Abstract
Peptidoglycan (PG) is a mesh-like heteropolymer made up of glycan chains cross-linked by short peptides and is the major scaffold of eubacterial cell walls, determining cell shape, size, and chaining. This structure, which is required for growth and survival, is located outside of the cytoplasmic membrane of bacterial cells, making it highly accessible to antibiotics. Penicillin-binding proteins (PBPs) are essential for construction of PG and perform transglycosylase activities to generate the glycan strands and transpeptidation to cross-link the appended peptides. The β-lactam antibiotics, which are among the most clinically effctive antibiotics for the treatment of bacterial infections, inhibit PBP transpeptidation, ultimately leading to cell lysis. Despite this importance, the discrete functions of individual PBP homologues have been difficult to determine. These major gaps in understanding of PBP activation and macromolecular interactions largely result from a lack of tools to assess the functional state of specific PBPs in bacterial cells. We have identified β-lactones as a privileged scaffold for the generation of PBP-selective probes and utilized these compounds for imaging of the essential proteins, PBP2x and PBP2b, in Streptococcus pneumoniae. We demonstrated that while PBP2b activity is restricted to a ring surrounding the division sites, PBP2x activity is present both at the septal center and at the surrounding ring. These spatially separate regions of PBP2x activity could not be detected by previous activity-based approaches, which highlights a critical strength of our PBP-selective imaging strategy.
Graphical Abstract
The integrity of the bacterial cell wall, known as peptidoglycan (PG), is dependent upon a complex structure. Efforts to inhibit PG biosynthesis have yielded many antibacterial agents, and although resistance has become a significant factor in antibiotic efficacy, opportunities clearly remain for the identification of novel antibiotic targets through a more complete understanding of the proteins that dictate cell wall construction.1 One major antibiotic target is the penicillin-binding proteins (PBPs), membrane-anchored enzymes involved in the PG polymerization (transglycosylation) and cross-linking (transpeptidation) steps required for cell wall synthesis (Figure 1a).1 As the targets of the β-lactam antibiotics, which inhibit their transpeptidase activity, the PBPs have been therapeutically significant for many decades. Despite this importance, the discrete functions of individual PBP homologues have been difficult to determine because each enzyme is often dispensable for growth, possibly due to functional redundancy, and because of a lack of tools to assess the functional state of the PBPs in live bacterial cells.
Figure 1.
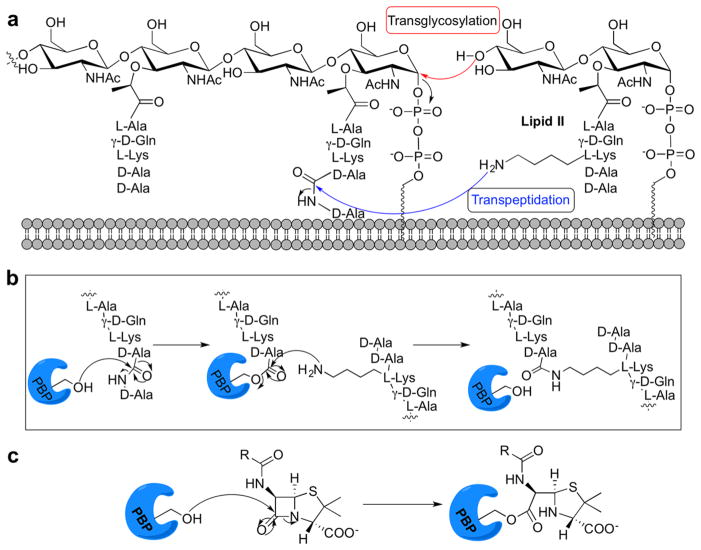
Cell wall biosynthesis requires the penicillin-binding proteins. (a) The PBPs link lipid II subunits with transglycosylase and transpeptidase reactions. (b) Mechanism of transpeptidation; PBPs form an acyl-enzyme intermediate, resulting in cleavage of a D-Ala residue. A neighboring group intercepts this activated intermediate to yield cross-linked peptidoglycan. (c) Active site serine attacks the β-lactam ring, leading to covalent modification of the PBP.
The PBPs are categorized into three classes based on their functional capacity. Class A high-molecular weight (HMW) PBPs are bifunctional proteins with transglycosylase (TG) and transpeptidase (TP) activities. Class B HMW PBPs are monofunctional TPs. The major class C or low molecular weight (LMW) PBPs are remodeling D,D-carboxypeptidases that remove ultimate D-Ala residues from PG peptides, modulating cross-linking between glycan chains.2 All PBPs possess a catalytic serine in their peptidase domain, which is required for substrate turnover and covalently modified by the β-lactam antibiotics (Figure 1b and c).3 The hydrolysis of the β-lactam complex is slow, allowing these molecules to occupy the active site for extended periods and prevent the enzyme from catalyzing further reactions, leading to cell lysis.4
Since it was determined that penicillin V acts as a global PBP inhibitor, β-lactams have been used as probes to gain insight into bacterial physiology.5,6 A standard strategy is tagging with a radio- or fluorophore-labeled penicillin (BOCILLIN-BODIPY FL, Boc-FL; 1 in Figure 2), followed by gel-based separation and detection7–9 to provide information about the catalytic activity of the PBPs.3,10 However, penicillin-based compounds label all PBPs, preventing discrete characterization of each homologue. Single PBPs can be studied with fluorescently labeled protein constructs; however, artificial fusions can perturb protein concentration, function, or localization.11,12 Moreover, PBP protein localization does not provide information about activity state. Alternatively, small molecule-conjugated fluorophores avoid the need for genetic manipulation and enable temporal resolution. For example, nascent PG can be imaged with fluorophore-conjugated vancomycin (Van-FL) and ramoplanin, which label PG biosynthetic precursors in various Gram-positive bacteria.13 Additionally, D-amino acid analogs that bear either a fluorophore or a bioorthogonal handle have been incorporated into the stem peptide during PG synthesis (fluorescent D-amino acids; FDAAs).14–17 FDAAs are being utilized in many investigations18 including our work to study the spatial separation of the PG synthesis machines19,20 and lack of PG turnover and recycling21 in Streptococcus pneumoniae. However, their nonspecificity for individual PBPs can only indicate regions of the cell where there is TP activity.21
Figure 2.
