Abstract
Alzheimer disease (AD) is a frequent and devastating neurodegenerative disease in humans, but still no curative treatment has been developed. Although many explicative theories have been proposed, precise pathophysiological mechanisms are unknown. Due to the importance of astrocytes in brain homeostasis they have become interesting targets for the study of AD. Changes in astrocyte function have been observed in brains from individuals with AD, as well as in AD in vitro and in vivo animal models. The presence of amyloid beta (Aβ) has been shown to disrupt gliotransmission, neurotransmitter uptake, and alter calcium signaling in astrocytes. Furthermore, astrocytes express apolipoprotein E and are involved in the production, degradation and removal of Aβ. As well, changes in astrocytes that precede other pathological characteristics observed in AD, point to an early contribution of astroglia in this disease. Astrocytes participate in the inflammatory/immune responses of the central nervous system. The presence of Aβ activates different cell receptors and intracellular signaling pathways, mainly the advanced glycation end products receptor/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, responsible for the transcription of pro-inflammatory cytokines and chemokines in astrocytes. The release of these pro-inflammatory agents may induce cellular damage or even stimulate the production of Aβ in astrocytes. Additionally, Aβ induces the appearance of oxidative stress (OS) and production of reactive oxygen species and reactive nitrogen species in astrocytes, affecting among others, intracellular calcium levels, NADPH oxidase (NOX), NF-κB signaling, glutamate uptake (increasing the risk of excitotoxicity) and mitochondrial function. Excessive neuroinflammation and OS are observed in AD, and astrocytes seem to be involved in both. The Aβ/NF-κB interaction in astrocytes may play a central role in these inflammatory and OS changes present in AD. In this paper, we also discuss therapeutic measures highlighting the importance of astrocytes in AD pathology. Several new therapeutic approaches involving phenols (curcumin), phytoestrogens (genistein), neuroesteroids and other natural phytochemicals have been explored in astrocytes, obtaining some promising results regarding cognitive improvements and attenuation of neuroinflammation. Novel strategies comprising astrocytes and aimed to reduce OS in AD have also been proposed. These include estrogen receptor agonists (pelargonidin), Bambusae concretio Salicea, Monascin, and various antioxidatives such as resveratrol, tocotrienol, anthocyanins, and epicatechin, showing beneficial effects in AD models.
Keywords: astrocytes, Alzheimer’s disease, neuroinflammation, oxidative stress, NF-κB pathway, neurodegeneration
Introduction
The loss of cognitive abilities induced by the development of dementia represents one of the main pathological burdens in humans, critically interfering with social and occupational activities. According to the World Alzheimer Report, over 46 million people live with dementia worldwide, totaling an estimated cost of US $818 billion in 2015, expecting to rise up to $1 trillion by 2018 (Prince et al., 2015). The elevated economic and social impact of dementia has been considered as a public health priority by the World Health Organization (Frankish and Horton, 2017). Many types of dementia with varied pathophysiological mechanisms have been described, but the most frequent in humans is AD accounting for 50–70% of all cases (Querfurth and LaFerla, 2010). Characteristically, AD has been divided in early-onset AD (<65 years) and late-onset AD, with the latter representing around 90% of AD-affected individuals (Mendez, 2017; Pierce et al., 2017). The development of early-onset AD has been related to an altered genetic background, explained primarily by autosomal dominant mutations in APP (MIM #104760), Presenilin 1 (PSEN1) (MIM #104311), and Presenilin 2 (PSEN2) (MIM #600759) genes (Lanoiselée et al., 2017). Whereas a complete explanation for the development of late-onset AD (also commonly referred to as sporadic AD) remains obscure, despite the many risk factors associated with this pathology. Among these factors are included: genetic, such as the presence of the APoE 𝜀4 allele, environmental, and several modifiable lifestyle factors (Killin et al., 2016; Van Cauwenberghe et al., 2016; Vos et al., 2017).
The main pathological hallmarks of AD are the presence of extracellular Aβ plaques, intraneuronal neurofibrillary tangles primarily composed of hyperphosphorylated tau, and brain atrophy, together with increased brain neuroinflammation (Raskin et al., 2015; Bronzuoli et al., 2016). Although many theories have been proposed to explain the pathogenesis of AD, the most widely accepted is the amyloid hypothesis, which states that Aβ dyshomeostasis is responsible for the cognitive phenotype of the disease, acting upstream and contributing to other molecular and cellular alterations observed in this condition (Selkoe and Hardy, 2016). Aβ peptide is obtained from the serial cleavage of APP, first through the action of BACE-1 also referred to as beta-secretase, and posteriorly through the gamma-secretase complex (Carroll and Li, 2016; Yan, 2017). The gamma-secretase complex, which also acts on the notch pathway, is composed of four subunits: presenilin (1 or 2), nicastrin, anterior pharynx-defective 1 (APH-1), and presenilin enhancer 2 (PEN2); with presenilin being the most actively studied and related to AD, as it contains the catalytic subunit of the complex (Ahn et al., 2010). Alterations in the cleaving process of APP produce abnormal lengthy species of the Aβ peptide which are deleterious to the brain cellular environment. These Aβ species have been reported to exhibit different profiles of toxicity, and among them, the soluble forms seem to be more neurotoxic than the fibrillary (aggregated) forms. In particular, the oligomeric form of the soluble Aβ1-42 is considered to be highly harmful (Wang Z.-X. et al., 2016).
The pathological study of brains from individuals with AD has revealed the presence of both neuroinflammation and OS (Lue et al., 1996; Ansari and Scheff, 2010). The precise mechanistic basis leading to the development of these changes in AD is not clear, and the debate of whether they are a causative factor or a consequence of the disease is still open. Despite the discussion, accruing evidence support a direct relationship between Aβ abnormal production and the development and/or maintenance of neuroinflammation and OS (Guerriero et al., 2016).
Plenty receptors and carriers have been reported to interact with the different presentations of Aβ, although it seems that depending on the structure of Aβ (monomer, oligomer, fibrillary), some promote clearance or degradation while others mediate the neurotoxic effects through uptake and accumulation (Jarosz-Griffiths et al., 2016). Aβ interacts and binds to several cellular-expressed pattern recognition receptors in astrocytes and microglia, initiating an innate immune response (Minter et al., 2016). Accordingly, components of innate immunity and complement cascade have been considered risk factors for the development of AD and have been associated with abnormal clearing or deposition of Aβ; in particular, variants in the genes complement receptor 1 (CR1) (Zhu et al., 2015), CD33 (Walker et al., 2015), and TREM2 (Yeh et al., 2016). Also, it has been shown that Aβ species, such as Aβ1-42, are able to induce the release of several proinflammatory cytokines and agents, including IL-1β, IL-6, NO, and TNFα, from glial cells (Lindberg et al., 2005; Hou et al., 2011). The precise intracellular signaling pathways involved in the proinflammatory and OS responses in neuronal and non-neuronal cells in AD are still not clear, although the NF-κB pathway has been reported to become activated in both settings (Shi et al., 2016).
Astrocytes are important CNS resident cells involved in numerous physiological aspects. Similar to neurons, astrocytes represent a heterogeneous population of cells depicting diverse functional and morphological characteristics (Ben Haim and Rowitch, 2017). Astrocytes express several markers that allow them to be distinguished from neurons and other glial cells, including GFAP, calcium-binding protein S100B, glutamine synthetase, and Aldh1L1 (Sofroniew and Vinters, 2010). Astrocytes are key for the maintenance of homeostatic balance and participate in processes such as neurotransmitter uptake and recycling, gliotransmitter release, neuroenergetics, inflammation, modulation of synaptic activity, ionic balance, and maintenance of BBB, among others (Magistretti and Allaman, 2015; Iglesias et al., 2017; Vasile et al., 2017). Precisely, due to this wide array of functional properties, astrocytes have become interesting targets for the study and treatment of numerous brain pathologies. In AD, several reports have shown that astrocytes contribute to cellular and functional degeneration, disrupting glial–neuronal and glial–vascular signaling (Acosta et al., 2017).
The aim of this paper is to review the relevant aspects concerning a possible role of astrocytes in the neuroinflammatory and OS changes observed in AD. As well, we will discuss novel neuroprotective and therapeutic measures highlighting the importance of astrocytes in AD pathology.
Astrocytes and Alzheimer’s Disease
Different studies have shown that the cooperative activity between glia and neurons results in the modulation of cognitive functions (Perea et al., 2009; Fields et al., 2014). Neuron–glial interactions actively control synaptic plasticity and neurotransmission. The concept of “tripartite synapse” refers to this cellular network involving both presynaptic and postsynaptic neurons, as well as astrocytes (Araque et al., 1999; Perea et al., 2009). Numerous gliotransmitters released from astrocytes control synaptic plasticity in different brain structures (Yang et al., 2003; Pascual et al., 2005; Panatier et al., 2006) such as cortex (Ding et al., 2007) and hippocampus (Araque et al., 1998; Jourdain et al., 2007), and are involved in the modulation of memory and learning processes. The interruption of astrocyte’s functions and hence in glia transmission, may result in different neuropsychiatric disorders (Rajkowska et al., 1999; Cohen-Gadol et al., 2004; Fellin et al., 2004; Webster et al., 2005), as well as neurodegenerative diseases, including AD (Forman et al., 2005; Halassa et al., 2007).
Calcium Dysregulation
A pathological increase in the amount of Aβ can induce functional and morphological changes in glial cells, including calcium dysregulation. In fact, microglia and astrocytes are activated close to senile plaques to internalize and break down Aβ (Mohamed and Posse de Chaves, 2011). This cellular activation may result in an inflammatory response and OS, playing a dual role in the pathophysiology of AD with both detrimental and neuroprotective results. Inflammatory mediators (i.e., bradykinin) may increase intracellular calcium concentration via nicotinic receptors and PI3K–Akt pathway in cultured astrocytes (Makitani et al., 2017). Tau has also been connected with astrocytes in AD, as Aβ was shown to bind the CaSR in human astrocytes, activating intracellular signaling which induced the production and release of phosphorylated Tau (Chiarini et al., 2017).
Amyloid beta has been shown to disrupt gliotransmission by enhancing calcium signaling in astrocytes (Lee et al., 2014). This calcium/gliotransmission alteration could underlie an important role of astrocytes in AD pathology. Actually, astrocytic calcium levels are abnormal in several models of AD as both acute and chronic exposure to Aβ elevates baseline calcium levels in cultured astrocytes (Haughey and Mattson, 2003; Alberdi et al., 2013; Lim et al., 2013). This calcium is partially released from intracellular sources such as the endoplasmic reticulum (Toivari et al., 2011). In addition, Aβ interacts with several types of surface receptors in astrocytes which leads to calcium entry, including purinergic receptor P2Y1 (Delekate et al., 2014), nicotinic receptors (α7-nAChRs) (Xiu et al., 2005; Lee et al., 2014), and glutamate metabotropic receptor mGluR5 (Grolla et al., 2013; Ronco et al., 2014). For instance, hippocampal astrocytes exposed to Aβ increased the frequency of NMDA receptor-mediated slow inward currents, together with calcium elevations mediated through α7nAChR activation (Pirttimaki et al., 2013). Aβ-induced dysfunction of NMDA receptors in astrocytes disrupts neuron–glial signal transmission with dramatic consequences on neuronal homeostasis, synaptic transmission, and plasticity. Therefore, neurotoxicity and selective neurodegeneration may be explained by Aβ simultaneous interaction with several receptors and neurotransmitter systems in the context of astrocyte calcium dysregulation.
Glutamatergic Dysfunction and Excitotoxicity
In AD, it has been shown that Aβ can interrupt glutamate uptake capacity and astrocytic calcium signaling (Vincent et al., 2010; Matos et al., 2012). Also, an increase in the expression of astrocytic Tau from aged transgenic animals leads to a decline in GLT activity and therefore in subsequent neurodegeneration (Komori, 1999; Dabir et al., 2004). Some studies have demonstrated in ex vivo astrocyte preparations that Aβ1-42 decreases the expression of GLT-1 and GLAST, two major GLTs in astroglia, via adenosine A2A receptors (de Vivo et al., 2010; Matos et al., 2012). Therefore, disruption in the clearance of excitatory neurotransmitters and increased levels of Aβ and Tau from astrocytes seem to be involved in the neuronal excitotoxicity observed in AD.
Glutamate NMDA and AMPA receptors have been related to the physiopathology of AD (Parameshwaran et al., 2008). Different studies have identified the expression of functional NMDA receptors in astrocytes (Kommers et al., 2002; Lalo et al., 2006) involved in cerebral vasodilatation, synaptic transmission, and neuronal–glial signaling (Verkhratsky and Kirchhoff, 2007; Palygin et al., 2010; Parfenova et al., 2012). Hence, Aβ-induced dysfunction in the expression and function of glutamate receptors in astrocytes, mainly in NMDA receptors, can interfere with neuronal–glial communication (Mota et al., 2014). The cellular excitotoxicity produced by the excessive stimulation of NMDA receptors in neurons and astrocytes has been shown to be reduced with the use of MK801 and memantine (NMDA receptor antagonists) (Lee et al., 2010). Furthermore, due its possible therapeutic role in neurodegenerative diseases including AD, a recent antagonist (UBP141) with preferential effects on astroglial NMDA receptors has been developed (Palygin et al., 2010, 2011). A better comprehension of the differences between neuronal and glial NMDA receptors may provide key elements for the development of novel therapeutics which primarily or selectively target astrocytic function. As well, Aβ can induce glutamate release from astrocytes resulting in an extrasynaptic activation of NMDA receptors. In this case, nitromemantine, which selectively inhibits extrasynaptic NMDA receptors, may protect against Aβ-induced synaptic dysfunction in the hippocampus (Talantova et al., 2013). Additionally, nitromemantine may prevent the synapse-destroying effects of Aβ/α7-nAChR signaling (Dal Prà et al., 2015).
Therefore, using astrocytic signaling as a possible target for drug development may have a therapeutic function in AD’s prevention and control. The antiepileptic drug levetiracetam has shown to reverse synaptic dysfunction as well as memory and learning deficits in human APP (hAPP) transgenic mice (Sanchez et al., 2012). Moreover, a retrospective observational study has shown clinical benefits of levetiracetam in early AD (Vossel et al., 2013). One way this drug may act is increasing glutamate and GABA transporters in astrocytes (Ueda et al., 2007). Chronic administration of levetiracetam may attenuate glutamate excitotoxicity and increase inhibitory neurotransmission. This molecular mechanism involving astrocytes may result in a reduction of cognitive abnormalities in AD.
Aβ Clearance
Astrocytes also participate in the degradation and removal of Aβ as they express different types of proteases involved in the enzymatic cleaving of Aβ. The metalloendopeptidases NEP, IDE, and ECE1 and ECE2 have been reported to be expressed in astrocytes, and are involved in the degradation of monomeric Aβ species (although NEP also hydrolyze oligomeric forms) (Mulder et al., 2012; Ries and Sastre, 2016). It has been proposed that the modification from “natively folded-active” to “aggregated-inactive” form of IDE and NEP may be a relevant pathological mechanism in late-onset AD (Dorfman et al., 2010). Astrocytes also express and secrete several MMPs, including MMP-2 and MMP-9, which degrade both monomeric and fibrillar extracellular forms of Aβ (Ries and Sastre, 2016). Furthermore, it was found in APP/presenilin 1 transgenic mice that astrocytes surrounding Aβ plaques increased the expression of both MMP-2 and MMP-9 (Yan et al., 2006; Yin et al., 2006).
Apolipoprotein E is primarily produced by astrocytes in the CNS and has been proposed to play a major role in AD. In mice, ApoE(-/-) astrocytes have been shown to fail to respond or internalize Aβ deposits to the same extent as do wild-type astrocytes (Koistinaho et al., 2004). As well, mice astrocytes expressing the ApoE 𝜀4 allele were less effective eliminating Aβ plaques than those astrocytes expressing the ApoE 𝜀3 allele (Simonovitch et al., 2016). Astrocytes derived from human induced pluripotent stem cells (iPSC) which expressed the ApoE 𝜀4 allele failed to support neuronal neurotrophic functions such as survival and synaptogenesis (Zhao et al., 2017). As the presence of ApoE 𝜀4 allele is considered a major risk factor in AD while the presence of ApoE 𝜀2 allele is considered a protective factor, a differential regulation of these isoforms regarding the presence of Aβ and associated responses such as neuroinflammation has been proposed (Dorey et al., 2017).
