Abstract
Objective: Fruit of Phyllanthus emblica Linn. (PE) is widely consumed as a functional food and used as a folk medicine due to its remarkable nutritional and pharmacological effects. Mitomycin C (MMC) and cisplatin (cDDP) are the most widely used forms of chemotherapeutic drug, but their clinical use is limited by their genotoxicity to normal cells. We aimed to determine whether PE has potential to reduce the genotoxicity, while improving the anticancer effect, of MMC and cDDP. Methods: Cell proliferation was evaluated using the trypan blue exclusion assay and colony-forming assay. Genomic instability (GIN) was measured using the cytokinesis-block micronucleus assay. Results: Co-treatment (72 h) with PE at 20–320 μg/ml significantly enhanced the efficacy of MMC (0.05 μg/ml) and cDDP (1 μg/ml) against Colo205 colorectal cancer cells (P<0.05), and at 80–320 μg/ml significantly decreased MMC-and cDDP-induced GIN and multinucleation in normal colonic NCM460 cells (P<0.05). PE significantly decreased the mitotic index (P<0.01), blocked mitotic progression (P<0.05), and promoted apoptosis (P<0.01) in MMC-and cDDP-treated NCM460 cells, suggesting that PE-mediated inhibition of mitosis and induction of apoptosis may limit the division and survival of highly damaged cells. Also, PE was found to inhibit the clonal expansion of MMC-and cDDP-treated NCM460 cells (P<0.05) and decrease the heterogeneity of the surviving clones. Conclusions: PE potentiates the anticancer efficacy of MMC and cDDP, while preventing their genotoxicity and inhibiting clonal expansions of unstable genomes in normal cells. These data suggest that PE has the potential to reduce the risk of secondary cancers induced by chemotherapeutics.
Keywords: Phyllanthus emblica, Mitomycin C, Cisplatin, Genomic instability, Cytokinesis-block micronucleus assay
1. Introduction
Cancer is a major public health problem worldwide and is the leading cause of death in economically developed countries (Torre et al., 2015). It has been estimated that about 14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide (Torre et al., 2015). Despite our rapidly growing knowledge about cancer, its treatment is still largely based on the use of chemotherapeutic drugs (Cheung-Ong et al., 2013). Mitomycin C (MMC) and cisplatin (cis-diamminedichloroplatinum, cDDP) were two of the earliest forms of chemotherapeutic drug and are still the most widely used (Deans and West, 2011). They crosslink DNA on the opposite strands of the double helix and form interstrand crosslinks (Cheung-Ong et al., 2013), and subsequently block transcription and replication by inhibiting DNA strand separation (Deans and West, 2011). Therefore, MMC and cDDP can induce cell cycle arrest or apoptosis in rapidly proliferating cell populations. However, since the actions of MMC and cDDP are not tumor-specific, they are deleterious to normal cells which have a high rate of turnover (Aronson, 2010; Cheung-Ong et al., 2013).
The genotoxicity of MMC and cDDP to normal cells has detrimental outcomes for survivors of chemotherapy. Long-term exposure to MMC can result in transgenerational genomic instability (GIN) in mice, raising a concern of delayed transgenerational effects in the children of survivors of anticancer therapy (Glen and Dubrova, 2012). Moreover, cDDP-induced GIN in normal cells is long-lasting (Dertinger et al., 2014) and can promote pro-oncogenic mutations in cancer-chemotherapy survivors. This may lead to long-term and delayed side effects such as an increased risk of secondary cancers (Kempf and Ivankovic, 1986; Travis et al., 1999), and may also predispose survivors to various nonmalignant diseases (e.g. reproductive disorders) (van den Belt-Dusebout et al., 2007; Meadows et al., 2009). Thus, there is an urgent need for new chemoprotective agents that can diminish the unwanted genotoxicity of chemotherapeutics in normal cells but maintain efficacy against tumors.
Unfortunately, clinical approaches that aim to specifically reduce chemotherapeutics-induced GIN are rare (Aronson, 2010; Cheung-Ong et al., 2013) and attempts to eliminate genotoxicity by using isolated compounds have largely been unsatisfactory. Chinese herbal medicines composed of multiple biologically active compounds are widely used to counteract the side-effects of chemotherapy in patients being treated for cancer in China (Ruan et al., 2006; Lam et al., 2010; Li et al., 2013). Phyllanthus emblica Linn. (PE, syn. Emblica officinalis Gaertn.) of Euphorbiaceae family is a fruiting plant distributed in tropical and subtropical areas of India, China, Thailand, Indonesia, and the Malay Peninsula. The fruit of PE is an important dietary source of vitamin C, minerals, and amino acids (Mirunalini and Krishnaveni, 2010). Moreover, it contains many known medicinally relevant polyphenols such as gallic acid, ellagic acid, quercetin, and geraniin. Many parts of PE plants, including the fruit, flower, seed, leaf, root, and bark, have been widely used in various Asia folk medicinal systems for thousands of years. Extracts from PE are thought to have numerous beneficial properties, including antioxidant, anticancer, anti-diabetic, and anti-inflammatory properties, and to protect multiple organs, including the brain, heart, liver, kidney, and stomach (Luo et al., 2011; Iamsaard et al., 2014; Mathai et al., 2015).
Previously, we found that the extract of PE fruit has the potential to suppress proliferation and promote apoptosis in human colorectal cancer (CRC) cells by inducing a catastrophic level of GIN (Guo et al., 2013). Meanwhile, PE shows no obvious cytotoxicity to normal colon epithelial cells and actually protects against the spontaneous GIN in them (Guo and Wang, 2016). These results demonstrate that PE possesses a high selectivity against cancer cells. However, no studies have examined whether PE can protect normal human cells from MMC-and cDDP-induced GIN. The aim of this study was to address this issue by using colon mucosal epithelial cell line NCM460 as an in vitro model. The use of colon mucosal epithelial cell lines is an appropriate model for this study for several reasons: (1) the gastrointestinal tract appears to be the main target organ for the toxic effects of chemotherapeutic drugs (Eng, 2010; Lam et al., 2010); (2) colon mucosal epithelial cells are highly sensitive to the genotoxicity of chemotherapeutic drugs due to the intrinsic factors such as low ability to repair DNA damage and a higher proliferation rate (Aronson, 2010; Cheung-Ong et al., 2013); and (3) CRC is one of the most common cancers in developed countries (Torre et al., 2015) and the mucosal layer typically is the origin of CRC and GIN is linked to its initiation and progression (Lengauer et al., 1997; Li and Lai, 2009). Moreover, NCM460 cells were used because they were spontaneously immortalized (Moyer et al., 1996). This property makes NCM460 valuable in analysis of many cellular functions, in particular those related to genomic integrity, since virus-transformed cells are associated with spontaneous GIN that differs from their normal counterpart.