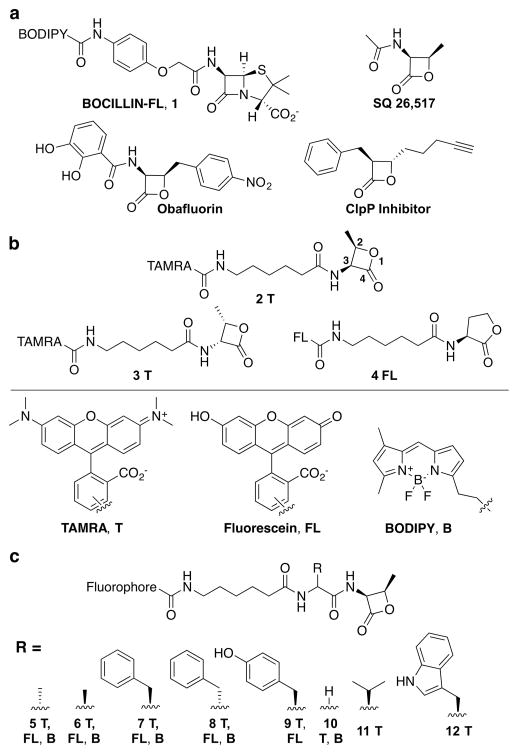
β-lactone-based probe library. (a) Structures of BOCILLIN-FL and three lactone-containing molecules with antibacterial activity. (b) Lactone probes bearing one of three fluorophores: 5(6)-carboxytetramethylrhodamine (TAMRA, T), 5(6)-fl uorescein (FL), and boron-dipyrromethene (BODIPY, B). These probes include a stereochemical mimic of biologically active β-lactams (2R,3S-β-lactone; 2T, FL, B), the opposite isomer (2S,3R-β-lactone; 3T, B), and a five-membered ring δ-lactone (4FL). (c) Amino acid components were incorporated to yield probes with functional groups found in the natural peptidoglycan substrate, D-Ala (5), and the opposite stereoisomer, L-Ala (6), or in β-lactam antibiotics, L-Phe (7), D-Phe (8), and L-Tyr (9), and Gly to assess the importance of the side chain (10). Two additional hydrophobic amino acids were also incorporated, L-Val (11) and L-Trp (12).
To dissect the distinct roles of the enzymes responsible for PG synthesis, small molecules that selectively target individual PBPs in an activity-dependent fashion are required. Several groups have synthesized β-lactam-based probes for in vitro protein labeling, largely yielding compounds that label both PBPs and other bacterial proteins.22,23 We previously reported that PBP-selective imaging probes could be obtained by derivatization of an antibiotic, cephalosporin C.24 Our studies focused on S. pneumoniae, an ovoid-shaped Gram-positive bacteria that causes serious diseases such as pneumonia, bacteremia, and meningitis, and is the major cause of worldwide childhood mortality from infectious disease.25 In addition to being clinically significant, S. pneumoniae is an important model of PG biosynthesis as it possesses a relatively simple PBP complement with three class A PBPs (PBP1a, PBP1b, and PBP2a), two class B PBPs (PBP2x and PBP2b), and one class C PBP (PBP3 or DacA; D,D-carboxypeptidase). Molecules based on cephalosporin C were utilized in combination with Boc-FL to separate the catalytic activity of PBP1b from that of PBP1a, PBP2x, PBP2a, and PBP2b. Intriguingly, these studies indicated that different populations of PBPs may be active at discrete locations during division. Clearly, tools that facilitate deeper examination of this issue will be instrumental in teasing apart the complex biology of the PBP family.
Probes designed to target individual PBP homologues require the identification of scaffolds that selectively inhibit each enzyme. We recently reported evaluation of 20 commercially available β-lactams from five classes of clinically utilized compounds for selective PBP inhibition in S. pneumoniae and Escherichia coli.26,27 Several compounds may provide the foundation for generation of selective probes; however, many PBPs are poorly inhibited by existing β-lactams. To address this challenge, we have identified a comparatively simple β-lactone-scaffold that can be utilized to generate PBP-selective imaging reagents. We have applied these probes to the examination of PBP activity in S. pneumoniae, with a focus on the essential homologues, PBP2x and PBP2b.
RESULTS AND DISCUSSION
β-Lactones (2-oxetanones) have been shown to covalently modify enzymes.28 The most well-known β-lactone, tetrahydrolipstatin (THL; Orlistat), is a long-term antiobesity drug that functions by irreversibly inhibiting a lipase.29 Natural products containing this moiety were first reported ~50 years ago;30,31 however, only a small number are known to possess antibiotic activity, including obafluorin,32 SQ 26,517,30,33 hymeglusin,31 and THL (Figure 2a).34 Recent work has illuminated several classes of enzymes involved in β-lactone biosynthesis,35–37 but the bacterial targets of β-lactone activity remain largely undetermined. Obafluorin protects mice infected with S. pyogenes and causes cell elongation in E. coli, suggesting cell wall biosynthesis inhibition. It was also the first example of a β-lactone substrate of a β-lactamase enzyme.38 A study using THL-based probes revealed several lipase enzymes as potential antibacterial targets of this molecule in Mycobacterium bovis BCG.34 Synthetic β-lactones have been shown to inhibit proteins involved in metabolism, antibiotic resistance, the β-lactamase PBP4* in Bacillus subtilis, and a virulence-associated protein, caseinolytic protease P (ClpP).39–42 Of particular interest to us was the determination that a D,D-carboxypeptidase is one of 13 targets of a β-lactone probe in Mycobacterium smegmatis (CplP inhibitor; Figure 2a).42
β-Lactone Compound Library Design and Assessment
Given the potential of the β-lactone to interact with active site serine residues, including those in β-lactamases and a carboxypeptidase,38,39,42 and the structural similarity of several of these compounds to the β-lactam antibiotics, we postulated that this scaffold could be optimized to create PBP-selective probes. Assessment of the natural product SQ 26,51743 indicated that it is a weak inhibitor of PBP2a (Figure 2a; SI Figure S1). Examination of the corresponding deacylated lactone, as well as the other cis isomer of this electrophilic core, demonstrated no PBP inhibition (SI Figure S2). Together, these data indicate that the β-lactones do not cause nonspecific PBP acylation and have the potential to inhibit these proteins.
We designed molecules with an amide group proximal to the electrophilic carbonyl and the ring substituents in the cis conformation to mimic SQ 26,517 and the β-lactam antibiotics (Figure 2a and 2T in b; lactone ring numbering indicated). For diversification, we appended an amino acid moiety, such as D-Ala to mimic the natural substrate or Phe to mimic the side chain of penicillin, onto this core and a fluorophore for visualization (Figure 2c). Probes were synthesized by adaptation of a known solution phase route (SI Scheme S1).44 The library contained 24 molecules, six that display a lactone coupled directly to a fluorophore [Figure 2b; 2–4; TAMRA (T), BODIPY-FL (B), or fluorescein (FL)], six compounds with substrate-like side chains (Figure 2c; 5 and 6), eight compounds with side chains to mimic groups found in β-lactam antibiotics (7–9), two functionalized with glycine to assess the role of the amino acid side chain in protein labeling (10), and two with alternative hydrophobic side chains (L-Val and L-Trp, 11 and 12).
