Abstract
Exophiala spinifera and Exophiala dermatitidis (Fungi: Chaetothyriales) are black yeast agents potentially causing disseminated infection in apparently healthy humans. They are the only Exophiala species producing extracellular polysaccharides around yeast cells. In order to gain understanding of eventual differences in intrinsic virulence of the species, their clinical profiles were compared and found to be different, suggesting pathogenic strategies rather than coincidental opportunism. Ecologically relevant factors were compared in a model set of strains of both species, and significant differences were found in clinical and environmental preferences, but virulence, tested in Galleria mellonella larvae, yielded nearly identical results. Virulence factors, i.e., melanin, capsule and muriform cells responded in opposite direction under hydrogen peroxide and temperature stress and thus were inconsistent with their hypothesized role in survival of phagocytosis. On the basis of physiological profiles, possible natural habitats of both species were extrapolated, which proved to be environmental rather than animal-associated. Using comparative genomic analyses we found differences in gene content related to lipid metabolism, cell wall modification and polysaccharide capsule production. Despite the fact that both species cause disseminated infections in apparently healthy humans, it is concluded that they are opportunists rather than pathogens.
Keywords: black yeast, capsule, Exophiala species, virulence, pathogenicity, opportunism, physiology, Galleria mellonella
Introduction
The black yeast genus Exophiala (Fungi, order Chaetothyriales) contains about 40 species, 17 of which have been reported from human infections (De Hoog et al., 2000b; Revankar and Sutton, 2010). Some species are notorious agents of deep and disseminated human infection, in debilitated but also in healthy individuals (Revankar et al., 2002). In contrast to frequent statements in the literature, Exophiala black yeasts are not common saprobes on plant debris but are selected by domesticated environments where conditions for microbial growth are relatively hostile. Their survival strategy has been referred to as polyextremotolerant (Gostincar et al., 2011).
Of all Exophiala species, Exophiala dermatitidis (E. dermatitidis) and Exophiala spinifera (E. spinifera) are associated with the most severe infections, which in systemic cases have high mortality rates of up to 80% (Rajam et al., 1958; Crosby et al., 1989; Campos-Takaki and Jardim, 1994; de Hoog et al., 2005; Radhakrishnan et al., 2010; Li et al., 2011; Patel et al., 2013; Hu et al., 2014; Wang et al., 2015; Chen et al., 2016). As a possible explanation of their relatively high virulence compared to other Exophiala species, the occurrence of extracellular polysaccharide on yeast cells has been mentioned, masking the cells for human phagocytes upon tissue invasion (Yurlova and de Hoog, 2002). Exophiala dermatitidis has a global distribution in the domesticated environment, but cases of deep phaeohyphomycosis are nearly exclusively found in East Asia (Revankar et al., 2002; Kantarcioglu et al., 2004). In Europe the fungus occurs as a respiratory colonizer in patients with cystic fibrosis (Kondori et al., 2011). In contrast to many other opportunistic fungi its frequency seems to be relatively unaffected by the growing hospitalized populations of patients with compromised immunity. Extended searches for the fungus in the natural environment yielded feces of frugivorous tropical animals as a possible niche, while prevalence in soil and plant debris was close to zero (Sudhadham et al., 2008). The species is however commonly found in indoor wet cells such as bathing facilities and dishwashers (Matos et al., 2002; Gümral et al., 2015) and other human-made environments such as creosoted railway sleepers (Gumral et al., 2014). These habitats are characterized by (i) high temperatures, (ii) osmotic stress, (iii) acidic or alkaline conditions, and (iv) toxicity along with (v) low nutrient availability. It has been speculated that such strongly selective environments may drive their evolution toward human pathogenicity (Gostincar et al., 2011; Dogen et al., 2013b; Zupancic et al., 2016).
Exophiala spinifera is rare, both in humans and in the environment. Disseminated infections may have a fatal outcome and were prevalently observed in immunocompetent children and adolescents, while in the elderly infections tend to remain as (sub) cutaneous lesions, taking a mild course despite underlying disorders (de Hoog et al., 1999). The species has not been reported from CF lungs. Its environmental occurrence displays a rather scattered picture.
The ecological differences between E. dermatitidis and E. spinifera are intriguing. Both are characterized by the production of extracellular slimes, which may be either in the form of a well-delimited capsule or of diffusely exuded exopolysaccharides (EPS). The capsular material was reported around very young cells of E. spinifera and acid mucopolysaccharides were observed around yeast cells of E. dermatitidis (Yurlova and de Hoog, 2002). In general, capsular material is a key determinant of virulence, as extracellular polysaccharides have a significant role in adherence, impairment of phagocytosis and to reduce complement-mediated killing (Nishimura and Miyaji, 1983). If the two species are opportunists without pathogenic strategies, the average clinical course of both is expected to be similar, i.e., dependent on host conditions and route of infection. Alternatively, the striking differences between the two species have to be explained by their environmental behavior. In the present study our systematic approach involves growth, morphology of invasive phases, multilocus sequencing, and physiology, while relative virulence was determined in a Galleria mellonella larvae model. In addition, we assessed the genomes of E. spinifera and E. dermatitidis in order to provide gene information on the physiological variations observed between the species.
Materials and methods
Literature search
Keywords “Exophiala spinifera,” “Exophiala dermatitidis,” and “Wangiella dermatitidis” were used in PubMed to search for English literature including research articles, reviews and case reports published until January 2017. In addition, research databases available at Westerdijk Institute were consulted.
Strains studied
A global set of 48 E. spinifera isolates (26 clinical, 22 environmental) and 47 E. dermatitidis isolates (28 clinical, 19 environmental) were available for study (Table 1). Strains were obtained from the Research Center for Medical Mycology at Peking University and the Centraalbureau voor Schimmelcultures (housed at Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands) from 1997 to 2016. Representatives of genotypes A1, A2, A3, genotype B, and genotype C of E. dermatitidis were included (Table 1). Data of prevalence of the two species were abstracted from a research database on black yeasts at Westerdijk Institute, comprising ITS and part of TEF1 sequenced items of E. spinifera and E. dermatitidis, respectively.
Table 1.
Strains analyzed of E. spinifera and E. dermatitidis.
x, Strains used in subsequent experiments (physiology and Galleria model).
Twenty E. spinifera strains and twenty E. dermatitidis strains (Table 1), representing maximum ecological and geographical variation, were selected for physiology testing and the G. mellonella virulence model. Identity of strains was verified by sequencing.
DNA extraction
Genomic DNA was obtained from strains grown for 7–14 day on MEA at 24°C. All cultures were handled within a class II biological safety cabinet. Extraction was followed by the cetyltrimethylammonium bromide (CTAB) protocol according to CBS. Quality and quantity of isolated DNA was verified on a NanoDrop ND-1000 Spectrophotometer using ND-1000 v3.3.0 software (Coleman Technologies, Wilmington, DE, USA). Samples were stored at −20°C.
DNA amplification and sequencing
The following nuclear genes were amplified by PCR: rDNA internal transcribed spacer region (ITS), partial transcriptional elongation factor 1 subunit α (TEF1-α), and β-tubulin (TUB). ITS of the rDNA operon was amplified with ITS1 and ITS4. Partial β-tubulin (TUB), covering the variable 5′-end containing four small introns, was amplified with TUB2a and TUB2b, the partial gene TEF1-α with EF1-728F and EF1-986R primer set. Conditions for amplifications of all genes were as described by de Hoog et al. (2011). Sequencing was done with an ABI3730 automatic sequencer (Applied Biosystems, Foster City, CA, USA) and sequence data were adjusted by SeqManPro (DNAStar, Madison, WI, USA). GenBank accession numbers are given in Table 1. We selectively submitted some of the sequences per clade.
Alignment and phylogenetic reconstruction
For phylogenetic reconstructions with different loci resulting in different degrees of resolution, appropriate reference sequences were obtained from GenBank. Multiple sequence alignments were created with online Mafft v7 using automatic alignment strategy. Alignments were reviewed and corrected manually. Ambiguously aligned regions, long gaps and introns were removed from the alignments using BioEdit v7.1.3.0. Phylogenetic reconstructions were done for each locus using maximum likelihood (ML) implemented in Mega v6.06, and MrBayes trees were done via the Cipres portal (http://www.phylo.org/). Mega v6.06 selected K2+G as the most appropriate model of DNA substitution for ML analysis. Support for the internodes was assessed by bootstrap analysis from 1,000 replicates. Trees were viewed and edited with Mega v6.06, FigTree v1.4.2 and Adobe Illustrator CS6.
Morphology
Slides were made by Shear's mounting medium without pigments. Micrographs were taken using a Nikon Eclipse 80i microscope and DS Camera Head DS-Fi1/DS-5 m/DS-2Mv/DS-2MBW using NIS-Element freeware package (Nikon Europe, Badhoevedorp, The Netherlands). Dimensions were taken with the Nikon Eclipse 80i measurement module on slides and the mean and standard deviation were calculated from measurements of 40–50 conidia.
Twenty E. spinifera and twenty E. dermatitidis strains were tested for the production of muriform cells known as the invasive phase of human chromoblastomycosis. Strains were incubated at 25 and 37°C for 1 week in liquid acidic medium (30 g glucose, 3 g NaNO3, 0.01 g FeSO4·7H2O, 0.265 g NH4Cl, 0.003 g thiamin, 1 mM CaCl2 in 1 L dH2O, pH adjusted at 2.5 with HCl) shaken at 150 r.p.m (Karuppayil and Szaniszlo, 1997).
Capsule
Strains were maintained on Potato Dextrose Agar (PDA) slants, inoculated on fresh PDA plates at 24 and 37°C and incubated for 7 days. Presence of extracellular polysaccharide was verified regularly during 2–7 days of growth and capsular sizes were measured with negative staining in India ink (Yurlova and de Hoog, 2002). All tests were performed three times in duplicate. Numerical values are the means of at least 20 determinations.
Physiology
All tests were done with 20-selected E. spinifera and 20 E. dermatitidis strains. Cardinal growth temperatures were determined on 2% malt extract agar (MEA; Difco). Plates were incubated at 15–45°C with 3°C intervals in the dark for 2 weeks; plates contained double quantities of medium and were sealed to prevent drying out. Colony diameters were measured for a selection of 20 E. spinifera strains and 20 E. dermatitidis strains based on phylogenetic results and references. In addition, growth responses at 40 and 45°C were recorded. To evaluate whether 40 and 45°C were fungicidal, the cultures were returned to 24°C after 2 weeks and incubated for two additional weeks. Experiments consisted of three simultaneous replicates for each isolate; the entire procedure was repeated once.
Lipases were tested with Tween 80 opacity test medium (TOTM) according to Slifkin (Slifkin, 2000), incubating Petri dishes at 24°C for 14 day. Proteolysis was tested with Bromocresol purple-milk solids-glucose agar (BCP-MS-G) medium (Fischer and Kane, 1971; Summerbell et al., 1988) using colony fragments as inoculum. After incubation at 24°C for 14 d, color changes of the medium were recorded. Haemolytic activity was evaluated by culturing isolates on blood agar (BioMérieux, Marcy-l'Étoile, France) for 14 d at 24°C. Positive reaction is a clear ring of hemolysis around the colony. Production of urease was determined in Christensen's urea broth after incubation at 24 and 37°C for 8 and 24 h, with a final check after 7 day. Acid productions was tested on Custer's chalk medium including 5% glucose and 0.5% calcium carbonate after incubation at 25°C for 2 weeks.
Oxygenic stress was evaluated with cultures in YEPD agar medium with hydrogen peroxide to reach concentrations of 3, 6, 9, and 12 mM after sterilization. Growth rates and morphology were recorded. Cycloheximide 0.2% (Sigma-Aldrich, Zwijndrecht, The Netherlands) tolerance was evaluated by growing isolates on SGA with and without cycloheximide incubated at 24 and 37°C for 2 wks. Osmotolerance was tested with YPD basic medium with 20, 40 and 60% sucrose. Halotolerance was tested with complete medium with 2.5, 5, and 10% NaCl and MgCl2.
Protein family classification
For functional annotation of protein sets corresponding to E. dermatitidis CBS 525.76 and E. spinifera CBS 899.68, sequences were retrieved from the Black Yeast Genome Database (Moreno et al., 2017). Genes correlated with capsule production were predicted by identifying orthologs using the OrthoMCL pipeline, previously described in Cryptococcus neoformans (Gish et al., 2016). Lipase family classification was performed based on the top BLAST hit (cut-off e-value > 1e−15) to the lipase-engineering database (Barth et al., 2004). Genes involved in the nitrogen metabolism were predicted mapping selected urease genes known from previously work (Carlini and Ligabue-Braun, 2016). Catalase/peroxidases genes were predicted via top BLAST hit (cut-off e-value > 1e−15) to the PeroxiBase (Fawal et al., 2013). Cell wall associated genes and potential peptidases were derived from the Black Yeast Genome Database (Moreno et al., 2017).
Virulence in Galleria mellonella
Conidia cells were harvested from MEA-grown cultures incubated for 7 days at 33°C. Conidia were counted in a Bürker-Turk counting chamber and a standardized conidial suspension was made. This suspension consisted of 107–104 conidia per larvae. To verify the number of colony forming units, 40 μL of 103 and 102 inoculums were placed on SGA plates with gentamycin. Plates were incubated for 14 days at 37°C and colonies were counted. To inject the larvae, 40 μL of the conidia suspension was injected into the haemocoel of G. mellonella larvae via the last left proleg with an insulin micro-syringe. As a control in each experiment, one G. mellonella group was injected with PBS only. Each strain was tested in three independent experiments in separate weeks. Viability of the larvae was checked daily. Any cocoons formed, were disregarded and left out of the equations. Values were presented as the mean obtained from the three separate experiments and differences were analyzed with the Mann-Whitney U-test; statistical significance was set at P < 0.05 using GraphPad Prism 5 (GraphPad, La Jolla, CA, USA) and SPSS 19.0 (IBM, USA) softwares.
Results
Habitats of E. dermatitidis and E. spinifera
Exophiala dermatitidis is a relatively widespread fungus in the environment, although mostly occurring in low abundance. An extensive ecological study was performed by Sudhadham et al. (2008), who searched for the species in numerous natural and domestic environments and detected the species in fruit samples, feces of frugivorous birds, and natural hot springs, but particularly in steam baths and on creosoted railway sleepers. Other studies (Dogen et al., 2013a; Gümral et al., 2015; Zupancic et al., 2016) confirmed the prevalence in bathing facilities, on railway sleepers, and in dishwashers. Common fungal habitats as plant debris and soil were consistently negative or nearly so. Thus, the fungus seems selected by either toxic, or by hot, moist and nutritionally poor environments.
Exophiala spinifera is a rare fungus in the environment. We analyzed almost all strains globally available in the literature and the research database available at Westerdijk Institute (Table 1). Strains originated from plant materials with high sugar content, such as pineapple, maize, sugarcane, rotten cactus, and apple juice. The species was particularly prevalent on decomposing scales of babassu coconuts (Nascimento et al., 2017), which are rich in lipids, terpenes and aromatic hydrocarbons (Figure 1). Creosoted wood, bathing facilities and dishwashers, but also soil and plant debris were consistently negative. Thus, the fungus seems selected by somewhat osmotic environments; its prevalence in the coconut habitat requires further study.