AD and Astrocyte Imaging
One of the most important research areas in AD is related to the development of biomarkers. Although several types of biomarkers have been explored, there is still not one that specifically diagnose, differentiate, and predict the rate of decline between populations of cognitively healthy/preclinical dementia, mild cognitive impaired and AD individuals (Fiandaca et al., 2014; Salvatore et al., 2015; Huynh and Mohan, 2017). Due to their fundamental role in CNS homeostasis, astrocytes could be considered as possible targets for tracking and studying in vivo changes in AD as well as serving as a biomarker for the disease.
L-Deprenyl is a selective inhibitor of the enzyme MAO-B, predominantly found on astrocytes (Levitt et al., 1982). This compound has been successfully used in vitro and in vivo to study the distribution and activity of MAO-B through different techniques including quantitative autoradiography and positron emission tomography (PET) (Kumlien et al., 1992; Arakawa et al., 2017). In postmortem samples from individuals with AD, the activity of both MAO-B and the binding of [3H]L-deprenyl was found to be increased in many brain regions (Jossan et al., 1991). In addition, MAO-B has been found to be increased during reactive astrocytosis in neurodegenerative conditions (Ekblom et al., 1993, 1994). As astrocytes produce MAO-B and this enzyme is increased during reactive astrocytosis, which is a process observed in AD, it seemed plausible to use L-dyprenyl as a marker of astrocytosis in this condition.
Accruing evidence seems to support the use of L-deprenyl in AD. A comparative study using PET between one of the currently accepted biomarkers for AD, the 11C-Pittsburgh Compound B, and (11)C-deuterium-L-deprenyl, concluded that the latter provided non-redundant information on both functional and pathologic aspects of the disease (Rodriguez-Vieitez et al., 2016a). Furthermore, L-deprenyl has provided valuable information about the stage of progression of AD. In a human study, the highest binding for (11)C-deuterium-L-deprenyl was observed in Braak I–II (initial AD stages), whereas it decreased with the most advanced Braak stages (Gulyás et al., 2011). Similar results have been obtained in other studies using (11)C-deuterium-L-deprenyl, where astrocytosis is prominent at the initial phases, even at preclinical stages, and then declines as the disease progresses (Carter et al., 2012; Schöll et al., 2015; Rodriguez-Vieitez et al., 2016b). In addition, a similar laminar binding pattern for tau and [3H]L-deprenyl at the temporal lobe was recently demonstrated, suggesting tau deposits and astrocytic inflammatory processes are closely related in AD (Lemoine et al., 2017). All these results point to an early contribution of astrocytes in AD pathology.
GABA–Glutamine Cycle
Neurons and astrocytes work in a coordinated way throughout different metabolic pathways to synthesize and release glutamate and GABA (Bak et al., 2006). At inhibitory synapses this pathway is called the GABA–glutamine cycle and it depends on GABA transporters and a multi-enzyme machinery that coordinates this process (i.e., GABA transaminase, glutamate decarboxylase, and glutamine synthetase) (Bak et al., 2006; Hertz, 2013). In AD, the processes related to GABA–glutamine cycle and GABA release from astrocytes seem to be altered. The glutamine–glutamate/GABA cycle consists of the transfer of glutamine from astrocytes to glutamatergic and GABAergic neurons. This process depends on glutamine synthetase and the tricarboxylic acid cycle (Walls et al., 2015). A reduction in pyruvate carboxylation, glutamine levels, and tricarboxylic acid cycle turnover in GABAergic neurons and astrocytes was shown in the transgenic rat AD model, McGill-R-Thy1-APP (Nilsen et al., 2014). Similarly, reduced expression of glutamine synthetase in postmortem AD brain samples indicates a profound alteration in neurotransmitter and protein synthesis, as well as metabolic dysfunction (Robinson, 2000). Astrocytes may produce and release GABA, with a main role on hippocampal synaptic plasticity function during memory processing. Increased activity of glutamate decarboxylase enzyme was found in glial synaptosomes obtained from the cortex of APP/TS1 transgenic mice, suggesting Aβ plaques stimulate GABA synthesis from astrocytes (Mitew et al., 2013). Reactive astrocytes from APP/PS1 transgenic mice have also been shown to produce GABA involving MAO-B, and release it through the bestrophin 1 channel, in an aberrant manner (Jo et al., 2014). In the same study, the suppression of GABA production or release from astrocytes completely restored the cognitive deficits and impairments in synaptic plasticity observed in the mice. Under physiological conditions, astrocytic GABA exerts a disinhibitory action at the perforant path to dentate gyrus neurons via GABAB receptors on interneurons. However, in the APPswe/PSEN1dE9 mice, it has been shown an inhibitory action of astrocytic GABA by targeting GABAA receptors in glutamatergic terminals (Yarishkin et al., 2015). These results provide a useful specific GABAergic target aimed at memory impairment reduction in AD. Alterations in the metabolic functions of astrocytes and consequently in glutamate and GABA–glutamine cycles may help explain cognitive disorders in AD (Le Prince et al., 1995; Robinson, 2000; Nilsen et al., 2014). Neurotransmitter transporters and effectors together with GABA-metabolizing enzymes are of special interest in drug development regarding therapeutical options for GABA-related neurological dysfunctions such as AD (Sarup et al., 2003; Nava-Mesa et al., 2014; Mutis et al., 2017; Sánchez-Rodríguez et al., 2017). Although special attention should be taken regarding the differential functional roles of neuronal and glial neurotransmitter transporters and overlying GABA/glutamate metabolic pathways in the development of high selective cell-specific drugs, in order to avert pharmacological interactions and unexpected side effects.
Metabolic Compromise
The metabolic cooperation between astrocytes and neurons is essential to the brain functioning. The energy metabolism of neurons depends on blood oxygen supply but also on astrocytic glucose transporters, mainly GLUT1 (Morgello et al., 1995). In addition, astrocytes may convert glycogen to lactate during periods of higher activity of the nervous system (Falkowska et al., 2015). Both in vivo and in vitro studies indicate that astrocytes participate in the regulation of cerebral blood flow according to neuronal activity and metabolic demand (Magistretti and Pellerin, 1999; Magistretti, 2006). Therefore, astrocytes are key to guarantee an adequate coupling between brain activity and metabolic supply. Several studies have shown reduced cerebral glucose metabolism in early stages of AD and correlation with symptoms severity (Desgranges et al., 1998; Mosconi et al., 2005, 2006, 2008). As mentioned early, Aβ affects neuronal excitability and it also may reduce astrocytic glycolytic capacity (Soucek et al., 2003; Schubert et al., 2009) and reduce the neurovascular unit function (Acosta et al., 2017; Kisler et al., 2017). Moreover, reductions in GLUT1 and lactate transporters in astrocyte cultures derived from transgenic AD mice have been reported (Merlini et al., 2011). In AD, the resulting metabolic dysfunction may alter the overall oxidative neuronal microenvironment (Mosconi et al., 2008). The chronic sustained effect of diminished lactate supply, increased neuronal activity, and reduced neurovascular coupling, underlines the OS increase during AD. Therefore, astrocytes are crucial players either acting as protectors against OS or participating in the progression of AD. The specific role of astrocytes on inflammatory response and OS damage will be reviewed in the next sections.
Neuroinflammation, Alzheimer’s Disease, and Astrocytes
Inflammation is a protective physiological response necessary to regulate processes associated with damage mechanisms in the organism. Several actions related to general inflammatory activities include protection against microorganisms, tissue repair, and removal of cellular debris. The CNS possesses some characteristics that differentiate the immune and inflammatory activities of the brain and spinal cord from those occurring in the rest of the body. Mainly, these differences arise through the presence of the BBB, which restricts the pass of leukocytes into the brain parenchyma, and also due to the cellular interactions of microglia and astrocytes, responsible for most of the immune/inflammatory CNS responses (Ransohoff et al., 2015). Although neuroinflammation arises innately as a protective mechanism when injury is present in the CNS, alteration in any of the components of this response may compromise the cellular microenvironment and become noxious to the brain. Many neurodegenerative conditions, including AD, have been associated with the presence of abnormal neuroinflammation (Ransohoff, 2016).
The role of astrocytes in neuroinflammation has been highlighted in the past years with many observations both in vivo and in vitro depicting the importance of these glial cells in this process (Colombo and Farina, 2016). In fact, an increase in the expression of GFAP is commonly considered as a hallmark of neuroinflammation in many neurodegenerative conditions, including AD (Millington et al., 2014). Astrocytes, together with microglia, react to a diverse range of pro- and anti-inflammatory agents (Sofroniew, 2014). Depending on the cytokine, astrocytes modify their phenotype to either activated or deactivated state. Increased levels of INF-γ, IL-1β, IL-6, and TNFα induce astrocytes to adopt a classical activation state (increased activation of NF-κB pathway, production of ROS and NO, and release of IL-1β, IL-6, and TNFα), while increased levels of IL-4 and IL-13 induce an alternative activation (increased secretion of IL-4 and decreased production of ROS and NO); oppositely, high levels of IL-10 and TGF-β induce astrocytic deactivation (reduced immune surveillance and proinflammatory signaling) (Dá Mesquita et al., 2016).
Furthermore, the reactive state of astrocytes may also depend on the source of injury (neuroinflammation or ischemia), indicating the complex range of responses these cells are capable to produce (Zamanian et al., 2012). In a recent paper, a new classification of reactive astrocytes was proposed, designating A1 those astrocytes that developed a neurotoxic phenotype and A2 those that depicted neurotrophic and neuroprotective characteristics (Liddelow et al., 2017). The authors also reported that the presence of IL-1α, TNFα, and C1q (all three released from microglia) promoted the appearance of A1 astrocytes and that this phenotype was found to be predominant in brain tissue from AD patients. These findings raise a number of questions regarding the manner in which the brain deals with different types of injuries and specifically how astrocytes and astrocytic-cellular interactions induce either a protective or harmful profile. An increase in A1 astrocytes seems to occur in AD, but still is not clear if Aβ induces this phenotype or if another specific agent is involved in this reaction. Nonetheless, it has been reported that the interaction of Aβ with astrocytes induces a pro-inflammatory profile and even astrogliosis (Batarseh et al., 2016).
RAGE, Astrocytes, and Amyloid Beta
Amyloid beta has been reported to interact with numerous cellular receptors and astrocytes express a large amount of them, including TLRs, scavenger receptors, glycoprotein receptors, lipoprotein receptors, RAGE, acetylcholine receptors, complement and chemokine receptors, T-cell receptors, and mannose receptor, among others (Farfara et al., 2008). Binding of Aβ to different types of receptors seems to depend on the Aβ peptide form (monomer or fibrillar). For example, IR- and SEC-R bind monomeric forms of Aβ, scavenger receptor CD36 and glycoprotein receptors lactadherin, and CD47 prefers fibrillary Aβ, while RAGE, ApoE, and nAChR α7nAChR bind both monomer and fibrillar forms (Verdier et al., 2004). The specific outcome of all these complex Aβ-astrocytic interactions is still under research, as the precise intracellular and intercellular communication changes prompted by the different types of Aβ acting on these receptors is yet to be elucidated. Despite the gaps in knowledge, the activation of some receptors, in particular RAGE, has been reported to induce proinflammatory changes in astrocytes when exposed to Aβ (González-Reyes and Graciela Rubiano, 2016).
Advanced glycation end products receptor is a multiligand pattern-recognition receptor, member of the immunoglobulin superfamily with a variety of isoforms present in brain cells (Ding and Keller, 2005). In addition to Aβ and several AGE, RAGE can bind DNA-binding protein HMGB1/amphoterin (Hori et al., 1995) and S100/calgranulins (Hofmann et al., 1999). The main intracellular pathway activated through RAGE is the NF-κB pathway (Tóbon-Velasco et al., 2014), although it can also activate other downstream pathways including Cdc42-Rac, p21ras and MAPK, JNK, and ERK (González-Reyes and Graciela Rubiano, 2016). Furthermore, astrocytes have been reported to adopt a phagocytic profile capable of engulfing Aβ, mediated by CD36, CD47, and RAGE receptors (Jones et al., 2013). Apart from Aβ, the interaction of astrocytic RAGE with other ligands, such as S100B, may also be involved in AD neuroinflammation (Cirillo et al., 2015). These findings point to the interaction of RAGE/NF-κB pathway in astrocytes as an important factor in the development or maintenance of inflammation in AD.
Astrocytes and NF-κB Pathway
The transcription factor NF-κB is currently considered as an important agent related to neuroinflammation in AD (Shi et al., 2016). NF-κB is known to be mainly activated by two pathways, the canonical (or classical) and the non-canonical (or alternative) (Nakajima and Kitamura, 2013). The canonical pathway involves activation of various receptors including RAGE and cytokine receptors, such as TNF receptor, IL-1 receptor, and the TLR family. These will induce the further activation of many downstream agents, in special IKKs alpha (IKKα) and beta (IKKβ), and NEMO (in charge of the degradation of the cytoplasmic inhibitor IKBα), and the subsequent complexes (mainly RelA) that act as transcription factors in the nucleus (Marcu et al., 2010; Wan and Lenardo, 2010). The non-canonical pathway, also known as the NEMO-NF-κB-independent pathway, occurs when NF-κB is activated by specific recruitment of TRAF2 and TRAF3, and involves p52 and RelB (Morgan and Liu, 2011). Both canonical (Wang et al., 2013) and non-canonical (Akama and Van Eldik, 2000) activation of NF-κB has been observed in astrocytes stimulated with Aβ, but still is not clear which type predominates in AD or if a differential NF-κB activation is related to the stage of the disease. It has been reported that most of the cytokines and chemokines produced by non-stimulated and activated astrocytes are direct targets of the NF-κB pathway, suggesting a central role of this factor in the proinflammatory (neurotoxic) and immunoregulatory (neuroprotective) actions of astrocytes in the CNS (Choi et al., 2014). Also, NF-κB is involved in other functions such as neuronal survival, differentiation, apoptosis, neurite outgrowth, and synaptic plasticity, all found to be altered in AD (Mémet, 2006).
In astrocytes and microglia, the activation of NF-κB due to Aβ stimulation leads to the production of the pro-inflammatory cytokines IL-1β, IL-6, iNOS, and TNFα (Bales et al., 1998; Akama and Van Eldik, 2000; Hou et al., 2011). In rats treated with Aβ1-42 oligomers, it was shown that COX-2, IL-1β, and TNFα were expressed in reactive astrocytes surrounding the Aβ-injection site and in nearby blood vessels, as well was found co-localization of NF-κB proteins with GFAP and COX-2 (Carrero et al., 2012). In primary astrocytic and mixed astrocytic-neuronal cell cultures from rats, the use of minocycline, an anti-inflammatory agent, reduced astrocytic inflammatory responses together with a decrease in neuronal loss, caspase-3 activation, and caspase-3-truncated Tau species in neurons (Garwood et al., 2011). Minocycline has been shown to inhibit the NF-κB signaling pathway in spinal rat astrocytes (Song et al., 2016). Other reports of beneficial outcomes due to regulation of the NF-κB signaling pathway in astrocytes were reviewed by Colombo and Farina (2016). Although an exaggerated neuroinflammatory response is observed in AD, an absolute suppression of the NF-κB signaling pathway may be undesirable and even worsen the pathological condition. In APPswe/PS1dE9 transgenic mice, the suppression of NF-κB attenuated astrogliosis in the hippocampus and cortex of the animals but increased the amount of Aβ1-42, suggesting a role of astrocytic-mediated neuroinflammation in the clearance of Aβ (Zhang et al., 2009). As well, the clinical evidence for the use of non-steroidal anti-inflammatory drugs (NSAIDS) in AD patients has not proven to be of benefit (Aisen et al., 2008; Szekely et al., 2008; Beeri et al., 2012; Alzheimer’s Disease Anti-inflammatory Prevention Trial Research Group, 2013).
