/melatonin/MT1 receptor axis could be a potential target for novel treatments of cholestatic liver injury.
4.6 Vascular endothelial growth factors and their receptors
Vascular endothelial growth factor (VEGF) is a protein family of growth factors including VEGF-A, -B, -C, -D, and -E [81, 82]. Three VEGF receptors (VEGFR-1 to -3) have been identified to date, and it is known that VEGF-A interacts with VEGFR-2 and VEGF-C interacts with VEGFR-3 [83, 84]. A previous study has shown that cholangiocytes express VEGF-A and -C as well as VEGFR-2 and -3, and these VEGF-A/VEGFR-2 and VEGF-C/VEGFR-3 axes are upregulated during BDL in rats [85]. This study has also demonstrated that administration of VEGF-A/C induces cholangiocyte proliferation by elevating intracellular IP3 levels and ERK1/2 phosphorylation. AANAT expression is correlated with VEGF-A/C expression and AANAT overexpression reduces VEGF-A/C expression in vitro, and AANAT Vivo-Morpholino increases VEGF-A/C expression in vivo [86]. Pancreatic duodenal homeobox-1 (PDX-1) is a transcription factor and expression levels of PDX-1 are upregulated in cholangiocytes isolated from BDL rats [87]. Expression levels of PDX-1 are associated with VEGF expression in cholangiocytes and siRNA transfection for PDX-1 inhibits VEGF expression [87]. Another study has demonstrated that PDX-1+/− mice show attenuated bile duct mass and liver fibrosis during BDL compared to wild-type [88]. These studies suggest that the PDX-1/VEGF-A/C/VEGFR-2/3 axis is associated with cholangiocyte proliferation and liver fibrosis in cholestatic liver diseases.
4.7 Galanin and galanin receptor 1
Galanin is a neuropeptide that interacts with three G protein-coupled receptors: galanin receptor 1 (GalR1), GalR2 and GalR3 [89]. Serum levels of galanin and GalR1 expression in cholangiocytes are elevated in BDL rats [90]. Administration of galanin induces bile duct hyperplasia in normal rats and galanin Vivo-Morpholino attenuates BDL-induced bile duct hyperplasia and fibrosis [90]. Although there are limited numbers of studies, the galanin/GalR1 axis may be associated with cholestatic liver injury.
4.8 Receptors activated by bile acids
As mentioned above, bile acids induce cholangiocyte proliferation. During cholestasis, accumulated bile acids stimulate cholangiocytes leading to bile duct hyperplasia. TGR5 (also known as G protein-coupled bile acid receptor 1, GPBAR1) is a specific receptor for bile acids. It is known that activation of TGR5 is associated with intracellular cAMP elevation in various cells [91]. Several mutations of the TGR5 gene have been identified from human PCS patients suggesting an association between TGR5 and cholangiopathies [92]. In cholangiocytes, the majority of expressed TGR5 is localized on the primary cilium [93]. A recent study has shown that taurolithocholic acid and other TGR5 agonists induce cholangiocyte proliferation via ERK1/2 phosphorylation [94]. This study has also demonstrated that TGR5−/− mice show reduced bile duct hyperplasia during BDL as well as cholic acid feeding compared to wild-type or control feeding suggesting that TGR5 is required for cholestasis- or bile acid-induced cholangiocyte proliferation. Another study, however, has demonstrated that TGR5 agonists inhibit cholangiocyte proliferation [95]. Further studies are required to elucidate the mechanisms of regulation of cholangiocyte proliferation by bile acids and TGR5 activation.
Sphingosine 1-phosphate receptor 2 (S1PR2) is activated by bile acids as well as TGR5, and accumulating evidence suggests that S1PR2 signaling is associated with biliary diseases [96]. A recent study has shown that S1PR2 expression is elevated in cholangiocytes during BDL, and taurocholate induces cholangiocyte proliferation by ERK1/2 activation and enhanced S1PR2 expression [97]. This study has also demonstrated that S1PR2−/− mice show reduced bile acid secretion, bile duct mass, and liver fibrosis during BDL. These studies suggest that bile acids followed by activation of receptors may be an important signaling pathway in pathogenesis of cholestatic liver diseases.