In this study, we firstly tested the potential of PE to enhance the efficacy of MMC and cDDP against Colo205 CRC cells. Secondly, we evaluated the inhibitory effects of PE on MMC-and cDDP-induced GIN and multinucleation in NCM460 cells. Thirdly, we investigated the effects of PE on the mitotic index, mitotic progression, and apoptosis induction in MMC-and cDDP-treated NCM460 cells. Finally, we examined the potential of PE to prevent the clonal expansion of genome-damaged NCM460 cells.
2. Experimental methods
2.1. Preparation of PE extract
Dried fruits of PE were provided by the Yunnan Phytopharmaceutical Co., Ltd. (Kunming, China). A sample of 50 g of mashed PE fruit was kept in 500 ml of distilled water for 2 h and then boiled for 10 min and allowed to cool to room temperature for 30 min. This procedure was repeated twice to ensure maximum extraction. The supernatant was filtered through 0.45-μm filters (Merck Millipore, MA, USA) and concentrated through lyophilisation. A stock solution of PE was prepared by dissolving the powder in RPMI 1640 medium (Gibco, NY, USA) at 5 mg/ml. The solution was filtered through a 0.22-μm pore size hydrophilic polyethersulfone membrane (Merck Millipore, MA, USA) and stored at −20 °C.
2.2. Chemicals
MMC and cDDP were purchased from Sigma-Aldrich (MO, USA) and dissolved in RPMI 1640 medium at concentrations of 0.1 and 1.0 mg/ml, respectively. The stock solutions were thawed at 4 °C and diluted to the concentration specified for the medium immediately before use.
2.3. Cell line and cell culture
The NCM460 cell line was obtained from INCELL (San Antonio, TX, USA). Colo205 was obtained from the Cell Bank of the Kunming Institute of Zoology (Chinese Academy of Sciences, Kunming, China). NCM460 and Colo205 cells were maintained as monolayers in 75-cm2 flasks in RPMI 1640 medium (Gibco, NY, USA) supplemented with 10% (v/v) newborn calf serum (Gibco, NY, USA), 1% (v/v) penicillin (5000 IU/ml)/streptomycin (5 mg/ml) solution (Gibco, NY, USA), and 1% (v/v) L-glutamine (2 mmol/L) (Sigma, MO, USA), and kept at 37 °C in a humidified atmosphere containing 5% CO2.
2.4. Tests of cell viability and colony forming ability
After treatment, both rounded-up and attached cells were harvested by trypsinization and washed twice with phosphate buffer saline (PBS, pH 7.2). Cell viability was tested by trypan blue exclusion. Cell suspensions were stained with trypan blue (Boster, Wuhan, China) for 2 min and then counted using a hemocytometer. Each experiment was repeated twice. Five hundred cells per well (in 6-well plates) were seeded in triplicate into fresh medium for the determination of colony forming ability. After 14 d of incubation, colonies were fixed with ethanol, stained with Giemsa (San’ersi, Shanghai, China), and counted (only aggregates of >30 cells were scored as colonies), and their survival was calculated as a percentage relative to the untreated control.
2.5. Cytokinesis-block micronucleus assay
The cytokinesis-block micronucleus (CBMN) assay was performed essentially as described previously (Guo and Wang, 2016; Guo et al., 2017). NCM460 cells were treated by PE alone or in combination with MMC (0.05 μg/ml) or cDDP (1 μg/ml) for 72 h. After the treatment periods ended, the medium was aspirated and cells were washed three times with PBS. To artificially induce binucleated cells (BNCs), the cells were placed in fresh medium containing cytochalasin B (Sigma-Aldrich, MO, USA; 1.5 μg/ml). The cytochalasin B was rinsed out with PBS after a further 24 h and cells were detached from plates with trypsin to generate a single-cell suspension. To ensure accuracy in identifying BNCs, cells were centrifuged using of a cytospin apparatus (Xiangyi, Hunan, China) at 800 r/min for 5 min to glass slides with the final density being kept between 0.5×105 and 1×105 cells per slide. After drying briefly in air, the slides were fixed in 100% methanol at −20 °C for 15 min and air-dried. Fixed cells were then stained with 5% (v/v) Giemsa (San’ersi, Shanghai, China) in PBS (pH 6.8). The slides were destained twice in ddH2O and air-dried, and then a cover slip was applied. Before scoring, slides were stored protected from light. Stained slides were encoded to ensure a blind microscopic analysis, and the code was not removed until the whole microscopic analysis was finished.
To determine the frequency of micronuclei (MN), nucleoplasmic bridges (NPBs), or nuclear buds (NBs), at least 1000 interphase BNCs with well-separated nuclei were scored based on criteria previously described (Fenech et al., 2003) using an Olympus 1000 CX21 microscope (Tokyo, Japan). The capacity of PE to reduce MMC-or cDDP-induced MN, NPBs, and NBs was calculated according to a formula adapted from Waters et al. (1990): R=[A−B−(C−D)]/(A−C)×100%, where R is the percentage of reduction; A is the frequency of MN, NPBs, or NBs after treatment with MMC or cDDP; B is the frequency of MN, NPBs, or NBs after treatment with PE and MMC or cDDP; C is the frequency of MN, NPBs, or NBs in the negative control; and D is the frequency of MN, NPBs, or NBs after treatment with PE.
In addition, at least 500 cells were scored for the nuclear division index (NDI), an estimated measure of the proliferative status of the viable cell fraction. The NDI was calculated as: NDI=(M 1+2M 2+3M ≥3)/N, where M 1, M 2, and M ≥3 represent the number of cells with one, two, and ≥three nuclei, respectively, and N is the total number of viable cells scored.