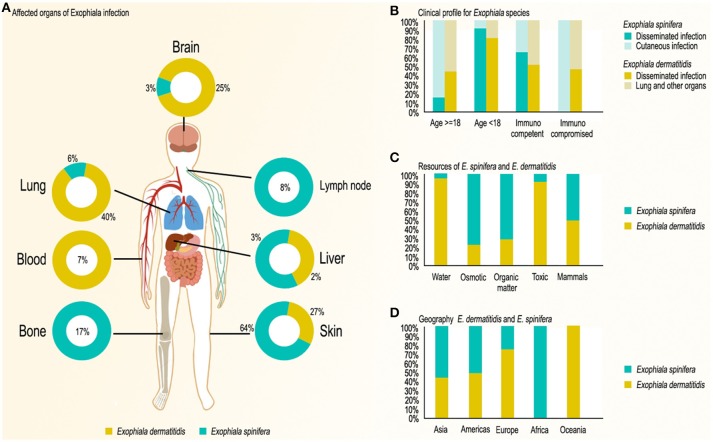
Figure 1.
Infection and distribution characteristics of E. spinifera and E. dermatitidis based on published case reports. (A) Organs affected; (B) Main clinical profiles of Exophiala species; (C) Environmental sources of Exophiala species; (D) Geography of Exophiala species.
In the human patient, E. dermatitidis occurs on (sub) cutaneous locations causing otitis externa, keratitis, and onychomycosis (Matsumoto et al., 1993; De Hoog et al., 2000a), but is more frequently found systemically, regularly colonizing the lungs of patients with cystic fibrosis (CF) (Horre et al., 2004; Chotirmall and McElvaney, 2014) and the intestinal tract of debilitated patients (Matos et al., 2002; de Hoog et al., 2005). Until January 2017, 77 cases (Table 2) have been reported in the English literature; 39 cases (Table 2) are single systemic infections (excluding pseudoepidemics). Life-threatening systemic infections occur in patients underlying disorders but also in otherwise healthy individuals (Alabaz et al., 2009) (51%, 39/77) particularly in those of Asian descent (Sudhadham et al., 2008) (62%, 24/39). Among the 39 systemic infections, 14 cases showed neurotropism while none was osteotropic; 34 of the cases concerned immunocompetent patiens. Two case series concerned injection of contaminated fluids into the bloodstream of patients, where neurotropism was frequently observed (Matsumoto et al., 1993). A case a fatality rate of over 80% was noted in systemic infections (Patel et al., 2013; Table 2).
Table 2.
Overview of published cases due to E. spinifera and E. dermatitidis.
| First author/year | Age/Sex | Country | Initial site | Cutaneous | Extra-cutaneous | Immuno-suppression | Treatment | Outcome | Disease type |
|---|---|---|---|---|---|---|---|---|---|
| E. SPINIFERA | |||||||||
| Rajam 1958 | 7/M | India | Face | Disseminated | Bone | No | Penicillin, streptomycin, nystatin, isoniazid | Death | CBM |
| Nielsen 1968 | 72/F | USA | Face | Unique | No | No | Surgery | CR | PHM |
| Padhye 1983 | 6/M | El Salvador | Face | Disseminated | Lung | No | AMB, KTC, 5-FC, ITC | PR | PHM |
| Padhye1984 | 60/M | USA | Arm | Regional | No | Yes | KTC, 5-FC | CR | PHM |
| Lacaz 1984 | 5/F | Brazil | Cutaneous | Unknown | Brain | Unknown | AMB | Unknown | PHM |
| Kotylo 1989 | 62/F | USA | Finger | Unique | No | Yes | Surgery, ITC | CR | PHM |
| Barba-Gomez 1992 | 49/M | Mexico | Finger | Unique | No | No | ITC, liquid nitrogen | CR | CBM |
| Mirza 1993 | 13/M | Pakistan | Unknown | Disseminated | No | No | AMB, 5-FC | PR | PHM |
| Padhye 1996 | 62/M | USA | Finger | Regional | No | Yes | 5-FC, ITC, surgery | PR, relapse | CBM |
| Campos-Takaki 1994 | 12/M | Brazil | Face | Disseminated | Bone, LAD | No | AMB | Death | PHM |
| Oba 2000 | 66/F | Japan | Arm | Unique | No | No | ITC, heat therapy | PR | PHM |
| Rajendran 2003 | 12/F | India | Face | Disseminated | LAD | No | ITC | PR | PHM |
| Negroni 2004 | 32/F | Argentina | Face | Few, distant | Bone, LAD | Yes | ITC, AMB, PZC, surgery | CR | PHM |
| Dutriaux 2005 | 59/F | France1 | Leg | Few, regional | No | Yes | VRC | PR | PHM |
| Takahara 2005 | 85/F | Japan | Arm | Regional | No | Yes | ITC, minocycline | CR | PHM |
| Develoux 2006 | 58/M | Senegal | Leg | Regional | No | No | TBF | Failure | CBM |
| Tomson 2006 | 78/M | Pakistan | Arm | Unique | No | No | ITC | Stability | CBM |
| Singal 2008 | 10/M | India | Leg | Disseminated | No | No | ITC, FCZ, TBF, cryo | Failure | PHM |
| Baubion 2008 | 73/M | France | Arm and leg | Multiple, Regional | No | Yes | ITC | Death | PHM |
| Chandler 2008 | 8/M | India | Leg | Disseminated | LAD | No | ITC, TBF | Failure | PHM |
| Harris 2009 | 49/M | USA | Leg | Few, regional | No | Yes | ITC | CR | PHM |
| Lin 2010 | 67/F | China | Scalp | Unknown | Unknown | Unknown | ITC | CR | PHM |
| Radhakrishnan 2010 | 20/F | India | Neck | Disseminated | Hepatic | No | KTC | Death | PHM |
| Li 2011 | 22/F | China | Face | Disseminated | Bone | No | Unknown | Death | PHM |
| Li 2011 | 9/M | China | Face | Disseminated | Bone | No | 5-FC | Death | PHM |
| Badali 2012 | 55/M | India | Arm | Few, distant | No | No | Surgery | CR | PHM |
| Lin 2012 | 27/F | China | Leg | Multiple, regional | No | Yes | FLC | Death | PHM |
| Singh 2012 | 26/M | India | Face | Multiple, regional | No | No | ITC | PR | PHM |
| Daboit 2012 | 80/M | Brazil | Hand | Unique | No | No | ITC | CR | PHM |
| Badali 2012 | 55/M | India | Arm | Few, distant | No | No | Surgery | CR | PHM |
| Lanternier 2015 | 26/F | Iran | Face | Disseminated | Lungs | CARD9 | FCZ, ITC, VRC | PR, relapse | PHM |
| Wang 2015 | 14/F | China | Trunk | Few, distant | No | No | ITC, ITC, TBF | CR | PHM |
| Wang 2015 | 23/F | China | Finger | Few, regional | No | No | 5-FC | Lost to follow-up | PHM |
| Bohelay 2016 | 76/M | France | Finger | Unique | No | Yes | Surgery, ITC | Relapse, CR | PHM |
| Srinivas 2016 | 12/M | India | Foot | Disseminated | Bone and brain | No | ITC, VRC | CR | CBM |
| Wendy 2016 | 45/M | Brazil | Purulent subcutaneous cyst | Regional | No | Yes | ITC | CR | PHM |
| E. DERMATITIDIS: | |||||||||
| Hiruma 1993 | 24/M | Japan | Brain abscess | Disseminated | Brain | None | MCZ, 5-FC, AMPH-B, KTC | Death | PHM |
| Lye 1993 | 39/M | Singapore | Peritonitis | Disseminated | Peritonitis | Peritoneal dialysis | Catheter removal, FLC | NM | Peritonitis |
| Blaschke-Hellmessen 1994 | 3/M | Germany | Fungemia | Disseminated | blood | AML | Catheter removal, AMPB, 5-FC | Death | Acute leukemia |
| Ajanee 1996 | 70/M | Singapore | Brain abscess | Disseminated | Brain | None | AMB, Op | Death | PHM |
| Woollons 1996 | 58/F | UK | Phaeohyphomycosis | Regional | No | RA, steroid | ITC, Op | Cure | Lung cancer |
| Nachman 1996 | 3/M | USA | Fungemia | Disseminated | Brain | HIV | Catheter removal, AMB, ITC | Death | PHM |
| Chang 2000 | 28/M | Korea | Meningitis, brain abscess | Disseminated | Brain | None | AMB, Op | Death | PHM |
| Vlassopoulos 2001 | 53/F | Greece | Peritonitis | Disseminated | Peritonitis | Peritoneal dialysis | Catheter removal, FLC | Cure | Peritonitis |
| Diemert 2001 | 29/F | Canada | Pneumonia | Regional | NO | Cystic fibrosis | AMB, ITC, VRC | Cure | Cystic fibrosis |
| Liou 2002 | 62/M | Taiwan | Lymphadinitis | Disseminated | Unknown | AML | AMB, ITC | Relapse | Lymphadinitis |
| Myoken 2003 | 39/F | Japan | Invasive stomatitis | Disseminated | Peritonitis | AML | ITC, AMB | Cure | Stomatitis |
| Greig 2003 | 55/F | UK | Peritonitis | Disseminated | Peritonitis | Peritoneal dialysis | Catheter removal, AMB | Cure | Peritonitis |
| Tseng 2005 | 58/F | Taiwan | Fungemia | Disseminated | Blood | Lung cancer | Catheter removal, AMB | Cure | Catheter-related fungaemia |
| Mukaino 2006 | 54/F | Japan | Pneumonia | Regional | No | Bronchiectasis | MCZ, nebulized AMB | Death | Pulmonary disorder |
| Taj-Aldeen 2006 | 54/F | Netherlands | Pneumonia | Regional | No | DM, systemic cancer | FLC, ITC, AMB | Cure | Pneumonia |
| Ozawa 2007 | 81/F | Japan | Pneumonia | Regional | No | None | FLC, ITC | Cure | Pneumonia |
| Alabaz 2009 | 8/M | Turkey | Systemic phaeohyphomycosis | Disseminated | Unknown | None | AMB, VRC | Death | PHM |
| Chang 2009 | 3/M | China | Brain abscess, meningitis | Disseminated | Brain | None | AMB, FLC, ITC | Death | PHM |
| Hong 2009 | 11/F | Korea | Liver chirosis | Disseminated | Liver | None | VRC, liver transplant | Death | Liver cirrhosis |
| Oztas 2009 | 24/F | Turkey | Systemic phaeohyphomycosis | Disseminated | Unknown | None | AMB, VRC | Cure | PHM |
| Griffard 2010 | 16/F | USA | Pneumonia | Regional | No | Cystic fibrosis | ITC, VRC | Relapse | Pneumonia |
| Bulloch 2011 | 86/F | USA | Lung nodule | Regional | No | Dementia | VRC | Cure | Lung nodule |
| Russo 2010 | 17/M | Argentina | Phaeohyphomycosis | Regional | No | None | ITC, Op | Cure | PHM |
| Suzuki 2012 | 65/M | Japan | Lung nodule | Regional | No | Multiple myeloma | VRC, Op | Cure | Lung nodule |
| Li 2010 | 19/F | China | Meningitis | Disseminated | Brain | None | NM | Death | Meningitis |
| Li 2010 | 30/F | China | Meningitis | Disseminated | Brain | None | NM | Death | Meningitis |
| Li 2010 | 3/M | China | Meningitis | Disseminated | Brain | None | AMB, 5-FC | Death | Meningitis |
| Alabaz 2009 | 8/M | China | hepatic lesions | Disseminated | Lymph node | None | AMB, VRC | Death | Lymph node |
| CDC 2002 | 77/F | USA | Meningitis | Disseminated | Brain | Contaminated injectable steroids | AMB, VRC, 5-FC | Death | Meningitis |
| CDC 2002 | 61/F | USA | Meningitis | Disseminated | Brain | Contaminated injectable steroids | VRC | Cure | Meningitis |
| CDC 2002 | 71/F | USA | Meningitis | Disseminated | Brain | Contaminated injectable steroids | NM | NM | Meningitis |
| CDC 2002 | 65/F | USA | Meningitis | Disseminated | Brain | Contaminated injectable steroids | NM | NM | Meningitis |
| CDC 2002 | 52/F | USA | Meningitis | Disseminated | Brain | Contaminated injectable steroids | NM | NM | Meningitis |
| Greig 2003 | 55/M | USA | Peritonitis | Disseminated | Yes | CAPD | AMB, ITC | Cure | Peritonitis |
| Liou 2002 | 62/F | China | Lymph-adenitis | Disseminated | Yes | AML | AMB, ITC, FLC | Cure | Lymphadenitis |
| Kerkmann 1999 | 19/F | Germany | Chronic otitis | Disseminated | Yes | NM | NYS | Cure | Chronic otitis |
| Kabel 1994 | 5/M | Netherlands | Intravascular | Disseminated | Yes | No | ITC | Cure | AML |
| Kusenbach 1992 | 6/F | Germany | Pneumonia | Regional | Yes | No | ITC | Cure | Cystic fibrosis |
| Kenney 1992 | 21/F | USA | Systemic | Disseminated | Yes | No | AMB, FLC, KTC | Cure | Chronic granulomatous disease |
| Ventin 1987 | 63/M | Germany | Systemic | Disseminated | Yes | Intravenous drug abuse | AMB | Death | Valvular aortal prosthesis |
| Patel 2013 | 50/F | India | Invasive | Disseminated | No | NO | AMB, FLC, VRC, ITC | Cure | Native-valve endocarditis |
| Woollons 1996 | 58/M | UK | Subcutaneous | Regional | No | Steroid injection | ITC, skin grafting, surgery | Cure | Painful nodules on right hand |
| Crosby 1989 | 60/M | USA | Subcutaneous | Regional | No | NO | Op | Cure | Subcutaneous |
| Scott 1986 | 74/M | Australia | Subcutaneous | Regional | No | Burn wood | Op | Cure | PHM |
| Patel 2006 | 52/M | USA | Superficial | Regional | No | Keratomilensis | NATA, ITC, FLC | Cure | Keratitis |
| Benaoudia 1999 | 31/M | France | Superficial | Regional | No | Keratoplasty | Steroids, ITC | Cure | Keratitis |
| Pospisil 1990 | 51/F | Czechia | Superficial | Regional | No | NM | BFC | Cure | Melanonychia |
| Pospisil 1990 | 35/M | Czechia | Superficial | Regional | No | RecklingHausen | AMB, iodine, vitamins | NM | Keratitis |
| Matsumoto 1993 | 42/M | Japan | Nail | Regional | No | No | ITC | Cure | Toe nail |
| Matsumoto 1992 | 51/F | Japan | PHM | Regional | No | Diabetes | BFC | Cure | Toe nail |
| Sood 2014 | 21/M | India | Cerebral | Disseminated | Brain | NM | Op, AMB, VRC | Cure | Ear abscess |
| Ajanee 1996 | 70/M | Pakistan | Invasive | Disseminated | Brain | None | AMB, Op | Death | Brain abscess |
| Simpson 1995 | 53/F | UK | Peritoneal dialysis | Disseminated | Yes | Peritoneal dialysis | Catheter removal, FLC | Cure | Peritonitis |
| Haase 1990 | 54/F | Germany | Pneumonia | Regional | No | None | MCZ, nebulized AMB | Cure | Pneumonia |
| Crosby 1989 | 60/M | USA | Endophthalmitis | Regional | No | None | ITC, Op | Cure | Endophthalmitis |
| Hu 2014 | 8/M | China | Lung, CNS | Disseminated | No | None | 5-FC, AMB, VRC | Cure | Pneumonia |
| Lanternier 2014 | 6/F | France | Brain, liver | Disseminated | Brain | CARD9- | NM | Relapse | NM |
| Chen 2016 | 78/M | China | Forearm | Regional | No | None | ITC | Cure | PHM |
| Watanabe 1961 | 41/M | Japan | Cheek | Regional | No | None | NM | Cure | PHM |
| Shimazono 1963 | 30/F | Japan | Brain | Disseminated | Brain | None | NM | Death | PHM |
| Sugawara 1964 | 15/M | Japan | Cheek | Disseminated | liver, gall bladder | Trauma | NM | Death | PHM |
| Tsai 2005 | 19/F | China | Cholelithiasis | Disseminated | Brain | Cholelithiasis | NM | Death | PHM |
| Harada 1989 | 15/M | Japan | Cutaneous | Disseminated | Lung | Cutaneous | NM | Death | PHM |
| Hohl 1983 | 65/M | USA | Subcutaneous | Regional | No | None | NM | Cure | PHM |
| Levenson 1984 | 29/M | USA | Cornea | Regional | No | Diabetes | NM | Cure | PHM |
| Crosby 1989 | 55/F | USA | Subcutaneous | Regional | No | Angina pectoris | NM | Cure | PHM |
| Barenfanger 1989 | 79/F | USA | Lung | Regional | No | Polymyositis | NM | Cure | PHM |
| Sharkey 1990 | 58/M | USA | Subcutaneous | Regional | No | Bronchiectasis | NM | Cure | PHM |
| Sharkey 1990 | 77/M | USA | Skin | Regional | No | Vasculitis | NM | Cure | PHM |
| Margo 1990 | 75/F | USA | Eye | Regional | No | Rheumatoid | NM | Cure | PHM |
| Crosby 1989 | 55/F | USA | Polymyositis | Disseminated | Blood | Polymyositis | NM | Death | PHM |
| Matsumoto 1990 | 34/M | Japan | Subcutaneous | Regional | No | Leukemia | NM | Cure | PHM |
| Myoken 2003 | 39/F | Japan | Gingiva | Regional | No | Leukemia | Op | Cure | PHM |
| Matsumoto 1992 | 51/F | Japan | Toe nail | Regional | No | None | BFC | Cure | PHM |
| Griffard 2010 | 16/F | USA | Acute respiratory exacerbations | Disseminated | Yes | Cystic fibrosis | VRC | Relapse | PHM |
| Diemert 2001 | 29/F | Canada | Lung | Disseminated | Yes | Cystic fibrosis | AMB, VRC | Cure | Invasive pulmonary |
| Kenney 1992 | 21/F | India | Cerebral | Disseminated | Brain | None | AMB, Op | Cure | Systemic infection |
Seventy seven E. dermatitidis cases and 36 E. spinifera cases have been reported in the English literature; main clinical characteristics are included.