4.9 Yes-associated protein
Yes-associated protein (YAP) is a transcriptional regulator for development, repair and proliferation of liver cells [98]. A recent study has demonstrated that patients with biliary atresia (BA) show bile duct hyperplasia and enhanced YAP expression in cholangiocytes compared to non-BA patients, and hence YAP expression levels in bile ducts can be a useful biomarker for diagnosis of BA [99]. Another study has shown that YAP expression in bile ducts is upregulated in human PSC and PBC patients, and YAP−/− mice show attenuated cholangiocyte proliferation and bile duct hyperplasia during BDL [100]. YAP is a major mediator of the Hippo signaling pathway, and a recent study has shown that the loss of Hippo-YAP signaling enhances the expression of Notch signaling pathway receptor Notch2 (Wu, et al. in press #2). This study has also demonstrated that the loss of both Hippo and Notch signaling results in excessive bile duct development suggesting that Hippo-YAP signaling is associated with Notch signaling and bile duct development. These studies suggest that YAP signaling is associated with cholangiocyte proliferation and bile duct development although further studies are required to understand the detailed mechanisms and functional roles of YAP signaling in cholangiopathies. Figure 3 illustrates regulations of cholangiocyte responses by messengers and their receptors.
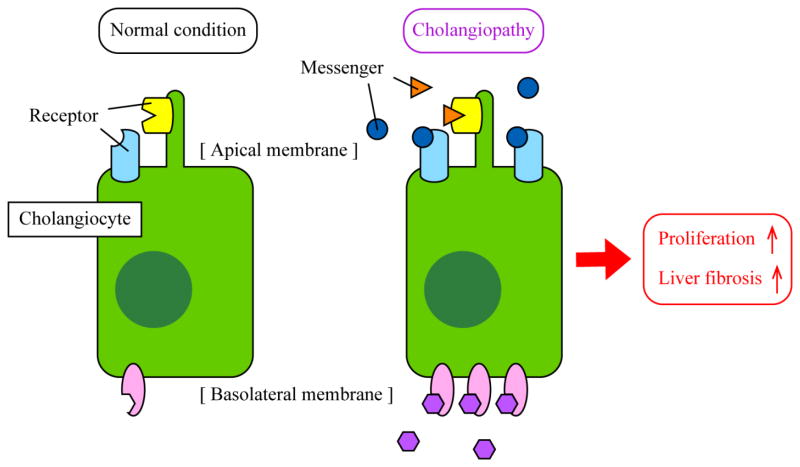
Figure 3. Working model of cholangiocyte responses during cholangiopathies.
Cholangiocytes are quiescent in healthy conditions (left). During cholestatic liver injury, expression levels of messengers such as SCT and SP as well as corresponding receptors such as SR and NK-1R are elevated in cholangiocytes (right). Binding of messengers to receptors in an autocrine/paracrine manner triggers signaling pathways for proliferation and fibrogenesis leading to bile duct hyperplasia and liver fibrosis. Although specific or primary locations are not identified for all proteins, some proteins such as TGR5 are located on primary cilia or apical membrane, and some proteins such as SR and CFTR are located on the basolateral membrane.
5 Regulation of cholangiocyte responses by extracellular vesicles
Extracellular vesicles (EVs) are membrane-bound vesicles secreted by various cell types. Recent studies suggest that EVs may play an important role in liver diseases [101, 102]. EVs contain various functionally active mediators including proteins, mRNAs and microRNAs (miRNAs). Secreted EVs can be transferred from donor cells to recipient cells and regulate pathophysiological events by delivering cargo mediators. Physiology and functions of cholangiocytes are regulated by other cholangiocytes or other liver cells during cholestatic liver injury. For example, Kupffer cells secrete inflammatory cytokines such as IL-6 during biliary damage and elevated IL-6 stimulates cholangiocytes to proliferate [103, 104]. Cholangiocyte proliferation and response could be regulated by EVs secreted from other cholangiocytes or liver cells. Cholangiocytes have primary cilia, which are chemosensory organelles, to detect and maintain bile homeostasis [105]. As described above, bile acids induce cholangiocyte proliferation and bile acid receptor TGR5 is expressed primarily in cilia in cholangiocytes. A previous study has shown that bile contain EVs and those biliary EVs interact with cholangiocyte cilia to downregulate cholangiocyte proliferation [106]. Another study has demonstrated that this downregulation of cholangiocyte proliferation caused by biliary EVs is dependent on TGR5 located on primary cilia [95]. These findings suggest that cholangiocyte response and proliferation may be regulated by EVs and their cargo mediators. EV transfer and pathophysiological regulation may be performed between cholangiocytes or cholangiocytes and other liver cells in an autocrine or paracrine manner. Further studies are required to elucidate mediators and detailed mechanisms of cholangiocyte regulation by EVs during cholestatic liver diseases.
