Abstract
Juvenile social play behavior is one of the earliest sexually differentiated behaviors to emerge. In rats, as with most other species that play, males engage in more rough-and-tumble play compared to females. Exposure to early life adversity is a major driver of adult health and can manifest differently in males and females. However, the effects of adverse early life exposure on play behavior in the juvenile period are poorly understood. To address this, male and female neonatal rats were exposed to predator odor (PO), for 5 min/day on PN1-PN3. At the time of exposure to PO, both male and female pups suppressed ultrasonic vocalization and displayed more freezing behavior. Circulating corticosterone increased in males immediately following PO exposure but not in females. The enduring effects of PO exposure were opposite in males compared to females in that PO exposed males decreased social play, while PO exposed females increased play behavior compared to same sex controls. PO exposure did not significantly affect cell genesis in the neonatal dentate gyrus of either sex. PO exposure did not affect anxiety-like behavior assessed in the juvenile period or in adulthood, nor did it affect social interactions in adulthood. This work provides new insight into how sex may interact with adverse early life events to contribute to development of the social consequences of such exposures.
Keywords: Early life adversity, Predator odor, Development, Sex differences, Corticosterone, Social play, Anxiety
1. Introduction
In most species, early social interactions occur in the context of same-aged conspecific social play behavior. In rats, this play behavior is most commonly manifested as rough-and-tumble play or play fighting (Pellis and Pellis, 1997, 1998). Play-fighting is goal-directed towards play-biting an opposing participant and frequently involves wrestling-like behaviors, as animals attempt to dominate one another (Aldis, 1975). Rough-and-tumble play in the juvenile rat is sexually differentiated, as males exhibit a higher frequency of play events compared to females, and this is determined by neonatal testosterone (Meaney and Stewart, 1981; Olioff and Stewart, 1978; Thor and Holloway, 1986). The sex difference in juvenile social play behavior provides a route to assess how external factors may interact with sex to effect social behavior in a non-reproductive context.
Adverse early life events greatly influence subsequent development and can have lasting implications for adult mental and physical health (Anda et al., 2006; Felitti et al., 1998; Heim and Nemeroff, 2001; Schilling et al., 2007). Stressful environments in early life harmfully influence behavior and broad physiological functions. Frequently these consequences persist as permanent pathology. Early life adversity is particularly important in mediating the risk for neuropsychiatric disorders in adulthood including many which have significant social contributions (Kendler et al., 2002; Morgan et al., 2007). A number of factors interact to shape how early life adversity may manifest as differential vulnerabilities to later-life pathologies. The nature of the early life adverse event, an individual's genetic background and sex are among these factors (Kundakovic et al., 2013).
Investigations into the consequences of early life adversity have left both social and sex-specific sequelae understudied. Among the limited studies to probe these areas, results are contradictory. Adversity in early life, modeled through maternal separation in male pups, induces increased evasion of play contacts, suggesting feminized patterns of play (Arnold and Siviy, 2002), as well as a shift towards more aggressive play (Veenema and Neumann, 2009). Others find few sex-specific effects as a result of early stressful exposures (Zimmerberg and Sageser, 2011). Thus no consensus has emerged on the ramifications of early life adversity on juvenile social play and the role of sex in mediating potential differences has rarely been considered.
Multiple brain areas contribute to execution of juvenile social play behavior. Lesion of the cortex, nucleus accumbens, hypothalamus and amygdala all decrease social play (Vanderschuren et al., 1997). The amygdala is also crucial for developmental organization, as local implant of steroids increase masculinized play (Meaney and McEwen, 1986; Tönjes et al., 1989). The amygdala continues to contribute to social behaviors throughout life including social recognition and interaction (Kling and Brothers, 1992), aggression (De Vries and Buijs, 1983; Davidson, 2000), sexual behavior (Newman, 1999) and maternal behavior (Nephew and Bridges, 2008; Bosch and Neumann, 2010; Nephew et al., 2010). Other brain regions are important for development of appropriate social behaviors in later life as well, including the hippocampus. The hippocampus is comprised of distinct regions including Ammon's horn and the dentate gyrus. The principle cells of the dentate gyrus, the granule cells, are primarily produced during the first two weeks of postnatal life (Schlessinger et al., 1975). The abundant period of postnatal neurogenesis of this region allows for early life experiences to modify granule cell production. Exposure of rat pups to stressful experiences early in life suppresses the production of granule neurons in the developing dentate gyrus (Tanapat et al., 1998). Pharmacologic lesions of the ventral hippocampus during this vulnerable postnatal period produce deficits in social interaction and social memory and increase displays of aggression in the juvenile period and adulthood (Becker et al., 1999; Sams-Dodd et al., 1997; Becker and Grecksch, 2000). These findings suggest that environmental exposures during periods of hippocampal development could influence granule cell formation and affect the outcome of a behavior dependent on this brain region.
Social deficits exist at the core of several neuropsychiatric disorders (Couture, 2006; Pelcovitz et al., 1994; Segrin, 2000). Among these disorders the prevalence, presentation, or a combination thereof, is greatly influenced by the biological variable of sex (Bao and Swaab, 2010). Natural variations in juvenile social play behavior between males and females provide a spectrum upon which to assess the impact of adverse early life event exposure. Correlates of early life stress are significantly influenced by the type of exposure (Zimmerberg and Sageser, 2011). Both neonatal isolation from the dam and predator odor exposure are commonly used models of stressful exposures (Zovkic and Sweatt, 2013). Exposure to live predators or cues indicative of the threat of predation impact a wide range of species. While responses are largely species-specific, nearly all consist of increased stress reactivity (St-Cyr and McGowan, 2015). Rodents primarily depend on olfaction to detect predators (Takahashi, 2014). Exposure of rodents to predator odor elicits an unconditioned fear response (Takahashi et al., 2008). Early life exposure of rat pups to predator odor significantly affects later life behaviors including fear responding (Hacquemand et al., 2010; Ayers et al., 2016). The impact of this early postnatal exposure to predator odor can also differentially affect male and female offspring (Mashoodh et al., 2009). To date, the consequences of early postnatal predator odor on later juvenile social play has not been examined. This study seeks to explore the sex specific consequences of early predator odor exposure on later juvenile social play in order to gain insight into the etiology of disrupted social behavior in neuropsychiatric disease.
2. Methods and materials
2.1. Animals
Timed pregnant Sprague-Dawley rats (Harlan) mated in our facility were allowed to deliver normally under standard laboratory conditions. The morning pups were found in the nest was designated as the day of birth (PN0). Pups were individually identified on PN0 by injection into the footpad with India ink. On PN21 animals were weaned and housed in groups consisting of two to three individuals of the same sex and exposure in polycarbonate cages (20 × 40 × 20 cm) with corncob bedding under a reverse 12:12-h light/dark cycle. Food and water were ad libitum. All breeding and experimental procedures were approved by the Institutional Care and Use Committee at the University of Maryland, Baltimore and performed in accordance with national animal care and use guidelines.