In contrast, E. spinifera is rarely involved in infections limited to the skin. Some cases (Rajam et al., 1958; Barba-Gomez et al., 1992; Padhye et al., 1996; Develoux et al., 2006; Tomson et al., 2006; Srinivas et al., 2016) were reported as chromoblastomycosis, although typical muriform cells were mostly lacking. Fatal systemic infections are known; 36 cases have been recorded in English and Chinese literature (Rajam et al., 1958; Nishimura and Miyaji, 1983; Padhye et al., 1983, 1984; Mirza et al., 1993; Campos-Takaki and Jardim, 1994; de Hoog et al., 1999; Rajendran et al., 2003; Negroni et al., 2004; Dutriaux et al., 2005; Baubion et al., 2008; Singal et al., 2008; Fothergill et al., 2009; Harris et al., 2009; Radhakrishnan et al., 2010; Li et al., 2011; Badali et al., 2012; Daboit et al., 2012; Lin et al., 2012; Bohelay et al., 2016; Silva et al., 2017; Table 2). Among the 12 systemic infections, all cases concerned immunocompetent patients. Osteotropism was mentioned in 5 cases, neurotropism in one case. An Asian predilection was notable (83%, 10/12). The fungus has not been encountered in CF lungs.
Geography
Exophiala dermatitidis is known from natural habitats from tropical regions and has been reported in Brazil (Reiss and Mok, 1979), Nigeria (Muotoe-Okafor and Gugnani, 1993), and Thailand (Sudhadham et al., 2008; Zeng and De Hoog, 2008). In the domestic, man-made environment and in patients the fungus has a global distribution (Sudhadham et al., 2008; Zeng and De Hoog, 2008). Numerous isolates have been recorded from e.g., the following countries: Germany (Horre et al., 2004), Italy, Japan (Hiruma et al., 1993), Slovenia (Zalar et al., 2011), Sweden (Kondori et al., 2011), The Netherlands (de Hoog et al., 2005), Turkey (Dogen et al., 2013b), and USA (Zeng et al., 2007), covering several continents. E. spinifera was found to have a similar global distribution judging from literature and 48 available strains, with a slight bias to (sub) tropical regions: Argentina, Brazil, China, Colombia, Germany, India, Italy, Mexico, Papua New Guinea, Senegal, Thailand, and USA (Figure 1). Most of the isolates concerned patient materials.
Diversity
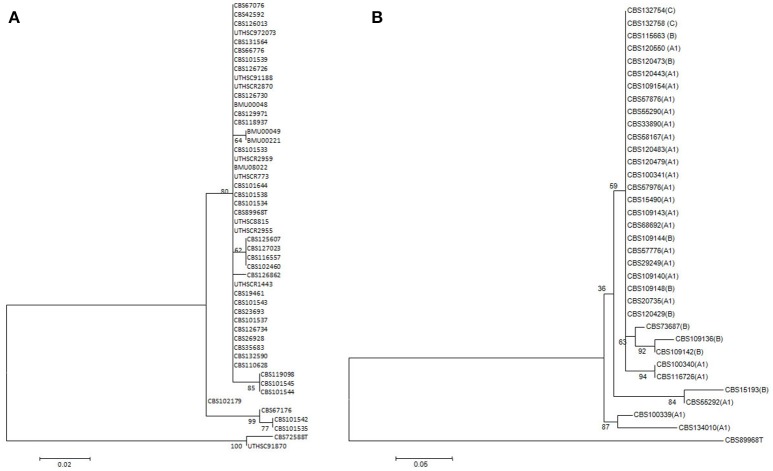
Exophiala dermatitidis is known to have three main genotypes in ITS, viz. A, B, and C (Rath et al., 1997; Sudhadham et al., 2010), of which genotype A has been subdivided further (Rath et al., 1997). Single-gene trees of TEF1 and TUB showed that there is random variation among partitions. This indicates that the published ITS haplotypes represent different variants of a single species. Haplotype diversity (Rozas and Rozas, 1995) for E. dermatitidis was as follows, TEF1: h = 10, Hd = 0.4825; TUB: h = 12, Hd = 0.6626, ITS: h = 10, Hd = 0.7077. The same parameters for E. spinifera were: TEF1: h = 10, Hd = 0.4541, TUB: h = 11, Hd = 0.8415, ITS: h = 12, Hd = 0.7606. No consistent difference was found in the multilocus tree between clinical and environmental strains, indicating that isolates from patients are likely to have an environmental origin. The nearest neighbor is Exophiala phaeomuriformis, which differs phenotypically by having a lower maximum growth temperature (Matos et al., 2003).
The 48 strains of E. spinifera, when sequenced for ITS, TUB and TEF1 did not show bootstrap-supported subclusters, and clustering was not concordant between genes. No consistent difference was found between clinical and environmental strains, which may indicate that the isolates from patients are likely to have an environmental origin (Figure 2). The nearest neighbor is Exophiala exophialae, represented by three strains differing in concordant ITS and TEF1 sequences.
Figure 2.
Dendrograms based on sequences of TEF1 region of rDNA gene of E. spinifera and E. dermatitidis. Dendrograms are constructed using Maximum likelihood method with correction of Kimura 2 parameter (K2P) in the Bionumerics package. Bootstrap support calculated using 1,000 replicates. Exophiala oligosperma CBS 725.88 and UTHSC 91-870 were used as outgroup in the E. spinifera tree, E. spinifera CBS 899.68 in the E. dermatitidis tree. (A) E. spinifera. (B) E. dermatitidis. Scale bars represent the estimated number of base substitutions per site.
Pathogenic traits
Results are presented in Table 3. Both species produce extracellular slime around 5–7 day-old budding cells at 24°C. In E. spinifera this occurs in the form of capsules of 5–6 μm width, of equal dimensions in environmental and clinical strains. The EPS produced under similar conditions in E. dermatitidis was diffused. Comparative genomic analyses using E. spinifera, E. dermatitidis, and C. neoformans, a model organism for capsule studies, revealed few differences between the black yeasts. Among the 62 genes correlated with the production and regulation of the polysaccharide capsule in C. neoformans, 45 and 42 orthologous genes were found in E. spinifera and E. dermatitidis, respectively (Table 4). Contrary to E. spinifera, where homologs of the alpha glucan synthase gene (AGS1) was found, in E. dermatitidis this homolog was absent, which might lead to slower growth, sensitivity to temperature and lack of structured capsule polysaccharide on budding cell surfaces (Reese et al., 2007). In addition, homologs corresponding to WSP1, a protein with multiple functions including production of the polysaccharide capsule and secretion of the enzyme urease (Shen et al., 2011), were identified in E. spinifera but seemed to be lost in the studied strain of E. dermatitidis.
Table 3.
Physiological and other phenotypic test results of 20 selected strains of each species.
| Accession number | PST (mM) | 5% NaCl | 10% NaCl | 5% MgCl2 | 10% MgCl2 | Cycloheximide | Lipolysis | Proteolysis | Hemolysis | Urease | 20% Sucrose | 40% Sucrose | 60% Sucrose | Cell shape 24°C | Cell shape 37°C | Capsule/EPS 24°C | Capsule/EPS 37°C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. SPINIFERA | |||||||||||||||||
| CBS 101533 | 9 | + | − | + | + | ++ | + | + | − | ++ | ++ | + | − | Meristematic | Meristematic | C | − |
| CBS 101539 | 9 | + | − | + | + | ++ | + | + | + | w | ++ | + | − | Meristematic | Yeast | C | − |
| CBS 116557 | 9 | + | − | + | + | ++ | + | + | − | + | ++ | + | − | Hyphae | Yeast | C | − |
| CBS 425.92 | 12 | + | − | + | + | ++ | + | + | − | w | ++ | + | − | Meristematic | Yeast | C | − |
| CBS 667.76 | 9 | + | − | + | + | ++ | + | + | − | ++ | ++ | − | − | Meristematic | Meristematic/Hyphae | C | − |
| CBS 670.76 | 9 | + | − | + | + | ++ | + | + | + | − | ++ | − | − | combination | Hyphae | C | − |
| CBS 126013 | 12 | + | − | + | + | ++ | + | + | + | ++ | ++ | − | − | Meristematic | Yeast | C | − |
| CBS 127023 | 12 | + | − | + | + | ++ | + | + | + | ++ | ++ | + | − | Meristematic | Yeast | C | − |
| CBS126726 | 9 | + | − | + | + | ++ | + | + | + | w | ++ | − | − | Meristematic | Yeast | C | − |
| CBS 131564 | 12 | + | − | + | + | ++ | + | + | + | ++ | ++ | − | − | Yeast | Yeast | C | − |
| CBS 101543 | 9 | + | − | + | + | ++ | + | + | + | ++ | ++ | − | − | Yeast | Yeast | C | − |
| CBS 102179 | 9 | + | − | + | + | ++ | + | + | − | ++ | ++ | + | − | Meristematic | Yeast | C | − |
| CBS 119098 | 9 | + | − | + | + | ++ | + | + | − | ++ | ++ | + | − | Meristematic | Meristematic | C | − |
| BMU 00048 | 9 | + | + | + | + | ++ | + | + | + | − | ++ | + | − | Meristematic | Meristematic | C | − |
| BMU 00049 | 12 | + | − | + | + | ++ | + | + | − | ++ | ++ | + | − | Hyphae | Hyphae/Meristematic | C | − |
| CBS 125607 | 12 | + | − | + | + | ++ | + | − | + | W | ++ | + | − | Hyphae/Meristematic | Meristematic | C | − |
| CBS 129971 | 9 | + | − | + | + | ++ | + | + | − | − | ++ | + | − | Meristematic | Hyphae | C | − |
| CBS 269.28 | 6 | + | − | + | + | ++ | + | + | − | ++ | ++ | + | − | Meristematic | Hyphae | C | − |
| CBS 899.68 | 9 | + | − | + | + | ++ | + | + | − | − | ++ | + | − | Meristematic | Meristematic | C | − |
| CBS 194.61 | 12 | + | − | + | + | ++ | + | + | + | ++ | ++ | + | − | Meristematic | Meristematic | C | − |
| E. DERMATITIDIS | |||||||||||||||||
| CBS 134010 | 6 | + | − | + | − | + | − | − | − | ++ | ++ | + | − | Meristematic | Yeast/Hyphae | E | E |
| CBS 120483 | 9 | + | − | + | − | + | − | − | − | − | ++ | − | − | Meristematic | Yeast/Hyphae | E | E |
| CBS 552.90 | 6 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | − | − |
| CBS 525.76 | 6 | + | − | + | − | + | − | + | − | W | ++ | + | − | Yeast | Yeast | E | E |
| CBS 292.49 | 6 | + | − | + | − | + | − | + | − | − | ++ | + | − | Meristematic | Meristematic | E | E |
| CBS 120443 | 6 | + | − | + | − | + | − | − | − | − | ++ | + | − | Meristematic | Yeast | E | E |
| CBS 120550 | 6 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | E | E |
| CBS 578.76 | 9 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast/Hyphae | E | E |
| CBS 115663 | 9 | + | − | + | − | + | − | − | − | − | ++ | + | − | Meristematic | Meristematic | E | E |
| CBS 207.35 | 9 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | E | E |
| CBS 686.92 | 6 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | E | E |
| CBS 120429 | 6 | + | − | + | − | + | − | − | − | W | ++ | − | − | very slow growth | E | E | |
| CBS 120473 | 6 | + | − | + | − | + | − | − | − | ++ | ++ | + | − | Yeast | Yeast | E | E |
| CBS 120472 | 9 | + | − | + | − | + | − | − | − | − | ++ | + | − | Meristematic | Hyphae | E | E |
| CBS 109144 | 9 | + | − | + | − | + | − | − | − | W | ++ | + | − | Meristematic | Meristematic | E | E |
| CBS 109149 | 9 | + | − | + | − | + | − | − | − | − | ++ | − | − | Yeast/Hyphae | Yeast/Hyphae | E | − |
| CBS 132754 | 9 | + | − | + | − | + | − | − | − | − | ++ | − | − | Yeast | Yeast | E | − |
| CBS 123474 | 6 | + | − | + | − | + | − | − | − | − | ++ | − | − | Meristematic | Meristematic | E | − |
| CBS 132758 | 3 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | E | − |
| CBS 109154 | 9 | + | − | + | − | + | − | − | − | − | ++ | + | − | Yeast | Yeast | E | − |
EPS, extracellular polysaccharide; PST, Peroxide stress tolerance (maximum concentration tolerated). W or w, weak positive; +, positive; ++, strong positive; −, negative.