6 Cholangiocyte regulation by microRNAs
Accumulating evidence suggests that miRNAs could be the key mediators for regulation of cholangiocyte response. During SCT-induced proliferation, miRNAs 125b and let7a, which regulate VEGF expression, are downregulated and Vivo-Morpholino for these miRNAs exacerbate bile duct hyperplasia in BDL mice by elevating VEGF expression and cholangiocyte proliferation [52]. Another study has demonstrated that miR-124 is downregulated during BDL and this leads to cholangiocyte proliferation in an IL-6-dependent mechanism [107]. This study has also found that miR-200 families are upregulated in BDL mice. A recent study has demonstrated that human PSC patients show elevated miR-200b expression in the liver compared to healthy individuals and miR-200b Vivo-Morpholino attenuates bile duct hyperplasia and liver fibrosis in Mdr2−/− mice (Wu et al. in press #1). Another study has also demonstrated that miR-200b expression is upregulated during GnRH-induced cell proliferation suggesting an association between miR-200b and cholangiocyte responses in cholangiopathies [66]. As described above, the PDX-1/VEGF-A/C axis is associated with cholangiocyte proliferation and liver fibrosis. A recent study has demonstrated that upregulation of miR-7a is associated with VEGF-A/C expression and bile duct hyperplasia during biliary damage [108]. Several miRNAs have been identified as candidate miRNAs that are associated with cholestatic liver diseases [109]. Expression of these miRNAs can be regulated endogenously or by EVs in an autocrine or paracrine manner. Further studies are required to elucidate detailed mechanisms of miRNA-mediated cholangiocyte responses.
7 Conclusion and future perspectives
Cholangiocytes are the main target cells in cholestatic liver diseases and current studies have shown that various pathways and axes are associated with cholangiocyte proliferation and response to biliary damage. These pathways and axes could be potential therapeutic targets for novel treatments. It is largely unclear, however, how these axes are regulated and what mediators are involved. Although EVs and miRNAs may be involved in regulation of those axes, further studies are required.
Supplementary Material
Highlights.
Cholangiocytes are heterogeneous showing different responses to injury
Typical cholangiocyte responses are proliferation and fibrogenesis
The secretin/secretin receptor axis is important for cholangiocyte responses
There are several other pathways associated with cholangiocyte proliferation
Acknowledgments
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Career Scientist Award and a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng, and the NIH grants DK58411, DK07698, DK095291 and DK062975 to Drs. Alpini, Meng and Glaser. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
Abbreviation
- AANAT
arylalkylamine N-acetyltransferase
- AE2
Cl−/HCO3− anion exchanger 2
- ANIT
α-naphthylisothiocyanate
- BA
biliary atresia
- BDL
bile duct ligation
- cAMP
adenosine 3′,5′-cyclic monophosphate
- CCA
cholangiocarcinoma
- CCl4
carbon tetrachloride
- CFTR
cystic fibrosis transmembrane conductance regulator
- CaM
calmodulin
- CaMK
CaM-dependent kinase
- CN
calcineurin
- ER
endoplasmic reticulum
- EVs
extracellular vesicles
- GalR1
galanin receptor 1
- GnRH
gonadotropin-releasing hormone
- GnRHR1
GnRH receptor 1
- GPBAR1
G protein-coupled bile acid receptor 1
- IL
interleukin
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- miRNAs
microRNAs
- MT1
melatonin 1A
- NAFLD
non-alcoholic fatty liver diseases
- NASH
non-alcoholic steatohepatitis
- NFAT
nuclear factor of activated T-cells
- NK-1R
neurokinin-1 receptor
- PDX-1
pancreatic duodenal homeobox-1
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- SCT
secretin
- SP
substance P
- S1PR2
sphingosine 1-phosphate receptor 2
- SR
secretin receptor
- SSTR2
somatostatin receptor
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- YAP
Yes-associated protein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maroni L, Haibo B, Ray D, Zhou T, Wan Y, Meng F, Marzioni M, Alpini G. Functional and structural features of cholangiocytes in health and disease. Cell Mol Gastroenterol Hepatol. 2015;1:368–380. doi: 10.1016/j.jcmgh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 3.Tabibian JH, Masyuk AI, Masyuk TV, O’Hara SP, LaRusso NF. Physiology of cholangiocytes. Compr Physiol. 2013;3:541–565. doi: 10.1002/cphy.c120019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesage G, Glaser SS, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers RE, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Liedtke C, Luedde T, Sauerbruch T, Scholten D, Streetz K, Tacke F, Tolba R, Trautwein C, Trebicka J, Weiskirchen R. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 2013;6:19. doi: 10.1186/1755-1536-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte proliferation. Liver. 2001;21:73–80. doi: 10.1034/j.1600-0676.2001.021002073.x. [DOI] [PubMed] [Google Scholar]