2.6. Analyses of mitotic index and mitotic progression
Analyses of mitotic index and mitotic progression were performed essentially as described previously (Guo et al., 2017). After treatment, both rounded-up and attached cells were harvested, centrifuged to slides, fixed, and stained as described above. Each slide was coded and scored blindly. Cell mitotic activity was assessed by the frequency of mitotic figures in 500 randomly counted Giemsa-stained nuclei. Mitotic cells with condensed chromosomes were microscopically distinguished from interphase cells. For the analysis of mitotic progression, at least 100 mitotic cells were counted and the frequencies of prophases, prometaphase, metaphase, and ana-telophase cells were scored.
2.7. Apoptosis examination and multinucleation analysis
Analysis of apoptosis was performed on the same slides prepared for mitotic index analysis. In Giemsa-stained cells, nuclei of apoptotic cells were conspicuously different from those of viable cells. Cells with chromatin condensation and intact cytoplasmic and nuclear boundaries as well as cells exhibiting nuclear fragmentation into smaller bodies within an intact cytoplasmic membrane were classified as apoptotic. Multinucleated cells are defined as cells with two or more nuclei. The microscopic evaluation of the multinucleation index was performed on the same slides as those prepared for mitotic index analysis. Mono-and multinucleated cells were enumerated in an average of 50 fields counting at least 250 cells per slide when viewed at 1000×magnification.
2.8. Statistical analysis
The differences in observed values between cells exposed only to PE, MMC, or cDDP (positive control) and the untreated group (negative control), as well as between cells exposed to PE plus MMC or cDDP and cells treated only with MMC or cDDP were analyzed using one-way analysis of variance (ANOVA). First, Levene’s test was performed to examine the homogeneity of variances among the control and treated groups. When a significant effect (one-way ANOVA, P<0.05) was detected, post-hoc tests were then applied: Tukey’s test was used when the assumption of equality of variances held (P>0.05), and the Dunnett T3 test was used otherwise (P≤0.05). Regression analysis was carried out to determine the dose-response relationship. We considered as being significant only differences having a P-value (two-tailed) lower than 0.05. All statistical analyses were performed using SPSS 17.0 for windows (SPSS, Chicago, IL, USA).
3. Results
3.1. PE enhances the efficacy of MMC and cDDP against Colo205 CRC cells
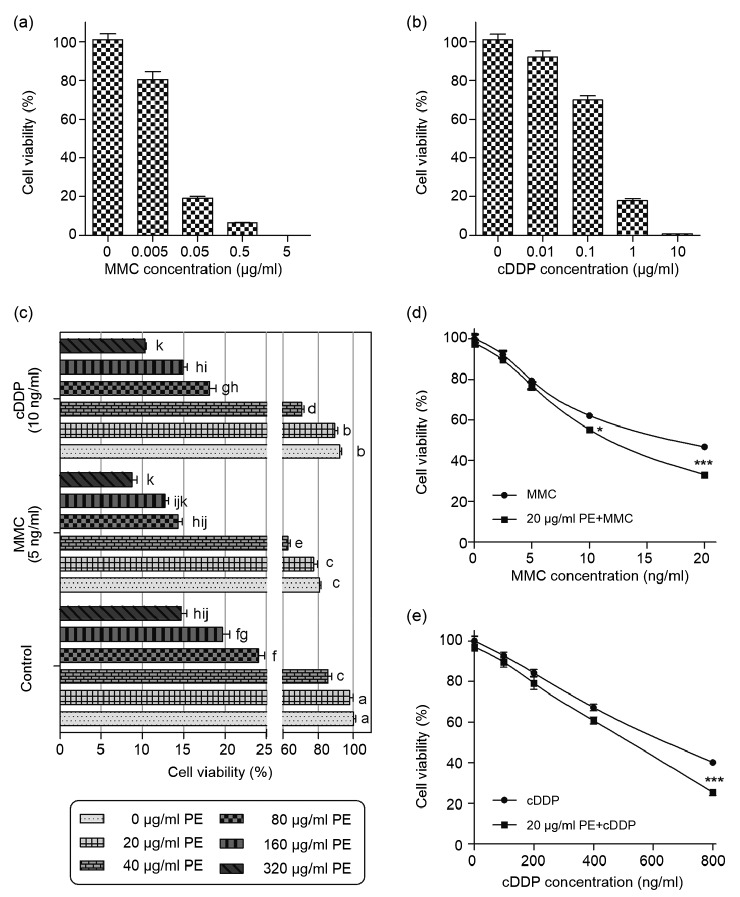
To obtain clinically relevant doses of MMC and cDDP for our studies, CRC cells were treated with various doses of MMC and cDDP for 72 h. The results showed that MMC at 0.05 μg/ml and cDDP at 1 μg/ml induced an over 80% reduction in Colo205 growth relative to corresponding controls (P<0.001; Figs. 1a and 1b). Because the toxicities of MMC and cDDP limit further dose escalation under clinical conditions, we chose 0.05 μg/ml and 1 μg/ml to mimic the clinically relevant concentrations of MMC and cDDP, respectively, for our experiments.
Fig. 1.
Effects of mitomycin C (MMC), cisplatin (cDDP), and P. emblica (PE) alone or in combination on cell growth of Colo205 cells
(a, b) Viability of Colo205 cells incubated with indicated doses of MMC (a) or cDDP (b) for 72 h. (c) Viability of Colo205 cells treated with PE either alone or in combination with MMC (5 ng/ml) or cDDP (10 ng/ml) for 72 h. (d, e) Viability of Colo205 cells treated with MMC (d) and cDDP (e) either alone or in combined with PE (20 μg/ml) for 72 h. Cell viability was expressed as a percentage of live cells relative to control cell suspensions treated with only solvent (medium). Cell numbers were determined by counting live cells on a hemacytometer with the inclusion of trypan blue. Data represent the mean of three independent experiments±standard error of mean (SEM), n=3. In (c), values that do not share the same letter are considered significantly different from each other (P<0.05). In (d) and (e), asterisks represent statistically significant differences from the group treated with MMC or cDDP alone: * P<0.05, *** P<0.001
To be able to use PE as a co-drug to prevent genotoxicity of MMC and cDDP, we firstly needed to evaluate its potential effects on the anti-cancer activity of MMC and cDDP. When Colo205 cells were exposed to the combination of PE (20–320 μg/ml) and MMC (5 ng/ml) or cDDP (10 ng/ml), greater inhibition of Colo205 proliferation was evident compared to cells exposed to PE alone (P<0.05; Fig. 1c). Moreover, PE consistently potentiated MMC-or cDDP-induced inhibition of Colo205 proliferation (P<0.05; Figs. 1d and 1e) at a concentration that had no single-agent effect (20 μg/ml). Together, these data demonstrate that PE enhances the efficacy of MMC and cDDP against CRC cells.