Table 4.
Polysaccharide capsule production related genes.
| C. neoformans-Gene ID | Name | Capsule phenotype | References | E. spinifera-Gene ID | E. dermatitidis-Gene ID |
|---|---|---|---|---|---|
| CNAG_00125 | CRG1 | Hyper | Wang et al., 2004 | PV08_00096T0 | HMPREF1120_07029T0 |
| CNAG_00268 | ILV2 | Hypo | Kingsbury et al., 2004 | PV08_11571T0 | HMPREF1120_02016T0 |
| CNAG_00375 | GCN5 | Hypo | ÒMeara et al., 2010a | PV08_07822T0 | HMPREF1120_05017T0 |
| CNAG_00396 | PKA1 | Hypo | D'Souza et al., 2001 | N/A | N/A |
| CNAG_00440 | SSN801 | Hyper | Liu et al., 2008 | PV08_01597T0 | HMPREF1120_01045T0 |
| CNAG_00570 | BCY1 | Hyper | D'Souza et al., 2001 | PV08_10852T0 | HMPREF1120_06501T0 |
| CNAG_00600 | CAP60 | Hypo | Chang and Kwon-Chung, 1998; Moyrand and Janbon, 2004 | N/A | N/A |
| CNAG_00697 | UGE1 | Hyper | Moyrand et al., 2007 | PV08_02096T0 | HMPREF1120_01443T0 |
| CNAG_00721 | CAP59 | Hypo | Chang and Kwon-Chung, 1994; Moyrand and Janbon, 2004 | N/A | N/A |
| CNAG_00746 | CAS35 | Hypo | Moyrand et al., 2004, 2007 | N/A | N/A |
| CNAG_00769 | PBS2 | Hyper | Bahn et al., 2005 | PV08_02653T0 | HMPREF1120_02538T0 |
| CNAG_01106 | VPH1 | Hypo | Erickson et al., 2001 | PV08_05620T0 | HMPREF1120_06087T0 |
| CNAG_01172 | PBX1 | Hypo | Liu et al., 2007 | N/A | N/A |
| CNAG_01371 | CRG2 | Hyper | Shen et al., 2008 | PV08_07821T0 | HMPREF1120_05016T0 |
| CNAG_01523 | HOG1 | Hyper | Bahn et al., 2005 | PV08_06527T0 | HMPREF1120_05833T0 |
| CNAG_01551 | GAT201 | Hypo | Liu et al., 2008 | PV08_04845T0 | HMPREF1120_00248T0 |
| CNAG_01626 | ADA2 | Hypo | Haynes et al., 2011 | PV08_00126T0 | HMPREF1120_06980T0 |
| CNAG_01654 | CAS34 | GXM defect | Moyrand et al., 2007 | N/A | N/A |
| CNAG_01727 | SSA1 | Hyper | Zhang et al., 2006 | PV08_02973T0 | HMPREF1120_02626T0 |
| CNAG_01845 | PKC1 | Hypo | Heung et al., 2005 | PV08_09341T0 | HMPREF1120_07353T0 |
| CNAG_01890 | MET6 | Hypo | Pascon et al., 2004 | PV08_04416T0 | HMPREF1120_05363T0 |
| CNAG_02029 | WSP1 | Hypo | Shen et al., 2011 | PV08_03067T0 | N/A |
| CNAG_02153 | TUP1 | Hyper | Lee et al., 2009 | PV08_11209T0 | HMPREF1120_04775T0 |
| CNAG_02215 | HAP3 | Hypo | Jung et al., 2010 | PV08_04195T0 | N/A |
| CNAG_02236 | PPG1 | Hypo | Gerik et al., 2005 | PV08_01602T0 | HMPREF1120_01040T0 |
| CNAG_02702 | GEF1 | Hypo | Zhu and Williamson, 2003 | PV08_01769T0 | HMPREF1120_01007T0 |
| CNAG_02797 | CPL1 | Hypo | Liu et al., 2008 | N/A | N/A |
| CNAG_02885 | CAP64 | Hypo | Chang et al., 1996; Moyrand and Janbon, 2004 | N/A | N/A |
| CNAG_03120 | AGS1 | Hypo | Reese et al., 2007 | PV08_03292T0 | N/A |
| CNAG_03202 | CAC1 | Hypo | Alspaugh et al., 2002 | PV08_06497T0 | HMPREF1120_06227T0 |
| CNAG_03322 | UXS1 | Xylosolation defect | Moyrand et al., 2002 | N/A | N/A |
| CNAG_03438 | HXT1 | Hyper | Chikamori and Fukushima, 2005 | N/A | N/A |
| CNAG_03582 | RIM20 | Hypo | ÒMeara et al., 2010b | PV08_05544T0 | HMPREF1120_02658T0 |
| CNAG_03670 | IRE1 | Hypo | Cheon et al., 2011 | PV08_04820T0 | HMPREF1120_00610T0 |
| CNAG_03818 | SSK1 | Hyper | Bahn et al., 2007 | PV08_04132T0 | HMPREF1120_04973T0 |
| CNAG_04162 | PKA2 | Hypo | D'Souza et al., 2001 | PV08_06181T0 | HMPREF1120_06255T0 |
| CNAG_04312 | MAN1 | Hypo | Wills et al., 2001 | PV08_06851T0 | HMPREF1120_08308T0 |
| CNAG_04505 | GPA1 | Hypo | Alspaugh et al., 1997 | PV08_06528T0 | HMPREF1120_05834T0 |
| CNAG_04730 | GPR4 | Hypo | Xue et al., 2006 | N/A | N/A |
| CNAG_04864 | CIR1 | Hypo | Jung et al., 2006 | PV08_09234T0 | HMPREF1120_00896T0 |
| CNAG_04952 | CPS1 | Hypo | Chang et al., 2006 | PV08_07536T0 | HMPREF1120_08949T0 |
| CNAG_04969 | UGD1 | Hypo | Griffith et al., 2004; Moyrand and Janbon, 2004 | PV08_08061T0 | HMPREF1120_03278T0 |
| CNAG_05081 | PDE1 | Hyper | Hicks et al., 2005 | N/A | N/A |
| CNAG_05139 | UGT1 | Hyper | Moyrand et al., 2007 | PV08_01002T0 | HMPREF1120_07559T0 |
| CNAG_05218 | ACA1 | Hypo | Bahn et al., 2004 | PV08_06686T0 | HMPREF1120_08701T0 |
| CNAG_05222 | NRG1 | Hypo | Cramer et al., 2006 | N/A | N/A |
| CNAG_05431 | RIM101 | Hypo | ÒMeara et al., 2010b | PV08_06048T0 | HMPREF1120_00699T0 |
| CNAG_05562 | PBX2 | Hypo | Liu et al., 2007 | N/A | N/A |
| CNAG_05563 | HOS2 | Hyper | Liu et al., 2008 | N/A | N/A |
| CNAG_05581 | CHS3 | Hyper | Baker et al., 2007, Banks et al., 2005 | PV08_01895T0 | HMPREF1120_07721T0 |
| CNAG_05703 | LRG1 | Hypo | Gerik et al., 2005 | PV08_01033T0 | HMPREF1120_07444T0 |
| CNAG_05817 | GMT1 | Hypo | Cottrell et al., 2007 | PV08_03821T0 | HMPREF1120_04904T0 |
| CNAG_06301 | SCH9 | Hyper | Wang et al., 2004 | PV08_11389T0 | HMPREF1120_01691T0 |
| CNAG_06591 | SET302 | Hyper | Liu et al., 2008 | N/A | N/A |
| CNAG_06808 | CPRa | Hypo | Chang et al., 2003 | N/A | N/A |
| CNAG_07408 | STE20 | Hypo | Wang et al., 2002 | PV08_01113T0 | HMPREF1120_05115T0 |
| CNAG_07470 | PDE2 | Hyper | Hicks et al., 2005 | PV08_05494T0 | HMPREF1120_02921T0 |
| CNAG_07554 | CAP10 | Hypo | Chang and Kwon-Chung, 1999; Moyrand and Janbon, 2004 | PV08_06099T0 | HMPREF1120_00655T0 |
| CNAG_07636 | CSR2 | Hyper | Baker et al., 2007, Banks et al., 2005 | PV08_01896T0 | HMPREF1120_07720T0 |
| CNAG_07680 | HAP5 | Hypo | Jung et al., 2010 | PV08_04111T0 | HMPREF1120_05506T0 |
| CNAG_07718 | CIN1 | Hypo | Shen et al., 2010 | PV08_01341T0 | HMPREF1120_00287T0 |
| CNAG_07937 | CAS1 | O-acetylation defect | Janbon et al., 2001 | PV08_08786T0 | N/A |
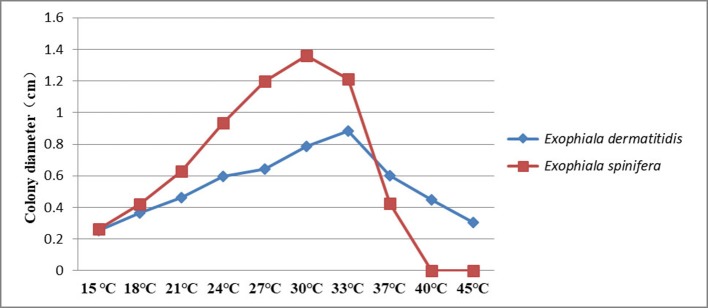
Both species had a broad range of thermotolerance (Figure 3). E. dermatitidis has an optimum at 33°C and still is able to grow at 45°C. E. spinifera has an optimum at 30°C, while its maximum growth temperature is 40°C. No growth is observed at 45°C; this temperature is fungistatic as about 50% of the strains showed regrowth when placed at 24°C.
Figure 3.
Thermotolerance of E. spinifera and E. dermatitidis. The averaged over 20 selected strains of each species are tested.
Hemolysis remained negative in all strains of E. dermatitidis and was positive in 50% of E. spinifera strains. E. dermatitidis showed slow acidification (yellow color change) and no proteolysis on Bromocresol purple-milk solids-glucose agar (BCP-MS-G). E. spinifera showed acidification within 1–2 weeks followed by a change to purple (alkaline) indicating casein decomposition (Figure 4). In general, growth velocity of E. spinifera was stimulated on protein medium. Nine strains of E. spinifera showed acidification of calcium carbonate medium, while only two E. dermatitidis were positive. Urease was positive in 25% of E. dermatitidis strains and in 80% of E. spinifera strains. The repertory of genes associated with nitrogen metabolism is composed by a single copy of the gene coding for urease (URE1) in both E. dermatitidis and E. spinifera (Table 5). In E. dermatididis, the homolog gene of URE1 shares 86% of BLASTP identity with that of the neurotropic black fungus Rhinocladiella mackenziei. The URE1 found in E. spinifera is highly conserved among other black yeasts members of the jeanselmei-clade, including Exophiala oligosperma (94% identity) and Exophiala xenobiotica (89% identity).
Figure 4.
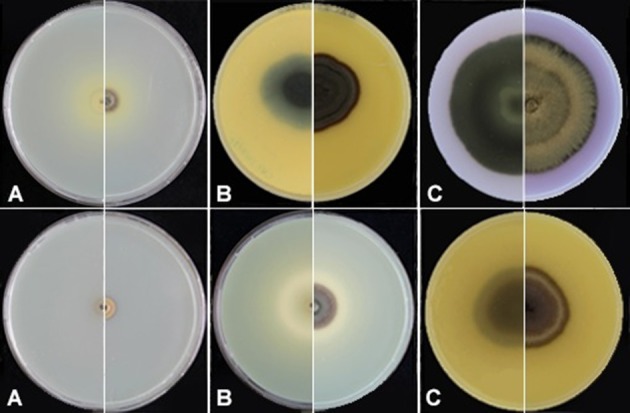
Proteolysis of E. spinifera strain CBS 125607 (upper panel) and E. dermatitidis strain CBS 120483 (lower panel). (A) Incubation for 1 week; (B) Incubation for 2.5 weeks; (C) Incubation for 1 month.
Table 5.
Ureases and their associated genes.
| Gene* | Z518_04527-UreF | Z518_02869-UreD | Z518_06116-UreG | Z518_07397-Nickel/cobalt transporter, high-affinity | Z518_09873-URE1 |
|---|---|---|---|---|---|
| E. dermatitidis | HMPREF1120_02686T0 | HMPREF1120_00803T0 | HMPREF1120_08744T0 | HMPREF1120_08266T0 | HMPREF1120_06619T0 |
| E. spinifera | PV08_05586T0 | PV08_01642T0 | PV08_07272T0 | PV08_07662T0 | PV08_10669T0 |
Reference genes extracted from R. mackenziei.
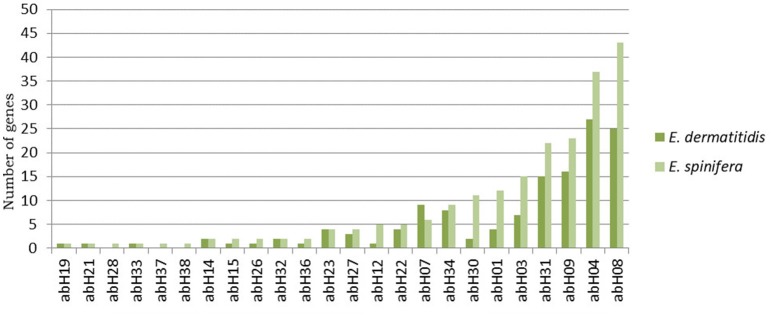
Lipolysis was positive in all E. spinifera strains and negative in E. dermatitidis strains. Genome-wide analyses revealed an abundance of lipases in both E. dermatididis and E. spinifera. Overall, the species possess 135 and 212 putative lipases, respectively. Twenty-one lipase families are conserved among these fungi, while three families seem to be specific to E. spinifera (Figure 5). The most abundant superfamily in E. spinifera was annotated ascytosolic hydrolases (abH08), including epoxide hydrolases and haloalkane dehalogenases. In E. dermatididis, the most abundant superfamily corresponds to Moraxella lipase 2-like (abH04). The superfamily abH04 consists of three families of bacterial esterases and lipases.
Figure 5.
Lipase composition in E. spinifera and E. dermatitidis. Twenty-one lipase families are conserved among these fungi, while three families seem to be specific to E. spinifera.
Both species grow well on 5% NaCl or MgCl2, while E. spinifera tolerated 10% MgCl2. No osmotolerance (60% sucrose) was observed. Cycloheximide tolerance was noted in both species, growth of E. spinifera being better than that of E. dermatitidis. Highest degrees of tolerance, with growth on SGA with 0.2% cycloheximide at 37°C, was observed in E. spinifera, while E. dermatitidis was somewhat inhibited. Growth at ambient temperature was with hyphae and yeast cells with a small number of isodiametrically inflating (meristematic) cells with dark and thick walls resembling muriform cells, but at 37°C yeast cells were preponderant. Incubated in acidic medium at 24 and 37°C, no septate muriform cells were produced, but dark, thick-walled yeast-like cells were prevalent. Comparative analyses of the cell wall genes in E. spinifera against the previously described gene set in E. dermatitidis revealed important differences between the species. In contrast to E. dermatididis, E. spinifera possesses multiple copies of genes involved in α-glucan metabolism (Table 6). For instance, the GT5 and GH13 genes (PV08_03291, PV08_03292, and PV08_03826), which were reported to be reduced in E. dermatitidis, were found in E. spinifera. These genes are required for the 1, 3-α-glucan synthesis. Conversely, all seven previously described chitin synthase genes were observed in both species (Table 6).