- 8.Park J, Gores GJ, Patel T. Lipopolysaccharide induces cholangiocyte proliferation via an interleukin-6-mediated activation of p44/p42 mitogen-activated protein kinase. Hepatology. 1999;29:1037–1043. doi: 10.1002/hep.510290423. [DOI] [PubMed] [Google Scholar]
- 9.O’Hara SP, Splinter PL, Trussoni CE, Gajdos GB, Lineswala PN, LaRusso NF. Cholangiocyte N-Ras protein mediates lipopolysaccharide-induced interleukin 6 secretion and proliferation. The Journal of biological chemistry. 2011;286:30352–30360. doi: 10.1074/jbc.M111.269464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L, Quezada M, Levine P, Han Y, McDaniel K, Zhou T, Lin E, Glaser S, Meng F, Francis H, Alpini G. Functional role of cellular senescence in biliary injury. Am J Pathol. 2015;185:602–609. doi: 10.1016/j.ajpath.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanuma Y, Sasaki M, Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2015;62:934–945. doi: 10.1016/j.jhep.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 13.Afroze S, Meng F, Jensen K, McDaniel K, Rahal K, Onori P, Gaudio E, Alpini G, Glaser SS. The physiological roles of secretin and its receptor. Ann Transl Med. 2013;1:29. doi: 10.3978/j.issn.2305-5839.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchitto A, Onori P, Renzi A, Carpino G, Mancinelli R, Alvaro D, Gaudio E. Recent advances on the mechanisms regulating cholangiocyte proliferation and the significance of the neuroendocrine regulation of cholangiocyte pathophysiology. Ann Transl Med. 2013;1:27. doi: 10.3978/j.issn.2305-5839.2012.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall C, Sato K, Wu N, Zhou T, Kyritsi T, Meng F, Glaser S, Alpini G. Regulators of Cholangiocyte Proliferation. Gene Expr. 2016 doi: 10.3727/105221616X692568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. The American journal of physiology. 1997;272:G1064–1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 17.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 18.Glaser S, Francis H, Demorrow S, Lesage G, Fava G, Marzioni M, Venter J, Alpini G. Heterogeneity of the intrahepatic biliary epithelium. World J Gastroenterol. 2006;12:3523–3536. doi: 10.3748/wjg.v12.i22.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22:227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 20.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, Francis HL, Dostal DE, Venter JK, DeMorrow S, Mancinelli R, Carpino G, Alvaro D, Kopriva SE, Savage JM, Alpini GD. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage GD, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. The American journal of physiology. 1997;272:G289–297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 22.Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. The American journal of physiology. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 23.Tag CG, Sauer-Lehnen S, Weiskirchen S, Borkham-Kamphorst E, Tolba RH, Tacke F, Weiskirchen R. Bile duct ligation in mice: induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J Vis Exp. 2015 doi: 10.3791/52438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afroze SH, Munshi MK, Martinez AK, Uddin M, Gergely M, Szynkarski C, Guerrier M, Nizamutdinov D, Dostal D, Glaser S. Activation of the renin-angiotensin system stimulates biliary hyperplasia during cholestasis induced by extrahepatic bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 2015;308:G691–701. doi: 10.1152/ajpgi.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf A, Meng F, Hargrove L, Kennedy L, Han Y, Francis T, Hodges K, Ueno Y, Nguyen Q, Greene JF, Francis H. Knockout of histidine decarboxylase decreases bile duct ligation-induced biliary hyperplasia via downregulation of the histidine decarboxylase/VEGF axis through PKA-ERK1/2 signaling. Am J Physiol Gastrointest Liver Physiol. 2014;307:G813–823. doi: 10.1152/ajpgi.00188.2014. [DOI] [PubMed] [Google Scholar]