Inflammatory Induction of Aβ in Astrocytes
Astrocytes not only are activated and induced to release chemokines and cytokines in the presence of Aβ, these cells also react to the presence of pro-inflammatory cytokines and even increase the production of Aβ in response. Therefore, the presence of inflammation is capable of increasing the production of Aβ. In addition, the development of neuroinflammation has also been related to cognitive changes in AD (Westin et al., 2012; Echeverria et al., 2016; Laurent et al., 2017).
Neuroinflammation in AD is characterized by the accumulation of cytokines such as IL-1β, IL-6, TNF-α, or TGF-β, which can contribute with cerebral amyloid deposition, augmentation of APP expression, Aβ formation, and subsequent recruitment and activation of microglial cells (Esler and Wolfe, 2001). In general, TNF-α, IL-1β, IFN-γ, L-6, and TGF-β are able to stimulate β-secretase and γ-secretase enzymatic activity through a JNK-dependent MAPK pathway, which cleaves APP and initiates Aβ formation (Liao et al., 2004). Astrocytes express and respond to a large scope of cytokines and chemokines suggesting a central role in the inflammatory-induced production of Aβ.
A study reported that a systemic immune challenge in wild-type mice during late gestation induced the development of AD-like pathology during aging, with animals displaying increased levels of hippocampal APP and altered Tau phosphorylation, together with microglia and astrocytic activation (Krstic et al., 2012). Additionally, it was shown that LPS-induced systemic inflammation in mice could contribute to cognitive impairment and increased expression of APP and Aβ1-42, associated with increased production of inflammatory mediators such as COX-2, IL-1, and iNOS (Lee et al., 2008). The same paper reported that these changes were accompanied with astrocytic activation. Other studies have found evidence, both in human and murine models, that inflammation induces the expression of Aβ. Primary astrocytes from mice, treated with a combination of TNFα and INF-γ, significantly increased levels of BACE1, APP, and Aβ1-40 (Zhao et al., 2011). In human primary astrocytes, treatment with INF-γ in combination with either TNFα or IL-1β induced the secretion of Aβ1-40 and Aβ1-42 (Blasko et al., 2000). Furthermore, TGF-β1 was found to induce overexpression of APP in astrocytes but not in neurons (Lesné et al., 2003). Cytokines seem to act on the 5′-untranslated region (5′-UTR) of the APP gene in astrocytes (Lahiri et al., 2003).
On the one hand, it seems that the presence of Aβ is able to induce the production and release of pro-inflammatory cytokines and chemokines from astrocytes, which could as well act in an autocrine manner to further induce the production of Aβ from astrocytes and possibly other cells. On the other hand, these results suggest that inflammation may be present at early stages (pre-clinical) of the disease or even that inflammation may be responsible for the appearance of pathological Aβ production and accumulation. Under both circumstances, astrocytes appear to be deeply involved in inflammatory changes observed in AD.
Astrocytes and Other Mechanisms of Neuroinflammation
Other factors related to astrocytes may contribute to the appearance or enhancement of neuroinflammation in AD. For example, the presence of elevated glucose levels (as found in diabetes) has been shown to increase neurotoxicity and the release of pro-inflammatory cytokines from primary human astrocytes (Bahniwal et al., 2017). A relation between AD and diabetes/metabolic syndrome has been explored previously (González-Reyes et al., 2016), as both Aβ and AGE bind to RAGE in astrocytes. As well, pro-inflammatory signaling in astrocytes may involve changes in the expression of the calcium-dependent phosphatase CaN, which has been shown to interact with another transcription factor involved in inflammatory responses, the NFAT (Acosta et al., 2017). Enhanced nuclear accumulation of CaN/NFAT was observed in human AD hippocampus and astrocytic cultures treated with Aβ (Abdul et al., 2009). In addition, it has been reported that Aβ deregulates calcium homeostasis via CaN and its downstream target NF-κB, as well as increasing NF-κB-dependent expression of mGluR5 and IP3R2 in astrocytes (Lim et al., 2013). Changes in mGluR5 and IP3 receptor expression have been reported in astrocytes surrounding amyloid plaques in a genetic mouse model of AD (Norris et al., 2005). Another possible factor contributing to the presence of neuroinflammation is the BBB, as in AD, it has been reported that the BBB occasionally loses its integrity (Chakraborty et al., 2017). This may be explained in part thanks to the accumulation of Aβ in brain blood vessels and also due to the associated vascular inflammation, allowing crossed communication between the peripheral immune system and the brain (Takeda et al., 2014). As astrocytes have a very important interaction with the BBB and its functional components are plausible to consider their involvement in BBB-associated neuroinflammatory changes in AD.
Uncontrolled neuroinflammation is a critical element in the progression of AD, impairing the normal function of the CNS. Astrocytes, together with microglia, are the main cells involved in the inflammation/immune responses of the CNS. The presence of Aβ activates different astrocytic cell receptors, mainly RAGE, inducing the activation of the inflammatory pathway NF-κB responsible for the transcription of numerous pro-inflammatory cytokines and chemokines in astrocytes. In addition, the presence of pro-inflammatory cytokines such as IL-1β can act on astrocytes stimulating the production of Aβ and perpetuating a pro-inflammatory profile in astrocytes. Astrocytes are key in the maintenance of the homeostatic balance of the CNS and use the mechanism of reactive astrogliosis as a defensive reaction (Pekny et al., 2016), therefore is fundamental to understand the pathophysiological process that causes astrocytes to convert from a protective agent into a cell that produces a maladaptive astrogliosis response in AD (Table 1).
Table 1.
Summary of studies reporting effects of Aβ or AD on pro-inflammatory and anti-inflammatory cytokines and chemokines in astrocytes.
| Experimental model | Pro-inflammatory agents | Anti-inflammatory agents | Reference |
|---|---|---|---|
| Primary culture of rat astrocytes treated with Aβ1-42 | ↑TNFα; ↑IL-1β; ↑IL-6; ↑COX-2; ↑PGE2; ↑IL-17; ↑INF-γ; ↑IP-10 | ↑IL-13 | Hu et al., 1998; Akama and Van Eldik, 2000; Garwood et al., 2011; Wang et al., 2013; Aguirre-Rueda et al., 2015 |
| Isolated rat astrocytes treated with Aβ25-35 | ↑TNFα; ↑IL-1β | Del Bo et al., 1995; Ayasolla et al., 2004 | |
| Primary cultures of rat astrocytes treated with Aβ1-40 | ↑IL-1β; ↑IL-6 | Bales et al., 1998 | |
| Mixed cultures of rat neurons and astrocytes treated with Aβ1-42 and Aβ25-35 | ↑TNFα | Masilamoni et al., 2005b | |
| Primary cultures of rat astrocytes treated with spherical aggregates of synthetic Aβ | ↑IL-1β | Todd and Garrard, 1979 | |
| In vivo i.c. infusion of Aβ1-42 oligomers in rats | ↑IL-1β; ↑TNFα; ↑COX-2 | Carrero et al., 2012 | |
| Primary culture of mice astrocytes treated with Aβ1-42 | ↑IL-1β; ↑IL-6; ↑TNFα | ↓TGF-β1 | Liu et al., 2009; Hou et al., 2011 |
| Brain sections of Tg2576 mice stained for GFAP | ↑IL-1β; ↑IL-6; ↑INF-γ; ↑IL-12 | ↑TGF- β1; ↑IL-10 | Mehlhorn et al., 2000; Apelt and Schliebs, 2001; Abbas et al., 2002 |
| In vivo i.c.v infusion of Aβ1-42 oligomers in mice | ↓TGF-β1 | Diniz et al., 2017 | |
| In vivo i.c.v infusion of Aβ1-42 in IL-32β transgenic mice | ↑IP-10; ↑GM-CSF | ↑IL-13 | Yun et al., 2015 |
| Astrocytoma human cell line U-373 MG treated with Aβ1-40 (co-treated with IL-1β) | ↑IL-6 | Gitter et al., 1995 | |
| Postmortem brain tissue from patients with AD, stained for GFAP (or other astrocytic marker) | ↑IL-1β; ↑IL-6; ↑IL-18 | Ojala et al., 2009; Bouvier et al., 2016 |
TNFα, tumor necrosis factor alpha; TGF-β1, transforming growth factor beta 1; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; GFAP, glial fibrillary acidic protein; IP-10, interferon gamma-induced protein 10; GM-CSF, granulocyte–macrophage colony-stimulating factor; i.c., intracerebral; i.c.v., intracerebroventricular.
Oxidative Stress, Alzheimer’s Disease, and Astrocytes
Oxidative stress is the result of a dysregulation between the amount of free and non-free radicals produced, including ROS and RNS. This can be attributed to the loss of homeostasis due to mitochondrial overproduction of oxidants over the production of antioxidants (Swomley and Butterfield, 2015). Among the most important ROS are the peroxyl radicals (ROO⋅), NO, the superoxide radical anion (), the hydroxyl radical OH⋅ and some other non-radical species such as peroxynitrite (ONOO-), single oxygen (O2), and hydrogen peroxide (H2O2) (Dasuri et al., 2013). ROS, as well as RNS, are produced under physiological conditions during the common metabolic pathways. These reactive species act on second messengers and subsequently may influence several signaling pathways in a positive or negative form, depending on the regulatory mechanism of its concentration, called redox regulation (Valko et al., 2007). Likewise, mitochondria are able to produce antioxidants which counteract the harmful effects of OS to maintain the balance between the production and detoxification of ROS. These antioxidants are classified in two main groups: enzymatic antioxidants such as SOD, catalase, antioxidant GSH, GPX, GSH reductase, and GSH-S-transferase, and non-enzymatic antioxidants such as GSH, thioredoxin, vitamins A, E, and C, flavonoids, and proteins like albumin and metallothionein (Valko et al., 2007; Halliwell, 2012).
Oxidative Stress and Alzheimer’s Disease
The development of OS in AD has been related to mitochondrial dysfunction, leading to superoxide overproduction ending in synaptic damage (Friedland-Leuner et al., 2014; Bhat et al., 2015). Mitochondrial dysfunction in AD seems to be linked to the increased presence of ROS and RNS (Islam, 2017). Müller et al. (2010) observed a decreased mitochondrial potential in transgenic Thy1-APP751SL mice. The same authors reported that increased intracellular Aβ production might trigger mitochondrial dysfunction quite early and independently of Aβ plaques and, that the accumulation of these alterations with aging lead to disruption of respiratory chain complexes (mainly III and IV) and significant reduction in the generation of NADH. The authors suggested that progressive increase in oxidant production together with a decrease in antioxidant components may conduce to the loss of brain homeostasis observed in AD. However, in AD, it has been demonstrated that prior to the appearance of senile plaques, brains present glucose hypometabolism due to abnormal oxidative metabolic routes in the mitochondria, which also induce increased ROS production and subsequent oxidative cell damage (Maruszak and Żekanowski, 2011). Additionally, variants of gene expression profiles in AD have shown downregulated expression of NeuroD6, which encodes a transcription factor involved in triggering antioxidant responses and the maintenance of the production of mitochondrial antioxidants (Uittenbogaard et al., 2010; Fowler et al., 2015). Also, RNA-Seq and microarray data analysis indicated a consistent downregulation of NeuroD6 in brains of individuals with AD, suggesting downregulation of NeuroD6 as a possible biomarker for AD (Satoh et al., 2014).
The role of ROS and OS in neurodegenerative diseases, including AD, is not entirely clear, although it has been observed that a modest level of oxidative RNA damage occurs during the process of aging in brain neurons, but a prominent level of oxidative RNA damage is present in vulnerable neurons which correspond to the earliest stage of cognitive decline in the transition from cognitively normal aging to AD (Nunomura et al., 2012). Furthermore, DNA bases are vulnerable to OS damage involving hydroxylation, protein carbonylation, and nitration in AD (García-Blanco et al., 2017). Changes in oxidative markers have been reported in brain regions such as hippocampus and inferior parietal cortex, which are also compromised in AD (Floyd and Hensley, 2002).
Brain is considered to be especially vulnerable to OS and susceptible to lipid peroxidation because of its high lipid and poly-unsaturated fatty acids content, and its low concentrations of antioxidants (Butterfield et al., 2013). In AD, neurotoxic effects of Aβ induce OS through lipid peroxidation, protein degradation, and amino acid oxidation, which in turn increase the production of ROS and RNS by positive feedback (Swomley and Butterfield, 2015). Alkenals, 4-hydroxynonenal (HNE), and 2-propenal (acrolein) are reactive products obtained from lipid peroxidation induced by Aβ. These agents can modify covalently some amino acids residues or change protein conformation, which in turn affects its function. Thus, a coupling between increased lipid peroxidation and structural modification of GLT-1 has been proposed, explained by increased HNE binding due to excessive Aβ1-42 (Butterfield et al., 2002). These events can compromise astrocyte function, inducing glutamate transport inhibition and increasing excitotoxicity to neurons in AD.
Astroglia Role in Oxidative Stress
Astrocytes seem to be involved in the processes leading to the appearance or maintenance of OS in AD. Abramov et al. (2004a), working in rat astrocytes, have shown that Aβ treatment increases intracellular-free calcium influx from the extracellular space, and induces changes in mitochondrial functions. These changes are associated with the activation of NOX due to Aβ interaction on the membrane, which, in turn, induces [Ca2+]i changes. The main changes leading to mitochondrial dysfunction in astrocytes are associated with mitochondrial depolarization, increased conductance, and presence of mitochondrial permeability transition pores (Duchen, 2000). A possible mechanism which explains why calcium influx is induced by Aβ into astrocytes could be related with the formation of calcium selective channels on the membrane, which seem capable of generating a different conductance (Abramov et al., 2004b). These channels have been shown to be formed by insertion of Aβ peptides in the membrane, and also are arranged in a structural configuration which requires a lesser content of cholesterol on the lipidic membrane (Kawahara and Kuroda, 2001; Arispe and Doh, 2002; Arispe et al., 2007).
Additionally, it has been found that Aβ1-42 oligomers are key factors on the induction of OS stress by astrocytes. Aβ1-42 oligomers binding to RAGE on astrocytes induce ROS production via NOX complex activation (Askarova et al., 2011). However, astrocytes are also able to trigger ERK1/2 pathways and cytosolic phospholipase A2 phosphorylation, independent of NOX activation, which in turn causes mitochondrial dysfunction by decreasing mitochondrial membrane potential, enhancement of NOX activity, and overproduction of ROS (Zhu et al., 2006). Astrocytes seem to be a primary target of Aβ, as this peptide induces various effects related to OS such as altered intracellular calcium signaling and calcium-dependent reduction in astrocytic GSH (Abramov et al., 2004b). Although this GSH depletion affects astrocytes, neurons are the cells that show a higher rate of cell death, suggesting that neurotoxicity reflects the neuronal dependence on astrocytes for antioxidant support. This could better be explained by the fact that astrocytes are the producers of the primary elements required for the production of GSH in neurons, such as glycine and cysteine (Abramov et al., 2003; Gandhi and Abramov, 2012). Regarding GSH, it has also been shown that in cultured astrocytes, a prolonged incubation with Aβ reduces induction of the transporter ABCC1 which is the main pathway for GSH release (Ye et al., 2015). Astrocytes play a central role in maintaining the neuronal integrity, nevertheless cytokines and neurotransmitters released by damaged or activated astrocytes may increase the neurotoxicity and vulnerability of neurons. During chronic OS, as observed in AD, the crosstalk communication between astrocytes and neurons is impaired resulting in disrupted memory consolidation. This compromise in memory formation is probably due to calcium overload and activation of MAPK pathways in astrocytes, which involve as well the JNK/SAPK pathways, and may conduce to anomalous deleterious signaling, including autophagic astroglial signals and apoptosis (Ishii et al., 2017).