2.1.1. Pup condition distribution
Each litter was culled to 10–12 pups and divided to contain equal numbers of male and female pups exposed to either control or predator odor conditions. Pups were assigned in this manner to distribute and thus control for differences in maternal care between dams. For assessment of freezing behavior and USV detection, 3 male and 3 female pups were exposed to maternal control bedding and 3 male and 2 female pups were exposed to predator bedding from a single litter. To determine plasma corticosterone following exposure, from two litters, 8 males and 6 females were exposed to maternal control bedding, while 7 males and 6 females were exposed to predator bedding. For BrdU analysis, from four litters, 10 males and 12 females were assigned to control conditions and 11 males and 11 females were exposed to predator odor. To identify effects of predator odor exposure on later life behaviors, 10 males and 10 females underwent exposure to maternal control bedding and 12 males and 12 females were exposed to predator odor. (See Fig. 1.)
Fig. 1.
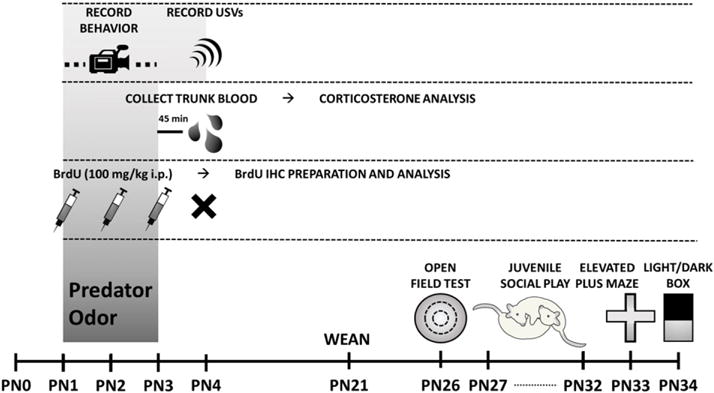
Timeline of the sequence of exposures and assessments for each cohort of animals.
2.2. Predator odor (PO) exposure
PO exposures occurred during the first three full days of life (PN1–3) for 5 min each day. Individually, newborn animals were placed in a chamber constructed from a perforated base (16.5 × 12.7 cm) raised 2.5 cm from the ground with 6.35 cm walls contained within a 27 × 22 × 25 cm Styrofoam box. The bottom of the Styrofoam box contained 2 in. of bedding from an adult male rat (on PN1 and PN3) or bedding from adult male and female ferrets (on PN2). Bedding was alternated over the course of the three days to prevent habituation to the predator odor. Placement of the pups in the base raised above the bedding ensured pups were exposed to the odor but did not come in contact with bedding and would not carry the odor back to the nest. Control animals were placed in a similar apparatus containing bedding from the maternal homecage. Pups were immediately returned to the dam and observed for any changes to maternal care. No alterations in care were evident following predator odor exposure.
2.2.1. Freezing during PO exposure
Behavior was recorded on a video camera during the three exposure days and later scored manually for immobility defined as cessation of body movement.
2.2.2. Ultrasonic vocalizations (USV) during PO exposure
The cohort of pups assessed for freezing behavior underwent a fourth day of exposure to predator odor and during the 5 minute exposure vocal emissions were recorded using an UltraSoundGate Condenser Microphone (CM16; Avisoft Bioacoustics) connected to an Avisoft UltraSoundGate 416 USB Audio device routed to a personal computer. Acoustic data were recorded with a sampling rate of 300,000 Hz in 16 bit format. For acoustical analysis, recordings were transferred to SASLab Pro (version 5.10; Avisoft Bioacoustics), and a fast Fourier transform was conducted (512 FFT length, 100% frame, Hamming window and 75% time window overlap). A high-pass finite impulse response filter was used to eliminate background noise below 20 kHz. An automated threshold-based algorithm (threshold: −50 dB) and a hold time mechanism (5 ms) were used to detect ultrasonic vocalizations (USVs) and calls also inspected manually to ensure that all USVs detected were legitimate calls. Those that were not were removed from analysis. The call parameters of call number and fundamental mean amplitude were quantified over the first 3 min of exposure.
2.3. Corticosterone levels following PO exposure
Animals were euthanized 45 min after the end of PO exposure on PN3 and trunk blood was collected, allowed to clot for 30 min and centrifuged at 5000 rpm for 10 min. After centrifugation, serum was diluted 1:1 and enzyme-linked immunosorbent assays were performed alongside the provided corticosterone standards (Assaypro, St. Charles, MO).
2.4. BrdU immunohistochemistry
Pups were injected with bromodeoxyuridine (BrdU; 100 mg/kg; Sigma Aldrich), a synthetic thymidine analog used to detect proliferating cells, immediately prior to PO exposure on PN1–3. The following day, pups were transcardially perfused with 0.9% saline and 4% paraformaldehyde. Brains were post-fixed for 48 h and allowed to sink in 30% sucrose prior to cryosectioning throughout the rostrocaudal extent of the hippocampus. Sections were mounted to slides and heated in 0.1 M citric acid (pH 6.0), rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 M HCl:PBS for 30 min, rinsed and incubated with mouse antibodies to BrdU (BD Biosciences diluted 1:500 in 0.5% Tween-20). The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200, Vector) for 60 min, rinsed, incubated with avidin–biotin complex (1:500; Vector), rinsed and reacted in 0.01% DAB. Slides were counterstained with cresyl violet, dehydrated, cleared and coverslipped. Unbiased stereology was used to estimate the number of BrdU+ cells in the hippocampus using the optical dissector method (West et al., 1991). StereoInvestigator software (MBFbioscience, Williston, VT) was used to delineate the granule cell layer of the dentate gyrus (DG) in each hemisphere. Analysis of BrdU + cells was conducted on the left and right hemisphere of 4 sections of the dorsal hippocampus and an estimation of total cells was generated.
2.5. Behavioral assessment
Behavior was assessed under indirect red-light illumination during the dark phase of the cycle between 3 and 7 h after lights off. Animals were first habituated to the room for 30 min on PN25 and then underwent open field testing on PN26, social play from PN27-32, elevated plus maze on PN33 and light/dark box on PN34. Animals were retested for elevated plus maze and light/dark box and tested in the social interaction test in adulthood. Juvenile behavior was analyzed in four total litters in two separate experimental cohorts separated by two months. In adulthood, the first cohort was examined for anxiety-like behavior and the second for social interaction.
2.5.1. Open field
The arena utilized was a circular opaque plastic tub 124.5 cm in dimeter with 92 cm walls open on top. Animals were placed in the northern edge of the arena and the behavior in the open field monitored by overhead camera for 5 min. The arena was cleaned between animals with 70% ethanol. Video recordings were analyzed in TopScan (CleverSys Inc.) for total distance traveled, entries into the center and duration in the center.
2.5.2. Social play behavior
Play behavior was conducted in a neutral arena (49 × 37 × 24 cm) with TEK-Fresh cellulose bedding (Harlan Laboratories). The first day of testing (PN27), animals were allowed to habituate to the arena for 10 min with their cagemates. Play behavior was assessed between sex and exposure-paired partners every day from PN28–32 for 10 min. All play partners were from separate litters and were not cagemates. During analysis, the frequency of pouncing, pinning, chasing, and boxing behaviors were scored for each individual and the total time engaged in play was scored for each pair.
2.5.3. Elevated plus maze
Rats were placed in the center of the maze (consisting of two opposing open arms/closed arms 102.5 × 12 cm; 72 cm from the ground), facing an open arm and allowed to explore the maze freely for 5 min. Exploration was recorded from an overhead camera and the time and entries into the open arm were scored by hand.