- 27.Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, Tanabe KK. Mouse model of carbon tetrachloride induced liver fibrosis: Histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeSage GD, Glaser SS, Marucci L, Benedetti A, Phinizy JL, Rodgers R, Caligiuri A, Papa E, Tretjak Z, Jezequel AM, Holcomb LA, Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. The American journal of physiology. 1999;276:G1289–1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 29.LeSage GD, Benedetti A, Glaser S, Marucci L, Tretjak Z, Caligiuri A, Rodgers R, Phinizy JL, Baiocchi L, Francis H, Lasater J, Ugili L, Alpini G. Acute carbon tetrachloride feeding selectively damages large, but not small, cholangiocytes from normal rat liver. Hepatology. 1999;29:307–319. doi: 10.1002/hep.510290242. [DOI] [PubMed] [Google Scholar]
- 30.Mancinelli R, Franchitto A, Gaudio E, Onori P, Glaser S, Francis H, Venter J, Demorrow S, Carpino G, Kopriva S, White M, Fava G, Alvaro D, Alpini G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancinelli R, Franchitto A, Glaser S, Meng F, Onori P, Demorrow S, Francis H, Venter J, Carpino G, Baker K, Han Y, Ueno Y, Gaudio E, Alpini G. GABA induces the differentiation of small into large cholangiocytes by activation of Ca(2+)/CaMK I-dependent adenylyl cyclase 8. Hepatology. 2013;58:251–263. doi: 10.1002/hep.26308. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- 33.Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, Lesage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 34.Marucci L, Alpini G, Glaser SS, Alvaro D, Benedetti A, Francis H, Phinizy JL, Marzioni M, Mauldin J, Venter J, Baumann B, Ugili L, LeSage G. Taurocholate feeding prevents CCl4-induced damage of large cholangiocytes through PI3-kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2003;284:G290–301. doi: 10.1152/ajpgi.00245.2002. [DOI] [PubMed] [Google Scholar]
- 35.Lesage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 36.Francis H, Glaser S, Demorrow S, Gaudio E, Ueno Y, Venter J, Dostal D, Onori P, Franchitto A, Marzioni M, Vaculin S, Vaculin B, Katki K, Stutes M, Savage J, Alpini G. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francis HL, Demorrow S, Franchitto A, Venter JK, Mancinelli RA, White MA, Meng F, Ueno Y, Carpino G, Renzi A, Baker KK, Shine HE, Francis TC, Gaudio E, Alpini GD, Onori P. Histamine stimulates the proliferation of small and large cholangiocytes by activation of both IP3/Ca2+ and cAMP-dependent signaling mechanisms. Lab Invest. 2012;92:282–294. doi: 10.1038/labinvest.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenzen R, Alpini G, Tavoloni N. Secretin stimulates bile ductular secretory activity through the cAMP system. The American journal of physiology. 1992;263:G527–532. doi: 10.1152/ajpgi.1992.263.4.G527. [DOI] [PubMed] [Google Scholar]
- 39.Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. The Journal of biological chemistry. 1992;267:15523–15529. [PubMed] [Google Scholar]
- 40.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, Lesage G, Akiba Y, Kaunitz JD, Ehrlich BE, Larusso NF, Nathanson MH. Cyclic AMP regulates bicarbonate secretion in cholangiocytes through release of ATP into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francis H, Glaser S, Ueno Y, Lesage G, Marucci L, Benedetti A, Taffetani S, Marzioni M, Alvaro D, Venter J, Reichenbach R, Fava G, Phinizy JL, Alpini G. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Mancinelli R, Onori P, Gaudio E, DeMorrow S, Franchitto A, Francis H, Glaser S, Carpino G, Venter J, Alvaro D, Kopriva S, White M, Kossie A, Savage J, Alpini G. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 44.Swulius MT, Waxham MN. Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alpini G, Franchitto A, Demorrow S, Onori P, Gaudio E, Wise C, Francis H, Venter J, Kopriva S, Mancinelli R, Carpino G, Stagnitti F, Ueno Y, Han Y, Meng F, Glaser S. Activation of alpha(1) -adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Kang X, Cao S, Cheng H, Wang D, Geng J. Calcineurin/NFATc1 pathway contributes to cell proliferation in hepatocellular carcinoma. Dig Dis Sci. 2012;57:3184–3188. doi: 10.1007/s10620-012-2255-8. [DOI] [PubMed] [Google Scholar]