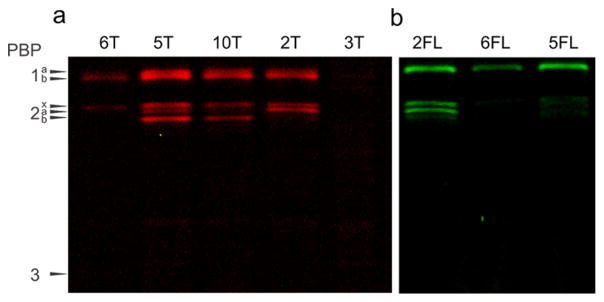
Assessment of both cis-functionalized analogs, (2R,3S)-β-lactone (2) and (2S, 3R)-β-lactone (3; Figure 2b), demonstrated that the stereochemistry of the substituents on the lactone ring is crucial for productive labeling. The compounds displaying these groups in the same orientation as is found in β-lactams, (2R, 3S)-β-lactone (2FL and 2T), labeled most PBPs (PBP1a, PBP1b, PBP2x, and PBP2a), while the opposite isomer labeled nothing ((2S,3R)-β-lactone, 3T; Figure 3a and b). We also found that lactone labeling of the PBPs is competitive with penicillin, indicating that these molecules are active-site directed, and that the resulting acylated protein complex is stable, as it is not displaced by subsequent incubation with penicillin V (SI Figure S3). The selectivity of this simple scaffold for the PBPs is remarkable given the relatively large number of protein classes tagged in previous studies with β-lactone-containing molecules.39–41,44 Indeed, these results are especially intriguing given that our probes lack an ionizable group to mimic the C-terminal D-Ala residue carboxylate in the native stem peptide, which is found in all clinically relevant β-lactam antibiotics.45 Finally, we also found that five-membered δ-lactones do not inhibit the PBPs (4FL and N-acyl homoserine lactones;46 SI Figure S4).
Figure 3.
Gel-based analysis of probes to examine ring geometry (2 and 3) and side chains that act as substrate mimetics (5 and 6). (a) TAMRA-functionalized probes. (2R,3S)-β-lactone (2T) and (2S,3R)-β-lactone (3T) showed different labeling profiles. The addition of glycine between the lactone and fluorophore group (10T) altered the labeling profile to include PBP2b but not PBP2a. The D-Ala-based probe (amino acid stereochemistry found in stem peptide; 5T) labeled all HMW PBPs, while the L-Ala-functionalized molecule was more selective (6T; PBP1b and PBP2x). (b) Fluorescein-functionalized probes. PBP labeling with 2FL and 6FL is comparable to the TAMRA probes. The D-Ala-based probe (5FL; PBP1b and PBP2x) tagged fewer PBPs than the TAMRA analog (5T). All probes were assessed at 5 μg/ mL.
We next assessed probes designed to resemble the stem peptide. The molecule containing a D-Ala residue and TAMRA (5T) labeled all five HMW PBPs, making it more general than compounds without a side chain (Gly derivative, 10T; HMW PBP2a not labeled) or those that lack an amino acid diversity element (Figure 3a; 2FL and 2T; HMW PBP2b not labeled). This may indicate that a similar binding pocket is accessed by D-Ala in the probe and in the natural peptide substrate. However, the fluorescein-functionalized probe (5FL) labeled only PBP1b and PBP2x, suggesting that the fluorophore component can also influence protein labeling (Figure 3b). This phenomenon was most commonly observed with BODIPY-FL-functionalized molecules, which often yielded substantially different profiles, including the labeling of non-PBP targets (e.g., Figure 4a 8B versus 8FL and 8T; SI Figures S5, S6, and S7).
Figure 4.
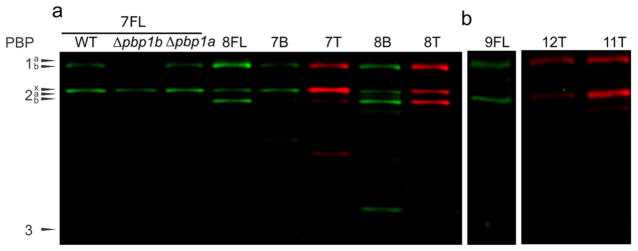
Gel-based analysis of probes containing side chains to mimic β-lactam antibiotics. (a) L-Phe-(2R,3S)-β-lactone (7T, B, and FL), D-Phe-(2R,3S)-β-lactone (8T, B, and FL) labeling was generally identical between FL and T variants, while BODIPY often increased the number of proteins labeled, including non-PBPs (see 8B). L-Phe-(2R,3S)-β-lactone-based probes (7) showed promise for assessment of PBP2x by use of the Δpbp1b strain. Gel-based analysis of S. pneumoniae wild-type (IU1945), Δpbp1a (E177), and Δpbp1b (E193) strains was performed to confirm specific labeling of PBP2x in the mutant strain. (b) Additional hydrophobic side chains were examined including L-Tyr (9FL), L-Val (11T), and L-Trp (12T), all of which labeled PBP1b and PBP2x. All probes were assessed at 5 μg/mL.
Next, we evaluated probes displaying hydrophobic side chains, several of which are found in β-lactam antibiotics, such as a phenyl group in penicillin G or the hydroxyphenyl displayed on amoxicillin. Resolution of PBP1a and PBP1b, which migrate very closely in the gels, was accomplished by utilizing Δpbp1a and Δpbp1b mutant strains (E177 and E193, respectively; SI Figure S8). The D-Phe derivative (8FL) tagged PBP1b, PBP2x, and PBP2b, while the L-Phe and L-Tyr FL derivatives (7FL and 9FL) labeled only PBP1b and PBP2x (Figure 4a and b). Indeed, a large number of probes, including those with alternative hydrophobic side chains, colabeled PBP1b and PBP2x (L-Ala, 6T and 6FL; D-Ala, 5FL; L-Phe, 7FL; L-Tyr, 9FL; L-Val, 11T; L-Trp, 12T). Overall, we found that PBP1b is labeled by all of the tested β-lactone probes, and several compounds coselectively labeled PBP1b and PBP2x. This may indicate that these proteins are tolerant of side chains with diverse size and configuration (SI Table S1 and Figures S9 and 10). Intriguingly, PBP2x is a common target of the β-lactams and is often coinhibited with PBP3,27 which was not labeled by any of the β-lactone probes. In contrast, PBP1b is among the less frequently targeted PBPs by β-lactam antibiotics, indicating that our probe library may be accessing a different region of binding space within the PBP active sites (comparisons of β-lactam and β-lactone inhibition trends in SI Figure S10). PBP2b, which is the least inhibited PBP with conventional β-lactam antibiotics, was labeled by three probe scaffolds (5T, 5FL 8T, 8FL, 10T). PBP1a and PBP2a labeling was only observed with compounds displaying a small side chain (PBP1a: 2FL, 2T, 5FL, 5T, 5B, 10T, and 10B; PBP2a: 2T, 2FL, 5T, and 5FL).