2.5.4. Light/dark box
Rats were placed in the dark zone of the L/D box for 5 min. The apparatus consisted of a 25.5 × 30.5 × 30.5 cm black polycarbonate carbonate compartment connected by a 5 × 5 cm opening to a 30.5 × 30.5 × 30.5 cm clear polycarbonate compartment with a 75 W light bulb suspended 90 cm above the floor. Time spent within and entries into the light zone were quantified by hand.
2.5.5. Social interaction test
Rats were placed in a plastic enclosure (80 × 80 cm) with a 5 × 5 grid of squares (16 × 16 cm). A small translucent perforated plastic box (20 × 20 cm) was placed against the center of one wall of the arena. Each test consisted of a target-absent trial followed by a target-present trial. During the target-absent trial, an individual test rat was placed in the center of the arena and, after 30 s of adaptation, the rat's movements were recorded by overhead video camera for 2.5 min. The rat was then temporarily removed from the arena and a novel juvenile (3-4 weeks old; always younger than the rat being tested) placed in the internal box at one end of the arena for the target present trial. The test rat was then returned to the center of the arena 2.5 min. Results were quantified as the percentage of time spent in the ‘interaction zone’ (the five grid squares immediately surrounding the plastic cage) as measured for both the ‘target-absent’ and ‘target-present’ trials. These percentages were then used to calculate an interaction ratio, which is the percent time spent in the ‘interaction zone’ during the ‘target-present’ trial divided by that of the ‘target-absent’.
3. Results
3.1. Neonatal behavioral response to predator odor exposure
Neonates exposed to predator odor emitted significantly fewer USV calls compared to those exposed to maternal bedding (F[1,7] = 0.3224, p = 0.0120; Fig. 2A). There was no sex difference in USV frequency (F[1,7] = 0.3224, p = 0.5879) and no interaction of sex with exposure (F[1,7] = 0. 0.2577, p = 0.6273). Of USVs emitted, those produced in the presence of predator odor showed a strong trend with a large effect size for being made at a lower amplitude (F[1,7] = 5.545, p = 0.0507; ; Fig. 2B). Animals exposed to PO also showed significantly elevated freezing behavior compared to those exposed to maternal bedding (F[1,7] = 15.17, p = 0.0059; Fig. 2C). Again no sex difference was observed in freezing (F[1,7] = 0.9783, p = 0.3556) and there was no interaction of sex with exposure (F[1,7] = 0.0.4758, p = 0.5125).
Fig. 2.
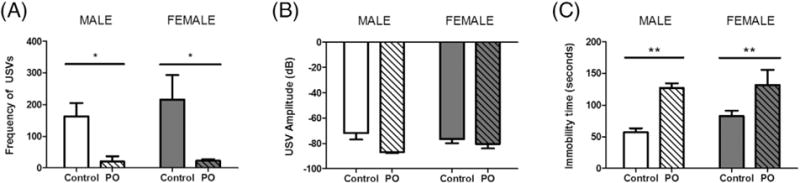
Rat pups modulate their behavior in response to the fearful stimulus of predator odor. Male and females pups were exposed to PO on PN1-PN3 for analysis of freezing behavior and on PN4 for detection of USV suppression. (A) Exposure to PO significantly reduced the number of USVs emitted in both males and females. (B) In both sexes, the amplitude of USVs produced in the presence of PO were marginally decreased with a large effect size (F[1,7] = 5.545, p = 0.0507; ). (C) When exposed to PO, both male and female pups increased freezing behavior. (Data expressed as mean ± SEM. Control male group, n = 3; PO male group, n = 2; control female group, n = 3; female group, n = 3; two-way ANOVA: *p < 0.05, **p < 0.01.)
3.2. Neonatal physiologic response to predator odor exposure
PO exposure increased serum corticosterone levels in males only (F[1,23] = 5.351, p = 0.0300, post-hoc t(13) = 2.203, p = 0.0462; Fig. 3A). Consistent with previous results, cellular proliferation in the granule cell layer of the dentate gyrus of the hippocampus was greater in males compared to females (F[1,40] = 7.179, p = 0.0107; Fig. 3B). There was a strong trend with a medium effect size for PO to reduce proliferation in both sexes (F[1,40] = 3.369, p = 0.0739; ). BDNF expression did not differ between groups (Fig. 3C).
Fig. 3.
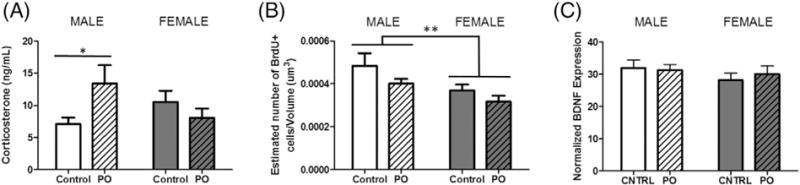
Corticosterone levels increased only in males following PO exposure. (A) There was no sex difference in corticosterone among animals exposed to maternal control bedding. In males, exposure to PO caused an increase in corticosterone, while female corticosterone did not change in response to the exposure. (B) As previously reported (Zhang et al., 2008; Bowers et al., 2010), there is a higher rate of cell proliferation in BrdU + cells in the dentate gyrus of neonatal males compared to females as indicated by BrdU + cell number. PO exposure resulted in a trend towards reduction of cellular proliferation in the dentate gyrus with a medium effect size (F[1,40] = 3.369, p = 0.0739; ). (C) Hippocampal BDNF protein did not significantly differ between any conditions. (Data expressed as mean ± SEM. Corticosterone assessment: control male group, n = 8; PO male group, n = 7; control female group, n = 6; female group, n = 6; BrdU analysis: control male group, n = 10; PO male group, n = 11; control female group, n = 12; PO female group, n = 11; twoway ANOVA and pairwise comparisons with Bonferroni correction: *p < 0.05, **p < 0.01.)
3.3. Juvenile social play following predator odor
Animals were tested for social play behavior from PN28-PN32 after PO exposure on PN1–3. As expected, control males spent more time engaged in play than females (F[1,40] = 8.175, p = 0.0067; Fig. 4A) and exhibited more play events overall (F[1,40] = 7.585, p = 0.0088; Fig. 4B). PO exposure had opposite effects on males and females for both time engaged in play (F[1,40] = 15.98, p = 0.0003) and frequency of play events (F[1,40] = 12.54, p = 0.0010). In males, previous PO exposure decreased time spent playing (t[20] = 2.687, p = 0.0142), as well as play event frequency (t[20] = 2.589, p = 0.0175), while females exposed to PO increased both (t[20] = 3.229, p = 0.0042; t[20] = 2.415, p = 0.0254, respectively). Compared to females, males also exhibited more specific play behaviors including pouncing (F[1,40] = 5.178, p = 0.0283; Fig. 4C), pinning (F[1,40] = 4.413, p = 0.0420; Fig. 4D), boxing (F[1,40] = 5.332, p = 0.0262; Fig. 4E) and chasing (F[1,40] = 8.901, p = 0.0048; Fig. 4F). Again, the effect of PO differentially effected the frequency of these specific play behaviors among males and females (pouncing: F[1,40] = 14.85, p = 0.004, pinning: F[1,40] = 13.81, p = 0.0006, boxing: F[1,40] = 17.50, p = 0.0002 and chasing F[1,40] = 8.375, p = 0.0061). Male animals exposed to PO decreased pouncing (t[20] = 2.341, p = 0.0297), pinning (t[20] = 2.657, p = 0.0151), boxing (t[20] = 3.193, p = 0.0046) and chasing (t[20] = 2.299, p = 0.0324). However, PO exposure to females resulted in an increase in play behaviors including pouncing (t[20] = 3.190, p = 0.0046), pinning (t[20] = 3.097, p = 0.0059) and boxing (t[20] = 3.101, p = 0.0056).