- 47.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feranchak AP, Doctor RB, Troetsch M, Brookman K, Johnson SM, Fitz JG. Calcium-dependent regulation of secretion in biliary epithelial cells: the role of apamin-sensitive SK channels. Gastroenterology. 2004;127:903–913. doi: 10.1053/j.gastro.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 49.Alpini G, Ulrich CD, 2nd, Phillips JO, Pham LD, Miller LJ, LaRusso NF. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. The American journal of physiology. 1994;266:G922–928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 50.Guerrier M, Attili F, Alpini G, Glaser S. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg Nutr. 2014;3:118–125. doi: 10.3978/j.issn.2304-3881.2014.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polak JM, Coulling I, Bloom S, Pearse AG. Immunofluorescent localization of secretin and enteroglucagon in human intestinal mucosa. Scand J Gastroenterol. 1971;6:739–744. doi: 10.3109/00365527109179946. [DOI] [PubMed] [Google Scholar]
- 52.Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, McDaniel K, Marzioni M, Invernizzi P, Ueno Y, Lai JM, Huang L, Standeford H, Alvaro D, Gaudio E, Franchitto A, Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glaser S, Lam IP, Franchitto A, Gaudio E, Onori P, Chow BK, Wise C, Kopriva S, Venter J, White M, Ueno Y, Dostal D, Carpino G, Mancinelli R, Butler W, Chiasson V, DeMorrow S, Francis H, Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazaridis KN, LaRusso NF. Primary Sclerosing Cholangitis. N Engl J Med. 2016;375:1161–1170. doi: 10.1056/NEJMra1506330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, Marzioni M, Alvaro D, Franchitto A, Gaudio E, Glaser S, Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-beta1 biliary secretion in mice. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)−/− mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 57.Lammert F, Wang DQ, Hillebrandt S, Geier A, Fickert P, Trauner M, Matern S, Paigen B, Carey MC. Spontaneous cholecysto- and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid-associated cholelithiasis. Hepatology. 2004;39:117–128. doi: 10.1002/hep.20022. [DOI] [PubMed] [Google Scholar]
- 58.Kennedy L, Wu N, Venter J, Meng F, Francis H, Zhou T, Glaser S, Alpini G. High fat diet-induced biliary lipoapoptosis, senescence, hepatic steatosis and fibrosis are reduced in secretin receptor knockout (SR−/−) mice. FASEB J. 2017;31:328.323. [Google Scholar]
- 59.Rameshwar P, Gascon P, Ganea D. Immunoregulatory effects of neuropeptides. Stimulation of interleukin-2 production by substance p. J Neuroimmunol. 1992;37:65–74. doi: 10.1016/0165-5728(92)90156-f. [DOI] [PubMed] [Google Scholar]
- 60.Trivedi M, Bergasa NV. Serum concentrations of substance P in cholestasis. Ann Hepatol. 2010;9:177–180. [PubMed] [Google Scholar]
- 61.Meng F, DeMorrow S, Venter J, Frampton G, Han Y, Francis H, Standeford H, Avila S, McDaniel K, McMillin M, Afroze S, Guerrier M, Quezada M, Ray D, Kennedy L, Hargrove L, Glaser S, Alpini G. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2014;306:G759–768. doi: 10.1152/ajpgi.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, Kopriva S, Chiasson V, DeMorrow S, Francis H, Meng F, Marzioni M, Franchitto A, Alvaro D, Supowit S, DiPette DJ, Onori P, Alpini G. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G297–305. doi: 10.1152/ajpgi.00418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan Y, Meng F, Wu N, Zhou T, Venter J, Francis H, Kennedy L, Glaser T, Bernuzzi F, Invernizzi P, Glaser S, Huang Q, Alpini G. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology. 2017 doi: 10.1002/hep.29138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enomoto M, Park MK. GnRH as a cell proliferation regulator: mechanism of action and evolutionary implications. Zoolog Sci. 2004;21:1005–1013. doi: 10.2108/zsj.21.1005. [DOI] [PubMed] [Google Scholar]
- 65.Ray D, Han Y, Franchitto A, DeMorrow S, Meng F, Venter J, McMillin M, Kennedy L, Francis H, Onori P, Mancinelli R, Gaudio E, Alpini G, Glaser SS. Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms. Am J Pathol. 2015;185:1061–1072. doi: 10.1016/j.ajpath.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kyritsi K, Meng F, Zhou T, Wu N, Venter J, Francis H, Kennedy L, Onori P, Franchitto A, Bernuzzi F, Invernizzi P, McDaniel K, Mancinelli R, Alvaro D, Gaudio E, Alpini G, Glaser S. Knockdown of Hepatic Gonadotropin-Releasing Hormone by Vivo-Morpholino Decreases Liver Fibrosis in Multidrug Resistance Gene 2 Knockout Mice by Down-Regulation of miR-200b. Am J Pathol. 2017 doi: 10.1016/j.ajpath.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, Rauser L, Lee SP, Lynch KR, Roth BL, O’Dowd BF. Discovery of a novel member of the histamine receptor family. Molecular pharmacology. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura A, Yamazaki K, Suzuki K, Sato S. Increased portal tract infiltration of mast cells and eosinophils in primary biliary cirrhosis. Am J Gastroenterol. 1997;92:2245–2249. [PubMed] [Google Scholar]