3.2. PE protects against MMC-and cDDP-induced GIN in NCM460 normal colonic cells
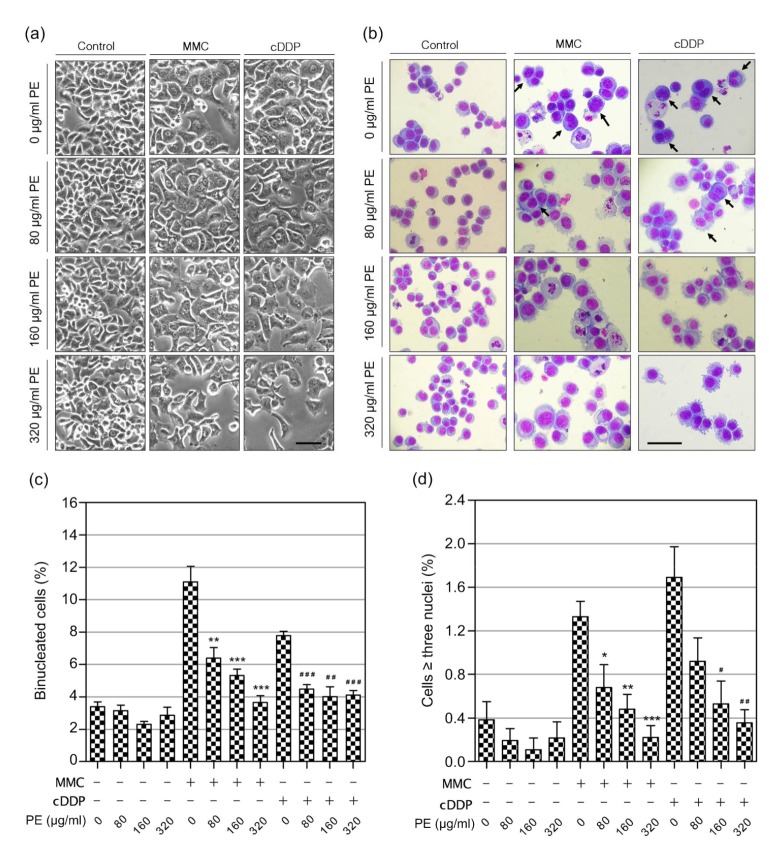
Because PE at doses beyond 40 μg/ml had dominate anticancer potential (Fig. 1c), we focused on PE at 80, 160, and 320 μg/ml for the following experiments. The effects of PE on MMC-and cDDP-induced GIN were investigated by CBMN assay. Photomicrographs of the different endpoints of GIN scored are shown in Fig. 2. The yields of both MMC-and cDDP-induced GIN in the presence or absence of PE obtained with the CBMN assay are presented in Table 1.
Fig. 2.
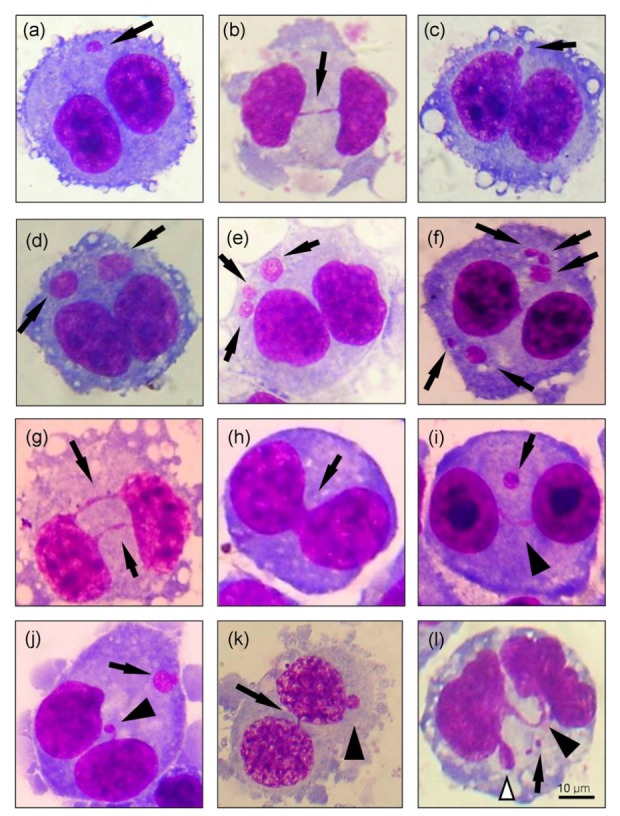
Photomicrographs of various endpoints used for evaluation of genomic instability in the cytokinesis-block micronucleus assay
Examples of binucleated cells (BNCs) induced by cytochalasin B, containing a micronucleus (MN, a), a nucleoplasmic bridge (NPB, b), or a nuclear bud (NB, c) are indicated by arrows. Examples of BNCs with severely damaged chromosomes included BNCs containing 2 MN (d), 3 MN (e), or more than 3 MN (f), as indicated by arrows; BNCs with 2 NPBs (g) or nuclear fusion (h), as indicated by arrows; and BNCs simultaneously harboring MN and NPBs (indicated by arrow and arrowhead, respectively; i), MN and NBs (indicated by arrow and arrowhead, respectively; j), NPBs and NBs (indicated by arrow and arrowhead, respectively; k), or MN, NPBs, and NBs (indicated by arrow, black arrowhead, and white arrowhead, respectively; l). Cells were stained with Giemsa, showing DNA in red and cytoplasm in blue (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
Table 1.