Fig. 4.
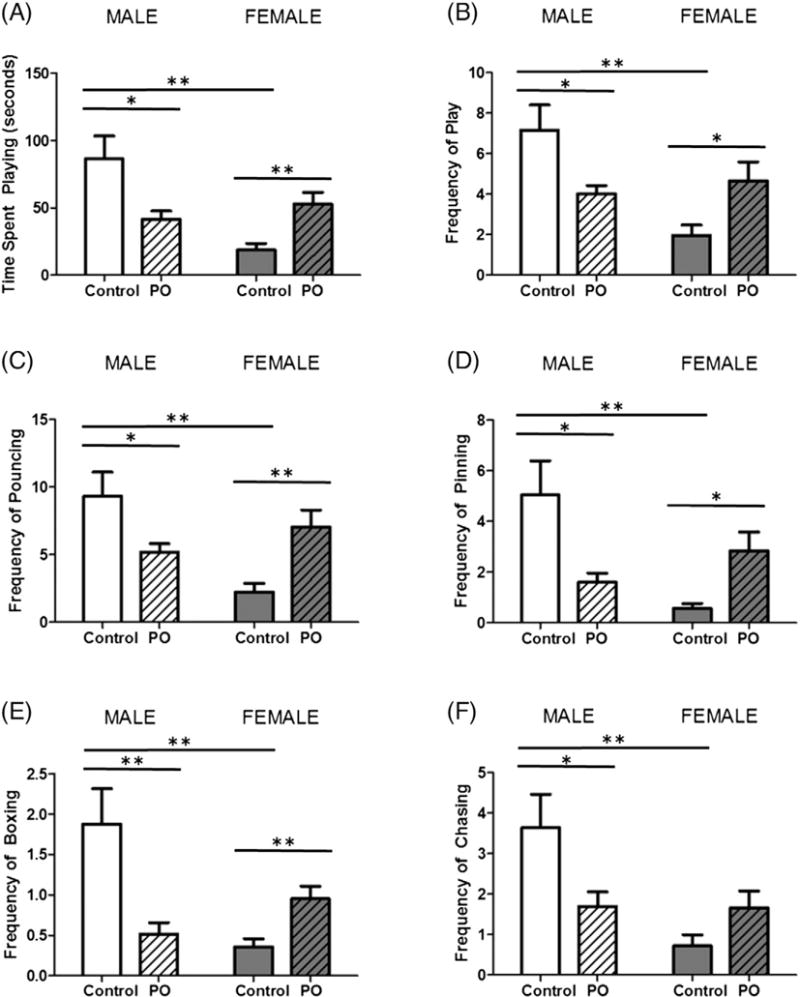
Neonatal PO exposure has opposite effects on later social play in males and females. Male and female pups that were exposed to PO were assessed for play behavior on PN28–PN32 with a consistent matched sex and exposure partner. (A) The average time spent playing per day was greater in males than females. Males exposed to PO engaged in less play and females more. (B) Males engaged in more play events per day than females. Males exposed to PO had a decreased frequency of play, while females exposed to PO increased play frequency. Analysis of specific play behaviors including pouncing (C), pinning (D), boxing (E) and (F) chasing revealed similar patterns, decreasing in males exposed to PO and increasing in PO exposed females. (Data expressed as mean ± SEM. Control male group, n = 10; PO male group, n = 12; control female group, n = 10; PO female group, n = 12; two-way ANOVA and pairwise comparisons with Bonferroni correction: *p < 0.05, **p < 0.01.)
3.4. Effect of predator odor exposure on juvenile anxiety-like behavior
In the OFT, all animals traveled the same distance (Fig. 5A), had the same number of bouts to the center 50% of the arena (Fig. 5B) and the same duration of time spent in the center compartment (Fig. 5C). In the EPM, time spent (Fig. 5D) and entries into the open arms (Fig. 5E) of the arena did not differ between animals. Lastly, the L/D box, also showed no effect of PO exposure, however, females spent more time in the light zone (F[1,40] = 10.81, p = 0.0022; Fig. 5F) and entered the light zone a greater number of times (F[1,40] = 10.24, p = 0.0028; Fig. 5G).
Fig. 5.
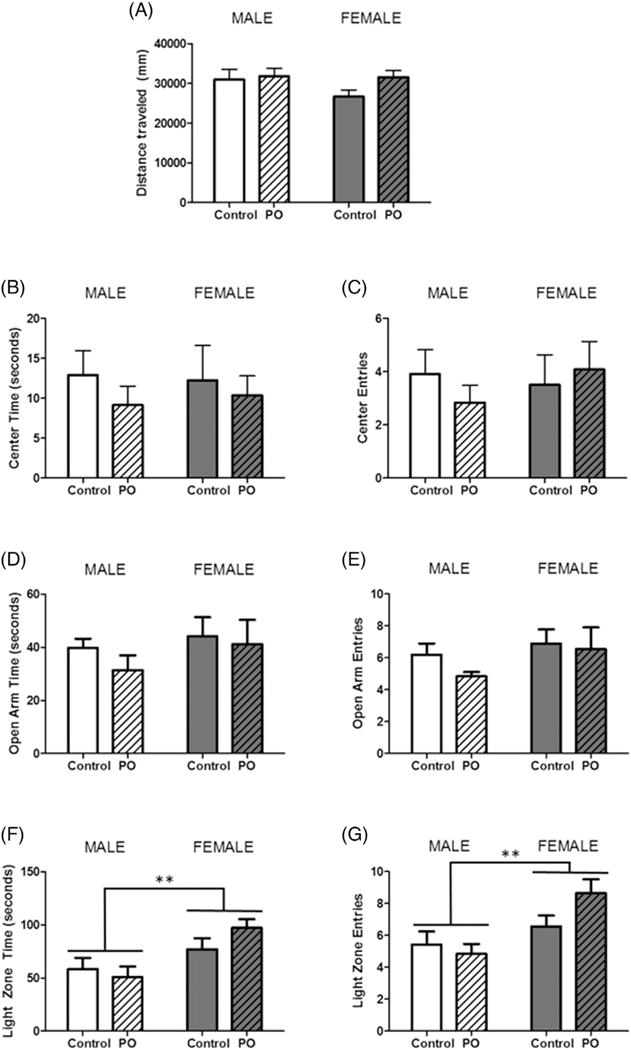
Exposure to predator odor during postnatal development does not affect anxiety-like behavior in adolescence. Male and female pups subject to PO exposure from PN1–PN3 and unexposed controls were assessed for anxiety-like behavior in the open field test on PN26, the elevated plus maze on PN33 and the light/dark box on PN34. (A) All animals traveled the same distance in the OFT and there were no differences in time spent in the center (B) or entries into the center (C). In the EPM, neither PO exposure nor sex altered (D) time spent in the open arms or (E) number of entries in the open arms. The L/D box, revealed no effect of predator odor exposure on (F) time spent in the light zone or (G) entries into the light zone, however females spent more time and entered into the light zone more than males. (Data expressed as mean ± SEM. Control male group, n = 10;PO male group, n = 12; control female group, n = 10; PO female group, n = 12; two-way ANOVA and pairwise comparisons with Bonferroni correction: **p < 0.01.)