To further examine the differences in the PBPs that may be responsible for the disparate labeling response, we compared the structures of PBP2x and PBP1a, the former being labeled by many probes and the latter tagged by only a small subset. Both proteins have been cocrystallized with tebipenem or biapenem, and we overlaid these structures for comparison of their active sites (2ZC3, PBP2x-biapenem; 2ZC4, PBP2x-tebipenem; 2ZC5, PBP1a-biapenem; 2ZC6, PBP1a-tebipenem; SI Figure S11).47 In PBP1a, a bulky residue can be found near C6 of the lactam core structure (i.e., F577), a position that we anticipate may be occupied by the diversity element in our probes. The presence of this large group may explain the requirement of a small side chain on the probes for productive binding. In contrast, PBP2x has an open cleft at this position, which is consistent with the observed tagging by a diversity of probes.
Although the only LMW PBP in S. pneumoniae, PBP3, is inhibited by the vast majority of β-lactam antibiotics,27 none of the β-lactone probes labeled this protein. It has been postulated that the facile inhibition of PBP3 can be partially attributed to the high catalytic efficiency of this D,D-carboxyendopeptidase as determined by the hydrolysis of the pseudo-substrate N-benzoyl-D-alanylmercaptoacetic acid (kcat/KM = 50 500 M−1s−1).48 In addition, although one might anticipate rapid hydrolysis of the resulting acylated protein species in this endopeptidase, previous work has shown that the deacylation rates of PBP3 and transpeptidase PBP1b, the most commonly labeled protein in these studies, are comparable when treated with [3H]benzylpenicillin (5.7 × 10−5 s−1 and 5.6 × 10−5 s−1, respectively).48 Thus, it is clear that neither catalytic efficiency nor hydrolysis of the resulting covalent species can explain the lack of PBP3 labeling with the β-lactone probes. Indeed, PBP3 and PBP2x are commonly inhibited to a similar extent by β-lactam antibiotics, a trend that was not observed in these studies. These data, along with the ability of the β-lactones to achieve PBP selectivity in the absence of a negatively charged substrate mimic, suggests that our probes may be accessing different binding space than the β-lactams.
PBP Imaging in S. pneumoniae with β-Lactone Probes
PBP2x is an essential HMW PBP in S. pneumoniae involved in septal PG synthesis.19,49,50 Mutations in the conserved motifs of PBP2x have been associated with β-lactam resistance; therefore, it is an important target for antibacterial agents.51–53 PBP2x has recently been shown to localize to septa of dividing S. pneumoniae cells using epitope tags or optimized GFP fusion constructs,19,50,54 corroborating its role in septal PG machinery. FDAA labeling of wild-type bacteria treated with methicillin to specifically inhibit PBP2x TP activity and in bacteria depleted for PBP2x also indicated that PBP2x migrates to the centers of septa in mid-to-late divisional cells.19 A PBP2x-selective activity-based probe is needed to directly corroborate the findings from these experiments by displaying only the active form of this PBP in live cells. Although we did not identify a probe that labels only PBP2x, we did uncover several compounds that label only PBP2x and PBP1b (SI Table S1), the latter of which can be deleted to yield a strain that is phenotypically indistinguishable from the wild-type organism.55 Thus, the Δpbp1b mutant (E193) provides the ideal platform in which to image PBP2x.
We utilized the 2R,3S-β-lactone-L-Phe-fluorescein (7FL) probe in imaging studies to localize PBP2x during the course of cell division (Figure 5). These images showed that PBP2x localized at the division septum as a ring in early-to-mid divisional cells. In mid-to-late divisional cells, PBP2x localized to both the constricting ring and a separate site at the center of the ring, as well as the equators of daughter cells. Finally, enzyme activity was constricted down to a single point at the division site before cell separation. This indicates that subpopulations of active PBP2x demonstrate different localization patterns during a single constriction event, which correlates well with recent reports of PBP2x localization,19,50 as well as a newly synthesized cell wall labeled by FDAAs (Figure 6). In previous reports, immunolabeling showed that PBP1a remained as a larger ring at later stages of cell division, while PBP2x concentrated to the center of the septa. Previous work using FDAAs strongly supported this finding but could not determine if any PBP2x remained in the outer division site ring because the FDAAs are incorporated by multiple PBPs. Clarification of this question highlights a critical strength of a PBP-selective, activity-based imaging strategy19,21 and emphasizes the need to understand how PBP dynamics are regulated. PBP2x has been demonstrated to interact with the serine-threonine kinase StkP, and the protein GpsB has recently been shown to modulate septal closure and PBP2x migration,20 but the exact mechanism behind PBP2x localization remains unclear. Since the 7FL probe can specifically label PBP2x, it may be an effective way to monitor changes in PBP2x localization in future studies of PBP dynamics.
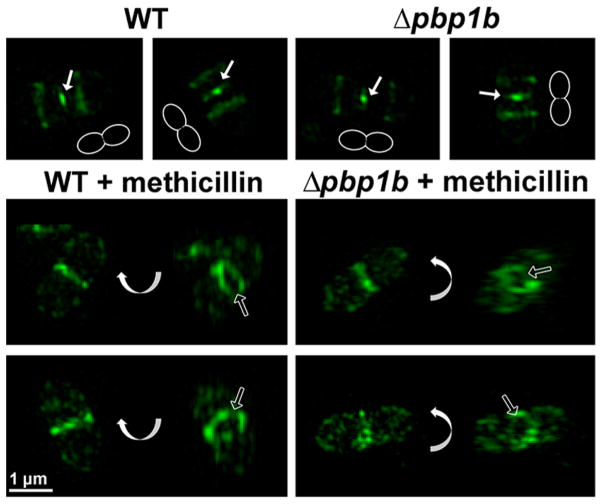
Figure 5.
7FL labeling of PBP2x with or without PBP1b and a PBP2x-inhibiting concentration of methicillin. Wild-type (IU1945) and Δpbp1b (E193) were grown and pretreated with methicillin (0.1 μg mL−1) for 20 min, where indicated. Cultures were then labeled with 7FL. Each image in the top row is a separate cell, and the outlines in each image in the top row demonstrate the orientation of the cell. The bottom two rows consist of pairs of images of the same cell, but one image has been rotated around the indicated axis. 7FL labeling does not change in the absence of PBP1b, and methicillin-treated cells are elongated and lack septal labeling compared to the untreated controls. Solid arrows point to central septal labeling, and empty arrows highlight empty rings after methicillin pretreatment. These images are representative of ~40 mid-to-late division cells for each condition from two biological replicates.
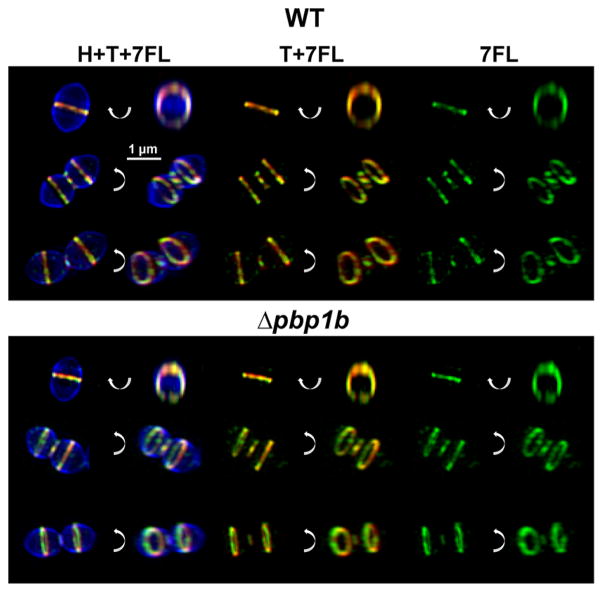
Figure 6.