Oxidative Stress and the Connection with Neuroinflammation
During neuroinflammation, increased concentrations of ROS/RNS may lead to the activation of the transcription factor NF-κB which induces the overexpression of NO synthases in astrocytes and microglia, in particular NOX2 and iNOS, resulting in peroxynitrite production by superoxide and NO reaction producing neuronal damage (Saha and Pahan, 2006; Brown, 2007; Morgan and Liu, 2011). Moreover, NF-κB activation induces the expression of COX-2 and cytosolic phospholipase A2, which in turn stimulate the generation of prostaglandins, promoting inflammation and OS (Hsieh and Yang, 2013). Castegna et al. (2003) reported that the formation of peroxynitrite ONOO- leads to protein nitration in enzymes, such as alpha and gamma enolases, implicated in brain glucose metabolism. Thus, the signaling pathway NF-κB, which is also heavily involved in inflammatory reactions, has been proposed to be involved in OS, as a direct crosstalk between ROS and NF-κB has been reported (Turillazzi et al., 2016). In AD, the presence of chronic OS alters the protective physiological role of the NF-κB transcription pathway, which normally promotes cell survival and prevents apoptosis and necrosis, through modulation of the JNK signaling pathway (Morgan and Liu, 2011). In astrocytes, it was reported that under certain conditions, IL-1β may act stimulating astrocytic GSH production, and potentially, augmenting total antioxidant capacity in the brain, via an NF-κB-dependent process (He et al., 2015). In this way, NF-κB pathway has been associated with both pro-oxidant and antioxidant roles. In AD, an alteration of this pro- and anti-oxidant role of NF-κB in astrocytes seems to be present, tending toward a pervasive pro-oxidative and pro-inflammatory profile (Table 2).
Table 2.
Summary of studies showing astrocytic-induced OS in several models of AD.
| Experimental model | Prooxidative | Antioxidative | Reference |
|---|---|---|---|
| Isolated cortical astrocytes; hippocampal neurons and astrocytes in coculture, and preparations of astrocytes from rat hippocampus (Aβ25-35, Aβ1-42). | ↑NOX; mitochondrial depolarization; ↑ROS | ↓GSH | Abramov et al., 2004a |
| Primary culture of cortical rat astrocytes (Aβ1-42). | ↑NOX; ↑ROS; mitochondrial depolarization; ↑iNOS | ↓Cu/Zn SOD; =MnSOD | Hu et al., 1998; Zhu et al., 2006; Askarova et al., 2011; Bungart et al., 2014; Aguirre-Rueda et al., 2015 |
| Mixed cultures of rat hippocampal neurons and astrocytes. Isolated cortical astrocytes (Aβ25-35, Aβ1-42). | ↓GSH | Abramov et al., 2003 | |
| Cultured cortical rat astrocytes (fibrillar, oligomeric, and scrambled Aβ1-42). | ↑iNOS and NO-derived peroxynitrite | Akama et al., 1998; Hu et al., 1998 | |
| Postmortem brains from AD patients. APP23 transgenic mice. APP induced by electrolytic cortical lesion. | ↑eNOS and iNOS | Lüth et al., 2001 | |
| AD postmortem brains. nNOS immunocytochemistry in reactive astrocytes. | ↑nNOS | Simic et al., 2000 | |
| Primary astrocyte culture derived from C57BL/6 (Aβ1-42) and derived from 5xFAD mice. | Acute ↑GSH after monomeric Aβ. Induction of ABCC1 was reduced in 6-month old 5xFAD mice. | Ye et al., 2015 | |
| Intrahippocampal injection of Aβ25-35. GFAP measured by ELISA in rats. | =iNOS | ↓Catalase | Sohanaki et al., 2016 |
| Cultured cortical rat astrocytes (Aβ25-35). | ↑NOX | ↓Catalase; ↓SOD, ↓GSH; ↑GST | Jeong et al., 2005 |
| Isolated rat astrocyte cultures (Aβ25-35). | ↑iNOS; ↑ROS; ↑RNS | ↑MnSOD | Ayasolla et al., 2004 |
| Mixed cultures of mice neurons and astrocytes. Isolated mice astrocytes (Aβ25-35; Aβ1-42). | ↑ROS; ↑RNS | ↓SOD; ↓GSH; ↓Catalase | Masilamoni et al., 2005b |
| AD postmortem human cerebral cortex and hippocampus. Immunocytochemistry for GFAP. | ↑MnSOD; ↓Cu/Zn SOD | Furuta et al., 1995; Maeda et al., 1997 |
ROS, reactive oxygen species; RNS, reactive nitrogen species; iNOS, inducible nitric oxide synthase; eNOS, endothelial nitric oxide synthase; nNOS, neuronal form of nitric oxide synthase; GSH, glutathione, GST, glutathione-S-transferase; GFAP, glial fibrillary acidic protein.
Novel anti-Alzheimer’s drugs will need to consider the selective modulation of astrocyte activity in order to reduce pro-inflammatory signaling as well as to attenuate OS and diminish excitotoxicity (Figure 1). Taking into account the complex physiopathology of AD, a deep knowledge about dysfunctional astrocyte intracellular pathways evoked by Aβ opens the possibility for the design of new effective multi-target directed drugs.
FIGURE 1.
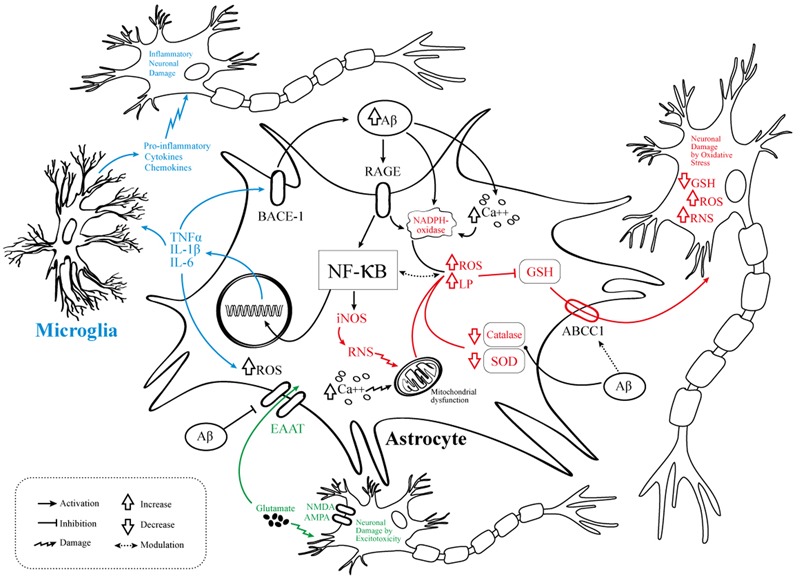
Pathophysiological events involving astrocytes in AD. Schematic representation of the molecular mechanisms linking the NF-κB pathway activation to AD pathogenesis. OS, abnormal neuroinflammatory response, and excitotoxic neuronal damage are related to several pathways of astrocyte dysfunction. In black are the elements common to the three mechanisms, namely the Aβ/RAGE/NF-kB interaction. In blue are the elements related to neuroinflammation. In red the elements related to OS. In green the elements related to neurotoxicity/excitotoxicity. Aβ, amyloid-beta; RAGE, receptor for advanced glycation products; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; iNOS, inducible nitric oxide synthase; RNS, reactive nitrogen species, ROS, reactive oxygen species; LP, lipid peroxidation; TNFα, tumor necrosis factor alpha; IL, interleukin; GSH, glutathione; SOD, superoxide dismutase; EAAT, excitatory amino acid transporter; BACE1, beta-secretase 1; NMDA, N-methyl-D-aspartate receptor; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NADPH oxidase, nicotinamide adenine dinucleotide phosphate oxidase; Ca++, calcium; ABCC1, ATP-binding cassette subfamily C member 1.
Novel Neuroprotective and Therapeutic Measures in Alzheimer’s Disease
Alzheimer’s disease is a major neurodegenerative disease affecting millions worldwide without a known curative treatment. Currently, only five drugs have been approved by the Food and Drug Administration (FDA) of the United States, three cholinesterase inhibitors (donepezil, galantamine, and rivastigmine), an NMDA receptor antagonist (memantine) and a combined donepezil–memantine drug. The cholinesterase inhibitors are approved for symptomatic treatment of mild-to-moderate stages of AD, while the NMDA antagonist is used for moderate-to-late stages. Regrettably none of these drugs is able to halt the progression of the disease and its uses are aimed at maximizing the quality of life of patients though broad symptom management (Caselli et al., 2017). Is therefore of great importance to design and develop new treatments which offer better therapeutic outcomes and disease-modifying responses to the patients with AD. Epidemiologic studies indicated that prolonged treatment with anti-inflammatory agents such as non-steroidal anti-inflammatory drugs could delay AD onset, as well as reduce disease rate progression (McGeer et al., 1990; Pasinetti, 2002; Cudaback et al., 2014). The inhibition of COX-mediated signaling pathways may reduce some inflammatory cytokines related with the physiopathology of AD. In addition, human studies confirm that OS plays a main role in the physiopathology of AD (Schrag et al., 2013; Chang et al., 2014). However, there are some controversies between observational studies and randomized controlled trials about the efficiency of antioxidative agents and anti-inflammatory drugs to reduce AD risk (Viña et al., 2004; Cudaback et al., 2014; Wang et al., 2015; Jiang et al., 2016). Many research groups and pharmaceutical companies have been developing new strategies to overcome the disease, but so far none of the Aβ-targeted phase three clinical trials reported has shown statistically significant benefit on its pre-specified clinical endpoints (Selkoe and Hardy, 2016). Many explanations may be offered for this lack of success, ranging from poorly designed trials to late interventions (irreversible modification of the disease due to advanced stage) but also, to the incomplete knowledge about the basic pathophysiological mechanisms of AD. As astrocytes have been shown to be involved in a diverse range of pathological changes observed in AD, they have been proposed as an interesting novel therapeutic target (Finsterwald et al., 2015). Because increased proinflammatory cytokines induced by Aβ are associated with enhanced production of free radicals in the astrocytes (Masilamoni et al., 2005a), new compounds with antioxidative and anti-inflammatory properties could reduce the effects of neurodegeneration in AD.
Various approaches involving astrocytes have been reported recently and some of them offer promising results in in vitro, animal, and preclinical models for the treatment of AD. In primary cortical rat astrocytes, the use of a light-generating nanoparticle attenuated Aβ-induced OS and inflammatory responses, through a reduction in the superoxide anion production and a lowering of IL-1β and iNOS expression (Bungart et al., 2014). Curcumin, a natural phenol obtained from plants and commonly used as a spice, has been proposed to be of benefit in AD, reducing Aβ formation and decreasing neurotoxicity in the brain (Lim et al., 2001; Yang et al., 2005; Cole et al., 2007). In a recent study using APP/PS1 transgenic mice and primary rat mixed neuronal/glial cultures, curcumin was reported to improve spatial memory deficits and promote cholinergic neuronal function in vivo, and in vitro, attenuated the inflammatory response of both microglia and astrocytes, acting through PPARγ, which inhibited the NF-κB signaling pathway in these cells (Liu et al., 2016). Activation of PPARγ with the use of the isoflavone phytoestrogen genistein showed an increase in the release of ApoE from primary astrocytes in an in vivo mouse model of AD (Bonet-Costa et al., 2016). In the same paper, the authors reported that treatment with genistein improved several cognitive features (hippocampal learning, recognition memory, implicit memory, and odor discrimination) as well as a reduction in the number and area of Aβ plaques. Neuroesteroids, such as progesterone, have been proposed to offer neuroprotection in neurodegenerative diseases including AD (Liu et al., 2013). In primary cultures of rat astrocytes, treatment with progesterone reduced Aβ-induced inflammatory responses (decreasing the production of IL-1β and TNFα), and also suppressed endoplasmic reticulum stress activation together with attenuation of PERK/elF2a signaling (Hong et al., 2016). In addition to polyphenols, many other natural phytochemicals have shown anti-inflammatory and immunosuppressive efficacy in AD models. For example, triptolide extract inhibit activation of microglia and astrocytes in the APP/PS1 transgenic mouse model of AD (Li et al., 2016). Punicalagin, a compound derived from pomegranate, not only may reduce neuroinflammation (lowering TNFα and ILβ) but also prevents OS through the reduction of iNOS, COX-2, and the production of ROS (Kim et al., 2017).
Other compounds with anti-amyloidogenic, antioxidative, and anti-inflammatory effects may have a potential role against dementia (Libro et al., 2016). For instance, the cannabinoid agonist WIN 55,212-2 has shown anti-inflammatory actions in primary cultured astrocytes after Aβ1-42 exposure. WIN pre-treatment prevented IL-1β, TNFα, and iNOS increase induced by Aβ. In addition, pretreatment with WIN prevented a decrease in copper/zinc-SOD induced by Aβ1-42 (Aguirre-Rueda et al., 2015). Cannabinoid receptor type 2 (CB2) agonist (MDA7) also reduced inflammation and also promoted clearance of amyloid plaques in the transgenic APP/PS1 mice model of AD (Wu et al., 2017). Astroglial hemichannel activity and inflammatory reactions evoked by Aβ25-35 were prevented by several cannabinoid receptor agonists such as WIN, 2-AG, and methanandamide (Gajardo-Gómez et al., 2017). Pantethine (B5 vitamine precursor) was able to modulate the astrocytic metabolic changes and inflammatory patterns induced by Aβ1-42 in astrocytes derived from the 5xFAD transgenic mouse model of AD (van Gijsel-Bonnello et al., 2017). In cultured cortical astrocytes, donepezil was shown to reduce inflammatory responses via nAChR and PI3K-Akt pathway, and to decrease intracellular ROS levels (Makitani et al., 2017). As mentioned early, donepezil is a cholinesterase inhibitor commonly used in AD patients.
Other strategies to reduce OS in AD models involve enhancers of antioxidative endogenous factors. For instance, pelargonidin (estrogen receptor agonist) increases catalase activity, reduces astrocyte activation in the hippocampus after Aβ25-35 exposure, and also prevents Aβ-induced spatial memory impairment (Sohanaki et al., 2016). Bambusae concretio Salicea treatment increases GSH-S-transferase and prevented catalase, SOD, GPX reduction, induced by Aβ25-35 in cultured astrocyte cells (Jeong et al., 2005). The novel compound Monascin is able to activate the expression of several antioxidative genes such as SOD-1, SOD-2, SOD-3, and Hsp16.2, and as a consequence reduced Aβ toxicity (Shi et al., 2016). In vitro and in vivo studies with exogenous antioxidative compounds such as resveratrol (Wang G. et al., 2016), tocotrienol (vitamin E form) (Ibrahim et al., 2017), anthocyanins (Rehman et al., 2017), and epicatechin (Cuevas et al., 2009) have shown beneficial effects in AD models. Finally, 3H-1,2-dithiole-3-thione, a potent free radical scavenger, is able to reduce ROS production in the AD cellular model N2a/APPswe (Wang et al., 2017).
Conclusion
Neuroinflammation and OS are part of the functional changes frequently observed in the brains of individuals with AD. Aβ has been shown to alter the normal dynamics of both inflammatory and antioxidant and prooxidant balance, promoting an unhealthy state for the brain and neuronal–glial communication networks. Astrocytes are involved in both inflammation and OS regulation in the CNS, and seem to have a central role in the basic pathophysiological aspects that surround this neurodegenerative disease. Although the precise relation between neuroinflammation, OS, astrocytes, and AD is still not clear, the evidence points toward an important participative role of the Aβ/NF-κB interaction in astrocytes as a critical agent in the pathological mechanism of AD. Despite the continuous efforts to develop a successful treatment for AD, there is still a gap in the knowledge of the precise etiological aspects of this disease which difficult the advance of therapeutics. Therefore, and due to the evidence presented in this review, is important to start considering astrocytes as a valuable novel therapeutic and neuroprotective target for future studies related to the treatment and mechanistic comprehension of AD.