Co-treatment with PE protected NCM460 cells against GIN induced by MMC and cDDP (n=3)
| Treatment (μg/ml) | MN (‰) | R of MN (%) | 1 MN (%) | 2 MN (%) | 3 MN (%) | ≥4 MN (%) | NPBs (‰) | R of NPBs (%) | 1 NPB (%) | ≥2 NPBs (%) | NF (%) |
| PE 0 | 36.28±1.69 | 85.89±3.24 | 12.04±2.73 | 1.54±0.71 | 0.52±0.35 | 5.45±0.50 | 94.23±2.69 | 4.81±2.26 | 0.96±0.96 | ||
| PE 80 | 21.08±1.61** | 85.68±5.94 | 14.31±5.94 | 0 | 0 | 3.33±0.46 | 100 | 0 | 0 | ||
| PE 160 | 15.34±0.61*** | 90.45±2.86 | 7.00±2.64 | 2.54±1.71 | 0 | 0.99±0.48*** | 100 | 0 | 0 | ||
| PE 320 | 39.95±3.38 | 83.14±1.90 | 12.96±1.78 | 3.17±1.52 | 0.72±0.72 | 2.46±0.49* | 100 | 0 | 0 | ||
| MMC 0.05+PE 0 | 312.63±30.91*** | 66.96±2.44 | 20.61±1.35 | 8.02±0.93 | 4.41±0.46 | 53.00±4.88*** | 81.29±2.11 | 12.72±2.45 | 5.99±1.14 | ||
| MMC 0.05+PE 80 | 301.58±14.38 | 53.49±2.05 | 23.01±2.08 | 12.06±0.08 | 11.44±0.70# | 26.49±5.70### | 51.29 | 83.62±1.13 | 13.35±2.24 | 3.03±3.03 | |
| MMC 0.05+PE 160 | 112.39±7.55### | 64.88 | 69.06±0.27 | 21.15±0.43 | 5.07±0.44 | 4.71±0.36 | 6.30±2.83### | 88.83 | 100 | 0 | 0 |
| MMC 0.05+PE 320 | 82.97±6.86### | 84.43 | 71.72±3.44 | 16.43±1.51 | 8.19±2.39 | 3.65±1.01 | 3.29±1.05### | 98.25 | 100 | 0 | 0 |
| cDDP 1+PE 0 | 328.33±27.41*** | 56.18±2.79 | 22.19±0.34 | 11.54±0.93 | 10.09±1.75 | 32.05±6.59*** | 76.83±4.79 | 15.26±4.14 | 10.76±2.43 | ||
| cDDP 1+PE 80 | 301.88±41.49 | 3.85 | 56.02±5.50 | 21.97±2.58 | 11.96±1.26 | 10.05±2.61 | 29.83±3.84 | 0.38 | 78.59±8.13 | 19.32±7.93 | 2.08±2.08 |
| cDDP 1+PE 160 | 72.21±3.42$$$ | 80.53 | 79.33±3.18$$ | 14.45±2.22 | 4.86±0.58$$ | 1.36±0.70$$$ | 13.17±3.32$ | 54.21 | 82.00±6.90 | 14.00±4.43 | 0 |
| cDDP 1+PE 320 | 55.16±7.02$$$ | 94.79 | 77.70±2.76$$ | 17.07±3.92 | 3.60±1.41$$$ | 1.63±0.58$$$ | 4.66±0.49$$$ | 91.73 | 100 | 0 | 0 |
|
| |||||||||||
|
| |||||||||||
| Treatment (μg/ml) | NBs (‰) | R of NBs (%) | MN+NPBs (‰) | R of MN+NPBs (%) | MN+NBs (‰) | R of MN+NBs (%) | NPBs+NBs (‰) | R of NPBs+NBs (%) | MN+NPBs+NBs (‰) | R of MN+NPBs+NBs (%) | |
|
| |||||||||||
| PE 0 | 5.05±0.56 | 0.30±0.13 | 0.68±0.32 | 0.30±0.13 | 0 | ||||||
| PE 80 | 3.82±1.10 | 0 | 0 | 0 | 0 | ||||||
| PE 160 | 1.39±0.29** | 0 | 0.28±0.28 | 0 | 0 | ||||||
| PE 320 | 7.54±0.59 | 0 | 0.54±0.27 | 0 | 0 | ||||||
| MMC 0.05+PE 0 | 29.20±3.36*** | 22.19±3.39*** | 9.14±2.23*** | 1.96±0.25*** | 1.79±0.29 | ||||||
| MMC 0.05+PE 80 | 19.21±1.62## | 36.27 | 16.23±4.37 | 25.86 | 9.61±0.31 | 1.32±0.33 | 20.48 | 0.99±0.57 | 55.31 | ||
| MMC 0.05+PE 160 | 9.28±2.59### | 67.33 | 2.98±2.07## | 86.39 | 4.31±0.88# | 52.36 | 0.33±0.33# | 80.12 | 0 | 100 | |
| MMC 0.05+PE 320 | 8.66±0.86### | 95.36 | 0.97±0.67### | 95.57 | 1.21±0.44## | 92.08 | 0 | 100 | 0 | 100 | |
| cDDP 1+PE 0 | 27.71±2.20*** | 14.44±4.54*** | 14.05±1.91*** | 1.58±0.50*** | 1.98±0.32 | ||||||
| cDDP 1+PE 80 | 14.90±1.48$$$ | 51.10 | 14.91±0.59 | 7.94±1.97$ | 40.61 | 0.99±0.57 | 22.66 | 1.00±0.58 | 50.51 | ||
| cDDP 1+PE 160 | 7.67±0.56$$$ | 72.29 | 3.53±1.43$ | 75.04 | 0.59±0.24$$$ | 97.68 | 0 | 100 | 0 | 100 | |
| cDDP 1+PE 320 | 7.60±0.28$$$ | 99.74 | 0.98±0.01$$ | 93.07 | 0.73±0.46$$$ | 98.59 | 0 | 100 | 0 | 100 | |
Values are the mean±standard error per 1000 binucleated cells. PE, Phyllanthus emblica; GIN, genomic instability; MMC, mitomycin C; cDDP, cisplatin; MN, micronuclei; NPBs, nucleoplasmic bridges; NBs, nuclear buds; NF, nuclear fusion; R, percentage of reduction.
P<0.05,
P<0.01,
P<0.001, significantly different from the negative control group;
P<0.05,
P<0.01,
P<0.001, significantly different from the MMC group;
P<0.05,
P<0.01,
P<0.001, significantly different from the cDDP group
There was a bell-shaped dose response to MN (Fig. 2a), NPBs (Fig. 2b), and NBs (Fig. 2c) when NCM460 cells were treated with PE alone (Table 1). The frequencies of MN, NPBs, and NBs after exposure to MMC or cDDP were significantly higher than those found in corresponding controls (P<0.001). These results demonstrate that colon mucosal epithelial cells are highly susceptible to MMC-and cDDP-induced GIN.