7FL labeling colocalizes with regions of new cell wall synthesis. Wild type (IU1945) and Δpbp1b (E193) cells were labeled for several generations with the FDAA 7-hydroxycoumarin-3-carboxylic acid 3-amino-D-alanine (HADA, H, pseudocolored blue), then for 5 min with the FDAA tetramethylrhodamine 3-amino-D-alanine (TADA, T, pseudocolored red), and finally with (2S,3R)-β-lactone-L-Phe-fluorescein (7FL, pseudocolored green) as detailed in the Materials and Methods. Each row has six views of the same cell, with one image in each pair rotated around the indicated axis. New cell wall synthesis and 7FL labeling colocalize at all stages of division. These images are representative of >40 cells at all stages of division for each condition from two biological replicates.
To confirm that the observed patterns were due exclusively to PBP2x labeling, cells were pretreated with methicillin, which specifically inhibits PBP2x in S. pneumoniae.50 Gel-based analysis confirmed that PBP2x labeling is dramatically decreased following methicillin treatment (SI Figure S12). 3D-SIM imaging of cells labeled with 7FL after pretreatment with methicillin (Figure 5) revealed elongated cells with a labeled ring at the division site, but with minimal or no constriction evident. Importantly, >98% of methicillin-treated late division cells (~40 cells examined) in both the wild-type and Δpbp1b strains displayed empty septal rings, in contrast with untreated controls from both strains that showed central septal labeling in 85% of wild-type and 73% of Δpbp1b cells (~40 cells examined). This indicates a lack of active PBP2x due to methicillin inhibition, which aligns with previous results56 and strongly supports the conclusion that septal 7FL labeling is due exclusively to binding of PBP2x. Any labeling at the division site after methicillin treatment is likely due to residual uninhibited PBP2x, since a small amount of PBP2x activity remains (SI Figure S12).
Dual Labeling Enables Visualization of Essential Proteins, PBP2x and PBP2b
PBP2b is the essential peripheral transpeptidase in S. pneumoniae.57 Similar to PBP2x, it is a primary penicillin-resistance determinant.58 In previous work, we showed that PBP2b localizes differently than FtsZ, which is the principal organizer of bacterial cell division.19 In early stages of S. pneumoniae division, FtsZ mediates the recruitment of division proteins, including PBP2b, to the midcell septal ring. At later stages of division, FtsZ migrates to the equators of the new daughter cells, while PBP2b remains at the closing septum.19 To complement this previous work, we sought to assess the activity of PBP2b throughout the bacterial cell cycle using the lactone probe 8T, which labels PBP1b, PBP2x, and PBP2b (Figure 7). When utilized alone, this probe yields a nearly identical labeling pattern (SI Figure S13) as 7FL (Figure 5) due to their shared targets. 8T labels the division site as a single ring in early division and labels the center of the division site, the constricting divisional ring, and the new equatorial rings of the future daughter cell in mid-to-late division. Finally, it constricts down to a single point at the division site just before the cells separate. Pretreatment with methicillin produced elongated cells with 8T-labeled divisional rings and minimal constriction. Importantly, >98% of late divisional cells treated with methicillin (~40 cells examined) in both the wild-type and Δpbp1b strains showed empty septal rings, where untreated strains showed 8T central septal labeling in 81% (wild-type) and 76% (Δpbp1b) of cells (~40 cells examined). This mirrors our results seen for 7FL and reinforces the idea that PBP2x alone is localized to the center of the constricting division site.
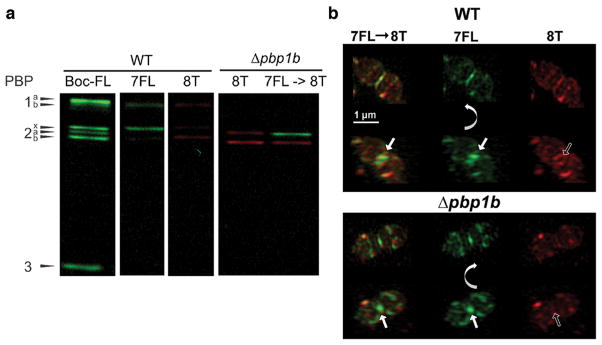
Figure 7.
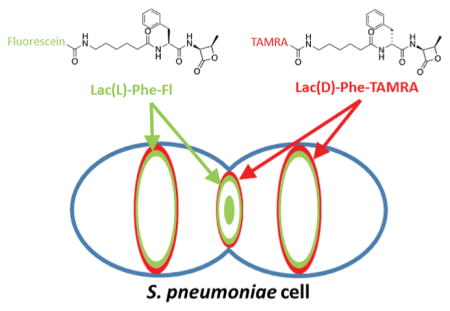
Dual labeling with (2R,3S)-β-lactone-L-Phe-fluorescein (7FL) followed by (2R,3S)-β-lactone-D-Phe-TAMRA (8T). (a) This strategy enables separate visualization of the activity of PBP2x (green) and PBP2b (red) in gel-based analysis. Both probes were used at 5 μg/mL for 20 min. (b) Labeling with 7FL followed by 8T reveals distinct PBP localization patterns. Cultures of wild-type (IU1945) and Δpbp1b (E193) were grown, labeled with 7FL, washed, labeled with 8T, and imaged. Each set is two rows of images of the same cell, with the bottom row rotated relative to the top row around the indicated axis. In both strains, 8T labeling is excluded from the septum and restricted to peripheral rings when the cells are labeled first with 7FL. Solid arrows highlight central septal labeling, and empty arrows highlight the exclusion of 8T labeling from the center of the division site. These images are representative of ~40 mid-to-late division cells for each condition from two biological replicates.