Author Contributions
RG-R conceived the review paper. RG-R, MN-M, and KV-S edited the final version of the manuscript. RG-R wrote the section: Introduction. RG-R and MN-M wrote the section: Astrocytes and Alzheimer’s Disease. RG-R and KV-S wrote the section: Neuroinflammation, Alzheimer’s Disease, and Astrocytes. KV-S, DA-S, and LM-M wrote the section: Oxidative Stress, Alzheimer’s Disease, and Astrocytes. MN-M wrote the section: Novel Neuroprotective and Therapeutic Measures in Alzheimer’s Disease. RG-R, MN-M, DA-S, and LM-M were involved in the construction of the figure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer ML and handling Editor declared their shared affiliation.
Abbreviations
- Aβ
amyloid beta
- AD
Alzheimer’s disease
- AGE
advanced glycation end products
- Aldh1L1
aldehyde dehydrogenase 1 family member L1
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APP
amyloid precursor protein
- ApoE
apolipoprotein E
- BACE-1
beta-site APP cleaving enzyme 1
- BBB
blood–brain barrier
- CaN
calcineurin
- CaSR
calcium sensing receptor
- CD
cluster of differentiation
- CNS
central nervous system
- COX
cyclooxygenase
- ECE
endothelin-converting enzyme
- ERK
extracellular regulated kinase
- GABA
gamma-aminobutyric acid
- GFAP
glial fibrillary acid protein
- GLAST
glutamate aspartate transporter
- GLT-1
glutamate transporter 1
- GPX
glutathione peroxidase
- GSH
glutathione
- HMGB1
high mobility group box-1
- IDE
insulin degrading enzyme
- IKBα
NF-κB inhibitor alpha
- IKK
IκB kinase
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IR
insulin receptor
- INF
interferon
- JNK
c-Jun N-terminal kinase
- MAO
monoamine oxidase
- MAPK
mitogen-activated protein kinase
- MMP
matrix metalloproteinase
- nAChR
nicotinic acetylcholine receptor
- NADH
reduced nicotinamide-adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NEMO
NF-κB essential modulator
- NEP
neprilysin
- NFAT
nuclear factor of activated T-cells
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthase
- 8-OHG
8-hydroxyguanosine
- OS
oxidative stress
- PI3K
phosphoinositide 3-kinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- RAGE
advanced glycation end products receptor
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SEC-R
serpin-enzyme complex receptor
- SOD
superoxide dismutase
- TGF-β
transforming growth factor beta
- TLR
Toll-like receptor
- TNFα
tumoral necrosis factor-alpha
- TRAF
tumor necrosis receptor-associated factor
- TREM2
triggering receptor expressed on myeloid cells 2
Footnotes
Funding. The review was funded in part by Universidad del Rosario.
References
- Abbas N., Bednar I., Mix E., Marie S., Paterson D., Ljungberg A., et al. (2002). Up-regulation of the inflammatory cytokines IFN-gamma and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP(SWE) transgenic mice. J. Neuroimmunol. 126 50–57. [DOI] [PubMed] [Google Scholar]
- Abdul H. M., Sama M. A., Furman J. L., Mathis D. M., Beckett T. L., Weidner A. M., et al. (2009). Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 29 12957–12969. 10.1523/JNEUROSCI.1064-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov A. Y., Canevari L., Duchen M. R. (2003). Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J. Neurosci. 23 5088–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov A. Y., Canevari L., Duchen M. R. (2004a). Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 24 565–575. 10.1523/JNEUROSCI.4042-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramov A. Y., Canevari L., Duchen M. R. (2004b). Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta 1742 81–87. 10.1016/j.bbamcr.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Acosta C., Anderson H. D., Anderson C. M. (2017). Astrocyte dysfunction in Alzheimer disease. J. Neurosci. Res. 95 2430–2447. 10.1002/jnr.24075 [DOI] [PubMed] [Google Scholar]
- Aguirre-Rueda D., Guerra-Ojeda S., Aldasoro M., Iradi A., Obrador E., Mauricio M. D., et al. (2015). WIN 55,212-2, agonist of cannabinoid receptors, prevents amyloid β1-42 effects on astrocytes in primary culture. PLOS ONE 10:e0122843. 10.1371/journal.pone.0122843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K., Shelton C. C., Tian Y., Zhang X., Gilchrist M. L., Sisodia S. S., et al. (2010). Activation and intrinsic gamma-secretase activity of presenilin 1. Proc. Natl. Acad. Sci. U.S.A. 107 21435–21440. 10.1073/pnas.1013246107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen P. S., Thal L. J., Ferris S. H., Assaid C., Nessly M. L., Giuliani M. J., et al. (2008). Rofecoxib in patients with mild cognitive impairment: further analyses of data from a randomized, double-blind, trial. Curr. Alzheimer Res. 5 73–82. [DOI] [PubMed] [Google Scholar]
- Akama K. T., Albanese C., Pestell R. G., Van Eldik L. J. (1998). Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc. Natl. Acad. Sci. U.S.A. 95 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama K. T., Van Eldik L. J. (2000). Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 275 7918–7924. [DOI] [PubMed] [Google Scholar]
- Alberdi E., Wyssenbach A., Alberdi M., Sánchez-Gómez M. V., Cavaliere F., Rodríguez J. J., et al. (2013). Ca2+-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12 292–302. 10.1111/acel.12054 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease Anti-inflammatory Prevention Trial Research Group (2013). Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT). Alzheimers Dement. 9 714–723. 10.1016/j.jalz.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M. A., Scheff S. W. (2010). Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 69 155–167. 10.1097/NEN.0b013e3181cb5af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelt J., Schliebs R. (2001). Beta-amyloid-induced glial expression of both pro- and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 894 21–30. [DOI] [PubMed] [Google Scholar]
- Arakawa R., Stenkrona P., Takano A., Nag S., Maior R. S., Halldin C. (2017). Test-retest reproducibility of [11C]-L-deprenyl-D2 binding to MAO-B in the human brain. EJNMMI Res. 7:54. 10.1186/s13550-017-0301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Parpura V., Sanzgiri R. P., Haydon P. G. (1998). Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur. J. Neurosci. 10 2129–2142. [DOI] [PubMed] [Google Scholar]
- Araque A., Parpura V., Sanzgiri R. P., Haydon P. G. (1999). Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 22 208–215. [DOI] [PubMed] [Google Scholar]
- Arispe N., Diaz J. C., Simakova O. (2007). Abeta ion channels. Prospects for treating Alzheimer’s disease with Abeta channel blockers. Biochim. Biophys. Acta 1768 1952–1965. 10.1016/j.bbamem.2007.03.014 [DOI] [PubMed] [Google Scholar]
- Arispe N., Doh M. (2002). Plasma membrane cholesterol controls the cytotoxicity of Alzheimer’s disease AbetaP (1-40) and (1-42) peptides. FASEB J. 16 1526–1536. 10.1096/fj.02-0829com [DOI] [PubMed] [Google Scholar]
- Askarova S., Yang X., Sheng W., Sun G. Y., Lee J. C.-M. (2011). Role of Aβ-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A2 activation in astrocytes and cerebral endothelial cells. Neuroscience 199 375–385. 10.1016/j.neuroscience.2011.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasolla K., Khan M., Singh A. K., Singh I. (2004). Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic. Biol. Med. 37 325–338. 10.1016/j.freeradbiomed.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Bahniwal M., Little J. P., Klegeris A. (2017). High glucose enhances neurotoxicity and inflammatory cytokine secretion by stimulated human astrocytes. Curr. Alzheimer Res. 14 731–741. 10.2174/1567205014666170117104053 [DOI] [PubMed] [Google Scholar]
- Bak L. K., Schousboe A., Waagepetersen H. S. (2006). The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 98 641–653. 10.1111/j.1471-4159.2006.03913.x [DOI] [PubMed] [Google Scholar]
- Bales K. R., Du Y., Dodel R. C., Yan G. M., Hamilton-Byrd E., Paul S. M. (1998). The NF-kappaB/Rel family of proteins mediates Abeta-induced neurotoxicity and glial activation. Brain Res. Mol. Brain Res. 57 63–72. [DOI] [PubMed] [Google Scholar]
- Batarseh Y. S., Duong Q.-V., Mousa Y. M., Al Rihani S. B., Elfakhri K., Kaddoumi A. (2016). Amyloid-β and astrocytes interplay in amyloid-β related disorders. Int. J. Mol. Sci. 17:338. 10.3390/ijms17030338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeri M. S., Schmeidler J., Lesser G. T., Maroukian M., West R., Leung S., et al. (2012). Corticosteroids, but not NSAIDs, are associated with less Alzheimer neuropathology. Neurobiol. Aging 33 1258–1264. 10.1016/j.neurobiolaging.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L., Rowitch D. H. (2017). Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci. 18 31–41. 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., et al. (2015). Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74 101–110. 10.1016/j.biopha.2015.07.025 [DOI] [PubMed] [Google Scholar]
- Blasko I., Veerhuis R., Stampfer-Kountchev M., Saurwein-Teissl M., Eikelenboom P., Grubeck-Loebenstein B. (2000). Costimulatory effects of interferon-gamma and interleukin-1beta or tumor necrosis factor alpha on the synthesis of Abeta1-40 and Abeta1-42 by human astrocytes. Neurobiol. Dis. 7 682–689. 10.1006/nbdi.2000.0321 [DOI] [PubMed] [Google Scholar]
- Bonet-Costa V., Herranz-Pérez V., Blanco-Gandía M., Mas-Bargues C., Inglés M., Garcia-Tarraga P., et al. (2016). Clearing amyloid-β through PPARγ/ApoE activation by genistein is a treatment of experimental Alzheimer’s disease. J. Alzheimers Dis. 51 701–711. 10.3233/JAD-151020 [DOI] [PubMed] [Google Scholar]
- Bouvier D. S., Jones E. V., Quesseveur G., Davoli M. A., A Ferreira T., Quirion R., et al. (2016). High resolution dissection of reactive glial nets in Alzheimer’s disease. Sci. Rep. 6:24544. 10.1038/srep24544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzuoli M. R., Iacomino A., Steardo L., Scuderi C. (2016). Targeting neuroinflammation in Alzheimer’s disease. J. Inflamm. Res. 9 199–208. 10.2147/JIR.S86958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. (2007). Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 35 1119–1121. 10.1042/BST0351119 [DOI] [PubMed] [Google Scholar]
- Bungart B. L., Dong L., Sobek D., Sun G. Y., Yao G., Lee J. C.-M. (2014). Nanoparticle-emitted light attenuates amyloid-β-induced superoxide and inflammation in astrocytes. Nanomedicine 10 15–17. 10.1016/j.nano.2013.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield D. A., Castegna A., Lauderback C. M., Drake J. (2002). Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 23 655–664. [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Swomley A. M., Sultana R. (2013). Amyloid β-peptide (1–42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid. Redox Signal. 19 823–835. 10.1089/ars.2012.5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero I., Gonzalo M. R., Martin B., Sanz-Anquela J. M., Arévalo-Serrano J., Gonzalo-Ruiz A. (2012). Oligomers of β-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B mechanism in the rat brain. Exp. Neurol. 236 215–227. 10.1016/j.expneurol.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Carroll C. M., Li Y.-M. (2016). Physiological and pathological roles of the γ-secretase complex. Brain Res. Bull. 126 199–206. 10.1016/j.brainresbull.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. F., Schöll M., Almkvist O., Wall A., Engler H., Långström B., et al. (2012). Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J. Nucl. Med. 53 37–46. 10.2967/jnumed.110.087031 [DOI] [PubMed] [Google Scholar]
- Caselli R. J., Beach T. G., Knopman D. S., Graff-Radford N. R. (2017). Alzheimer disease: scientific breakthroughs and translational challenges. Mayo Clin. Proc. 92 978–994. 10.1016/j.mayocp.2017.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castegna A., Thongboonkerd V., Klein J. B., Lynn B., Markesbery W. R., Butterfield D. A. (2003). Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J. Neurochem. 85 1394–1401. [DOI] [PubMed] [Google Scholar]
- Chakraborty A., de Wit N. M., van der Flier W. M., de Vries H. E. (2017). The blood brain barrier in Alzheimer’s disease. Vasc. Pharmacol. 89 12–18. 10.1016/j.vph.2016.11.008 [DOI] [PubMed] [Google Scholar]
- Chang Y.-T., Chang W.-N., Tsai N.-W., Huang C.-C., Kung C.-T., Su Y.-J., et al. (2014). The roles of biomarkers of oxidative stress and antioxidant in Alzheimer’s disease: a systematic review. Biomed Res. Int. 2014:182303. 10.1155/2014/182303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini A., Armato U., Gardenal E., Gui L., Dal Prà I. (2017). Amyloid β-exposed human astrocytes overproduce phospho-tau and overrelease it within exosomes, effects suppressed by calcilytic NPS 2143-further implications for Alzheimer’s therapy. Front. Neurosci. 11:217 10.3389/fnins.2017.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. S., Lee H. J., Lim I., Satoh J., Kim S. U. (2014). Human astrocytes: secretome profiles of cytokines and chemokines. PLOS ONE 9:e92325. 10.1371/journal.pone.0092325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo C., Capoccia E., Iuvone T., Cuomo R., Sarnelli G., Steardo L., et al. (2015). S100B inhibitor pentamidine attenuates reactive gliosis and reduces neuronal loss in a mouse model of Alzheimer’s disease. Biomed Res. Int. 2015:508342. 10.1155/2015/508342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gadol A. A., Pan J. W., Kim J. H., Spencer D. D., Hetherington H. H. (2004). Mesial temporal lobe epilepsy: a proton magnetic resonance spectroscopy study and a histopathological analysis. J. Neurosurg. 101 613–620. 10.3171/jns.2004.101.4.0613 [DOI] [PubMed] [Google Scholar]
- Cole G. M., Teter B., Frautschy S. A. (2007). Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 595 197–212. 10.1007/978-0-387-46401-5_8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E., Farina C. (2016). Astrocytes: key regulators of neuroinflammation. Trends Immunol. 37 608–620. 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Cudaback E., Jorstad N. L., Yang Y., Montine T. J., Keene C. D. (2014). Therapeutic implications of the prostaglandin pathway in Alzheimer’s disease. Biochem. Pharmacol. 88 565–572. 10.1016/j.bcp.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas E., Limón D., Pérez-Severiano F., Díaz A., Ortega L., Zenteno E., et al. (2009). Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur. J. Pharmacol. 616 122–127. 10.1016/j.ejphar.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Dá Mesquita S., Ferreira A. C., Sousa J. C., Correia-Neves M., Sousa N., Marques F. (2016). Insights on the pathophysiology of Alzheimer’s disease: the crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 68 547–562. 10.1016/j.neubiorev.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Dabir D. V., Trojanowski J. Q., Richter-Landsberg C., Lee V. M.-Y., Forman M. S. (2004). Expression of the small heat-shock protein alphaB-crystallin in tauopathies with glial pathology. Am. J. Pathol. 164 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Prà I., Chiarini A., Armato U. (2015). Antagonizing amyloid-β/calcium-sensing receptor signaling in human astrocytes and neurons: a key to halt Alzheimer’s disease progression? Neural Regen. Res. 10 213–218. 10.4103/1673-5374.152373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasuri K., Zhang L., Keller J. N. (2013). Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 62 170–185. 10.1016/j.freeradbiomed.2012.09.016 [DOI] [PubMed] [Google Scholar]
- de Vivo L., Melone M., Rothstein J. D., Conti F. (2010). GLT-1 promoter activity in astrocytes and neurons of mouse hippocampus and somatic sensory cortex. Front. Neuroanat. 3:31. 10.3389/neuro.05.031.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bo R., Angeretti N., Lucca E., De Simoni M. G., Forloni G. (1995). Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and beta-amyloid production in cultures. Neurosci. Lett. 188 70–74. [DOI] [PubMed] [Google Scholar]