3.5. Assessment of lasting effects of early PO exposure on adult behavior
In adulthood, females traveled a greater distance in the OFT compared to males (F[1,20] = 8.856, p = 0.0081; Fig. 6A. Those exposed to PO traveled less than control animals (F[1,20] = 4.677, p = 0.0443). Furthermore, females entered the center zone more often than males (F[1,20] = 4.906, p = 0.0399; Fig. 6B). PO exposed animals displayed fewer entries into this zone (F[1,20] = 4.677, p = 0.0443), but time spent in the center zone did not differ between groups (Fig. 6C). The interaction ratio of the social interaction test showed no significant effects of either variable (Fig. 6D). In the EPM, there were no significant differences with regards to the time spent in the open arms (Fig. 6E) nor were the entries into this compartment altered by either variable (Fig. 6F). Similarly in the L/D box, these variables did not significantly impact duration within (Fig. 6G) or entries into the light zone (Fig. 6H).
Fig. 6.
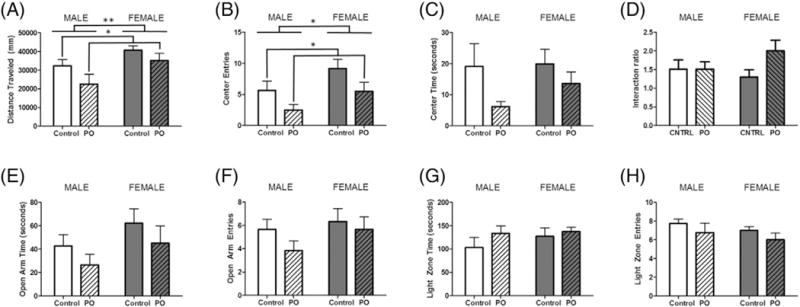
Adult social and anxiety-like behaviors are not affected by early PO exposure. Male and female pups exposed to PO were retested for social and anxiety-like behaviors in adulthood. (A) Distance traveled was greater among unexposed females and decreased in both males and females exposed to PO. (B) Unexposed females also displayed more entries in the center zone, whereas PO exposed male and females entered this zone less. (C) Time spent in the center zone did not significantly differ between groups. (D) The interaction ratio of the SIT showed no significant effects of either variable. In the EPM, groups did not differ with respect to (E) time spent in the open arms or (F) number of entries in the open arms of the elevated plus maze. Behavior in the L/D box showed no significant effect of any condition on (G) time spent in the light zone or (H) number of entries into the light zone of the light/dark box. (Data expressed as mean ± SEM. For OFT, EPM and L/D Box control male group, n = 6; PO male group, n = 6; control female group, n = 6; PO female group, n = 6; for SIT control male group, n = 4; PO male group, n = 6; control female group, n = 4; PO female group n = 6 two-way ANOVA.)
4. Discussion
Early adverse experiences induce long-lasting changes in brain development, impacting later behavior (Anda et al., 2006; Chugani et al., 2001; Felitti et al., 1998; Glaser, 2000; McGowan et al., 2009). Several experiences can qualify as adverse, however, most generally evoke fear. Some animal models of fear utilize exposure of prey to predator odor, as many species depend on olfaction for predator detection (Takahashi, 2014). The proximity of an unfamiliar adult male poses a threat to young rat pups because roaming males often intrude on areas occupied by other males and kill the offspring of resident females (Mennella and Moltz, 1988). Because unweaned rat pups frequently fall victim to infanticide by adult male rat intruders (Paul and Kupferschmidt, 1975), rat pups have developed the capacity to recognize and respond to adult male odor, thus making PO odor exposure a useful model for fear-invoking early adverse experiences.
In neonatal rat pups, USVs are elicited by removal of the pup from the nest and are thought to reflect a state of distress (Noirot, 1968). The emission of the ultrasonic sound solicits maternal attention, retrieval and return to the nest (Allin and Banks, 1972; Noirot, 1972; Smotherman et al., 1974). However, in order to remain inconspicuous and avoid predation, rat pups are able to inhibit or modulate USV production in the presence of stressful stimuli (Takahashi, 1992a, 1992b). USV suppression is accompanied by other defensive behaviors including an immature version of freezing (Wiedenmayer and Barr, 1998).
4.1. Exposure to predator odor reduced USVs and induced freezing in very young pups
In the current study, exposure to PO on PN1–3 increased freezing compared to littermates exposed to maternal cage bedding. Additionally, when tested on PN4, during PO exposure animals produced dramatically fewer USVs and those which were produced tended to be made at a lower amplitude. While we detected defensive behaviors from PN1–4, previous work suggests newborn pup do not have the ability to inhibit production of USVs or display freezing in the presence of an unfamiliar male rat but that instead these behaviors emerge at the end of the second postnatal week (Takahashi, 1992a, 1992b; Wiedenmayer and Barr, 1998). The delayed developmental emergence of these defensive mechanisms is thought to occur as a result of increased corticosterone following the end of the stress hyporesponsive period in the second week (SHRP; Moriceau et al., 2004; Takahashi, 1994; Takahashi and Rubin, 1993). Thus our detection of defensive behaviors early in development appears to be in conflict with reports that such behaviors do not emerge until later. However, in our experiment, USVs were only analyzed over the first 3 min of PO exposure, whereas previous experiments utilized 10 min recording sessions (Takahashi, 1992a). In these experiments, ACTH was elevated, suggesting pups at the younger age did recognize the proximity of the adult male to be threatening. Therefore the failure to detect USV suppression in the presence of the male may be due to the inability of the young pup to maintain the defensive response throughout the longer recording session used in previous studies. During extended periods away from the nest, young pups become cold and activate metabolic heat production, which increases oxygen consumption. Increased respiration stimulates USV production and potentially confounds assessment of USV suppression (Blumberg and Alberts, 1990). Our work suggests the need for re-evaluation of the developmental timecourse of defensive behaviors and the factors which modulate their emergence.
4.2. Exposure to predator odor increased corticosterone levels only in neonatal males
Despite its name, some stressors can elevate adrenal steroids during the hyporesponsive period (Sapolsky and Meaney, 1986; Tanapat et al., 1998; Viau et al., 1996). We found that PO exposure of neonatal rats on PN4 resulted in a significant increase in circulating corticosterone in males only, an effect also seen by others following exposure to adult males on PN5 (Tanapat et al., 1998). This finding has important implications for sex differences in the emergence of behaviors thought to rely on corticosterone secretion, but more research is necessary to confirm this result and understand the mechanism through which it is established.
4.3. Exposure to predator odor did not affect cell genesis in neonatal dentate gyrus
Early PO exposure significantly decreases proliferating cells in the granule cell layer of the dentate gyrus, likely through elevations in corticosterone (Gould et al., 1992; Tanapat et al., 1998). Despite elevation in corticosterone in males following PO exposure from PN1–3, in our study we did not detect a significant decrease in dentate gyrus proliferation, though data trended towards a decline. Unlike previous work which used a single dose of the cell division marker, we preformed daily BrdU injections immediately before PO exposures from PN1–3. Compensatory cell proliferation in days following exposure to the scent of an unfamiliar male rat may have masked any short-term effects.