- 69.Kennedy LL, Hargrove LA, Graf AB, Francis TC, Hodges KM, Nguyen QP, Ueno Y, Greene JF, Meng F, Huynh VD, Francis HL. Inhibition of mast cell-derived histamine secretion by cromolyn sodium treatment decreases biliary hyperplasia in cholestatic rodents. Lab Invest. 2014;94:1406–1418. doi: 10.1038/labinvest.2014.129. [DOI] [PubMed] [Google Scholar]
- 70.Jones H, Hargrove L, Kennedy L, Meng F, Graf-Eaton A, Owens J, Alpini G, Johnson C, Bernuzzi F, Demieville J, DeMorrow S, Invernizzi P, Francis H. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(−/−) mice. Hepatology. 2016;64:1202–1216. doi: 10.1002/hep.28704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hargrove L, Kennedy L, Demieville J, Jones H, Meng F, DeMorrow S, Karstens W, Madeka T, Greene J, Jr, Francis H. BDL-induced biliary hyperplasia, hepatic injury and fibrosis are reduced in mast cell deficient Kitw-sh mice. Hepatology. 2017 doi: 10.1002/hep.29079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. doi: 10.1023/a:1020107915919. [DOI] [PubMed] [Google Scholar]
- 73.Glaser S, Han Y, Francis H, Alpini G. Melatonin regulation of biliary functions. Hepatobiliary Surg Nutr. 2014;3:35–43. doi: 10.3978/j.issn.2304-3881.2013.10.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tahan G, Akin H, Aydogan F, Ramadan SS, Yapicier O, Tarcin O, Uzun H, Tahan V, Zengin K. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg. 2010;53:313–318. [PMC free article] [PubMed] [Google Scholar]
- 75.Ohta Y, Kongo M, Kishikawa T. Preventive effect of melatonin on the progression of alpha-naphthylisothiocyanate-induced acute liver injury in rats. J Pineal Res. 2003;34:185–193. doi: 10.1034/j.1600-079x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 76.Ohta Y, Kongo M, Kishikawa T. Melatonin exerts a therapeutic effect on cholestatic liver injury in rats with bile duct ligation. J Pineal Res. 2003;34:119–126. doi: 10.1034/j.1600-079x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 77.Glenister R, McDaniel K, Francis H, Venter J, Jensen K, Dusio G, Gaudio E, Glaser S, Meng F, Alpini G. Therapeutic actions of melatonin on gastrointestinal cancer development and progression. Transl Gastrointest Cancer. 2013;2 doi: 10.3978/j.issn.2224-4778.2012.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renzi A, Glaser S, Demorrow S, Mancinelli R, Meng F, Franchitto A, Venter J, White M, Francis H, Han Y, Alvaro D, Gaudio E, Carpino G, Ueno Y, Onori P, Alpini G. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol. 2011;301:G634–643. doi: 10.1152/ajpgi.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Renzi A, DeMorrow S, Onori P, Carpino G, Mancinelli R, Meng F, Venter J, White M, Franchitto A, Francis H, Han Y, Ueno Y, Dusio G, Jensen KJ, Greene JJ, Jr, Glaser S, Gaudio E, Alpini G. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57:1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clauss M. Molecular biology of the VEGF and the VEGF receptor family. Semin Thromb Hemost. 2000;26:561–569. doi: 10.1055/s-2000-13213. [DOI] [PubMed] [Google Scholar]
- 82.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 83.Larrivee B, Karsan A. Signaling pathways induced by vascular endothelial growth factor. Int J Mol Med. 2000;5:447–456. doi: 10.3892/ijmm.5.5.447. [DOI] [PubMed] [Google Scholar]
- 84.Zachary I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans. 2003;31:1171–1177. doi: 10.1042/bst0311171. [DOI] [PubMed] [Google Scholar]
- 85.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 86.Renzi A, Mancinelli R, Onori P, Franchitto A, Alpini G, Glaser S, Gaudio E. Inhibition of the liver expression of arylalkylamine N-acetyltransferase increases the expression of angiogenic factors in cholangiocytes. Hepatobiliary Surg Nutr. 2014;3:4–10. doi: 10.3978/j.issn.2304-3881.2014.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marzioni M, Saccomanno S, Candelaresi C, Rychlicki C, Agostinelli L, Shanmukhappa K, Trozzi L, Pierantonelli I, De Minicis S, Benedetti A. Pancreatic Duodenal Homeobox-1 de novo expression drives cholangiocyte neuroendocrine-like transdifferentiation. J Hepatol. 2010;53:663–670. doi: 10.1016/j.jhep.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 88.Marzioni M, Saccomanno S, Agostinelli L, Rychlicki C, De Minicis S, Pierantonelli I, Trauner M, Fickert P, Muller T, Shanmukhappa K, Trozzi L, Candelaresi C, Baroni GS, Benedetti A. PDX-1/Hes-1 interactions determine cholangiocyte proliferative response to injury in rodents: possible implications for sclerosing cholangitis. J Hepatol. 