- Delekate A., Füchtemeier M., Schumacher T., Ulbrich C., Foddis M., Petzold G. C. (2014). Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 5:5422. 10.1038/ncomms6422 [DOI] [PubMed] [Google Scholar]
- Desgranges B., Baron J. C., de la Sayette V., Petit-Taboué M. C., Benali K., Landeau B., et al. (1998). The neural substrates of memory systems impairment in Alzheimer’s disease. A PET study of resting brain glucose utilization. Brain 121(Pt 4) 611–631. [DOI] [PubMed] [Google Scholar]
- Ding Q., Keller J. N. (2005). Evaluation of rage isoforms, ligands, and signaling in the brain. Biochim. Biophys. Acta 1746 18–27. 10.1016/j.bbamcr.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Ding S., Fellin T., Zhu Y., Lee S.-Y., Auberson Y. P., Meaney D. F., et al. (2007). Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus. J. Neurosci. 27 10674–10684. 10.1523/JNEUROSCI.2001-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz L. P., Tortelli V., Matias I., Morgado J., Bérgamo Araujo A. P., Melo H. M., et al. (2017). Astrocyte transforming growth factor Beta 1 protects synapses against Aβ oligomers in Alzheimer’s disease model. J. Neurosci. 37 6797–6809. 10.1523/JNEUROSCI.3351-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey E., Bamji-Mirza M., Najem D., Li Y., Liu H., Callaghan D., et al. (2017). Apolipoprotein E isoforms differentially regulate Alzheimer’s disease and amyloid-β-induced inflammatory response in vivo and in vitro. J. Alzheimers Dis. 57 1265–1279. 10.3233/JAD-160133 [DOI] [PubMed] [Google Scholar]
- Dorfman V. B., Pasquini L., Riudavets M., López-Costa J. J., Villegas A., Troncoso J. C., et al. (2010). Differential cerebral deposition of IDE and NEP in sporadic and familial Alzheimer’s disease. Neurobiol. Aging 31 1743–1757. 10.1016/j.neurobiolaging.2008.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. (2000). Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 529(Pt 1) 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverria V., Yarkov A., Aliev G. (2016). Positive modulators of the α7 nicotinic receptor against neuroinflammation and cognitive impairment in Alzheimer’s disease. Prog. Neurobiol. 144 142–157. 10.1016/j.pneurobio.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Ekblom J., Jossan S. S., Bergström M., Oreland L., Walum E., Aquilonius S. M. (1993). Monoamine oxidase-B in astrocytes. Glia 8 122–132. 10.1002/glia.440080208 [DOI] [PubMed] [Google Scholar]
- Ekblom J., Jossan S. S., Oreland L., Walum E., Aquilonius S. M. (1994). Reactive gliosis and monoamine oxidase B. J. Neural Transm. Suppl. 41 253–258. [DOI] [PubMed] [Google Scholar]
- Esler W. P., Wolfe M. S. (2001). A portrait of Alzheimer secretases–new features and familiar faces. Science 293 1449–1454. 10.1126/science.1064638 [DOI] [PubMed] [Google Scholar]
- Falkowska A., Gutowska I., Goschorska M., Nowacki P., Chlubek D., Baranowska-Bosiacka I. (2015). Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci. 16 25959–25981. 10.3390/ijms161125939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfara D., Lifshitz V., Frenkel D. (2008). Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J. Cell. Mol. Med. 12 762–780. 10.1111/j.1582-4934.2008.00314.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T., Pascual O., Gobbo S., Pozzan T., Haydon P. G., Carmignoto G. (2004). Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43 729–743. 10.1016/j.neuron.2004.08.011 [DOI] [PubMed] [Google Scholar]
- Fiandaca M. S., Mapstone M. E., Cheema A. K., Federoff H. J. (2014). The critical need for defining preclinical biomarkers in Alzheimer’s disease. Alzheimers Dement. 10 S196–S212. 10.1016/j.jalz.2014.04.015 [DOI] [PubMed] [Google Scholar]
- Fields R. D., Araque A., Johansen-Berg H., Lim S.-S., Lynch G., Nave K.-A., et al. (2014). Glial biology in learning and cognition. Neuroscientist 20 426–431. 10.1177/1073858413504465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterwald C., Magistretti P. J., Lengacher S. (2015). Astrocytes: new targets for the treatment of neurodegenerative diseases. Curr. Pharm. Des. 21 3570–3581. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Hensley K. (2002). Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 23 795–807. [DOI] [PubMed] [Google Scholar]
- Forman M. S., Lal D., Zhang B., Dabir D. V., Swanson E., Lee V. M.-Y., et al. (2005). Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J. Neurosci. 25 3539–3550. 10.1523/JNEUROSCI.0081-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler K. D., Funt J. M., Artyomov M. N., Zeskind B., Kolitz S. E., Towfic F. (2015). Leveraging existing data sets to generate new insights into Alzheimer’s disease biology in specific patient subsets. Sci. Rep. 5:14324. 10.1038/srep14324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankish H., Horton R. (2017). Prevention and management of dementia: a priority for public health. Lancet 10.1016/S0140-6736(17)31756-7 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Friedland-Leuner K., Stockburger C., Denzer I., Eckert G. P., Müller W. E. (2014). Mitochondrial dysfunction: cause and consequence of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 127 183–210. 10.1016/B978-0-12-394625-6.00007-6 [DOI] [PubMed] [Google Scholar]
- Furuta A., Price D. L., Pardo C. A., Troncoso J. C., Xu Z. S., Taniguchi N., et al. (1995). Localization of superoxide dismutases in Alzheimer’s disease and Down’s syndrome neocortex and hippocampus. Am. J. Pathol. 146 357–367. [PMC free article] [PubMed] [Google Scholar]
- Gajardo-Gómez R., Labra V. C., Maturana C. J., Shoji K. F., Santibañez C. A., Sáez J. C., et al. (2017). Cannabinoids prevent the amyloid β-induced activation of astroglial hemichannels: a neuroprotective mechanism. Glia 65 122–137. 10.1002/glia.23080 [DOI] [PubMed] [Google Scholar]
- Gandhi S., Abramov A. Y. (2012). Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012:428010. 10.1155/2012/428010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Blanco A., Baquero M., Vento M., Gil E., Bataller L., Cháfer-Pericás C. (2017). Potential oxidative stress biomarkers of mild cognitive impairment due to Alzheimer disease. J. Neurol. Sci. 373 295–302. 10.1016/j.jns.2017.01.020 [DOI] [PubMed] [Google Scholar]
- Garwood C. J., Pooler A. M., Atherton J., Hanger D. P., Noble W. (2011). Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2:e167. 10.1038/cddis.2011.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitter B. D., Cox L. M., Rydel R. E., May P. C. (1995). Amyloid beta peptide potentiates cytokine secretion by interleukin-1 beta-activated human astrocytoma cells. Proc. Natl. Acad. Sci. U.S.A. 92 10738–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Reyes R. E., Aliev G., Ávila-Rodrigues M., Barreto G. E. (2016). Alterations in glucose metabolism on cognition: a possible link between diabetes and dementia. Curr. Pharm. Des. 22 812–818. [DOI] [PubMed] [Google Scholar]
- González-Reyes R. E., Graciela Rubiano M. (2016). Astrocyte’s RAGE: more than just a question of mood. Cent. Nerv. Syst. Agents Med. Chem. 10.2174/1871524916999160505105121 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Grolla A. A., Fakhfouri G., Balzaretti G., Marcello E., Gardoni F., Canonico P. L., et al. (2013). Aβ leads to Ca2+ signaling alterations and transcriptional changes in glial cells. Neurobiol. Aging 34 511–522. 10.1016/j.neurobiolaging.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Guerriero F., Sgarlata C., Francis M., Maurizi N., Faragli A., Perna S., et al. (2016). Neuroinflammation, immune system and Alzheimer disease: searching for the missing link. Aging Clin. Exp. Res. 29 821–831. 10.1007/s40520-016-0637-z [DOI] [PubMed] [Google Scholar]
- Gulyás B., Pavlova E., Kása P., Gulya K., Bakota L., Várszegi S., et al. (2011). Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem. Int. 58 60–68. 10.1016/j.neuint.2010.10.013 [DOI] [PubMed] [Google Scholar]
- Halassa M. M., Fellin T., Haydon P. G. (2007). The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13 54–63. 10.1016/j.molmed.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Halliwell B. (2012). Free radicals and antioxidants: updating a personal view. Nutr. Rev. 70 257–265. 10.1111/j.1753-4887.2012.00476.x [DOI] [PubMed] [Google Scholar]
- Haughey N. J., Mattson M. P. (2003). Alzheimer’s amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromol. Med. 3 173–180. [DOI] [PubMed] [Google Scholar]
- He Y., Jackman N. A., Thorn T. L., Vought V. E., Hewett S. J. (2015). Interleukin-1β protects astrocytes against oxidant-induced injury via an NF-κB-dependent upregulation of glutathione synthesis. Glia 63 1568–1580. 10.1002/glia.22828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. (2013). The glutamate-glutamine (GABA) cycle: importance of late postnatal development and potential reciprocal interactions between biosynthesis and degradation. Front. Endocrinol. 4:59. 10.3389/fendo.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., et al. (1999). RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97 889–901. [DOI] [PubMed] [Google Scholar]
- Hong Y., Wang X., Sun S., Xue G., Li J., Hou Y. (2016). Progesterone exerts neuroprotective effects against Aβ-induced neuroinflammation by attenuating ER stress in astrocytes. Int. Immunopharmacol. 33 83–89. 10.1016/j.intimp.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Hori O., Brett J., Slattery T., Cao R., Zhang J., Chen J. X., et al. (1995). The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 270 25752–25761. [DOI] [PubMed] [Google Scholar]
- Hou L., Liu Y., Wang X., Ma H., He J., Zhang Y., et al. (2011). The effects of amyloid-β42 oligomer on the proliferation and activation of astrocytes in vitro. In Vitro Cell. Dev. Biol. Anim. 47 573–580. 10.1007/s11626-011-9439-y [DOI] [PubMed] [Google Scholar]
- Hsieh H.-L., Yang C.-M. (2013). Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res. Int. 2013:e484613. 10.1155/2013/484613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Akama K. T., Krafft G. A., Chromy B. A., Van Eldik L. J. (1998). Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 785 195–206. [DOI] [PubMed] [Google Scholar]
- Huynh R. A., Mohan C. (2017). Alzheimer’s disease: biomarkers in the genome, blood, and cerebrospinal fluid. Front. Neurol. 8:102 10.3389/fneur.2017.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N. F., Yanagisawa D., Durani L. W., Hamezah H. S., Damanhuri H. A., Wan Ngah W. Z., et al. (2017). Tocotrienol-rich fraction modulates amyloid pathology and improves cognitive function in AβPP/PS1 mice. J. Alzheimers Dis. 55 597–612. 10.3233/JAD-160685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J., Morales L., Barreto G. E. (2017). Metabolic and inflammatory adaptation of reactive astrocytes: role of PPARs. Mol. Neurobiol. 54 2518–2538. 10.1007/s12035-016-9833-2 [DOI] [PubMed] [Google Scholar]
- Ishii T., Takanashi Y., Sugita K., Miyazawa M., Yanagihara R., Yasuda K., et al. (2017). Endogenous reactive oxygen species cause astrocyte defects and neuronal dysfunctions in the hippocampus: a new model for aging brain. Aging Cell 16 39–51. 10.1111/acel.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. T. (2017). Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 39 73–82. 10.1080/01616412.2016.1251711 [DOI] [PubMed] [Google Scholar]
- Jarosz-Griffiths H. H., Noble E., Rushworth J. V., Hooper N. M. (2016). Amyloid-β receptors: the good, the bad, and the prion protein. J. Biol. Chem. 291 3174–3183. 10.1074/jbc.R115.702704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J.-C., Yoon C.-H., Lee W.-H., Park K.-K., Chang Y.-C., Choi Y. H., et al. (2005). Effects of Bambusae concretio Salicea (Chunchukhwang) on amyloid beta-induced cell toxicity and antioxidative enzymes in cultured rat neuronal astrocytes. J. Ethnopharmacol. 98 259–266. 10.1016/j.jep.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Jiang T., Sun Q., Chen S. (2016). Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 147 1–19. 10.1016/j.pneurobio.2016.07.005 [DOI] [PubMed] [Google Scholar]
- Jo S., Yarishkin O., Hwang Y. J., Chun Y. E., Park M., Woo D. H., et al. (2014). GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 20 886–896. 10.1038/nm.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. S., Minogue A. M., Connor T. J., Lynch M. A. (2013). Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J. Neuroimmune Pharmacol. 8 301–311. 10.1007/s11481-012-9427-3 [DOI] [PubMed] [Google Scholar]
- Jossan S. S., Gillberg P. G., Gottfries C. G., Karlsson I., Oreland L. (1991). Monoamine oxidase B in brains from patients with Alzheimer’s disease: a biochemical and autoradiographical study. Neuroscience 45 1–12. [DOI] [PubMed] [Google Scholar]
- Jourdain P., Bergersen L. H., Bhaukaurally K., Bezzi P., Santello M., Domercq M., et al. (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 10 331–339. 10.1038/nn1849 [DOI] [PubMed] [Google Scholar]
- Kawahara M., Kuroda Y. (2001). Intracellular calcium changes in neuronal cells induced by Alzheimer’s beta-amyloid protein are blocked by estradiol and cholesterol. Cell. Mol. Neurobiol. 21 1–13. [DOI] [PubMed] [Google Scholar]
- Killin L. O. J., Starr J. M., Shiue I. J., Russ T. C. (2016). Environmental risk factors for dementia: a systematic review. BMC Geriatr. 16:175. 10.1186/s12877-016-0342-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. E., Hwang C. J., Lee H. P., Kim C. S., Son D. J., Ham Y. W., et al. (2017). Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 117 21–32. 10.1016/j.neuropharm.2017.01.025 [DOI] [PubMed] [Google Scholar]
- Kisler K., Nelson A. R., Montagne A., Zlokovic B. V. (2017). Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18 419–434. 10.1038/nrn.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., et al. (2004). Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 10 719–726. 10.1038/nm1058 [DOI] [PubMed] [Google Scholar]
- Kommers T., Rodnight R., Boeck C., Vendite D., Oliveira D., Horn J., et al. (2002). Phosphorylation of glial fibrillary acidic protein is stimulated by glutamate via NMDA receptors in cortical microslices and in mixed neuronal/glial cell cultures prepared from the cerebellum. Brain Res. Dev. Brain Res. 137 139–148. [DOI] [PubMed] [Google Scholar]
- Komori T. (1999). Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick’s disease. Brain Pathol. 9 663–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krstic D., Madhusudan A., Doehner J., Vogel P., Notter T., Imhof C., et al. (2012). Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflammation 9:151. 10.1186/1742-2094-9-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumlien E., Hilton-Brown P., Spännare B., Gillberg P. G. (1992). In vitro quantitative autoradiography of [3H]-L-deprenyl and [3H]-PK 11195 binding sites in human epileptic hippocampus. Epilepsia 33 610–617. [DOI] [PubMed] [Google Scholar]
- Lahiri D. K., Chen D., Vivien D., Ge Y.-W., Greig N. H., Rogers J. T. (2003). Role of cytokines in the gene expression of amyloid beta-protein precursor: identification of a 5’-UTR-binding nuclear factor and its implications in Alzheimer’s disease. J. Alzheimers Dis. 5 81–90. [DOI] [PubMed] [Google Scholar]
- Lalo U., Pankratov Y., Kirchhoff F., North R. A., Verkhratsky A. (2006). NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J. Neurosci. 26 2673–2683. 10.1523/JNEUROSCI.4689-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoiselée H.-M., Nicolas G., Wallon D., Rovelet-Lecrux A., Lacour M., Rousseau S., et al. (2017). APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: a genetic screening study of familial and sporadic cases. PLOS Med. 14:e1002270. 10.1371/journal.pmed.1002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent C., Dorothée G., Hunot S., Martin E., Monnet Y., Duchamp M., et al. (2017). Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain 140 184–200. 10.1093/brain/aww270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prince G., Delaere P., Fages C., Lefrançois T., Touret M., Salanon M., et al. (1995). Glutamine synthetase (GS) expression is reduced in senile dementia of the Alzheimer type. Neurochem. Res. 20 859–862. [DOI] [PubMed] [Google Scholar]