4.4. Exposure to predator odor had opposite effects in males and females on later juvenile social play
Male rats engage in a higher frequency of rough-and-tumble play events during the juvenile period, relative to females (Argue and McCarthy, 2015; Meaney and Stewart, 1981; Olioff and Stewart, 1978; Thor and Holloway, 1986). Androgen signaling through androgen receptors (ARs) plays a crucial role in masculinization of social play. Perinatal exposure of males to AR antagonists results in reductions in social play during the juvenile period (Hotchkiss et al., 2003; Meaney et al., 1983). Treatment during the neonatal critical period with dihydrotestosterone (DHT), the androgenic metabolite of testosterone, is sufficient for masculinization of social play behavior (Meaney and Stewart, 1981), but there is a role for the aromatized metabolite estradiol as well (Olesen et al., 2005).
Male animals exposed to PO on PN1–3 exhibited decreased social play when tested as juveniles over 3 weeks later, compared to unexposed littermates. This reduction was evident in global measures of time engaged in play and total play events, but decrements were also noted across all specific play behaviors analyzed including pouncing, pinning, boxing and chasing. Corticosterone injection to males on PN1–2 or PN3–4, but not later in development, causes similar reductions (Meaney et al., 1982). The temporal boundary for glucocorticoid action coincides with the period during which androgens masculinize play, suggesting that glucocorticoid action is mediated through suppression of androgen secretion or action (Beatty et al., 1981). While early corticosterone treatment does not alter plasma testosterone during the critical period (Meaney et al., 1982), glucocorticoids can decrease AR protein levels in cells that co-express both receptors (Burnstein et al., 1995). Both glucocorticoid and androgen receptors are expressed in regions of the brain implicated in regulation of juvenile social play (Beatty et al., 1982; Clayton et al., 1977; Meaney et al., 1981; Meaney et al., 1985). Therefore the reduction in male play following PO exposure may be accomplished through corticosterone-mediated downregulation of AR expression and disruption of signaling which is important for masculinization of juvenile social play.
While male animals that are exposed to neonatal PO respond with suppressed social play during the juvenile period, females increased their social play behavior. As with males, all parameters measured including both the time engaged in play and number of play events as well as frequency of specific behaviors of pinning, pouncing, boxing and chasing were affected.
The neurotransmitter dopamine has also been shown to be important for sexual differentiation of social play. Neonatal administration of dopamine agonist masculinizes social play (Götz et al., 1991; Olesen et al., 2005; Tönjes et al., 1989) and males have higher dopamine content in the early postnatal period across many brain regions (Balan et al., 2000; Connell et al., 2004; Lesage et al., 1996). Although dopamine is traditionally viewed as important for the processing of rewards (Wise and Rompre, 1989), it is more broadly implicated in attention to salient events, which can either be appetitive or aversive (Berridge and Robinson, 1998; Salamone et al., 1997). Consistent with this, global activation of the dopaminergic system is observed following stress (Abercrombie et al., 1989). Thus the stressful event of PO exposure could elevate dopamine and thereby masculinize play in females.
4.5. Exposure to predator odor had no effect on anxiety behaviors in juveniles or adults
Effects of neonatal PO exposure were limited to juvenile social play behavior and did not extend to other behaviors including juvenile and adult open field, elevated plus maze and light/dark box assessments, as well as, tests of adult social interaction. These findings suggest that early PO exposure exerts consequences that are specific to social play during the juvenile period. There is no comprehensive understanding as to why rats play, but there are several intriguing explanations. Juvenile social play is rewarding. Anticipation of play elicits 50 KHz ultrasonic vocalizations (Knutson et al., 1998), which reflect a positive emotional state in rats (Knutson et al., 2002). Furthermore, play can be used as a reward in maze learning (Humphreys and Einon, 1981; Normansell and Panksepp, 1990) and rats will develop condition place preferences for environments in which they have played (Calcagnetti and Schechter, 1992). Play also functions to shape adult behaviors including aggression, sexual behavior and development of coping with social challenges (Gerall et al., 1967; Van den Berg et al., 1999). Alterations evident in juvenile social play as a result of early predator odor exposure provide some insight into other behaviors that may be affected by the exposure and are related to the motivation for and the consequences of engagement in play during the juvenile period.
5. Conclusion
Early life adversity is a leading risk factor for development of several neuropsychiatric disorders including depression, anxiety, post-traumatic stress disorder and schizophrenia (Chapman et al., 2004; Heim and Nemeroff, 2001; Lu et al., 2008; Morgan et al., 2007). Additionally, adverse childhood experiences can alter the course of psychiatric illness by influencing time of onset, severity of symptoms and comorbid complications (Leverich et al., 2002; Lu et al., 2008). Social behavior is often altered in individuals with psychiatric illnesses (Couture, 2006; Pelcovitz et al., 1994; Segrin, 2000), most of which also display sex differences in prevalence of presentation (Bao and Swaab, 2010). This work establishes that exposure to stressful events early in development can result in sexually dimorphic effects on social play behavior in the juvenile period. Ultimately this creates a foundation on which to further explore how early stressful events interact with sex to produce socially-relevant sex specific consequences and disease manifestation.
Acknowledgments
The authors are grateful to J. Michael Bowers for his assistance in collection and analysis of USV data. This work was supported by the National Institutes of Health NIH/NINDS R01 NS050525 and NIH/NIMH R01 MH52716-018 to MMM.