2013;58:750–756. doi: 10.1016/j.jhep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 89.Fang P, Yu M, Guo L, Bo P, Zhang Z, Shi M. Galanin and its receptors: a novel strategy for appetite control and obesity therapy. Peptides. 2012;36:331–339. doi: 10.1016/j.peptides.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 90.McMillin M, Frampton G, Grant S, DeMorrow S. The Neuropeptide Galanin Is Up-Regulated during Cholestasis and Contributes to Cholangiocyte Proliferation. Am J Pathol. 2017;187:819–830. doi: 10.1016/j.ajpath.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hov JR, Keitel V, Laerdahl JK, Spomer L, Ellinghaus E, ElSharawy A, Melum E, Boberg KM, Manke T, Balschun T, Schramm C, Bergquist A, Weismuller T, Gotthardt D, Rust C, Henckaerts L, Onnie CM, Weersma RK, Sterneck M, Teufel A, Runz H, Stiehl A, Ponsioen CY, Wijmenga C, Vatn MH, Stokkers PC, Vermeire S, Mathew CG, Lie BA, Beuers U, Manns MP, Schreiber S, Schrumpf E, Haussinger D, Franke A, Karlsen TH. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keitel V, Ullmer C, Haussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785–789. doi: 10.1515/BC.2010.077. [DOI] [PubMed] [Google Scholar]
- 94.Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, Ullmer C, Knoefel WT, Herebian D, Mayatepek E, Haussinger D, Keitel V. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 95.Masyuk AI, Huang BQ, Radtke BN, Gajdos GB, Splinter PL, Masyuk TV, Gradilone SA, LaRusso NF. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1013–1024. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nagahashi M, Yuza K, Hirose Y, Nakajima M, Ramanathan R, Hait NC, Hylemon PB, Zhou H, Takabe K, Wakai T. The roles of bile acids and sphingosine-1-phosphate signaling in the hepatobiliary diseases. J Lipid Res. 2016;57:1636–1643. doi: 10.1194/jlr.R069286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Aoki H, Yang J, Peng K, Liu R, Li X, Qiang X, Sun L, Gurley EC, Lai G, Zhang L, Liang G, Nagahashi M, Takabe K, Pandak WM, Hylemon PB, Zhou H. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–2018. doi: 10.1002/hep.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen Q, Anders RA, Alpini G, Bai H. Yes-associated protein in the liver: Regulation of hepatic development, repair, cell fate determination and tumorigenesis. Dig Liver Dis. 2015;47:826–835. doi: 10.1016/j.dld.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 99.Gurda GT, Zhu Q, Bai H, Pan D, Schwarz KB, Anders RA. The use of Yes-associated protein expression in the diagnosis of persistent neonatal cholestatic liver disease. Hum Pathol. 2014;45:1057–1064. doi: 10.1016/j.humpath.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, Pan D, Anders RA. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology. 2016;64:2219–2233. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65:213–221. doi: 10.1016/j.jhep.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato K, Hall C, Glaser S, Francis H, Meng F, Alpini G. Pathogenesis of Kupffer Cells in Cholestatic Liver Injury. Am J Pathol. 2016;186:2238–2247. doi: 10.1016/j.ajpath.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 105.Masyuk AI, Masyuk TV, LaRusso NF. Cholangiocyte primary cilia in liver health and disease. Dev Dyn. 2008;237:2007–2012. doi: 10.1002/dvdy.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, Splinter PL, LaRusso NF. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiao Y, Wang J, Yan W, Zhou Y, Chen Y, Zhou K, Wen J, Wang Y, Cai W. Dysregulated miR-124 and miR-200 expression contribute to cholangiocyte proliferation in the cholestatic liver by targeting IL-6/STAT3 signalling. J Hepatol. 2015;62:889–896. doi: 10.1016/j.jhep.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 108.Marzioni M, Agostinelli L, Candelaresi C, Saccomanno S, De Minicis S, Maroni L, Mingarelli E, Rychlicki C, Trozzi L, Banales JM, Benedetti A, Baroni GS. Activation of the developmental pathway neurogenin-3/microRNA-7a regulates cholangiocyte proliferation in response to injury. Hepatology. 2014;60:1324–1335. doi: 10.1002/hep.27262. [DOI] [PubMed] [Google Scholar]
- 109.Marin JJ, Bujanda L, Banales JM. MicroRNAs and cholestatic liver diseases. Curr Opin Gastroenterol. 2014;30:303–309. doi: 10.1097/MOG.0000000000000051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.