- Lee J. W., Lee Y. K., Yuk D. Y., Choi D. Y., Ban S. B., Oh K. W., et al. (2008). Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J. Neuroinflammation 5:37. 10.1186/1742-2094-5-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Kosuri P., Arancio O. (2014). Picomolar amyloid-β peptides enhance spontaneous astrocyte calcium transients. J. Alzheimers Dis. 38 49–62. 10.3233/JAD-130740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.-C., Ting K. K., Adams S., Brew B. J., Chung R., Guillemin G. J. (2010). Characterisation of the expression of NMDA receptors in human astrocytes. PLOS ONE 5:e14123. 10.1371/journal.pone.0014123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine L., Saint-Aubert L., Nennesmo I., Gillberg P.-G., Nordberg A. (2017). Cortical laminar tau deposits and activated astrocytes in Alzheimer’s disease visualised by (3)H-THK5117 and (3)H-deprenyl autoradiography. Sci. Rep. 7:45496. 10.1038/srep45496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S., Docagne F., Gabriel C., Liot G., Lahiri D. K., Buée L., et al. (2003). Transforming growth factor-beta 1 potentiates amyloid-beta generation in astrocytes and in transgenic mice. J. Biol. Chem. 278 18408–18418. 10.1074/jbc.M300819200 [DOI] [PubMed] [Google Scholar]
- Levitt P., Pintar J. E., Breakefield X. O. (1982). Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc. Natl. Acad. Sci. U.S.A. 79 6385–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-M., Zhang Y., Tang L., Chen Y.-H., Gao Q., Bao M.-H., et al. (2016). Effects of triptolide on hippocampal microglial cells and astrocytes in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neural Regen. Res. 11 1492–1498. 10.4103/1673-5374.191224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y.-F., Wang B.-J., Cheng H.-T., Kuo L.-H., Wolfe M. S. (2004). Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 279 49523–49532. 10.1074/jbc.M402034200 [DOI] [PubMed] [Google Scholar]
- Libro R., Giacoppo S., Soundara Rajan T., Bramanti P., Mazzon E. (2016). Natural phytochemicals in the treatment and prevention of dementia: an overview. Molecules 21:518. 10.3390/molecules21040518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D., Iyer A., Ronco V., Grolla A. A., Canonico P. L., Aronica E., et al. (2013). Amyloid beta deregulates astroglial mGluR5-mediated calcium signaling via calcineurin and Nf-kB. Glia 61 1134–1145. 10.1002/glia.22502 [DOI] [PubMed] [Google Scholar]
- Lim G. P., Chu T., Yang F., Beech W., Frautschy S. A., Cole G. M. (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21 8370–8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg C., Selenica M.-L. B., Westlind-Danielsson A., Schultzberg M. (2005). Beta-amyloid protein structure determines the nature of cytokine release from rat microglia. J. Mol. Neurosci. 27 1–12. [DOI] [PubMed] [Google Scholar]
- Liu M.-H., Lin Y.-S., Sheu S.-Y., Sun J.-S. (2009). Anti-inflammatory effects of daidzein on primary astroglial cell culture. Nutr. Neurosci. 12 123–134. 10.1179/147683009X423274 [DOI] [PubMed] [Google Scholar]
- Liu S., Wu H., Xue G., Ma X., Wu J., Qin Y., et al. (2013). Metabolic alteration of neuroactive steroids and protective effect of progesterone in Alzheimer’s disease-like rats. Neural Regen. Res. 8 2800–2810. 10.3969/j.issn.1673-5374.2013.30.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-J., Li Z.-H., Liu L., Tang W.-X., Wang Y., Dong M.-R., et al. (2016). Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 7:261. 10.3389/fphar.2016.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L. F., Brachova L., Civin W. H., Rogers J. (1996). Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J. Neuropathol. Exp. Neurol. 55 1083–1088. [PubMed] [Google Scholar]
- Lüth H. J., Holzer M., Gärtner U., Staufenbiel M., Arendt T. (2001). Expression of endothelial and inducible NOS-isoforms is increased in Alzheimer’s disease, in APP23 transgenic mice and after experimental brain lesion in rat: evidence for an induction by amyloid pathology. Brain Res. 913 57–67. [DOI] [PubMed] [Google Scholar]
- Maeda M., Takagi H., Hattori H., Matsuzaki T. (1997). Localization of manganese superoxide dismutase in the cerebral cortex and hippocampus of Alzheimer-type senile dementia. Osaka City Med. J. 43 1–5. [PubMed] [Google Scholar]
- Magistretti P. J. (2006). Neuron-glia metabolic coupling and plasticity. J. Exp. Biol. 209 2304–2311. 10.1242/jeb.02208 [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Allaman I. (2015). A cellular perspective on brain energy metabolism and functional imaging. Neuron 86 883–901. 10.1016/j.neuron.2015.03.035 [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Pellerin L. (1999). Astrocytes couple synaptic activity to glucose utilization in the brain. News Physiol. Sci. 14 177–182. [DOI] [PubMed] [Google Scholar]
- Makitani K., Nakagawa S., Izumi Y., Akaike A., Kume T. (2017). Inhibitory effect of donepezil on bradykinin-induced increase in the intracellular calcium concentration in cultured cortical astrocytes. J. Pharmacol. Sci. 134 37–44. 10.1016/j.jphs.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Otero M., Olivotto E., Borzi R. M., Goldring M. B. (2010). NF-kappaB signaling: multiple angles to target OA. Curr. Drug Targets 11 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruszak A., Żekanowski C. (2011). Mitochondrial dysfunction and Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 35 320–330. 10.1016/j.pnpbp.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Masilamoni J. G., Jesudason E. P., Jesudoss K. S., Murali J., Paul S. F. D., Jayakumar R. (2005a). Role of fibrillar Abeta25-35 in the inflammation induced rat model with respect to oxidative vulnerability. Free Radic. Res. 39 603–612. 10.1080/10715760500117373 [DOI] [PubMed] [Google Scholar]
- Masilamoni J. G., Vignesh S., Kirubagaran R., Jesudason E. P., Jayakumar R. (2005b). The neuroprotective efficacy of alpha-crystallin against acute inflammation in mice. Brain Res. Bull. 67 235–241. 10.1016/j.brainresbull.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Matos M., Augusto E., Machado N. J., dos Santos-Rodrigues A., Cunha R. A., Agostinho P. (2012). Astrocytic adenosine A2A receptors control the amyloid-β peptide-induced decrease of glutamate uptake. J. Alzheimers Dis. 31 555–567. 10.3233/JAD-2012-120469 [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E., Rogers J., Sibley J. (1990). Anti-inflammatory drugs and Alzheimer disease. Lancet 335:1037. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G., Hollborn M., Schliebs R. (2000). Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int. J. Dev. Neurosci. 18 423–431. [DOI] [PubMed] [Google Scholar]
- Mémet S. (2006). NF-kappaB functions in the nervous system: from development to disease. Biochem. Pharmacol. 72 1180–1195. 10.1016/j.bcp.2006.09.003 [DOI] [PubMed] [Google Scholar]
- Mendez M. F. (2017). Early-onset Alzheimer disease. Neurol. Clin. 35 263–281. 10.1016/j.ncl.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini M., Meyer E. P., Ulmann-Schuler A., Nitsch R. M. (2011). Vascular β-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAβ mice. Acta Neuropathol. 122 293–311. 10.1007/s00401-011-0834-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington C., Sonego S., Karunaweera N., Rangel A., Aldrich-Wright J. R., Campbell I. L., et al. (2014). Chronic neuroinflammation in Alzheimer’s disease: new perspectives on animal models and promising candidate drugs. Biomed Res. Int. 2014:309129. 10.1155/2014/309129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter M. R., Taylor J. M., Crack P. J. (2016). The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 136 457–474. 10.1111/jnc.13411 [DOI] [PubMed] [Google Scholar]
- Mitew S., Kirkcaldie M. T. K., Dickson T. C., Vickers J. C. (2013). Altered synapses and gliotransmission in Alzheimer’s disease and AD model mice. Neurobiol. Aging 34 2341–2351. 10.1016/j.neurobiolaging.2013.04.010 [DOI] [PubMed] [Google Scholar]
- Mohamed A., Posse de Chaves E. (2011). Aβ internalization by neurons and glia. Int. J. Alzheimers Dis. 2011:127984. 10.4061/2011/127984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. J., Liu Z. (2011). Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21 103–115. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S., Uson R. R., Schwartz E. J., Haber R. S. (1995). The human blood-brain barrier glucose transporter (GLUT1) is a glucose transporter of gray matter astrocytes. Glia 14 43–54. 10.1002/glia.440140107 [DOI] [PubMed] [Google Scholar]
- Mosconi L., Pupi A., De Leon M. J. (2008). Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1147 180–195. 10.1196/annals.1427.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L., Sorbi S., de Leon M. J., Li Y., Nacmias B., Myoung P. S., et al. (2006). Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer’s disease. J. Nucl. Med. 47 1778–1786. [PubMed] [Google Scholar]
- Mosconi L., Tsui W.-H., De Santi S., Li J., Rusinek H., Convit A., et al. (2005). Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology 64 1860–1867. 10.1212/01.WNL.0000163856.13524.08 [DOI] [PubMed] [Google Scholar]
- Mota S. I., Ferreira I. L., Rego A. C. (2014). Dysfunctional synapse in Alzheimer’s disease - A focus on NMDA receptors. Neuropharmacology 76(Pt A) 16–26. 10.1016/j.neuropharm.2013.08.013 [DOI] [PubMed] [Google Scholar]
- Mulder S. D., Veerhuis R., Blankenstein M. A., Nielsen H. M. (2012). The effect of amyloid associated proteins on the expression of genes involved in amyloid-β clearance by adult human astrocytes. Exp. Neurol. 233 373–379. 10.1016/j.expneurol.2011.11.001 [DOI] [PubMed] [Google Scholar]
- Müller W. E., Eckert A., Kurz C., Eckert G. P., Leuner K. (2010). Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer’s disease–therapeutic aspects. Mol. Neurobiol. 41 159–171. 10.1007/s12035-010-8141-5 [DOI] [PubMed] [Google Scholar]
- Mutis J. A., Rodríguez J. H., Nava-Mesa M. O. (2017). Rapidly progressive cognitive impairment with neuropsychiatric symptoms as the initial manifestation of status epilepticus. Epilepsy Behav. Case Rep. 7 20–23. 10.1016/j.ebcr.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S., Kitamura M. (2013). Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic. Biol. Med. 65 162–174. 10.1016/j.freeradbiomed.2013.06.020 [DOI] [PubMed] [Google Scholar]
- Nava-Mesa M. O., Jiménez-Díaz L., Yajeya J., Navarro-Lopez J. D. (2014). GABAergic neurotransmission and new strategies of neuromodulation to compensate synaptic dysfunction in early stages of Alzheimer’s disease. Front. Cell. Neurosci. 8:167 10.3389/fncel.2014.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen L. H., Witter M. P., Sonnewald U. (2014). Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 34 906–914. 10.1038/jcbfm.2014.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris C. M., Kadish I., Blalock E. M., Chen K.-C., Thibault V., Porter N. M., et al. (2005). Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J. Neurosci. 25 4649–4658. 10.1523/JNEUROSCI.0365-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A., Tamaoki T., Motohashi N., Nakamura M., McKeel D. W., Tabaton M., et al. (2012). The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J. Neuropathol. Exp. Neurol. 71 233–241. 10.1097/NEN.0b013e318248e614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala J., Alafuzoff I., Herukka S.-K., van Groen T., Tanila H., Pirttilä T. (2009). Expression of interleukin-18 is increased in the brains of Alzheimer’s disease patients. Neurobiol. Aging 30 198–209. 10.1016/j.neurobiolaging.2007.06.006 [DOI] [PubMed] [Google Scholar]
- Palygin O., Lalo U., Pankratov Y. (2011). Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br. J. Pharmacol. 163 1755–1766. 10.1111/j.1476-5381.2011.01374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palygin O., Lalo U., Verkhratsky A., Pankratov Y. (2010). Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium 48 225–231. 10.1016/j.ceca.2010.09.004 [DOI] [PubMed] [Google Scholar]
- Panatier A., Theodosis D. T., Mothet J.-P., Touquet B., Pollegioni L., Poulain D. A., et al. (2006). Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125 775–784. 10.1016/j.cell.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Parameshwaran K., Dhanasekaran M., Suppiramaniam V. (2008). Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp. Neurol. 210 7–13. 10.1016/j.expneurol.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Parfenova H., Tcheranova D., Basuroy S., Fedinec A. L., Liu J., Leffler C. W. (2012). Functional role of astrocyte glutamate receptors and carbon monoxide in cerebral vasodilation response to glutamate. Am. J. Physiol. Heart Circ. Physiol. 302 H2257–H2266. 10.1152/ajpheart.01011.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O., Casper K. B., Kubera C., Zhang J., Revilla-Sanchez R., Sul J.-Y., et al. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310 113–116. 10.1126/science.1116916 [DOI] [PubMed] [Google Scholar]
- Pasinetti G. M. (2002). From epidemiology to therapeutic trials with anti-inflammatory drugs in Alzheimer’s disease: the role of NSAIDs and cyclooxygenase in beta-amyloidosis and clinical dementia. J. Alzheimers Dis. 4 435–445. [DOI] [PubMed] [Google Scholar]
- Pekny M., Pekna M., Messing A., Steinhäuser C., Lee J.-M., Parpura V., et al. (2016). Astrocytes: a central element in neurological diseases. Acta Neuropathol. 131 323–345. 10.1007/s00401-015-1513-1 [DOI] [PubMed] [Google Scholar]
- Perea G., Navarrete M., Araque A. (2009). Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 32 421–431. 10.1016/j.tins.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Pierce A. L., Bullain S. S., Kawas C. H. (2017). Late-onset Alzheimer disease. Neurol. Clin. 35 283–293. 10.1016/j.ncl.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Pirttimaki T. M., Codadu N. K., Awni A., Pratik P., Nagel D. A., Hill E. J., et al. (2013). α7 nicotinic receptor-mediated astrocytic gliotransmitter release: Aβ effects in a preclinical Alzheimer’s mouse model. PLOS ONE 8:e81828. 10.1371/journal.pone.0081828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M., Wimo A., Guerchet M., Ali G., Wu Y., Prina M. (2015). World Alzheimer Report, 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International (ADI). [Google Scholar]
- Querfurth H. W., LaFerla F. M. (2010). Alzheimer’s disease. N. Engl. J. Med. 362 329–344. 10.1056/NEJMra0909142 [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Miguel-Hidalgo J. J., Wei J., Dilley G., Pittman S. D., Meltzer H. Y., et al. (1999). Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry 45 1085–1098. [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M. (2016). How neuroinflammation contributes to neurodegeneration. Science 353 777–783. 10.1126/science.aag2590 [DOI] [PubMed] [Google Scholar]
- Ransohoff R. M., Schafer D., Vincent A., Blachère N. E., Bar-Or A. (2015). Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics 12 896–909. 10.1007/s13311-015-0385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin J., Cummings J., Hardy J., Schuh K., Dean R. A. (2015). Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr. Alzheimer Res. 12 712–722. 10.1186/s40478-014-0134-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman S. U., Shah S. A., Ali T., Chung J. I., Kim M. O. (2017). Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 54 255–271. 10.1007/s12035-015-9604-5 [DOI] [PubMed] [Google Scholar]