In order to target PBP2b specifically, 8T was utilized on cells first labeled with 7FL. Results of gel-based studies indicate that this combination tags PBP2x (green) and PBP2b (red) individually in the Δpbp1b mutant (Figure 7). 3D-SIM imaging of dual labeled cells revealed that 7FL displayed a labeling pattern similar to the single labeling results seen in Figure 5, as expected. However, labeling of 8T was excluded from the center of the division site in >83% of wild-type and Δpbp1b cells (~40 cells examined). Instead, it was restricted to the outer peripheral ring around the division site and the equatorial rings of future daughter cells, which have not yet begun to constrict. These results correlate well with previous work57 that demonstrated that PBP2b localization remained separate from and external to PBP2x during constriction, and we have now shown that active PBP2b follows this pattern as well and ruled out the possibility that methicillin treatment disrupts PBP2b localization. Importantly, this study is the first to demonstrate colocalization of specifically labeled PBPs confirmed to be enzymatically active, and our results agree well with previous reports on PBP localization in S. pneumoniae.19,49,50,54,59
β-Lactone Imaging Probes Show Antibacterial Activity
We previously reported PBP2x as the main killing target for β-lactam antibiotics in S. pneumoniae.27 Given the observed affinity of our β-lactone probes for this PBP, we hypothesized that they might possess an inhibitory effect on pneumococcal growth. We found the minimum inhibitory concentrations of 7FL and 8T probes (80 μg/mL), which interact with this protein, to be substantially lower than that of SQ 26,517, a mild antibacterial agent (500 μg/mL; Table S2).33
Conclusions and Summary
PG biosynthesis remains an outstanding target for new antibiotics; however, there are significant gaps in our knowledge about the mechanisms and control of PBP activity during this process. Continued development of tools to map the activities of the PBPs is a critical component to our understanding of bacterial growth and may lead to the identification of new therapeutic targets. Here, we report the generation of a library of compounds based upon a scaffold not previously known to target the PBPs, the β-lactones. These probes demonstrated distinct interaction patterns with the PBPs in comparison to β-lactam antibiotics and enabled us to examine PBP2x and PBP2b activity in live S. pneumoniae. We found that PBP2b and PBP2x colocalize as a single ring in early division, but during mid-to-late division, the central septal site contains PBP2x, while PBP2b remains at the outer division ring. This is the first demonstration of separate labeling of the activity state of these two essential proteins and confirms that PBP2b and PBP2x display distinct patterns of localization during constriction. As such, this work highlights the importance of the development of PBP-selective probes. Their combination with powerful cell biology tools will be essential for examination of bacterial function by evaluating the localization and activation of the PBPs in myriad eubacteria.
MATERIALS AND METHODS
Synthesis and characterization of probes and additional experimental protocols are discussed in detail in the Supporting Information.
Application of β-Lactone Probes to S. pneumoniae Cells
Specific settings and experimental details are discussed in the Supporting Information. S. pneumoniae IU1945, E177 (Δpbp1a), and E193 (Δpbp1b) cells were grown in Becton-Dickinson brain heart infusion (BHI) broth at 37 °C in an atmosphere of 5% CO2 to OD620 0.2. Cells from 1 mL of culture were resuspended in 50 μL of PBS containing 1–20 μg mL−1 of different β-lactone probes or 5 μg mL−1 of Boc-FL, washed with PBS, and digested by lysozyme and lysed by sonification. The membrane proteome was isolated by centrifugation at 4 °C and then resuspended in 100 μL of PBS. Protein concentration was adjusted at 2.5 mg mL−1. Samples were mixed with 4 × SDS-PAGE loading buffer and heated for 5 min at 90–95 °C, cooled to RT, and run on a 10% SDS-PAGE, and labeled proteins were visualized in gel and analyzed by ImageJ software (NIH).
For imaging experiments, the cells were washed with appropriate buffers to remove excess probe after the incubation period and resuspended in 15 μL of Vectashield Hardest Antifade (Vector Laboratories) and kept on ice. For fluorescence imaging, 1.2 μL of cell suspension was spotted onto a clean slide and covered with a coverslip and imaged via 3D-SIM.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants DP2OD008592 (E.E.C.), RO1GM113172 (M.E.W.), and RO1GM114315 (M.E.W.); a Pew Biomedical Scholar Award (E.E.C.); a Sloan Research Fellow Award (E.E.C.); and start-up funds from the University of Minnesota and Indiana University.
Footnotes
Author Contributions
S.S. performed gel-based analyses, synthesis, characterization, and concentration determination of probes and assisted with the manuscript. M.J.B. performed microscopy-based analyses and assisted with the manuscript. O.K. performed experiments for SI Figure S2, SI Figure S3, and SI Figure S14 and assisted with the manuscript. A.S. performed synthesis and characterization of probes and assisted with the manuscript. C.L.B. performed synthesis and characterization of probes. J.D.S. performed synthesis and assessment of SQ 26,517 (SI Figure S4). M.E.W. and E.E.C. led overall experimental design and assisted with data interpretation and manuscript writing.
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschem-bio.7b00614.
Experimental section including detailed procedures for probe synthesis, cell labeling, gel electrophoresis, and fluorescence imaging; chemical characterization data, including proton and carbon nuclear magnetic resonance (NMR) and high resolution mass (MS) spectra; supporting Figures 1–14, supporting Tables 1 and 2, and supporting Scheme 1 (PDF)
References
- 1.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:556–556. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 2.Macheboeuf P, Contreras-Martel C, Job V, Dideberg O, Dessen A. Penicillin binding proteins: key players in bacterial cell cycle and drug resistance processes. FEMS Microbiol Rev. 2006;30:673–691. doi: 10.1111/j.1574-6976.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 3.Waxman DJ, Strominger JL. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- 4.Gordon E, Mouz N, Duee E, Dideberg O. The crystal structure of the penicillin-binding protein 2x from Streptococcus pneumoniae and its acyl-enzyme form: implication in drug resistance. J Mol Biol. 2000;299:477–485. doi: 10.1006/jmbi.2000.3740. [DOI] [PubMed] [Google Scholar]
- 5.Falconer SB, Czarny TL, Brown ED. Antibiotics as probes of biological complexity. Nat Chem Biol. 2011;7:415–423. doi: 10.1038/nchembio.590. [DOI] [PubMed] [Google Scholar]