Table 6.
Cell wall modification pathway genes.
| Genes*: the key or differential genes in the cell wall modification pathway | E. dermatitidis | E. spinifera |
|---|---|---|
| Chitin synthase | ||
| CHS1 Class I, CHS2 class II | HMPREF1120_06816, HMPREF1120_07981 | PV08_00820, PV08_10842 |
| CHS3 Class III | HMPREF1120_06479 | PV08_10744 |
| CHS4 Class IV | HMPREF1120_07721 | PV08_01895 |
| CHS5 Class V, CHS7 Class VII | HMPREF1120_08776, HMPREF1120_08777 | PV08_07002, PV08_07003 |
| CHS6 Class VI | HMPREF1120_09115 | PV08_07085 |
| Chitin synthase like | HMPREF1120_01791 | |
| UDP-N-acetylglucosamine 6-dehydrogenase | HMPREF1120_01790 | PV08_06692 |
| Regulation of chitin synthase activity, by analogy to S. cerevisiae | ||
| SKT5 Activator of Chs3p during vegetative growth | HMPREF1120_07720 | PV08_01896 |
| Similarity with ScSkt5, activator of Chs3 | HMPREF1120_06335 | PV08_10210 |
| Similarity with ScSkt5, activator of Chs3 | HMPREF1120_05528 | PV08_11174 |
| BNI4 scaffold protein that tethers chitin synthase III (Chs3p) to the bud neck | HMPREF1120_05249 | PV08_03080 |
| ScCHS5 Similarity with ScChs5, component of exomer complex | HMPREF1120_05359 | PV08_04423 |
| ScCHS6 Similarity with ScChs6, component of exomer complex | HMPREF1120_01856 | PV08_11343 |
| Similarity with export control protein ScChs7 | HMPREF1120_00837 | PV08_09293 |
| ScCHS7 Similarity with export control protein ScChs7 | HMPREF1120_03003 | PV08_09547 |
| Chitin modification | ||
| Cda1/2 chitin deacetylase | HMPREF1120_08023 | PV08_03824 |
| Chitin degradation | ||
| ChiA GPI anchored class III chitinase | HMPREF1120_03399 | PV08_09305 |
| Class III chitinase | HMPREF1120_02334 | PV08_02554 |
| ChiB Class V chitinase | HMPREF1120_06669 | PV08_07861 |
| Class V chitinase | HMPREF1120_03714 | |
| Chitinase | HMPREF1120_04557 | PV08_03599 |
| Chitinase | HMPREF1120_07241 | |
| NagA Extracellular N-acetyl-beta-glucosaminidase | HMPREF1120_06035 | PV08_05624 |
| NagA | HMPREF1120_06285 | PV08_06135 |
| 1,3-a-glucan synthesis and processing | ||
| AgsB/A Catalytic subunits of the 1,3-a-glucan synthase complex (GT5 and GH13) - http://www.cazy.org/ | PV08_03291, PV08_03292, PV08_03826 | |
| HMPREF1120_08319 | PV08_06861 | |
| Putative amylase; similarity with H. capsulatum Amy1 | HMPREF1120_03460 | PV08_02428 |
| 1,3-b-glucan synthesis and processing | ||
| FksA Putative catalytic subunit 1,3-b-glucan synthase complex; ScFks1-like | HMPREF1120_03476 | PV08_10508 |
| ScSMI1 Putative regulatory component 1,3-b-glucan synthesis; ScKnr4-like | HMPREF1120_04893 | PV08_03822 |
| EngA Endo-1,3-b-glucanase (GH 81-family); ScEng1-like | HMPREF1120_09022 | PV08_06894 |
| Putative exo-1,3-b-glucanase family (GH 5); related to the ScExg1-family | HMPREF1120_04506 | PV08_03500 |
| HMPREF1120_06180 | PV08_06271 | |
| Putative exo-1,3-b-glucanase family (GH 55); related to Coniothyrium minitans exo-1,3-glucanase (Cmg1) | HMPREF1120_01556 | PV08_03602, PV08_11776 |
| HMPREF1120_05230 | ||
| Bgl2-family of putative 1,3-b-transglucosylases (GH 17) proposed to be involved | HMPREF1120_00547 | PV08_01318 |
| HMPREF1120_05209 | PV08_00062 | |
| HMPREF1120_06595 | PV08_10359 | |
| HMPREF1120_08449 | PV08_01193 | |
| HMPREF1120_04141 | PV08_08282 | |
| HMPREF1120_03066 | PV08_03009 | |
| HMPREF1120_08078 | PV08_10096 | |
| Crh1-family of putative transglycosidases (GH 16);involved in crosslinking b-glucan | HMPREF1120_04931 | PV08_03356 |
| HMPREF1120_00627 | PV08_04702 | |
| HMPREF1120_07927 | PV08_07455 | |
| HMPREF1120_02703 | PV08_05520 | |
| Gas-family of putative 1,3-b-transglucosylases (GH 72) proposed to be involved in connecting the emerging 1,3-b-glucan chains to the existing b-glucan | HMPREF1120_07283 | PV08_09503 |
| HMPREF1120_01763 | PV08_11789 | |
| HMPREF1120_03477 | PV08_10507 | |
| GelG 1,3-b-glucanosyltransferase | HMPREF1120_01682 | PV08_11613 |
| SunA Sun family, involved in septation, possibly b-glucosidase activity; | HMPREF1120_01649 | PV08_06721 |
| SunB | HMPREF1120_06902 | PV08_00042 |
| Kre6 Putative transglycosidase required for 1,6-b-glucan biosynthesis | HMPREF1120_01614 | PV08_06798 |
| CelA Similarity with cellulose synthases of the GT 2 family. Putatively involved in 1,3-b-/1,4-b-glucan synthesis | HMPREF1120_04699 | PV08_00315 |
| Mlg1 Mixed-linked glucanases in C. carbonum, hydrolyze 1,3-b-/1,4-b-glucans | HMPREF1120_05299 | |
| Mlg1 | HMPREF1120_02373 | PV08_09397 |
| Mlg1 | HMPREF1120_07765 | PV08_01870 |
| HMPREF1120_09051 | PV08_07166 | |
| Other cell wall biosynthesis proteins | ||
| Endo-mannanase family (GH 76) with a putative role in GPI-CWP incorporation; | HMPREF1120_04431 | PV08_03378 |
| HMPREF1120_03513 | PV08_10453 | |
| HMPREF1120_05522 | PV08_11178 | |
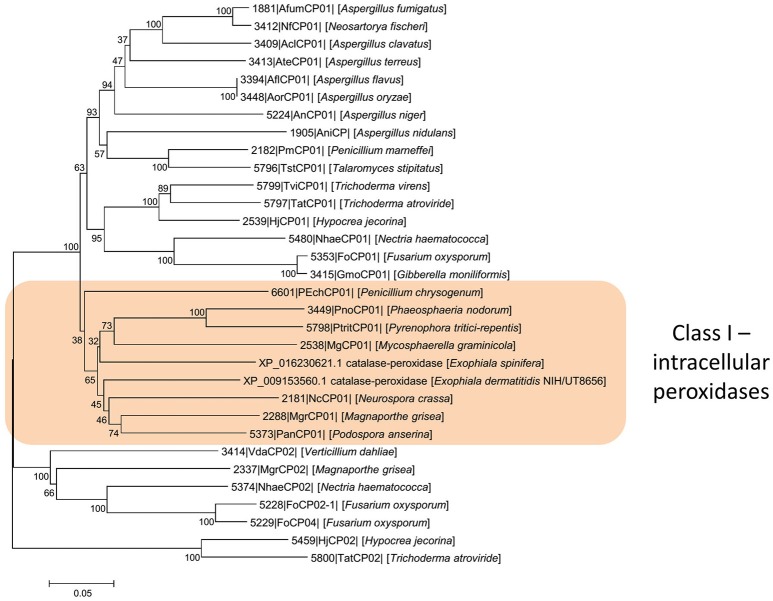
Exophiala dermatitidis tolerated 6–9 mM hydrogen peroxide, while the maximum concentration allowing growth of E. spinifera was 9–12 mM. With increasing H2O2-concentration, morphology of colonies changed from hyphae to yeast and cell walls tended to lose melanin (Figures 6, 7 and Tables 7, 8). In both species we predicted genes coding for the bifunctional catalase/peroxidase enzymes (PV08_11368 in E. spinifera and HMPREF1120_01299 in E. dermatitidis) that may reduce hydrogen peroxide. The genes carry the functional protein domain Haem peroxidase (IPR002016). Phylogenetic analyses indicated that it belongs to Class I, which includes intracellular peroxidases involved in cellular protection against toxic peroxides (Delort et al., 1989; Figure 8).
Figure 6.
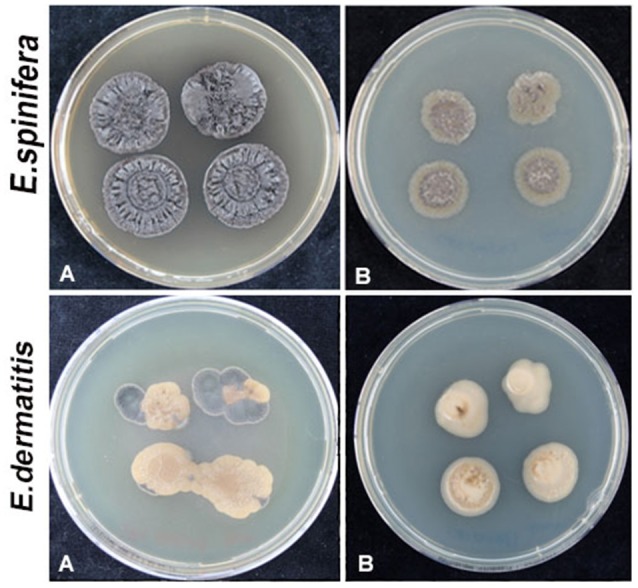
Peroxide tolerance for E. spinifera strain CBS 101542 and E. dermatitidis strain CBS 134010. Upper panel (A,B) melanized colonies of CBS 101542 subjected to CBS 101542 peroxide; Lower panel (A,B) colonies subjected to CBS 134010 peroxide showing loss of melanin.
Figure 7.
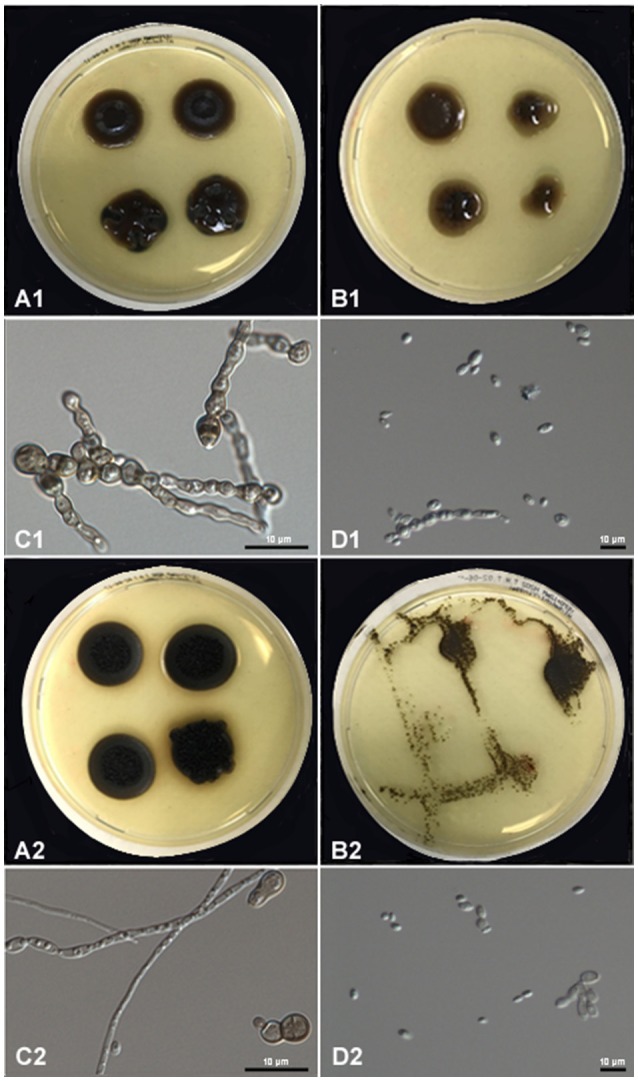
Peroxide tolerance of E. spinifera strain CBS 116557 (upper two panels) and E. dermatitidis strain CBS 115663 (lower two panels). (A) Show mycelial phase, (B) show yeast phase, (C) show muriform cell, (D) show yeast cell.
Table 7.
The assimilation responses of 40 strains to different compounds.
| No. | CBS number | Hydrogen peroxide | Tween-80 | Calcium carbonate | Urea | Sucrose | Sodium | Cycloheximide | Casein |
|---|---|---|---|---|---|---|---|---|---|
| E. DERMATITIDIS | |||||||||
| 1 | CBS134010 | + | − | − | + | + | + | + | − |
| 2 | CBS 120483 | + | − | + | − | + | + | + | − |
| 3 | CBS 552,90 | + | − | − | − | + | + | + | − |
| 4 | CBS207.35 | + | − | − | − | + | + | + | − |
| 5 | CBS525.76 | + | − | − | + | + | + | + | − |
| 6 | CBS292.49 | + | − | − | − | + | + | + | − |
| 7 | CBS 120443 | + | − | − | − | + | + | + | − |
| 8 | CBS 120550 | + | − | − | − | + | + | + | − |
| 9 | CBS 578,76 | + | − | − | − | + | + | + | − |
| 10 | CBS115663 | + | − | − | − | + | + | + | − |
| 11 | CBS 686,92 | + | − | − | − | + | + | + | − |
| 12 | CBS 120429 | + | − | − | + | + | + | + | − |
| 13 | CBS 120473 | + | − | + | + | + | + | + | − |
| 14 | CBS 120472 | + | − | − | − | + | + | + | − |
| 15 | CBS 109144 | + | − | − | + | + | + | + | − |
| 16 | CBS 109149 | + | − | − | − | + | + | + | − |
| 17 | CBS 132754 | + | − | − | − | + | + | + | − |
| 18 | CBS 123474 | + | − | − | − | + | + | + | − |
| 19 | CBS 132758 | + | − | − | − | + | + | + | − |
| 20 | CBS 109154 | + | − | − | − | + | + | + | − |
| E. SPINIFERA | |||||||||
| 1 | CBS 101533 | + | + | + | + | + | + | + | + |
| 2 | CBS 101539 | + | + | + | + | + | + | + | + |
| 3 | CBS 116557 | + | + | + | + | + | + | + | + |
| 4 | CBS 425.92 | + | + | − | + | + | + | + | + |
| 5 | CBS 669.76 | + | + | − | + | + | + | + | + |
| 6 | CBS 671.76 | + | + | − | + | + | + | + | + |
| 7 | CBS 126013 | + | + | + | − | + | + | + | + |
| 8 | CBS 127023 | + | + | + | + | + | + | + | + |
| 9 | CBS126726 | + | + | − | + | + | + | + | + |
| 10 | CBS 131564 | + | + | − | + | + | + | + | + |
| 11 | CBS 101543 | + | + | − | + | + | + | + | + |
| 12 | CBS 102179 | + | + | + | + | + | + | + | + |
| 13 | CBS 119098 | + | + | − | + | + | + | + | + |
| 14 | CBS 123468 | + | + | − | + | + | + | + | + |
| 15 | CBS 123469 | + | + | − | − | + | + | + | + |
| 16 | CBS 125607 | + | + | + | + | + | + | + | + |
| 17 | CBS 129971 | + | + | + | + | + | + | + | + |
| 18 | CBS 269.28 | + | + | − | − | + | + | + | + |
| 19 | CBS 899.68 | + | + | + | + | + | + | + | + |
| 20 | CBS 194.61 | + | + | − | − | + | + | + | + |
+, positive; −, negative.