- Ries M., Sastre M. (2016). Mechanisms of Aβ clearance and degradation by glial cells. Front. Aging Neurosci. 8:160 10.3389/fnagi.2016.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. R. (2000). Neuronal expression of glutamine synthetase in Alzheimer’s disease indicates a profound impairment of metabolic interactions with astrocytes. Neurochem. Int. 36 471–482. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vieitez E., Carter S. F., Chiotis K., Saint-Aubert L., Leuzy A., Schöll M., et al. (2016a). Comparison of early-phase 11C-deuterium-l-deprenyl and 11C-pittsburgh compound B PET for assessing brain perfusion in Alzheimer disease. J. Nucl. Med. 57 1071–1077. 10.2967/jnumed.115.168732 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Vieitez E., Saint-Aubert L., Carter S. F., Almkvist O., Farid K., Schöll M., et al. (2016b). Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain 139 922–936. 10.1093/brain/awv404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco V., Grolla A. A., Glasnov T. N., Canonico P. L., Verkhratsky A., Genazzani A. A., et al. (2014). Differential deregulation of astrocytic calcium signalling by amyloid-β, TNFα, IL-1β and LPS. Cell Calcium 55 219–229. 10.1016/j.ceca.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Saha R. N., Pahan K. (2006). Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid. Redox Signal. 8 929–947. 10.1089/ars.2006.8.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore C., Cerasa A., Battista P., Gilardi M. C., Quattrone A., Castiglioni I., et al. (2015). Magnetic resonance imaging biomarkers for the early diagnosis of Alzheimer’s disease: a machine learning approach. Front. Neurosci. 9:307 10.3389/fnins.2015.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P. E., Zhu L., Verret L., Vossel K. A., Orr A. G., Cirrito J. R., et al. (2012). Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. U.S.A. 109 E2895–E2903. 10.1073/pnas.1121081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez I., Temprano-Carazo S., Nájera A., Djebari S., Yajeya J., Gruart A., et al. (2017). Activation of G-protein-gated inwardly rectifying potassium (Kir3/GirK) channels rescues hippocampal functions in a mouse model of early amyloid-β pathology. Sci. Rep. 7:14658. 10.1038/s41598-017-15306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarup A., Larsson O. M., Schousboe A. (2003). GABA transporters and GABA-transaminase as drug targets. Curr. Drug Targets CNS Neurol. Disord. 2 269–277. [DOI] [PubMed] [Google Scholar]
- Satoh J.-I., Yamamoto Y., Asahina N., Kitano S., Kino Y. (2014). RNA-Seq data mining: downregulation of NeuroD6 serves as a possible biomarker for Alzheimer’s disease brains. Dis. Markers 2014:123165. 10.1155/2014/123165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M., Carter S. F., Westman E., Rodriguez-Vieitez E., Almkvist O., Thordardottir S., et al. (2015). Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci. Rep. 5:16404. 10.1038/srep16404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag M., Mueller C., Zabel M., Crofton A., Kirsch W. M., Ghribi O., et al. (2013). Oxidative stress in blood in Alzheimer’s disease and mild cognitive impairment: a meta-analysis. Neurobiol. Dis. 59 100–110. 10.1016/j.nbd.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Schubert D., Soucek T., Blouw B. (2009). The induction of HIF-1 reduces astrocyte activation by amyloid beta peptide. Eur. J. Neurosci. 29 1323–1334. 10.1111/j.1460-9568.2009.06712.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D. J., Hardy J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.-M., Han Y.-W., Han X.-H., Zhang K., Chang Y.-N., Hu Z.-M., et al. (2016). Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 366 127–134. 10.1016/j.jns.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Simic G., Lucassen P. J., Krsnik Z., Kruslin B., Kostovic I., Winblad B., et al. (2000). nNOS expression in reactive astrocytes correlates with increased cell death related DNA damage in the hippocampus and entorhinal cortex in Alzheimer’s disease. Exp. Neurol. 165 12–26. 10.1006/exnr.2000.7448 [DOI] [PubMed] [Google Scholar]
- Simonovitch S., Schmukler E., Bespalko A., Iram T., Frenkel D., Holtzman D. M., et al. (2016). Impaired autophagy in APOE4 astrocytes. J. Alzheimers Dis. 51 915–927. 10.3233/JAD-151101 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V. (2014). Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20 160–172. 10.1177/1073858413504466 [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Vinters H. V. (2010). Astrocytes: biology and pathology. Acta Neuropathol. 119 7–35. 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohanaki H., Baluchnejadmojarad T., Nikbakht F., Roghani M. (2016). Pelargonidin improves memory deficit in amyloid β25-35 rat model of Alzheimer’s disease by inhibition of glial activation, cholinesterase, and oxidative stress. Biomed. Pharmacother. 83 85–91. 10.1016/j.biopha.2016.06.021 [DOI] [PubMed] [Google Scholar]
- Song Z., Xiong B., Guan X., Cao F., Manyande A., Zhou Y., et al. (2016). Minocycline attenuates bone cancer pain in rats by inhibiting NF-κB in spinal astrocytes. Acta Pharmacol. Sin. 37 753–762. 10.1038/aps.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucek T., Cumming R., Dargusch R., Maher P., Schubert D. (2003). The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 39 43–56. [DOI] [PubMed] [Google Scholar]
- Swomley A. M., Butterfield D. A. (2015). Oxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomics. Arch. Toxicol. 89 1669–1680. 10.1007/s00204-015-1556-z [DOI] [PubMed] [Google Scholar]
- Szekely C. A., Green R. C., Breitner J. C. S., Østbye T., Beiser A. S., Corrada M. M., et al. (2008). No advantage of A beta 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology 70 2291–2298. 10.1212/01.wnl.0000313933.17796.f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Sato N., Morishita R. (2014). Systemic inflammation, blood-brain barrier vulnerability and cognitive/non-cognitive symptoms in Alzheimer disease: relevance to pathogenesis and therapy. Front. Aging Neurosci. 6:171. 10.3389/fnagi.2014.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M. W., Okamoto S., et al. (2013). Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. U.S.A. 110 E2518–E2527. 10.1073/pnas.1306832110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóbon-Velasco J. C., Cuevas E., Torres-Ramos M. A. (2014). Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol. Disord. Drug Targets 13 1615–1626. [DOI] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. (1979). Overall pathway of mononucleosome production. J. Biol. Chem. 254 3074–3083. [PubMed] [Google Scholar]
- Toivari E., Manninen T., Nahata A. K., Jalonen T. O., Linne M.-L. (2011). Effects of transmitters and amyloid-beta peptide on calcium signals in rat cortical astrocytes: fura-2AM measurements and stochastic model simulations. PLOS ONE 6:e17914. 10.1371/journal.pone.0017914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turillazzi E., Neri M., Cerretani D., Cantatore S., Frati P., Moltoni L., et al. (2016). Lipid peroxidation and apoptotic response in rat brain areas induced by long-term administration of nandrolone: the mutual crosstalk between ROS and NF-kB. J. Cell. Mol. Med. 20 601–612. 10.1111/jcmm.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y., Doi T., Nagatomo K., Tokumaru J., Takaki M., Willmore L. J. (2007). Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 1151 55–61. 10.1016/j.brainres.2007.03.021 [DOI] [PubMed] [Google Scholar]
- Uittenbogaard M., Baxter K. K., Chiaramello A. (2010). NeuroD6 genomic signature bridging neuronal differentiation to survival via the molecular chaperone network. J. Neurosci. Res. 88 33–54. 10.1002/jnr.22182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39 44–84. 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. (2016). The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 18 421–430. 10.1038/gim.2015.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gijsel-Bonnello M., Baranger K., Benech P., Rivera S., Khrestchatisky M., de Reggi M., et al. (2017). Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer’s disease: alleviation by pantethine. PLOS ONE 12:e0175369. 10.1371/journal.pone.0175369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasile F., Dossi E., Rouach N. (2017). Human astrocytes: structure and functions in the healthy brain. Brain Struct. Funct. 222 2017–2029. 10.1007/s00429-017-1383-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier Y., Zarándi M., Penke B. (2004). Amyloid beta-peptide interactions with neuronal and glial cell plasma membrane: binding sites and implications for Alzheimer’s disease. J. Pept. Sci. 10 229–248. 10.1002/psc.573 [DOI] [PubMed] [Google Scholar]
- Verkhratsky A., Kirchhoff F. (2007). NMDA receptors in glia. Neuroscientist 13 28–37. 10.1177/1073858406294270 [DOI] [PubMed] [Google Scholar]
- Viña J., Lloret A., Ortí R., Alonso D. (2004). Molecular bases of the treatment of Alzheimer’s disease with antioxidants: prevention of oxidative stress. Mol. Asp. Med. 25 117–123. 10.1016/j.mam.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Vincent A. J., Gasperini R., Foa L., Small D. H. (2010). Astrocytes in Alzheimer’s disease: emerging roles in calcium dysregulation and synaptic plasticity. J. Alzheimers Dis. 22 699–714. 10.3233/JAD-2010-101089 [DOI] [PubMed] [Google Scholar]
- Vos S. J. B., van Boxtel M. P. J., Schiepers O. J. G., Deckers K., de Vugt M., Carrière I., et al. (2017). Modifiable risk factors for prevention of dementia in midlife, late life and the oldest-old: validation of the LIBRA index. J. Alzheimers Dis. 58 537–547. 10.3233/JAD-161208 [DOI] [PubMed] [Google Scholar]
- Vossel K. A., Beagle A. J., Rabinovici G. D., Shu H., Lee S. E., Naasan G., et al. (2013). Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 70 1158–1166. 10.1001/jamaneurol.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. G., Whetzel A. M., Serrano G., Sue L. I., Beach T. G., Lue L.-F. (2015). Association of CD33 polymorphism rs3865444 with Alzheimer’s disease pathology and CD33 expression in human cerebral cortex. Neurobiol. Aging 36 571–582. 10.1016/j.neurobiolaging.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A. B., Waagepetersen H. S., Bak L. K., Schousboe A., Sonnewald U. (2015). The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem. Res. 40 402–409. 10.1007/s11064-014-1473-1 [DOI] [PubMed] [Google Scholar]
- Wan F., Lenardo M. J. (2010). The nuclear signaling of NF-kappaB: current knowledge, new insights, and future perspectives. Cell Res. 20 24–33. 10.1038/cr.2009.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Chen L., Pan X., Chen J., Wang L., Wang W., et al. (2016). The effect of resveratrol on beta amyloid-induced memory impairment involves inhibition of phosphodiesterase-4 related signaling. Oncotarget 7 17380–17392. 10.18632/oncotarget.8041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.-X., Tan L., Liu J., Yu J.-T. (2016). The essential role of soluble Aβ oligomers in Alzheimer’s disease. Mol. Neurobiol. 53 1905–1924. 10.1007/s12035-015-9143-0 [DOI] [PubMed] [Google Scholar]
- Wang H.-M., Zhang T., Huang J.-K., Sun X.-J. (2013). 3-N-butylphthalide (NBP) attenuates the amyloid-β-induced inflammatory responses in cultured astrocytes via the nuclear factor-κB signaling pathway. Cell. Physiol. Biochem. 32 235–242. 10.1159/000350139 [DOI] [PubMed] [Google Scholar]
- Wang J., Tan L., Wang H.-F., Tan C.-C., Meng X.-F., Wang C., et al. (2015). Anti-inflammatory drugs and risk of Alzheimer’s disease: an updated systematic review and meta-analysis. J. Alzheimers Dis. 44 385–396. 10.3233/JAD-141506 [DOI] [PubMed] [Google Scholar]
- Wang L., Wang M., Hu J., Shen W., Hu J., Yao Y., et al. (2017). Protective effect of 3H-1, 2-dithiole-3-thione on cellular model of Alzheimer’s disease involves Nrf2/ARE signaling pathway. Eur. J. Pharmacol. 795 115–123. 10.1016/j.ejphar.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Webster M. J., O’Grady J., Kleinman J. E., Weickert C. S. (2005). Glial fibrillary acidic protein mRNA levels in the cingulate cortex of individuals with depression, bipolar disorder and schizophrenia. Neuroscience 133 453–461. 10.1016/j.neuroscience.2005.02.037 [DOI] [PubMed] [Google Scholar]
- Westin K., Buchhave P., Nielsen H., Minthon L., Janciauskiene S., Hansson O. (2012). CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer’s disease. PLOS ONE 7:e30525. 10.1371/journal.pone.0030525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Hocevar M., Foss J. F., Bie B., Naguib M. (2017). Activation of CB2 receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. Eur. J. Pharmacol. 811 12–20. 10.1016/j.ejphar.2017.05.044 [DOI] [PubMed] [Google Scholar]
- Xiu J., Nordberg A., Zhang J.-T., Guan Z.-Z. (2005). Expression of nicotinic receptors on primary cultures of rat astrocytes and up-regulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide(1-42). Neurochem. Int. 47 281–290. 10.1016/j.neuint.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Yan P., Hu X., Song H., Yin K., Bateman R. J., Cirrito J. R., et al. (2006). Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J. Biol. Chem. 281 24566–24574. 10.1074/jbc.M602440200 [DOI] [PubMed] [Google Scholar]
- Yan R. (2017). Physiological functions of the β-site amyloid precursor protein cleaving enzyme 1 and 2. Front. Mol. Neurosci. 10:97 10.3389/fnmol.2017.00097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Lim G. P., Begum A. N., Ubeda O. J., Simmons M. R., Ambegaokar S. S., et al. (2005). Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 280 5892–5901. 10.1074/jbc.M404751200 [DOI] [PubMed] [Google Scholar]
- Yang Y., Ge W., Chen Y., Zhang Z., Shen W., Wu C., et al. (2003). Contribution of astrocytes to hippocampal long-term potentiation through release of D-serine. Proc. Natl. Acad. Sci. U.S.A. 100 15194–15199. 10.1073/pnas.2431073100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarishkin O., Lee J., Jo S., Hwang E. M., Lee C. J. (2015). Disinhibitory action of astrocytic GABA at the perforant path to dentate gyrus granule neuron synapse reverses to inhibitory in Alzheimer’s disease model. Exp. Neurobiol. 24 211–218. 10.5607/en.2015.24.3.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B., Shen H., Zhang J., Zhu Y.-G., Ransom B. R., Chen X.-C., et al. (2015). Dual pathways mediate β-amyloid stimulated glutathione release from astrocytes. Glia 63 2208–2219. 10.1002/glia.22886 [DOI] [PubMed] [Google Scholar]
- Yeh F. L., Wang Y., Tom I., Gonzalez L. C., Sheng M. (2016). TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron 91 328–340. 10.1016/j.neuron.2016.06.015 [DOI] [PubMed] [Google Scholar]
- Yin K.-J., Cirrito J. R., Yan P., Hu X., Xiao Q., Pan X., et al. (2006). Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 26 10939–10948. 10.1523/JNEUROSCI.2085-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H.-M., Kim J. A., Hwang C. J., Jin P., Baek M. K., Lee J. M., et al. (2015). Neuroinflammatory and amyloidogenic activities of IL-32β in Alzheimer’s disease. Mol. Neurobiol. 52 341–352. 10.1007/s12035-014-8860-0 [DOI] [PubMed] [Google Scholar]
- Zamanian J. L., Xu L., Foo L. C., Nouri N., Zhou L., Giffard R. G., et al. (2012). Genomic analysis of reactive astrogliosis. J. Neurosci. 32 6391–6410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Luhrs K. J., Ryff K. A., Malik W. T., Driscoll M. J., Culver B. (2009). Suppression of nuclear factor kappa B ameliorates astrogliosis but not amyloid burden in APPswe/PS1dE9 mice. Neuroscience 161 53–58. 10.1016/j.neuroscience.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Zhao J., Davis M. D., Martens Y. A., Shinohara M., Graff-Radford N. R., Younkin S. G., et al. (2017). APOE 𝜀4/𝜀4 diminishes neurotrophic function of human iPSC-derived astrocytes. Hum. Mol. Genet. 26 2690–2700. 10.1093/hmg/ddx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., O’Connor T., Vassar R. (2011). The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J. Neuroinflammation 8:150. 10.1186/1742-2094-8-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Lai Y., Shelat P. B., Hu C., Sun G. Y., Lee J. C.-M. (2006). Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J. Neurosci. 26 11111–11119. 10.1523/JNEUROSCI.3505-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.-C., Yu J.-T., Jiang T., Wang P., Cao L., Tan L. (2015). CR1 in Alzheimer’s disease. Mol. Neurobiol. 51 753–765. 10.1007/s12035-014-8723-8 [DOI] [PubMed] [Google Scholar]