- 6.Böttcher T, Sieber SA. β-Lactams and β-lactones as activity-based probes in chemical biology. MedChemComm. 2012;3:408–417. [Google Scholar]
- 7.Spratt BG, Pardee AB. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975;254:516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- 8.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother. 1999;43:1124–1128. doi: 10.1128/aac.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakaye B, Damblon C, Jamin M, Galleni M, Lepage S, Joris B, Marchand-Brynaert J, Frydrych C, Frere J-M. Synthesis, purification and kinetic properties of fluorescein-labelled penicillins. Biochem J. 1994;300:141–145. doi: 10.1042/bj3000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghuysen JM. The Bacterial DD-Carboxypeptidase-Transpeptidase Enzyme System. Part III. Interactions with Penicillin. University of Tokyo Press; Tokyo, Japan: 1977. [Google Scholar]
- 11.Margolin W. The price of tags in protein localization studies. J Bacteriol. 2012;194:6369–6371. doi: 10.1128/JB.01640-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swulius MT, Jensen GJ. The helical MreB cytoskeleton in Escherichia coli MC1000/pLE7 is an artifact of the N-Terminal yellow fluorescent protein tag. J Bacteriol. 2012;194:6382–6386. doi: 10.1128/JB.00505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiyanont K, Doan T, Lazarus MB, Fang X, Rudner DZ, Walker S. Imaging peptidoglycan biosynthesis in Bacillus subtilis with fluorescent antibiotics. Proc Natl Acad Sci U S A. 2006;103:11033–11038. doi: 10.1073/pnas.0600829103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, VanNieuwenhze MS. In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew Chem, Int Ed. 2012;51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegrist MS, Whiteside S, Jewett JC, Aditham A, Cava F, Bertozzi CR. D-amino acid chemical reporters reveal peptidoglycan dynamics of an intracellular pathogen. ACS Chem Biol. 2013;8:500–503. doi: 10.1021/cb3004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebar MD, May JM, Meeske AJ, Leiman SA, Lupoli TJ, Tsukamoto H, Losick R, Rudner DZ, Walker S, Kahne DE. Reconstitution of peptidoglycan cross-linking leads to improved fluorescent probes of cell wall synthesis. J Am Chem Soc. 2014;136:10874–10877. doi: 10.1021/ja505668f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pidgeon SE, Fura JM, Leon W, Birabaharan M, Vezenov D, Pires MM. Metabolic profiling of bacteria by unnatural C-terminated D-amino acids. Angew Chem, Int Ed. 2015;54:6158–6162. doi: 10.1002/anie.201409927. [DOI] [PubMed] [Google Scholar]
- 18.Bisson-Filho AW, Hsu YP, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, Brun YV, Garner EC. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science. 2017;355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsui HC, Boersma MJ, Vella SA, Kocaoglu O, Kuru E, Peceny JK, Carlson EE, VanNieuwenhze MS, Brun YV, Shaw SL, Winkler ME. Pbp2x localizes separately from Pbp2b and other peptidoglycan synthesis proteins during later stages of cell division of Streptococcus pneumoniae D39. Mol Microbiol. 2014;94:21–40. doi: 10.1111/mmi.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rued BE, Zheng JJ, Mura A, Tsui H-CT, Boersma MJ, Mazny JL, Corona F, Perez AJ, Fadda D, Doubravová L, Buriánkova K, Branny P, Massidda O, Winkler ME. Suppression and synthetic-lethal genetic relationships of ΔgpsB mutations indicate that GpsB mediates protein phosphorylation and penicillin-binding protein interactions in Streptococcus pneumoniae D39. Mol Microbiol. 2017;103:931–957. doi: 10.1111/mmi.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boersma MJ, Kuru E, Rittichier JT, VanNieuwenhze MS, Brun YV, Winkler ME. Minimal Peptidoglycan (PG) Turnover in Wild-Type and PG Hydrolase and Cell Division Mutants of Streptococcus pneumoniae D39 Growing Planktonically and in Host-Relevant Biofilms. J Bacteriol. 2015;197:3472–3485. doi: 10.1128/JB.00541-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staub I, Sieber SA. β-Lactams as selective chemical probes for the in vivo labeling of bacterial enzymes involved in cell wall biosynthesis, antibiotic resistance, and virulence. J Am Chem Soc. 2008;130:13400–13409. doi: 10.1021/ja803349j. [DOI] [PubMed] [Google Scholar]
- 23.Dargis M, Malouin F. Use of biotinylated beta-lactams and chemiluminescence for study and purification of penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1994;38:973–980. doi: 10.1128/aac.38.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocaoglu O, Calvo RA, Sham LT, Cozy LM, Lanning BR, Francis S, Winkler ME, Kearns DB, Carlson EE. Selective Penicillin-Binding Protein Imaging Probes Reveal Substructure in Bacterial Cell Division. ACS Chem Biol. 2012;7:1746–1753. doi: 10.1021/cb300329r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriques-Normark B, Tuomanen EI. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harbor Perspect Med. 2013;3:a010215. doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocaoglu O, Carlson EE. Profiling of β-Lactam Selectivity for Penicillin-Binding Proteins in Escherichia coli DC2. Antimicrob Agents Chemother. 2015;59:2785–2790. doi: 10.1128/AAC.04552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kocaoglu O, Tsui H-CT, Winkler ME, Carlson EE. Profiling of β-Lactam Selectivity for Penicillin-Binding Proteins in Streptococcus pneumoniae D39. Antimicrob Agents Chemother. 2015;59:3548–3555. doi: 10.1128/AAC.05142-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim DH, Park JI, Chung SJ, Park JD, Park NK, Han JH. Cleavage of beta-lactone ring by serine protease. Mechanistic implications. Bioorg Med Chem. 2002;10:2553–2560. doi: 10.1016/s0968-0896(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 29.Venukadasula PKM, Chegondi R, Maitra S, Hanson PR. A Concise, Phosphate-Mediated Approach to the Total Synthesis of (–)-Tetrahydrolipstatin. Org Lett. 2010;12:1556–1559. doi: 10.1021/ol1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells JS, Hunter JC, Astle GL, Sherwood JC, Ricca CM, Trejo WH, Bonner DP, Sykes RB. Distribution of beta-lactam and beta-lactone producing bacteria in nature. J Antibiot. 1982;35:814–821. doi: 10.7164/antibiotics.35.814. [DOI] [PubMed] [Google Scholar]
- 31.Aldridge DC, Giles D, Turner WB. Antibiotic 1233A: a fungal beta-lactone. J Chem Soc C. 1971;0:3888–3891. doi: 10.1039/j39710003888. [DOI] [PubMed] [Google Scholar]
- 32.Tymiak AA, Culver CA, Malley MF, Gougoutas JZ. Structure of obafluorin: an antibacterial beta-lactone from pseudomonas fluorescens. J Org Chem. 1985;50:5491–5495. [Google Scholar]
- 33.Parker WL, Rathnum ML, Liu WC. SQ 26,517 A beta-lactone produced by a Bacillus species. J Antibiot. 1982;35:900–902. doi: 10.7164/antibiotics.35.900. [DOI] [PubMed] [Google Scholar]