Table 8.
Responses of 20 strains of E. spinifera and 20 of E. dermatitidis upon peroxide challenge.
| No. of strains at max. concentration tolerated | 3 mM | 6 mM | 9 mM | 12 mM |
|---|---|---|---|---|
| E. spinifera | 0 | 1 | 10 | 9 |
| E. dermatitidis | 1 | 10 | 9 | 0 |
| Yeast conversion at max. concentration | + | − | ||
| E. spinifera | 11 | 9 | ||
| E. dermatitidis | 16 | 4 | ||
| Melanization at max. concentration | + | − | ||
| Exophiala spinifera | 15 | 5 | ||
| Exophiala dermatitidis | 4 | 16 | ||
+, positive; −, negative.
Figure 8.
Phylogenetic analysis of intracellular peroxidases. The analysis indicates that they belong to Class I, which includes intracellular peroxidases involved in cellular protection against toxic peroxides. Scale bars represent the estimated number of base substitutions per site.
Galleria mellonella virulence model
To determine if there was any difference in pathogenicity between E. dermatitidis and E. spinifera, we determined the LD50 in Galleria mellonella larvae at 37°C. No significant difference in the LD50 values between E. dermatitidis and E. spinifera was observed. However, we did notice a difference in death rates between the species. At an inoculum of 107 Colony Forming Units per larvae, all larvae infected with E. dermatitidis died before day 6, while at that time point still 30% of E. spinifera infected larvae were alive. It took till day 10 before all E. spinifera infected larvae died. This difference in time-to-death between the two species was significant (Log-Rank, p = 0.001) (Figure 9). Within each species also a difference was noted between larvae infected with environmental isolates or with isolates obtained from clinical cases. The time-to-death in G. mellonella larvae infected with clinical isolates of E. dermatitidis was shorter than in G. mellonella larvae infected with environmental isolates of the same species (Log-Rank, p = 0.006). The same was true for E. spinifera (Log-Rank, p = 0.0326).
Figure 9.
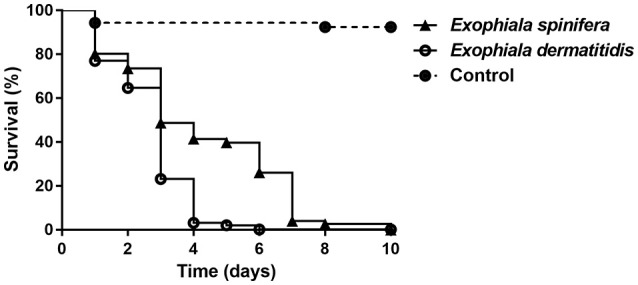
Galleria mellonella infection model of 10 strains for E. spinifera and 10 strains of E. dermatitidis tested at an inoculum density of 107 cells. No significant differences were observed between clinical and environmental strains.
Discussion
Melanized fungi of the order Chaetothyriales are frequently involved in human infection; the Atlas of Clinical Fungi lists 48 clinically relevant species. Nonetheless they are regarded as opportunists, as for only very few species a natural life cycle with an animal host has been suggested (Vicente et al., 2008). As opportunists, it is predicted that their invasive potential has to be explained from their environmental behavior. For example, agents of subcutaneous skin disease may be present on decaying thorns of prickly plants (Vicente et al., 2008), while hydrophilic yeast-like species can be carried by aerosols and are easily inhaled (Rath et al., 1997; Horre et al., 2004; Kondori et al., 2011). It is generally assumed that within these major categories of infection routes there is not much difference between species, the clinical course mainly being determined by portal of entrance and conditions of the host. In order to test this hypothesis, we compared two black yeast species with broad similarity in their growth form and infective ability, both being able to cause disseminated infections in immunocompetent humans. A simple overview of the literature learnt that the two species are clinically very different. E. dermatitidis is common as a pulmonary colonizer in patients with cystic fibrosis, where E. spinifera has never been observed.
The traumatic route may lead to subcutaneous infection. We found that both species tend to convert to yeast under hyperoxygen or temperature stress. Thus, mycetoma formation, as reported in Exophiala jeanselmei and exceptionally in Exophiala oligosperma, is less likely in E. dermatitidis and E. spinifera. Mycetoma grains are dense clumps of sterile hyphae which are not easily phagocytosed and provoke severe inflammation. Rather, the yeast conversion of E. dermatitidis and E. spinifera suggests potential dissemination in the bloodstream and is consistent with the clinical observation of disseminated infection.
Although both species are able to cause disseminated infections in otherwise apparently healthy hosts, E. dermatitidis regularly (36%) shows neurotropism, whereas this has never been observed in E. spinifera; in contrast, in the latter species some osteotropism (42%) has been mentioned (Rajam et al., 1958; Campos-Takaki and Jardim, 1994; Li et al., 2011; Srinivas et al., 2016).
The habitat choice of each fungus should explain observed types of opportunism on the human host (Vicente et al., 2008; Dogen et al., 2013b; Gumral et al., 2014). Our strain data show that the ecological differences between E. spinifera and E. dermatitidis are large. E. dermatitidis is found in habitats that are either toxic or poor in nutrients, suggesting evasion of microbial competition as a strategy of the fungus. In contrast, habitat choices of E. spinifera suggest some osmophily, while its regular presence on decaying scales of coconuts—a substrate exceptionally rich in black yeasts but where E. dermatitidis remained absent—was remarkable. Babassu coconut scales are rich in fatty acids and etheric oils; the relative abundance of lipase genes in E. spinifera is consistent with this habitat choice. E. dermatitidis is highly selected by hot and moist indoor facilities, particularly steam baths and dishwashers, where temperatures periodically are 60–90°C. Exophiala spinifera has not been observed in domestic environments, which not only indicates differences in ecological preference, but also in exposition to human hosts, e.g., inhalation by patients with cystic fibrosis.
In general E. spinifera was physiologically more active, as judged from its response to proteins, lipids, ureum, and acid production. The repertoire of protease families in E. spinifera and E. dermatitidis according to MEROPS classification (Teixeira et al., 2017) revealed striking protease family expansions in E. spinifera, such as the families M38, S09X and S33. These expansions are absent in E. dermatitidis and might be responsible for the differences in the protein degradation profile. Abundance of proteases at the expense of carbohydrate-active enzymes is often taken as an indication of vertebrate pathogenicity (El Kaoutari et al., 2013). Exophiala spinifera possesses multiple copies of genes involved in α-glucan metabolism, which is considered an essential virulence factor in chromoblastomycosis (Teixeira et al., 2017). Nonetheless the LD50 of E. spinifera was comparable to that of E. dermatitidis in the Galleria model, indicating no obvious difference in virulence between the two species. However, when larvae were infected with E. spinifera the time-to-death was prolonged compared to larvae infected with E. dermatitidis, indicating that at least in G. mellonella larvae E. spinifera was more virulent. Furthermore, the origin of the isolates appeared also to be important. In both E. dermatitidis and E. spinifera, a shorter time-to-death was obtained with clinical isolates compared to environmental isolates.
The species also showed more tolerance to cycloheximide and hydrogen peroxide. The response of both species to elevated oxygenic action was mostly by yeast conversion (Figure 7, Table 3) and loss of melanin (Figure 6, Table 8) rather than by the formation of muriform cells and melanization. This response is not in line with the expected pattern in agents of chromoblastomycosis. We therefore consider published cases of human chromoblastomycosis by E. dermatitidis or E. spinifera (Rajam et al., 1958; Li et al., 2011; Lanternier et al., 2015; Srinivas et al., 2016) as questionable. E. dermatitidis shows a higher degree of thermotolerance, as expressed in its prevalence in hot indoor wet cells. Temperatures in steam baths and dishwashers intermittently are far above the permissive temperature for growth. Tesei et al. (2015) demonstrated that the fungus upon hostile conditions turns down its metabolism rather than showing a physiological stress response, which might be a survival mechanism for the super-extreme. It is speculated that E. spinifera lacks this strategy, as it has never been isolated from indoor habitats with extreme temperatures.
In E. spinifera, two patient populations have been described, i.e., healthy children and adolescents on the one hand, and elderly patients on the other. Somewhat unexpectedly, the former group was associated with fatal disseminated infection, whereas the latter only developed mild disease with successful cure. In contrast, dissemination in E. dermatitidis mostly occurred in East-Asian immunocompetent patients, and involvement of cervical lymph nodes and central nervous system was frequently reported. The species has a global distribution in the domesticated environment (Sudhadham et al., 2008), and therefore it remains unexplained why this type of infection is nearly limited to East Asia.
In both species investigated, molecular diversity has been reported. E. dermatitidis has several ITS-based genotypes, but our study demonstrated that ribosomal variation did not correspond with variation in protein-coding genes, and thus the species can be regarded as a single biological entity, clearly separated from its nearest neighbor species E. phaeomuriformis. E. spinifera showed a nearly identical degree of intraspecific variation; only the distinction of a small cluster known as E. exophialae was supported in all partitions, confirming this group as a neighboring species. This led to the conclusion that E. dermatitidis and E. spinifera cannot be meaningfully subdivided, and no lineages with reduced gene flow seem to exist. The found degrees of variation in barcoding genes (ITS Hd = 0.76 and 0.71 in E. spinifera and E. dermatitidis, respectively) may be taken as a model for black yeasts. No ascosporulating sexual states have been observed in either of them—although non-sporulating fruiting bodies have been reported (Gueidan et al., 2008) in E. dermatitidis, and in both species only a single mating type (MAT1-2) has been observed (Teixeira et al., 2017), thus possibly propagation and evolution is largely by independent clones. All data indicate that variation in both species is limited to the strain level, and no predictive grouping is possible.
Strain-level variation did not show significant correlation between clinical and environmental origins. Phenotypic intraspecific variation was found e.g., in protease, urease and acid production, but none of these parameters could be linked (Table 3). Antifungal susceptibility shows some variability in both species (Badali et al., 2012), and this could not be linked to other types of variation either. Given the rather significant variation in susceptibility, it is recommended always to perform in vitro prior to therapy.
It is safe to say that neither species behaves as a human pathogen, i.e., having enhanced fitness by the use of human vectors. Rather, human infection is extremely coincidental, not playing a role in the evolution of the fungi, and therefore typically opportunistic in nature. However, our data show that even related opportunists sharing essential virulence factors in yeast phases and capsular budding cells may be clinically very different. Surprisingly, the majority of cases of both species occurred in patients without known immune disorder, and the frequency of black yeast infection does not seem to increase with growing populations of compromised patients. Possibly several of the disseminated cases were patients with hidden congenital immune defects such as CARD9 deficiency (Lanternier et al., 2015). Idiopathic invasive fungal infections should lead to a search for underlying inborn errors of immunity (Alcais et al., 2010; Casanova and Abel, 2013; Lanternier et al., 2015), but also to different sources of exposition and windows of opportunity for microbial growth.
Author contributions
YS carried out the literature search, strains collection, DNA extraction and sequencing, phylogenetic tree construction, physiology tests, Galleria mellonella virulence experiments, participated in the data analysis and drafted the manuscript. WL-vdS carried out the design of Galleria mellonella experiment and participated in the data analysis. LM carried out the genome data analysis and interpretation. BG participated in the data interpretation and analysis. RL and SdH participated in the design of the study, statistical analysis and manuscript revision and review. All authors read and approved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JAMÁ and handling Editor declared their shared affiliation.
Acknowledgments
We acknowledge Mickey Konings from Erasmus MC, University of Rotterdam for his work on the larvae experiment.
Footnotes
Funding. This work was supported by the international Cooperation and Exchanges Project (NSFC No. 81520108026) from National Natural Science Foundation of China.