Abbreviations
- AR
androgen receptor
- DHT
dihydrotestosterone
- EPM
elevated plus maze
- L/D box
light/dark box
- OFT
open field test
- PN
postnatal day
- PO
predator odor
- SIT
social interaction test
- USV
ultrasonic vocalization
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52(5):1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. http://dx.doi.org/10.1111/j.1471–4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Aldis Owen. Play-Fighting. Academic Press; New York: 1975. [Google Scholar]
- Allin JT, Banks EM. Functional aspects of ultrasound production by infant albino rats (Rattus norvegicus) Anim Behav. 1972;20(1):175–185. doi: 10.1016/s0003-3472(72)80189-1. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Giles WH. The enduring effects of abuse and related adverse experiences in childhood: a convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. http://dx.doi.org/m10.07/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argue KJ, McCarthy MM. Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol. Sex Differ. 2015;6(1) doi: 10.1186/s13293-015-0034-x. http://dx.doi.org/10.1186/s13293-015-0034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JL, Siviy SM. Effects of neonatal handling and maternal separation on rough-and-tumble play in the rat. Dev Psychobiol. 2002;41(3):205–215. doi: 10.1002/dev.10069. http://dx.doi.org/10.1002/dev.10069. [DOI] [PubMed] [Google Scholar]
- Ayers LW, Asok A, Blaze J, Roth TL, Rosen JB. Changes in dam and pup behavior following repeated postnatal exposure to a predator odor (TMT): a preliminary investigation in Long-Evans rats. Dev Psychobiol. 2016;58(2):176–184. doi: 10.1002/dev.21362. http://dx.doi.org/10.1002/dev.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan IS, Ugrumov MV, Calas A, Mailly P, Krieger M, Thibault J. Tyrosine hydroxylase-expressing and/or aromatic L-amino acid decarboxylase-expressing neurons in the mediobasal hypothalamus of perinatal rats: differentiation and sexual dimorphism. J Comp Neurol. 2000;425(2):167–176. doi: 10.1002/1096-9861(20000918)425:2<167::aid-cne1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscientist. 2010;16(5):550–565. doi: 10.1177/1073858410377005. http://dx.doi.org/10.1177/1073858410377005. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Traylor KL, Meaney MJ. Temporal boundary of the sensitive period for hormonal organization of social play in juvenile rats. Physiol Behav. 1981;26(2):241–243. doi: 10.1016/0031-9384(81)90017-2. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Traylor KL, Donegan JC, Godding PR. Septal lesions increase play fighting in juvenile rats. Physiol Behav. 1982;28(4):649–652. doi: 10.1016/0031-9384(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G. Social memory is impaired in neonatally ibotenic acid lesioned rats. Behav Brain Res. 2000;109(1):137–140. doi: 10.1016/s0166-4328(99)00163-1. [DOI] [PubMed] [Google Scholar]
- Becker A, Grecksch G, Bernstein HG, Höllt V, Bogerts B. Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology. 1999;144(4):333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic by-product of laryngeal braking? Behav Neurosci. 1990;104(5):808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31(5):883–891. doi: 10.1111/j.1460-9568.2010.07115.x. http://dx.doi.org/10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ. 2010;1(1):8. doi: 10.1186/2042-6410-1-8. http://dx.doi.org/10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstein KL, Maiorino CA, Dai JL, Cameron DJ. Androgen and glucocorticoid regulation of androgen receptor cDNA expression. Mol Cell Endocrinol. 1995;115(2):177–186. doi: 10.1016/0303-7207(95)03688-1. [DOI] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol Behav. 1992;51(4):667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–225. doi: 10.1016/j.jad.2003.12.013. http://dx.doi.org/10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. http://dx.doi.org/10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Clayton CJ, Grosser BI, Stevens W. The ontogeny of corticosterone and dexamethasone receptors in rat brain. Brain Res. 1977;134(3):445–453. doi: 10.1016/0006-8993(77)90821-6. [DOI] [PubMed] [Google Scholar]
- Connell S, Karikari C, Hohmann CF. Sex-specific development of cortical monoamine levels in mouse. Brain Res Dev Brain Res. 2004;151(1-2):187–191. doi: 10.1016/j.devbrainres.2004.03.008. http://dx.doi.org/10.1016/j.devbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Couture SM. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Supplement 1):S44–S63. doi: 10.1093/schbul/sbl029. http://dx.doi.org/10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Dysfunction in the neural circuitry of emotion regulation–a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. http://dx.doi.org/10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273(2):307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. http://dx.doi.org/10.1016/S0749–3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Gerall HD, Ward IL, Gerall AA. Disruption of the male rat’s sexual behaviour induced by social isolation. Anim Behav. 1967;15(1):54–58. doi: 10.1016/s0003-3472(67)80010-1. [DOI] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain—a review. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41(1):97–116. [PubMed] [Google Scholar]
- Götz F, Tönjes R, Maywald J, Dörner G. Short- and long-term effects of a dopamine agonist (lisuride) on sex-specific behavioural patterns in rats. Exp Clin Endocrinol. 1991;98(2):111–121. doi: 10.1055/s-0029-1211107. http://dx.doi.org/10.1055/s-0029-1211107. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12(9):3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacquemand R, Buron G, Pourié G, Karrer M, Jacquot L, Brand G. Effects of CO2 inhalation exposure on mice vomeronasal epithelium. Cell Biol Toxicol. 2010;26(4):309–317. doi: 10.1007/s10565-009-9143-9. http://dx.doi.org/10.1007/s10565-009-9143-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. http://dx.doi.org/10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Ostby JS, Vandenbergh JG, Gray LE. An environmental antiandrogen, vinclozolin, alters the organization of play behavior. Physiol Behav. 2003;79(2):151–156. doi: 10.1016/s0031-9384(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim Behav. 1981;29(1):259–270. http://dx.doi.org/10.1016/S0003-3472(81)80173-X. [Google Scholar]
- Kendler KS, Sheth K, Gardner CO, Prescott CA. Childhood parental loss and risk for first-onset of major depression and alcohol dependence: the time-decay of risk and sex differences. Psychol Med. 2002;32(7):1187–1194. doi: 10.1017/s0033291702006219. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. John Wiley & Sons; New York: 1992. pp. 353–377. [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology (Washington, DC: 1983) 1998;112(1):65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128(6):961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Lim S, Gudsnuk K, Champagne FA. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Frontiers in Psychiatry. 2013;4 doi: 10.3389/fpsyt.2013.00078. http://dx.doi.org/10.3389/fpsyt.2013.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage J, Bernet F, Montel V, Dupouy JP. Hypothalamic metabolism of neurotransmitters (serotonin, norepinephrine, dopamine) and NPY, and gonadal and adrenal activities, during the early postnatal period in the rat. Neurochem Res. 1996;21(1):87–96. doi: 10.1007/BF02527676. [DOI] [PubMed] [Google Scholar]
- Leverich GS, McElroy SL, Suppes T, Keck PE, Denicoff KD, Nolen WA, Post RM. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288–297. doi: 10.1016/s0006-3223(01)01239-2. http://dx.doi.org/10.1016/S0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- Lu W, Mueser KT, Rosenberg SD, Jankowski MK. Correlates of adverse childhood experiences among adults with severe mood disorders. Psychiatric Services (Washington, D.C) 2008;59(9):1018–1026. doi: 10.1176/ps.2008.59.9.1018. http://dx.doi.org/10.1176/appi.ps.59.9.1018. [DOI] [PubMed] [Google Scholar]