- 34.Ravindran MS, Rao SP, Cheng X, Shukla A, Cazenave-Gassiot A, Yao SQ, Wenk MR. Targeting lipid esterases in mycobacteria grown under different physiological conditions using activity-based profiling with tetrahydrolipstatin (THL) Mol Cell Proteomics. 2014;13:435–448. doi: 10.1074/mcp.M113.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christenson JK, Richman JE, Jensen MR, Neufeld JY, Wilmot CM, Wackett LP. β-Lactone Synthetase Found in the Olefin Biosynthesis Pathway. Biochemistry. 2017;56:348–351. doi: 10.1021/acs.biochem.6b01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaffer JE, Reck MR, Prasad NK, Wencewicz TA. β-Lactone formation during product release from a non-ribosomal peptide synthetase. Nat Chem Biol. 2017;13:737–744. doi: 10.1038/nchembio.2374. [DOI] [PubMed] [Google Scholar]
- 37.Wolf F, Bauer JS, Bendel TM, Kulik A, Kalinowski J, Gross H, Kaysser L. Biosynthesis of the β-lactone proteasome inhibitors belactosin and cystargolide. Angew Chem, Int Ed. 2017;56:6665–6668. doi: 10.1002/anie.201612076. [DOI] [PubMed] [Google Scholar]
- 38.Wells JS, Trejo WH, Principe PA, Sykes RB. Obafluorin, a novel beta-lactone produced by Pseudomonas fluorescens. Taxomony, fermentation and biological properties. J Antibiot. 1984;37:802–803. doi: 10.7164/antibiotics.37.802. [DOI] [PubMed] [Google Scholar]
- 39.Böttcher T, Sieber SA. Beta-lactones as privileged structures for the active-site labeling of versatile bacterial enzyme classes. Angew Chem, Int Ed. 2008;47:4600–4603. doi: 10.1002/anie.200705768. [DOI] [PubMed] [Google Scholar]
- 40.Böttcher T, Sieber SA. Beta-lactones as specific inhibitors of ClpP attenuate the production of extracellular virulence factors of Staphylococcus aureus. J Am Chem Soc. 2008;130:14400–14401. doi: 10.1021/ja8051365. [DOI] [PubMed] [Google Scholar]
- 41.Zeiler E, Braun N, Bottcher T, Kastenmuller A, Weinkauf S, Sieber SA. Vibralactone as a tool to study the activity and structure of the ClpP1P2 complex from Listeria monocytogenes. Angew Chem, Int Ed. 2011;50:11001–11004. doi: 10.1002/anie.201104391. [DOI] [PubMed] [Google Scholar]
- 42.Compton CL, Schmitz KR, Sauer RT, Sello JK. Antibacterial activity of and resistance to small molecule inhibitors of the ClpP Peptidase. ACS Chem Biol. 2013;8:2669–2677. doi: 10.1021/cb400577b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pu Y, Lowe C, Sailer M, Vederas JC. Synthesis, Stability and Antimicrobial Activity of (+)-Obafluorin and Related beta-Lactone Antibiotics. J Org Chem. 1994;59:3642–3655. [Google Scholar]
- 44.Wang Z, Gu C, Colby T, Shindo T, Balamurugan R, Waldmann H, Kaiser M, van der Hoorn RA. Beta-lactone probes identify a papain-like peptide ligase in Arabidopsis thaliana. Nat Chem Biol. 2008;4:557–563. doi: 10.1038/nchembio.104. [DOI] [PubMed] [Google Scholar]
- 45.Konaklieva MI. Molecular targets of β-lactam-based antibiotics: Beyond the usual suspects. Antibiotics. 2014;3:128–142. doi: 10.3390/antibiotics3020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh MA, Blackwell HE. Chemical probes of quorum sensing: from compound development to biological discovery. FEMS Microbiol Rev. 2016;40:774–794. doi: 10.1093/femsre/fuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamada M, Watanabe T, Baba N, Takeuchi Y, Ohsawa F, Gomi S. Crystal Structures of Biapenem and Tebipenem Complexed with Penicillin-Binding Proteins 2X and 1A from Streptococcus pneumoniae. Antimicrob Agents Chemother. 2008;52:2053–2060. doi: 10.1128/AAC.01456-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morlot C, Pernot L, Le Gouellec A, Di Guilmi AM, Vernet T, Dideberg O, Dessen A. Crystal structure of a peptidoglycan synthesis regulatory factor (PBP3) from Streptococcus pneumoniae. J Biol Chem. 2005;280:15984–15991. doi: 10.1074/jbc.M408446200. [DOI] [PubMed] [Google Scholar]
- 49.Berg KH, Stamsas GA, Straume D, Havarstein LS. Effects of low PBP2b levels on cell morphology and peptidoglycan composition in Streptococcus pneumoniae R6. J Bacteriol. 2013;195:4342–4354. doi: 10.1128/JB.00184-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Land AD, Tsui HC, Kocaoglu O, Vella SA, Shaw SL, Keen SK, Sham LT, Carlson EE, Winkler ME. Requirement of essential Pbp2x and GpsB for septal ring closure in Streptococcus pneumoniae D39. Mol Microbiol. 2013;90:939–955. doi: 10.1111/mmi.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hakenbeck R, Bruckner R, Denapaite D, Maurer P. Molecular mechanisms of beta-lactam resistance in Streptococcus pneumoniae. Future Microbiol. 2012;7:395–410. doi: 10.2217/fmb.12.2. [DOI] [PubMed] [Google Scholar]
- 52.Nagai K, Davies TA, Jacobs MR, Appelbaum PC. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumo-cocci. Antimicrob Agents Chemother. 2002;46:1273–1280. doi: 10.1128/AAC.46.5.1273-1280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maurer P, Todorova K, Sauerbier J, Hakenbeck R. Mutations in Streptococcus pneumoniae penicillin-binding protein 2x: importance of the C-terminal penicillin-binding protein and serine/threonine kinase-associated domains for beta-lactam binding. Microb Drug Resist. 2012;18:314–321. doi: 10.1089/mdr.2012.0022. [DOI] [PubMed] [Google Scholar]
- 54.Fleurie A, Manuse S, Zhao C, Campo N, Cluzel C, Lavergne JP, Freton C, Combet C, Guiral S, Soufi B, Macek B, Kuru E, VanNieuwenhze MS, Brun YV, Di Guilmi AM, Claverys JP, Galinier A, Grangeasse C. Interplay of the serine/threonine-kinase StkP and the paralogs DivIVA and GpsB in pneumococcal cell elongation and division. PLoS Genet. 2014;10:e1004275. doi: 10.1371/journal.pgen.1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Land AD, Winkler ME. The requirement for pneumococcal MreC and MreD is relieved by inactivation of the gene encoding PBP1a. J Bacteriol. 2011;193:4166–4179. doi: 10.1128/JB.05245-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsui H-CT, Land AD, Kocaoglu O, Vella S, Carlson EE, Winkler ME, Shaw SL, Keen SK, Sham L-T. Requirement of essential Pbp2x and GpsB for septal ring closure in Streptococcus pneumoniae D39. Mol Microbiol. 2013;90:939–955. doi: 10.1111/mmi.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsui H-C, Zheng JJ, Magallon AN, Ryan JD, Yunck R, Rued BE, Bernhardt TG, Winkler ME. Suppression of a deletion mutation in the gene encoding essential PBP2b reveals a new lytic transglycosylase involved in peripheral peptidoglycan synthesis in Streptococcus pneumoniae D39. Mol Microbiol. 2016;100:1039–1065. doi: 10.1111/mmi.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of beta-lactam antibiotics. Antimicrob Agents Chemther. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peters K, Schweizer I, Beilharz K, Stahlmann C, Veening JW, Hakenbeck R, Denapaite D. Streptococcus pneumoniae PBP2x mid-cell localization requires the C-terminal PASTA domains and is essential for cell shape maintenance. Mol Microbiol. 2014;92:733–755. doi: 10.1111/mmi.12588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.