References
- Alabaz D., Kibar F., Arikan S., Sancak B., Celik U., Aksaray N., et al. (2009). Systemic phaeohyphomycosis due to Exophiala (Wangiella) in an immunocompetent child. Med. Mycol. 47, 653–657. 10.1080/13693780802715815 [DOI] [PubMed] [Google Scholar]
- Alcais A., Quintana-Murci L., Thaler D. S., Schurr E., Abel L., Casanova J. L. (2010). Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann. N.Y. Acad. Sci. 1214, 18–33. 10.1111/j.1749-6632.2010.05834.x [DOI] [PubMed] [Google Scholar]
- Alspaugh J., Perfect J., Heitman J. (1997). Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 11, 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh J., Pukkila-Worley R., Harashima T., Cavallo L., Funnell D., Cox G., et al. (2002). Adenylyl cyclase functions downstream of the G-alpha protein Gpa1 and controls mating and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell. 1, 75–84. 10.1128/EC.1.1.75-84.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badali H., Chander J., Bayat M., Seyedmousavi S., Sidhu S., Rani H., et al. (2012). Multiple subcutaneous cysts due to Exophiala spinifera in an immunocompetent patient. Med. Mycol. 50, 207–213. 10.3109/13693786.2011.603367 [DOI] [PubMed] [Google Scholar]
- Bahn Y., Hicks J., Giles S., Cox G., Heitman J. (2004). Adenylyl cyclaseassociated protein AcaI regulates virulence and differentiation of Cryptococcus neoformans via the cyclic AMP-protein kinase a cascade. Eukaryot. Cell. 3, 1476–1491. 10.1128/EC.3.6.1476-1491.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn Y., Kojima K., Cox G., Heitman J. (2005). Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol. Biol. Cell. 16, 2285–2300. 10.1091/mbc.E04-11-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn Y.-S., Geunes-Boyer S., Heitman J. (2007). Ssk2 mitogen-activated protein kinase kinase kinase governs divergent patterns of the stress activated Hog1 signaling pathway in Cryptococcus neoformans. Eukaryot. Cell 6, 2278–2289. 10.1128/EC.00349-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L. G., Specht C. A., Donlin M. J., Lodge J. K. (2007). Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 6, 855–867. 10.1128/EC.00399-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks I. R., Specht C. A., Donlin M. J., Gerik K. J., Levitz S. M., Lodge J. K. (2005). A chitin synthase and its regulator protein are critical for chitosan production and growth of the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4, 1902–1912. 10.1128/EC.4.11.1902-1912.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barba-Gomez J. F., Mayorga J., McGinnis M. R., Gonzalez-Mendoza A. (1992). Chromoblastomycosis caused by Exophiala spinifera. J. Am. Acad. Dermatol. 26, 367–370. [DOI] [PubMed] [Google Scholar]
- Barth S., Fischer M., Schmid R. D., Pleiss J. (2004). The database of epoxide hydrolases and haloalkane dehalogenases: one structure, many functions. Bioinformatics 20, 2845–2847. 10.1093/bioinformatics/bth284 [DOI] [PubMed] [Google Scholar]
- Baubion E., Desbois N., Durox H., Riaux A., Deschamp L., Derancourt C., et al. (2008). Ulcerated nodular lesions on extremities of patient undergoing treatment for glioblastoma on French Caribbean island of Martinique. Med. Trop. 68, 537–540. [PubMed] [Google Scholar]
- Bohelay G., Robert S., Bouges-Michel C., Gerin M., Levy A., Fain O., et al. (2016). Subcutaneous phaeohyphomycosis caused by Exophiala spinifera in a European patient with lymphoma: a rare occurrence case report and literature review. Mycoses 59, 691–696. 10.1111/myc.12515 [DOI] [PubMed] [Google Scholar]
- Campos-Takaki G. M., Jardim M. L. (1994). Report of chronic subcutaneous abscesses caused by Exophiala spinifera. Mycopathologia 127, 73–76. [DOI] [PubMed] [Google Scholar]
- Carlini C. R., Ligabue-Braun R. (2016). Ureases as multifunctional toxic proteins: a review. Toxicon 110, 90–109. 10.1016/j.toxicon.2015.11.020 [DOI] [PubMed] [Google Scholar]
- Casanova J. L., Abel L. (2013). The genetic theory of infectious diseases: a brief history and selected illustrations. Annu. Rev. Genom. Hum. Genet. 14, 215–243. 10.1146/annurev-genom-091212-153448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Kwon-Chung K. J. (1994). Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell Biol. 14, 4912–4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Kwon-Chung K. J. (1998). Isolation of the third capsule-associated gene, CAP60, required for virulence in Cryptococcus neoformans. Infect. Immun. 5, 2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Kwon-Chung K. J. (1999). Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 18, 5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Jong A., Huang S., Zerfas P., Kwon-Chung K. J. (2006). CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans. Infect. Immun. 74, 3930–3938. 10.1128/IAI.00089-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Miller G. F., Kwon-Chung K. J. (2003). Importance of a developmentally regulated pheromone receptor of Cryptococcus neoformans for virulence. Infect. Immun. 71, 4953–4960. 10.1128/IAI.71.9.4953-4960.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. C., Penoyer L. A., Kwon-Chung K. J. (1996). The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 64, 1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Zhang J., Dong Z., Wang F. (2016). Cutaneous phaeohyphomycosis caused by Exophiala dermatitidis: a case report and literature review. Indian J. Dermatol. Venereol. Leprol. 82, 173–177. 10.4103/0378-6323.171013 [DOI] [PubMed] [Google Scholar]
- Cheon S. A., Jung Chen K.-W., Heitman Y.-L. J., Bahn Y.-S., Kang H. A. (2011). Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 7:e1002177. 10.1371/journal.ppat.1002177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikamori M., Fukushima K. (2005). A new hexose transporter from Cryptococcus neoformans: molecular cloning and structural and functional characterization. Fungal Genet. Biol. 42, 646–655. 10.1016/j.fgb.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Chotirmall S. H., McElvaney N. G. (2014). Fungi in the cystic fibrosis lung: bystanders or pathogens? Int. J. Biochem. Cell Biol. 52, 161–173. 10.1016/j.biocel.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Cottrell T. R., Griffith C. L., Liu H., Nenninger A. A., Doering T. L. (2007). The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot. Cell 6, 776–785. 10.1128/EC.00015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer K. L., Gerrald Q. D., Nichols C. B., Price M. S., Alspaugh J. A. (2006). Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot. Cell 5, 1147–1156. 10.1128/EC.00145-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby J. H., O'Quinn M. H., Steele J. C., Jr., Rao R. N. (1989). Fine-needle aspiration of subcutaneous phaeohyphomycosis caused by Wangiella dermatitidis. Diagn. Cytopathol. 5, 293–297. [DOI] [PubMed] [Google Scholar]
- Daboit T. C., Duquia R. P., Magagnin C. M., Mendes S. D., Castrillon M. R., Steglich R., et al. (2012). A case of Exophiala spinifera infection in Southern Brazil: molecular identification and antifungal susceptibility. Med. Mycol. 1, 72–75. 10.1016/j.mmcr.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog G. S., Matos T., Sudhadham M., Luijsterburg K. F., Haase G. (2005). Intestinal prevalence of the neurotropic black yeast Exophiala (Wangiella) dermatitidis in healthy and impaired individuals. Mycoses 48, 142–145. 10.1111/j.1439-0507.2004.01083.x [DOI] [PubMed] [Google Scholar]
- de Hoog G. S., Poonwan N., Gerrits van den Ende A. H. G. (1999). Taxonomy and pathogenicity of Exophiala spinifera and its relationship to E. jeanselmei. Stud. Mycol. 43, 133–142. [Google Scholar]
- De Hoog G. S., Queiroz-Telles F., Haase G., Fernandez-Zeppenfeldt G., Attili Angelis D., Gerrits Van Den Ende A. H., et al. (2000a). Black fungi: clinical and pathogenic approaches. Med. Mycol. 38(Suppl. 1), 243–250. 10.1080/mmy.38.s1.243.250 [DOI] [PubMed] [Google Scholar]
- De Hoog G. S., Guarro J., Figueras M. J., Gené J. (2000b). Atlas of Clinical Fungi, 2nd, Edn. Baarn; Reus: Centraalbureau voor Schimmelcultures; Universitat Rovira I Virgili. [Google Scholar]
- de Hoog G. S., Vicente V. A., Najafzadeh M. J., Harrak M. J., Badali H., Seyedmousavi S. (2011). Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia 27, 46–72. 10.3767/003158511X614258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delort J., Dumas J. B., Darmon M. C., Mallet J. (1989). An efficient strategy for cloning 5′ extremities of rare transcripts permits isolation of multiple 5′-untranslated regions of rat tryptophan hydroxylase mRNA. Nucleic. Acids Res. 17, 6439–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Develoux M., Dieng M. T., Ndiaye B., Raphenon G., Lepers J. P. (2006). Chromomycosis caused by Exophilia spinifera in Sahelian Africa. Ann. Dermatol. Vener. 133, 68–69. [DOI] [PubMed] [Google Scholar]
- Dogen A., Kaplan E., Ilkit M., de Hoog G. S. (2013a). Massive contamination of Exophiala dermatitidis and E. phaeomuriformis in railway stations in subtropical Turkey. Mycopathologia 175, 381–386. 10.1007/s11046-012-9594-z [DOI] [PubMed] [Google Scholar]
- Dogen A., Kaplan E., Oksuz Z., Serin M. S., Ilkit M., de Hoog G. S. (2013b). Dishwashers are a major source of human opportunistic yeast-like fungi in indoor environments in Mersin, Turkey. Med. Mycol. 51, 493–498. 10.3109/13693786.2012.738313 [DOI] [PubMed] [Google Scholar]
- D'Souza C. A., Alspaugh J. A., Yue C., Harashima T., Cox G. M., Perfect J. R., et al. (2001). Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell Biol. 9, 3179–3191. 10.1128/MCB.21.9.3179-3191.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutriaux C., Saint-Cyr I., Desbois N., Cales-Quist D., Diedhou A., Boisseau-Garsaud A. M. (2005). Subcutaneous phaeohyphomycosis due to Exophiala spinifera in a renal transplant recipient. Ann. Dermatol. Venereol. 132, 259–262. [DOI] [PubMed] [Google Scholar]
- El Kaoutari A., Armougom F., Gordon J. I., Raoult D., Henrissat B. (2013). The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 11, 497–504. 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- Erickson T., Liu L., Gueyikian A., Zhu X., Gibbons J., Williamson P. R. (2001). Multiple virulence factors of Cryptococcus neoformans are dependent on VPH1. Mol. Microbiol. 42, 1121–1131. 10.1046/j.1365-2958.2001.02712.x [DOI] [PubMed] [Google Scholar]
- Fawal N., Li Q., Savelli B., Brette M., Passaia G., Fabre M., et al. (2013). PeroxiBase: a database for large-scale evolutionary analysis of peroxidases. Nucleic. Acids Res. 41, D441–D444. 10.1093/nar/gks1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. B., Kane J. (1971). The detection of contamination in Trichophyton rubrum and Trichophyton mentagrophytes. Mycopathologia 43, 169–180. [DOI] [PubMed] [Google Scholar]
- Fothergill A. W., Rinaldi M. G., Sutton D. A. (2009). Antifungal susceptibility testing of Exophiala spp.: a head-to-head comparison of amphotericin B, itraconazole, posaconazole and voriconazole. Med. Mycol. 47, 41–43. 10.1080/13693780802512451 [DOI] [PubMed] [Google Scholar]
- Gerik K. J., Donlin M. J., Soto C. E., Banks A. M., Banks I. R., Maligie M. A., et al. (2005). Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 58, 393–408. 10.1111/j.1365-2958.2005.04843.x [DOI] [PubMed] [Google Scholar]
- Gish S. R., Maier E. J., Haynes B. C., Santiago-Tirado F. H., Srikanta D. L., Ma C. Z., et al. (2016). Computational analysis reveals a key regulator of cryptococcal virulence and determinant of host response. mBio 7, e00313–e00316. 10.1128/mBio.00313-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostincar C., Lenassi M., Gunde-Cimerman N., Plemenitas A. (2011). Fungal adaptation to extremely high salt concentrations. Adv. Appl. Microbiol. 77, 71–96. 10.1016/B978-0-12-387044-5.00003-0 [DOI] [PubMed] [Google Scholar]
- Griffith C. L., Klutts J. S., Zhang L., Levery S. B., Doering T. L. (2004). UDPglucose dehydrogenase plays multiple roles in the biology of the pathogenic fungus Cryptococcus neoformans. J. Biol. Chem. 279, 51669–51676. 10.1074/jbc.M408889200 [DOI] [PubMed] [Google Scholar]
- Gueidan C., Villaseñor C. R., de Hoog G. S., Gorbushina A. A., Untereiner W. A., Lutzoni F. (2008). A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud. Mycol. 61, 11–19. 10.3114/sim.2008.61.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gümral R., Özhak-Baysan B., Tümgör A., Saraçli M. A., Yildiran S. T., Ilkit M., et al. (2015). Dishwashers provide a selective extreme environment for human-opportunistic yeast-like fungi. Fungal Divers 76, 1–9. 10.1007/s13225-015-0327-8 [DOI] [Google Scholar]
- Gumral R., Tumgor A., Saracli M. A., Yildiran S. T., Ilkit M., de Hoog G. S. (2014). Black yeast diversity on creosoted railway sleepers changes with ambient climatic conditions. Microb. Ecol. 68, 699–707. 10.1007/s00248-014-0459-5 [DOI] [PubMed] [Google Scholar]
- Harris J. E., Sutton D. A., Rubin A., Wickes B., De Hoog G. S., Kovarik C. (2009). Exophiala spinifera as a cause of cutaneous phaeohyphomycosis: case study and review of the literature. Med. Mycol. 47, 87–93. 10.1080/13693780802412611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. C., Skowyra M. L., Spencer S. J., Gish S. R., Williams M., Held E. P., et al. (2011). Toward an integrated model of capsule regulation in Cryptococcus neoformans. PLoS Pathog. 12:e1002411 10.1371/journal.ppat.1002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heung L. J., Kaiser A. E., Luberto C., Del Poeta M. (2005). The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J. Biol. Chem. 280, 28547–28555. 10.1074/jbc.M503404200 [DOI] [PubMed] [Google Scholar]
- Hicks J. K., Bahn Y.-S., Heitman J. (2005). Pde1 phosphodiesterase modulates cyclic AMP levels through a protein kinase A-mediated negative feedback loop in Cryptococcus neoformans. Eukaryot. Cell 4, 1971–1981. 10.1128/EC.4.12.1971-1981.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma M., Kawada A., Ohata H., Ohnishi Y., Takahashi H., Yamazaki M., et al. (1993). Systemic phaeohyphomycosis caused by Exophiala dermatitidis. Mycoses 36, 1–7. [DOI] [PubMed] [Google Scholar]
- Horre R., Schaal K. P., Siekmeier R., Sterzik B., de Hoog G. S., Schnitzler N. (2004). Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration 71, 360–366. 10.1159/000079640 [DOI] [PubMed] [Google Scholar]
- Hu B., Li S., Hu H., Chen T., Guo X., Zhang Z., et al. (2014). Central nervous system infection caused by Exophiala dermatitidis in a case and literature review. Zhonghua er ke za zhi 52, 620–624. [PubMed] [Google Scholar]
- Janbon G., Himmelreich U., Moyrand F., Improvisi L., Dromer F. (2001). Cas1p is a membrane protein necessary for the O-acetylation of the Cryptococcus neoformans capsular polysaccharide. Mol. Microbiol. 42, 453–467. 10.1046/j.1365-2958.2001.02651.x [DOI] [PubMed] [Google Scholar]
- Jung W. H., Saikia S., Hu G., Wang J., Fung C. K., D'Souza C., et al. (2010). HapX positively and negatively regulates the transcriptional response to iron deprivation in Cryptococcus neoformans. PLoS Pathog. 6:e1001209. 10.1371/journal.ppat.1001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W. H., Sham A., White R., Kronstad J. W. (2006). Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 4:e410. 10.1371/journal.pbio.0040410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarcioglu A. S., de Hoog G. S. (2004). Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 47, 4–13. [DOI] [PubMed] [Google Scholar]
- Karuppayil S. M., Szaniszlo P. J. (1997). Importance of calcium to the regulation of polymorphism in Wangiella (Exophiala) dermatitidis. J. Med. Vet. Mycol. 35, 379–388. [DOI] [PubMed] [Google Scholar]