The frequencies of MN, NPBs, and NBs in samples treated with PE and MMC or cDDP were lower than those in cultures treated with MMC or cDDP alone (Table 1). In particular, PE at 320 μg/ml decreased MMC-or cDDP-induced NPBs and NBs to a level comparable to those of the corresponding negative controls (medium). In addition, PE could protect cells against severe GIN from MMC and cDDP. In cDDP-treated cells, 44% of micronucleated BNCs harbored two or more MN (Figs. 2d–2f; Table 1). This was decreased to less than 22% by 160 and 320 μg/ml PE (P<0.01; Table 1). PE was also found to have potential to reduce the percentages of BNCs containing two or more NPBs (Fig. 2g) and nuclear fusion (Fig. 2h) in MMC-or cDDP-treated cells (P<0.05; Table 1). Moreover, PE had a remarkable ability to reduce the frequencies of BNCs simultaneously expressing MN and NPBs (P<0.05; Fig. 2i), MN and NBs (P<0.05; Fig. 2j), NPBs and NBs (P<0.05; Fig. 2k), and MN, NPBs, and NBs (P<0.05; Fig. 2l) induced by MMC and cDDP (Table 1). Note that PE at 320 μg/ml decreased the frequencies of these endpoints to a level comparable to corresponding negative controls (P>0.05). From these results, it was evident that co-treatment of NCM460 cells with PE and chemotherapeutics led to a significant decrease in GIN induced by the latter.
3.3. PE protects against MMC-and cDDP-induced multinucleation in NCM460 cells
Because multinucleation/polyploidy is a genetically unstable intermediate state that will lead to massive GIN in the cell’s offsprings (Storchova and Kuffer, 2008), we set out to determine the potential of PE for protecting against MMC-and cDDP-induced multinucleation. NCM460 cells not treated with MMC or cDDP reached a confluence of 90% after 72 h, with cells showing a typical epithelial-like morphology (Fig. 3a). MMC-and cDDP-treated cell showed enlarged cell and nuclear size, flattened cell morphology, and the appearance of multinucleated cell forms (Figs. 3a and 3b), indicating the existence of binucleate tetraploid/near-tetraploid and multinucleate polyploidy cells, caused by cytokinesis failure. When combined with PE, we found that PE significantly reduced the generation of BNCs in MMC-and cDDP-treated NCM460 cells (P<0.01; Fig. 3c), indicating that PE may reduce MMC-and cDDP-induced cytokinesis failure. Moreover, we found that PE could significantly prevent NCM460 cells from becoming tri-and tetra-nucleated cells upon MMC and cDDP exposure (P<0.05; Fig. 3d), suggesting that PE may protect against MMC-and cDDP-induced multipolar division.
Fig. 3.
Effects of Phyllanthus emblica (PE) on mitomycin C (MMC)-or cisplatin (cDDP)-induced multinucleation
NCM460 cells were incubated with PE alone or in combination with MMC or cDDP for 72 h before the analysis of multinucleation. (a) Representative phase-contrast images of NCM460 cells treated with PE, MMC, cDDP or in combination for 72 h. (b) Morphology of NCM460 cells treated with PE, MMC, cDDP or in combination for 72 h. Representative photomicrographs of cells stained with Giemsa dye are shown. Note that there are many multinucleated cells in MMC-and cDDP-treated cells (arrows). Bars (a and b), 100 μm. Quantification of the binucleation (c) and multinucleation (≥three nuclei; d) frequency after treatment with PE, MMC, cDDP or in combination for 72 h. The symbols “+” and “–” represent cells treated with or without the indicated components, respectively. Data represent the mean±SEM from at least three independent experiments. * P<0.05; ** P<0.01; *** P<0.001 vs. the MMC-treated group; # P<0.05; ## P<0.01; ### P<0.001 vs. the cDDP-treated group
3.4. PE prevents clonal expansions of MMC-and cDDP-treated NCM460 cells
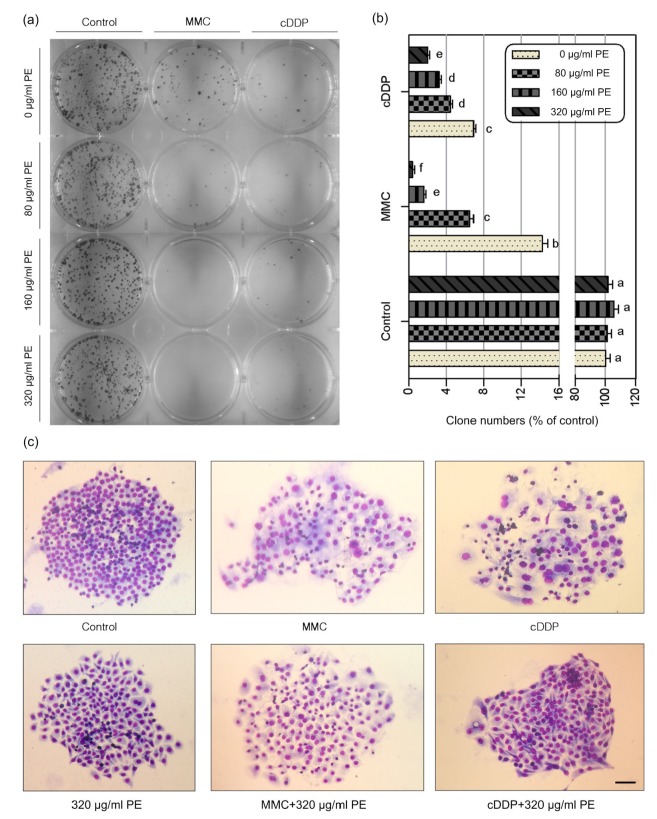
Besides GIN, the clonal expansion of a genetically unstable cell in a premalignant neoplasm will have an associated risk of progression to cancer (Maley et al., 2004). Thus, we set out to examine whether PE could prevent clonal expansion of genetically unstable cells derived from MMC-or cDDP-exposure. The colony forming ability of NCM460 cells in samples treated with PE alone was not significantly different from the control (medium) (Figs. 4a and 4b). These data demonstrate that PE at doses up to 320 μg/ml did not inhibit the proliferation and colony forming ability of NCM460 cells.
Fig. 4.