- Mashoodh R, Sinal CJ, Perrot-Sinal TS. Predation threat exerts specific effects on rat maternal behaviour and anxiety-related behaviour of male and female offspring. Physiol Behav. 2009;96(4–5):693–702. doi: 10.1016/j.physbeh.2009.01.001. http://dx.doi.org/10.1016/j.physbeh.2009.01.001. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonté B, Szyf M, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. http://dx.doi.org/10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Neonatal-androgens influence the social play of prepubescent rats. Horm Behav. 1981;15(2):197–213. doi: 10.1016/0018-506x(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Dodge AM, Beatty WW. Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiol Behav. 1981;26(3):467–472. doi: 10.1016/0031-9384(81)90175-x. http://dx.doi.org/10.1016/0031-9384(81)90175-X. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Beatty WW. The influence of glucocorticoids during the neonatal period on the development of play-fighting in Norway rat pups. Horm Behav. 1982;16(4):475–491. doi: 10.1016/0018-506x(82)90054-x. http://dx.doi.org/10.1016/0018-506X(82)90054-X. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37(2):85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Jensen LK, McGinnis MY, McEwen BS. Nuclear and cytosolic androgen receptor levels in the limbic brain of neonatal male and female rats. Brain Res. 1985;355(2):179–185. doi: 10.1016/0165-3806(85)90039-2. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, McEwen BS. Testosterone Implants into the Amygdala during the Neonatal Period Masculinize the Social Play of Juvenile Female Rats. Brain Res. 1986;398(2):324–328. doi: 10.1016/0006-8993(86)91492-7. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Moltz H. Infanticide in rats: male strategy and female counter-strategy. Physiol Behav. 1988;42(1):19–28. doi: 10.1016/0031-9384(88)90254-5. http://dx.doi.org/10.1016/0031-9384(88)90254-5. [DOI] [PubMed] [Google Scholar]
- Moriceau Stephanie, Roth Tania L, Okotoghaide Terri, Sullivan Regina M. Corticosterone Controls the Developmental Emergence of Fear and Amygdala Function to Predator Odors in infant Rat Pups. int J Dev Neurosci. 2004;22(5-6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Kirkbride J, Leff J, Craig T, Hutchinson G, McKenzie K, Fearon P. Parental separation, loss and psychosis in different ethnic groups: a case-control study. Psychol Med. 2007;37(4):495–503. doi: 10.1017/S0033291706009330. http://dx.doi.org/10.1017/S0033291706009330. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav. 2008;91(1):77–83. doi: 10.1016/j.pbb.2008.06.013. http://dx.doi.org/10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58(1):102–106. doi: 10.1016/j.neuropharm.2009.06.032. http://dx.doi.org/10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. http://dx.doi.org/10.1111/j.1749-6632.1999.tb09271.x (1 ADVANCING FRO). [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds in young rodents. II. Changes with age in albino rats. Anim Behav. 1968;16(1):129–134. doi: 10.1016/0003-3472(68)90123-1. [DOI] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5(4):371–387. doi: 10.1002/dev.420050410. http://dx.doi.org/10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev Psychobiol. 1990;23(1):75–83. doi: 10.1002/dev.420230108. http://dx.doi.org/10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Jessen HM, Auger CJ, Auger AP. Dopaminergic activation of estrogen receptors in neonatal brain alters progestin receptor expression and juvenile social play behavior. Endocrinology. 2005;146(9):3705–3712. doi: 10.1210/en.2005-0498. http://dx.doi.org/10.1210/en.2005-0498. [DOI] [PubMed] [Google Scholar]
- Olioff M, Stewart J. Sex differences in the play behavior of prepubescent rats. Physiol Behav. 1978;20(2):113–115. doi: 10.1016/0031-9384(78)90060-4. [DOI] [PubMed] [Google Scholar]
- Paul L, Kupferschmidt J. Killing of conspecific and mouse young by male rats. J Comp Physiol Psychol. 1975;88(2):755–763. http://dx.doi.org/10.1037/h0076440. [Google Scholar]
- Pelcovitz D, Kaplan S, Goldenberg B, Mandel F, Lehane J, Guarrera J. Post-traumatic stress disorder in physically abused adolescents. J Am Acad Child Adolesc Psychiatry. 1994;33(3):305–312. doi: 10.1097/00004583-199403000-00002. http://dx.doi.org/10.1097/00004583-199403000-00002. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The prejuvenile onset of play fighting in laboratory rats (Rattus norvegicus) Dev Psychobiol. 1997;31(3):193–205. [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: aschema for neurobehavioral analyses. Neurosci Biobehav Rev. 1998;23(1):87–101. doi: 10.1016/s0149-7634(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21(3):341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132(3):303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11(1):65–76. doi: 10.1016/s0006-8993(86)80190-1. http://dx.doi.org/10.1016/0165-0173(86)90010-X. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. 2007;7:30. doi: 10.1186/1471-2458-7-30. http://dx.doi.org/10.1186/1471-2458-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159(2):149–175. doi: 10.1002/cne.901590202. http://dx.doi.org/10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Segrin C. Social skills deficits associated with depression. Clin Psychol Rev. 2000;20(3):379–403. doi: 10.1016/s0272-7358(98)00104-4. http://dx.doi.org/10.1016/S0272-7358(98)00104-4. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12(1):55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- St-Cyr S, McGowan PO. Programming of stress-related behavior and epigenetic neural gene regulation in mice offspring through maternal exposure to predator odor. Front Behav Neurosci. 2015;9 doi: 10.3389/fnbeh.2015.00145. http://dx.doi.org/10.3389/fnbeh.2015.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK. Developmental expression of defensive responses during exposure to conspecific adults in preweanling rats (Rattus norvegicus) J Comp Psychol. 1992a;106(1):69–77. doi: 10.1037/0735-7036.106.1.69. (Washington, D.C.: 1983) [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav. 1992b;52(3):493–498. doi: 10.1016/0031-9384(92)90336-z. http://dx.doi.org/10.1016/0031-9384(92)90336-Z. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Rubin WW. Corticosteroid Induction of Threat-Induced Behavioral Inhibition in Preweanling Rats. Behav Neurosci. 1993;107(5):860–866. doi: 10.1037//0735-7044.107.5.860. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Organizing Action of Corticosterone on the Development of Behavioral Inhibition in the Preweanling Rat. Brain Res Dev Brain Res. 1994;81(1):121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Front Behav Neurosci. 2014;8:72. doi: 10.3389/fnbeh.2014.00072. http://dx.doi.org/10.3389/fnbeh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi LK, Chan MM, Pilar ML. Predator odor fear conditioning: current perspectives and new directions. Neurosci Biobehav Rev. 2008;32(7):1218–1227. doi: 10.1016/j.neubiorev.2008.06.001. http://dx.doi.org/10.1016/j.neubiorev.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16(3-4):235–239. doi: 10.1016/s0736-5748(98)00029-x. http://dx.doi.org/10.1016/S0736-5748(98)00029-X. [DOI] [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Social play soliciting by male and female juvenile rats: effects of neonatal androgenization and sex of cagemates. Behav Neurosci. 1986;100(2):275–279. doi: 10.1037//0735-7044.100.2.275. [DOI] [PubMed] [Google Scholar]
- Tönjes R, Götz F, Maywald J, Dörner G. Influence of a dopamine agonist (lisuride) on sex-specific behavioural patterns in rats. II. Long-term effects. Exp Clin Endocrinol. 1989;94(1-2):48–54. doi: 10.1055/s-0029-1210879. http://dx.doi.org/10.1055/s-0029-1210879. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev Psychobiol. 1999;34(2):129–138. [PubMed] [Google Scholar]
- Vanderschuren LJ, Niesink RJ, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci Biobehav Rev. 1997;21(3):309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Maternal separation enhances offensive play-fighting, basal corticosterone and hypothalamic vasopressin mRNA expression in juvenile male rats. Psychoneuroendocrinology. 2009;34(3):463–467. doi: 10.1016/j.psyneuen.2008.10.017. http://dx.doi.org/10.1016/j.psyneuen.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Meaney MJ. Changes in plasma adrenocorticotropin, corticosterone, corticosteroid-binding globulin, and hippocampal glucocorticoid receptor occupancy/translocation in rat pups in response to stress. J Neuroendocrinol. 1996;8(1):1–8. doi: 10.1111/j.1365-2826.1996.tb00680.x. http://dx.doi.org/10.1111/j.1365-2826.1996.tb00680.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased Stereological Estimation of the Total Number of Neurons in the Subdivisions of the Rat Hippocampus Using the Optical Fractionator. The Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer CP, Barr GA. Ontogeny of defensive behavior and analgesia in rat pups exposed to an adult male rat. Physiol Behav. 1998;63(2):261–269. doi: 10.1016/s0031-9384(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. http://dx.doi.org/10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27(4):791–800. doi: 10.1111/j.1460-9568.2008.06073.x. http://dx.doi.org/10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerberg B, Sageser KA. Comparison of two rodent models of maternal separation on juvenile social behavior. Frontiers in Psychiatry. 2011;2:39. doi: 10.3389/fpsyt.2011.00039. http://dx.doi.org/10.3389/fpsyt.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic IB, Sweatt JD. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology. 2013;38(1):77–93. doi: 10.1038/npp.2012.79. http://dx.doi.org/10.1038/npp.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]