- Kingsbury J. M., Yang Z., Ganous T. M., Cox G. M., McCusker J. H. (2004). Cryptococcus neoformans Ilv2p confers resistance to sulfometuron methyl and is required for survival at 37 degrees C and in vivo. Microbiology. 150, 1547–1558. 10.1099/mic.0.26928-0 [DOI] [PubMed] [Google Scholar]
- Kondori N., Gilljam M., Lindblad A., Jonsson B., Moore E. R., Wenneras C. (2011). High rate of Exophiala dermatitidis recovery in the airways of patients with cystic fibrosis is associated with pancreatic insufficiency. J. Clin. Microbiol. 49, 1004–1009. 10.1128/JCM.01899-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanternier F., Barbati E., Meinzer U., Liu L., Pedergnana V., Migaud M., et al. (2015). Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J. Infect. Dis. 211, 1241–1250. 10.1093/infdis/jiu412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Chang Y. C., Varma A., Kwon-Chung K. J. (2009). Regulatory diversity of TUP1 in Cryptococcus neoformans. Eukaryot. Cell 8, 1901–1908. 10.1128/EC.00256-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. M., Li R. Y., de Hoog G. S., Sudhadham M., Wang D. L. (2011). Fatal Exophiala infections in China, with a report of seven cases. Mycoses 54, e136–e142. 10.1111/j.1439-0507.2010.01859.x [DOI] [PubMed] [Google Scholar]
- Lin Y. P., Li W., Yang Y. P., Huang W. M., Fan Y. M. (2012). Cutaneous phaeohyphomycosis caused by Exophiala spinifera in a patient with systemic lupus erythematosus. Lupus 21, 548–551. 10.1177/0961203311428460 [DOI] [PubMed] [Google Scholar]
- Liu O. W., Chun C. D., Chow E. D., Chen C., Madhani H. D. (2008). Systematic genetic analysis of virulence in the human fungal pathogen Cryptococcus neoformans. Cell 135, 174–188. 10.1016/j.cell.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu O. W., Kelly M. J. S., Chow E. D., Madhani H. D. (2007). Parallel beta-helix proteins required for accurate capsule polysaccharide synthesis and virulence in the yeast Cryptococcus neoformans. Eukaryot. Cell 6, 630–640. 10.1128/EC.00398-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos T., de Hoog G. S., de Boer A. G., de Crom I., Haase G. (2002). High prevalence of the neurotrope Exophiala dermatitidis and related oligotrophic black yeasts in sauna facilities. Mycoses 45, 373–377. [DOI] [PubMed] [Google Scholar]
- Matos T., Haase G., de Hoog G. S. (2003). Molecular diversity of oligotrophic and neurotropic members of the black yeast genus Exophiala, with accent on E. dermatitidis. Antonie van Leeuwenhoek 83, 293–303. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Matsuda T., McGinnis M. R., Ajello L. (1993). Clinical and mycological spectra of Wangiella dermatitidis infections. Mycoses 36, 145–155. [DOI] [PubMed] [Google Scholar]
- Mirza S. H., Hannan A., Ahmad A., Ahmad M. (1993). Subcutaneous phaeohyphomycosis. J. Infect. 27, 75–78. [DOI] [PubMed] [Google Scholar]
- Moreno L. F., Feng P., Weiss V. A., Vicente V. A., Stielow J. B., de Hoog S. (2017). Phylogenomic analyses reveal the diversity of laccase-coding genes in Fonsecaea genomes. PloS ONE 12:e0171291. 10.1371/journal.pone.0171291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyrand F., Janbon G. (2004). UGD1, encoding the Cryptococcus neoformans UDP-glucose dehydrogenase, is essential for growth at 37 degrees C and for capsule biosynthesis. Eukaryot. Cell 3, 1601–1608. 10.1128/EC.3.6.1601-1608.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyrand F., Chang Y. C., Himmelreich U., Kwon-Chung K. J., Janbon G. (2004). Cas3p belongs to a seven-member family of capsule structure designer proteins. Eukaryot. Cell 3, 1513–1524. 10.1128/EC.3.6.1513-1524.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyrand F., Fontaine T., Janbon G. (2007). Systematic capsule gene disruption reveals the central role of galactose metabolism on Cryptococcus neoformans virulence. Mol. Microbiol. 3, 771–781. 10.1111/j.1365-2958.2007.05695.x [DOI] [PubMed] [Google Scholar]
- Moyrand F., Klaproth B., Himmelreich U., Dromer F., Janbon G. (2002). Isolation and characterization of capsule structure mutant strains of Cryptococcus neoformans. Mol. Microbiol. 45, 837–849. 10.1046/j.1365-2958.2002.03059.x [DOI] [PubMed] [Google Scholar]
- Muotoe-Okafor F. A., Gugnani H. C. (1993). Isolation of Lecythophora utabilis and Wangiella dermatitidis from the fruit eating bat, Eidolon helvum. Mycopathologia 122, 95–100. [DOI] [PubMed] [Google Scholar]
- Nascimento M. M., Vicente V. A., Bittencourt J. V., Gelinski J. M., Prenafeta-Boldú F. X., Romero-Güiza M., et al. (2017). Diversity of opportunistic black fungi on babassu coconut shells, a rich source of esters and hydrocarbons. Fungal Biol. 121, 488–500. 10.1016/j.funbio.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Negroni R., Helou S. H., Petri N., Robles A. M., Arechavala A., Bianchi M. H. (2004). Case study: posaconazole treatment of disseminated phaeohyphomycosis due to Exophiala spinifera. Clin. Infect. Dis. 38, e15–e20. 10.1086/380840 [DOI] [PubMed] [Google Scholar]
- Nishimura K., Miyaji M. (1983). Defense mechanisms of mice against Exophiala dermatitidis infection. Mycopathologia 81, 9–21. [DOI] [PubMed] [Google Scholar]
- ÒMeara T. R., Hay C., Price M. S., Giles S., Alspaugh J. A. (2010a). Cryptococcus neoformans histone acetyltransferase Gcn5 regulates fungal adaptation to the host. Eukaryot. Cell 9, 1193–1202. 10.1128/EC.00098-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÒMeara T., Norton D., Price M., Hay C., Clements M., Nichols C., et al. (2010b). Interaction of Cryptococcus neoformans Rim101 and protein kinase A regulates capsule. PLoS Pathog 6:e1000776 10.1371/journal.ppat.1000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhye A. A., Ajello L., Chandler F. W., Banos J. E., Hernandez-Perez E., Llerena J., et al. (1983). Phaeohyphomycosis in El Salvador caused by Exophiala spinifera. Am. J. Trop. Med. Hyg. 32, 799–803. [DOI] [PubMed] [Google Scholar]
- Padhye A. A., Hampton A. A., Hampton M. T., Hutton N. W., Prevost-Smith E., Davis M. S. (1996). Chromoblastomycosis caused by Exophiala spinifera. Clin. Infect. Dis. 22, 331–335. [DOI] [PubMed] [Google Scholar]
- Padhye A. A., Kaplan W., Neuman M. A., Case P., Radcliffe G. N. (1984). Subcutaneous phaeohyphomycosis caused by Exophiala spinifera. Sabouraudia 22, 493–500. [PubMed] [Google Scholar]
- Pascon R. C., Ganous T. M., Kingsbury J. M., Cox G. M., McCusker J. H. (2004). Cryptococcus neoformans methionine synthase: expression analysis and requirement for virulence. Microbiology 150, 3013–3023. 10.1099/mic.0.27235-0 [DOI] [PubMed] [Google Scholar]
- Patel A. K., Patel K. K., Darji P., Singh R., Shivaprakash M. R., Chakrabarti A. (2013). Exophiala dermatitidis endocarditis on native aortic valve in a postrenal transplant patient and review of literature on E. Dermatitidis infections. Mycoses 56, 365–372. 10.1111/myc.12009 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan D., Jayalakshmi G., Madhumathy A., Banu S. T., Geethalakshmi S., Sumathi G. (2010). Subcutaneous phaeohyphomycosis due to Exophiala spinifera in an immunocompromised host. Indian J. Med. Microbiol. 28, 396–399. 10.4103/0255-0857.71838 [DOI] [PubMed] [Google Scholar]
- Rajam R. V., Kandhari K. C., Thirumalachar M. J. (1958). Chromoblastomycosis caused by a rare yeast like dematiaceous fungus. Mycopathol. Mycol. Appl. 9, 5–19. [DOI] [PubMed] [Google Scholar]
- Rajendran C., Khaitan B. K., Mittal R., Ramam M., Bhardwaj M., Datta K. K. (2003). Phaeohyphomycosis caused by Exophiala spinifera in India. Med. Mycol. 41, 437–441. [DOI] [PubMed] [Google Scholar]
- Rath P. M., Muller K. D., Dermoumi H., Ansorg R. (1997). A comparison of methods of phenotypic and genotypic fingerprinting of Exophiala dermatitidis isolated from sputum samples of patients with cystic fibrosis. J. Med. Microbiol. 46, 757–762. [DOI] [PubMed] [Google Scholar]
- Reese A. J., Yoneda A., Breger J. A., Beauvais A., Liu H., Griffith C. L., et al. (2007). Loss of cell wall alpha(1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Mol. Microbiol. 63, 1385–1398. 10.1111/j.1365-2958.2006.05551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss N. R., Mok W. Y. (1979). Wangiella dermatitidis isolated from bats in Manaus, Brazil. Sabouradia 17, 213–218. [PubMed] [Google Scholar]
- Revankar S. G., Sutton D. A. (2010). Melanized fungi in human disease. Clin. Microbiol. Rev. 23, 884–928. 10.1128/CMR.00019-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar S. G., Patterson J. E., Sutton D. A., Pullen R., Rinaldi M. G. (2002). Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infec. Dis. 34, 467–476. 10.1086/338636 [DOI] [PubMed] [Google Scholar]
- Rozas J., Rozas R. (1995). DnaSP, DNA sequence polymorphism: an interactive program for estimating population genetics parameters from DNA sequence data. CABIOS 11, 621–625. [DOI] [PubMed] [Google Scholar]
- Shen G., Wang Y.-L., Whittington A., Li L., Wang P. (2008). The RGS protein Crg2 regulates pheromone and cyclic AMP signaling in Cryptococcus neoformans. Eukaryot. Cell 7, 1540–1548. 10.1128/EC.00154-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Whittington A., Wang P. (2011). Wsp1, a GBD/CRIB domain-containing WASP homolog, is required for growth, morphogenesis, and virulence of Cryptococcus neoformans. Eukaryotic. Cell 10, 521–529. 10.1128/EC.00274-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Whittington A., Song K., Wang P. (2010). Pleiotropic function of intersectin homologue Cin1 in Cryptococcus neoformans. Mol. Microbiol. 76, 662–676. 10.1111/j.1365-2958.2010.07121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva W. C., Goncalves S. S., Santos D. W., Padovan A. C., Bizerra F. C., Melo A. S. (2017). Species diversity, antifungal susceptibility and phenotypic and genotypic characterisation of Exophiala spp. infecting patients in different medical centres in Brazil. Mycoses 60, 328–337. 10.1111/myc.12597 [DOI] [PubMed] [Google Scholar]
- Singal A., Pandhi D., Bhattacharya S. N., Das S., Aggarwal S., Mishra K. (2008). Pheohyphomycosis caused by Exophiala spinifera: a rare occurrence. Int. J. Dermatol. 47, 44–47. 10.1111/j.1365-4632.2007.03430.x [DOI] [PubMed] [Google Scholar]
- Slifkin M. (2000). Tween 80 opacity test responses of various Candida species. J. Clin. Microbiol. 38, 4626–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S. M., Gowda V. K., Mahantesh S., Mannapur R., Shivappa S. K. (2016). Chromoblastomycosis associated with bone and central nervous involvement system in an immunocompetent child caused by Exophiala Spinifera. Indian J. Dermatol. 61, 324–328. 10.4103/0019-5154.182425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhadham M., de Hoog G. S., Menken S. B., Gerrits van den Ende A. H., Sihanonth P. (2010). Rapid screening for genotypes as possible markers of virulence in the neurotropic black yeast Exophiala dermatitidis using PCR-RFLP. J. Microbiol. Meth. 80, 138–142. 10.1016/j.mimet.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Sudhadham M., Prakitsin S., Sivichai S., Chaiyarat R., Dorrestein G. M., Menken S. B., et al. (2008). The neurotropic black yeast Exophiala dermatitidis has a possible origin in the tropical rain forest. Stud. Mycol. 61, 145–155. 10.3114/sim.2008.61.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell R. C., Rosenthal S. A., Kane J. (1988). Rapid method for differentiation of Trichophyton rubrum, Trichophyton mentagrophytes, and related dermatophyte species. J. Clin. Microbiol. 26, 2279–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M. M., Moreno L. F., Stielow B. J., Muszewska A., Hainaut M., Gonzaga L., et al. (2017). Exploring the genomic diversity of black yeasts and relatives (Chaetothyriales, Ascomycota). Stud. Mycol. 86, 1–28. 10.1016/j.simyco.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesei D., Marzban G., Marchetti-Deschmann M., Tafer H., Arcalis E., Sterflinger K. (2015). Protein functional analysis data in support of comparative proteomics of the pathogenic black yeast Exophiala dermatitidis under different temperature conditions. Data Brief 5, 372–375. 10.1016/j.dib.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomson N., Abdullah A., Maheshwari M. B. (2006). Chromomycosis caused by Exophiala spinifera. Clin. Exper. Dermato. 31, 239–241. 10.1111/j.1365-2230.2005.02006.x [DOI] [PubMed] [Google Scholar]
- Vicente V. A., Attili-Angelis D., Pie M. R., Queiroz-Telles F., Cruz L. M., Najafzadeh M. J., et al. (2008). Environmental isolation of black yeast-like fungi involved in human infection. Stud. Mycol. 61, 137–144. 10.3114/sim.2008.61.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang C., Shen Y., Lv G., She X., Zeng R., et al. (2015). Phaeohyphomycosis caused by Exophiala spinifera: an increasing disease in young females in mainland China? Two case reports and review of five cases reported from mainland China. Mycoses 58, 193–196. 10.1111/myc.12295 [DOI] [PubMed] [Google Scholar]
- Wang P., Cox G. M., Heitman J. (2004). A Sch9 protein kinase homologue controlling virulence independently of the cAMP pathway in Cryptococcus neoformans. Curr. Genet. 46, 247–255. 10.1007/s00294-004-0529-1 [DOI] [PubMed] [Google Scholar]
- Wang P., Nichols C. B., Lengeler K. B., Cardenas M. E., Cox G. M., et al. (2002). Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1, 257–272. 10.1128/EC.1.2.257-272.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. A., Roberts I. S., Del Poeta M., Rivera J., Casadevall A., Cox G. M., et al. (2001). Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol. Microbiol. 40:610–620. 10.1046/j.1365-2958.2001.02401.x [DOI] [PubMed] [Google Scholar]
- Xue C., Bahn Y.-S., Cox G. M., Heitman J. (2006). G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol. Biol. Cell 17, 667–679. 10.1091/mbc.E05-07-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurlova N. A., de Hoog G. S. (2002). Exopolysaccharides and capsules in human pathogenic Exophiala species. Mycoses 45, 443–448. 10.1046/j.1439-0507.2002.00807.x [DOI] [PubMed] [Google Scholar]
- Zalar P., Novak M., de Hoog G. S., Gunde-Cimerman N. (2011). Dishwashers–a man-made ecological niche accommodating human opportunistic fungal pathogens. Fungal Biol. 115, 997–1007. 10.1016/j.funbio.2011.04.007 [DOI] [PubMed] [Google Scholar]
- Zeng J. S., De Hoog G. S. (2008). Exophiala spinifera and its allies: diagnostics from morphology to DNA barcoding. Med. Mycol. 46, 193–208. 10.1080/13693780701799217 [DOI] [PubMed] [Google Scholar]
- Zeng J. S., Sutton D. A., Fothergill A. W., Rinaldi M. G., Harrak M. J., de Hoog G. S. (2007). Spectrum of clinically relevant Exophiala species in the United States. J. Clin. Microbiol. 45, 3713–3720. 10.1128/JCM.02012-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Hacham M., Panepinto J., Hu G., Shin S., Zhu X., et al. (2006). The Hsp70 member, Ssa1, acts as a DNA-binding transcriptional co-activator of laccase in Cryptococcus neoformans. Mol. Microbiol. 62, 1090–1101. 10.1111/j.1365-2958.2006.05422.x [DOI] [PubMed] [Google Scholar]
- Zhu X., Williamson P. R. (2003). A CLC-type chloride channel gene is required for laccase activity and virulence in Cryptococcus neoformans. Mol. Microbiol. 50, 1271–1281. 10.1046/j.1365-2958.2003.03752.x [DOI] [PubMed] [Google Scholar]
- Zupancic J., Novak Babic M., Zalar P., Gunde-Cimerman N. (2016). The Black Yeast Exophiala dermatitidis and other selected opportunistic human fungal pathogens spread from dishwashers to kitchens. PLoS ONE 11:e0148166. 10.1371/journal.pone.0148166 [DOI] [PMC free article] [PubMed] [Google Scholar]