Effects of Phyllanthus emblica (PE) on the colony-formation ability of mitomycin C (MMC) or cisplatin (cDDP)-treated cells
NCM460 cells were treated with PE alone or in combination with MMC (0.05 μg/ml) or cDDP (1 μg/ml) for 72 h. Five hundred cells per well were seeded into fresh medium for 14 d for determining their colony formation ability. (a) Representative images of Giemsa-stained colonies formed 14 d after drugs removed. (b) Quantification of the percentage of clonogenic survival of the indicated groups. Results shown are the mean±SEM of three independent experiments. Values that do not share the same letter are considered significantly different from each other (P<0.05). (c) Representative morphology of colonies from the indicated groups. Bar: 100 μm
As expected, the colony forming ability of NCM460 cells was significantly reduced (P<0.001) by MMC and cDDP, by about 86% and 93%, respectively (Figs. 4a and 4b). These results indicated that MMC and cDDP induced a long-lasting cytotoxicity to normal cells. However, in cells treated by combined MMC or cDDP and PE, the colony forming ability was reduced further as the concentration of PE increased (P<0.01; Figs. 5a and 4b). In addition, we found that compared to clones formed from control cells, clones from MMC-and cDDP-treated cells did not compact into a tight sheet but instead formed a wide, loose sheet and showed temporal alterations in cell morphology associated with profound changes in size and increased nuclear/cytoplasmic ratio. The resulting clones contained substantial numbers of giant mononucleated cells and multinucleated cells (Fig. 4c). In contrast, clones formed from cells treated with the combination of PE and MMC or cDDP had a regular cell size, a decreased level of multinucleation, and a compact morphology (Fig. 4c). Together, these results suggest that PE could prevent the clonal expansion of genome-damaged cells induced by MMC or cDDP and decrease the intercellular heterogeneity in surviving clones.
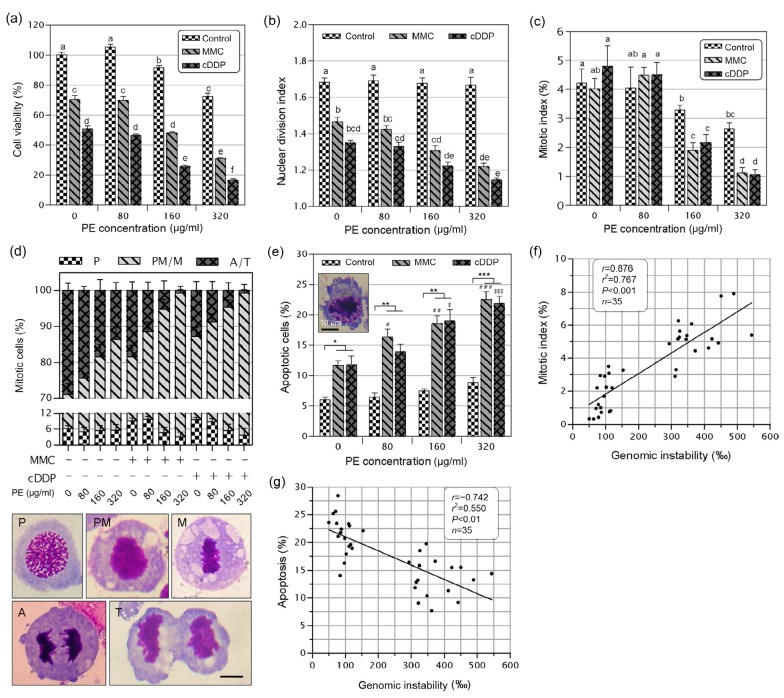
Fig. 5.
Effects of Phyllanthus emblica (PE) on proliferation, mitotic index, and apoptosis of mitomycin C (MMC)-or cisplatin (cDDP)-treated cells
NCM460 cells were incubated with PE alone or in combination with MMC (0.05 μg/ml) or cDDP (1 μg/ml) for 72 h before the cell viability (a), nuclear division index (b), mitotic index (c), mitotic progression (d), and apoptosis (e) were determined. (d) Cells were observed in all stages of mitosis and were classified into the different mitotic stages as depicted in the bottom images. P: prophase; PM: prometaphase; M: metaphase; A: anaphase; T: telophase. The insert in (e) shows a representative image of apoptotic cell. Figures (f) and (g) show the relationship between genomic instability and mitotic index (f) and apoptosis (g), respectively. Results shown in (a–e) are the mean±SEM of at least three independent experiments. Bars (d and e): 10 μm. Values in (a–c) that do not share the same letter are considered significantly different from each other (P<0.05). In (e), * P<0.05, ** P<0.01, *** P<0.001; # P<0.05, ## P<0.01, ### P<0.001 vs. the MMC-treated group; $ P <0.05; $$ P<0.01, $$$ P<0.001 vs. the cDDP-treated group
3.5. PE decreases the mitotic index, blocks mitotic progression, and promotes apoptosis in MMC-and cDDP-treated NCM460 cells
Although PE at 80–320 μg/ml was highly cytotoxic to Colo205 cells (Fig. 1c), it was only slightly cytotoxic to NCM460 cells (Fig. 5a). Notably, 320 μg/ml PE inhibited cell proliferation by up to 85% in Colo205 cells (Fig. 1c) but by only 28% in NCM460 cells (Fig. 5a). These data suggest that PE has a high selectivity to target CRC cells.
A statistically significant (P<0.05) decrease in proliferation was found in samples treated with PE plus MMC or cDDP in comparison with cultures treated with MMC or cDDP alone (Fig. 5a). This finding was further supported by NDI (Fig. 5b) and mitotic index (Fig. 5c) data. We also monitored the capacity of PE to modify the distribution of cells in different phases of mitosis. We found a significant increase in the frequencies of prometaphases and metaphases with a concomitant decrease in ana-telophases in cells treated with a combination of PE and MMC or cDDP, when compared to that of cells treated by MMC or cDDP alone (P<0.05; Fig. 5d). This result indicates that PE forces MMC-and cDDP-treated cells to spend longer time in prometaphase and metaphase, possibly in trying to repair the aberrant mitosis.
In addition, the frequency of apoptotic cells was significantly increased after treatment with MMC or cDDP (P<0.05). For MMC and cDDP, the frequency of apoptosis observed with 80, 160, and 320 μg/ml PE was significantly greater than that observed after treatment with PE alone (P<0.01, P<0.01, and P<0.001, respectively; Fig. 5e). This result suggests that combined treatment with PE could increase the apoptogenic effects of MMC and cDDP.
We found that GIN (the frequency of BNCs harboring any biomarker of MN, NPBs, or NBs) was positively correlated with the mitotic index (r=0.876, P<0.001; Fig. 5f) and negatively correlated with apoptosis (r=−0.74, P<0.001; Fig. 5g). These data suggest that preventing mitosis exit and promoting apoptosis might contribute, at least in part, to the chemoprotective action of PE.
4. Discussion
DNA cross-linking agents like MMC and cDDP were one of the earliest, and are still the most widely used forms of chemotherapeutic drug (Deans and West, 2011). However, a potential hazard, so far only scarcely investigated, is uncontrolled adverse genotoxicity in non-cancerous tissues (Cheung-Ong et al., 2013), which will lead to an increased risk of secondary cancers in survivors (Kempf and Ivankovic, 1986; Travis et al., 1999). In this context, strategies to protect against MMC-or cDDP-induced genotoxicity are of clinical interest. We have reported previously that PE can protect against spontaneous GIN in NCM460 cells (Guo and Wang, 2016). In this study, we therefore focused on the potential protective activity of PE against GIN induced by MMC and cDDP. We provided compelling evidence showing that PE can diminish the genotoxicities of MMC and cDDP on normal colon epithelial cells but still potentiate efficacy against the CRC cells.
As DNA reactive genotoxins, MMC and cDDP are known for their ability to induce DNA interstrand crosslinks after bio-activation (Cheung-Ong et al., 2013). These simple lesions can readily induce point mutations, chromosome breakage, amplifications, deletions, and translocations, through processing of the damaged base either spontaneously or by various DNA repair pathways (Brüsehafer et al., 2014). Thus, a wide spectrum of GIN events can be produced by MMC and cDDP. To measure these GIN events, we used the well-regarded CBMN assay, in which the numerical and structural chromosomal aberrations can be detected by using biomarkers of MN, NPBs, and NBs (Fenech, 2006; 2007). MN may result from whole chromosomal loss or chromosomal fragmentation. NPBs are thought to be sensitive indicators of: (1) chromosome rearrangements originating from dicentric chromosomes that result either from misrepair of chromosome breaks or from telomere-to-telomere end fusions, and (2) failed chromosome decatenation when one sister chromatid persistently adheres to its sibling as late as anaphase (Guo et al., 2013). NBs are a direct manifestation of the nuclear elimination of amplified DNA or chromatin (Fenech et al., 2011). The simultaneous expression of MN, NPB, and NB is an indirect measurement of breakage-fusion-bridge cycles (Fenech, 2007).
For a substance to be classified as antigenotoxic, it must first be evaluated for genotoxicity. In our study, the two concentrations of PE (80 and 160 μg/ml) could actually reduce GIN level when compared with the baseline control. These results were consistent with our previous observations (Guo and Wang, 2016). Moreover, PE at 320 μg/ml showed no significant effect on the frequency of GIN in NCM460 cells.
We found that co-treatment of NCM460 cells with PE could decrease GIN induced from MMC and cDDP, confirming that PE is a good chemoprotective agent. Coupled with our previous results, the antigenotoxic activity of PE may result from the prevention of mitosis exit by activating the spindle assembly checkpoint (Guo and Wang, 2016). Based on the previous reports, the antigenotoxic activity of PE may also be attributable to the inhibition of cytochrome P450 (Sharma et al., 2000; Banu et al., 2004), a reductase involved in the bio-activation of MMC, thereby reducing MMC bio-activation. In addition, we found that PE could prevent the generation of MMC-and cDDP-induced BNCs. The underlying molecular mechanisms may be: (1) the reduction of MN and NPBs, thereby reducing the frequency of spontaneous furrow regression caused by chromatin localized to the cleavage plane (Pan et al., 1984); (2) the prevention of defects in cytokinetic apparatus in NCM460 cells (Guo and Wang, 2016).
Chemotherapy using MMC and cDDP is largely associated with an increased risk of secondary cancers that may manifest decades after treatment (Kempf and Ivankovic, 1986; Travis et al., 1999), but the molecular basis of this process remains elusive. Clonal evolution is a model devised to explain the emergence of multidrug resistance and relapse after initial efficient chemotherapy (Greaves and Maley, 2012). This model can also be used to explain the emergence of secondary cancers in chemotherapy survivors. After a chemotherapy challenge, individual normal cells will have distinct proliferative states, depending on the balance of oncogenic and tumor suppressive genes in the genome. Most of these cells will die in the following few days; however, an undefined number of cells will gain the capability to proliferation and clonal expansion. Among the surviving clones, only those with large size and marked heterogeneity of genetic lesions are more likely to progress to cancer because they have a greater selective advantage against cancer-suppressive mechanisms (Maley et al., 2004). Therefore, the combination of GIN and clonal expansion is a predictor of chemotherapy-induced secondary cancers, and interventions that limit the emergence of GIN cells and their clonal expansion may reduce the risk of progression to cancer
In this study, we found clones formed from MMC-and cDDP-treated NCM460 cells exhibited intercellular heterogeneity in cell size and GIN, indicating the risk of oncogenic transformation after exposure to MMC and cDDP. Moreover, our findings that PE protected against MMC-and cDDP-induced GIN and inhibited their clonal expansion indicated that PE may be a therapeutic drug that suppresses the progression of MMC-and cDDP-treated normal cells towards the malignant state (secondary cancer).
Our data also showed that the combination of PE and MMC or cDDP was more efficacious than either drug alone in inhibiting the proliferation of Colo205 CRC cells. These results agreed with the finding that a PE has synergistic effect with doxorubin and cDDP against human hepatocellular carcinoma and lung cancer cells (Pinmai et al., 2008), as well as the finding that PE acts synergistically with cDDP to reduce cell proliferation of human ovarian cancer cells (De et al., 2013). These results suggest that PE can enhance the anticancer potential of MMC and cDDP in a broad range of cancer types. However, the mechanisms involved in the interaction between chemotherapeutic drugs and PE still remain unclear. We have found that PE could elevate the GIN rate in human CRC cell lines Colo320 (Guo et al., 2013) and Colo205 (unpublished data). Although GIN is a hallmarker of cancer cells and is crucial to cancer evolution (Heng et al., 2013), elevating GIN level in cancer cells is a way to kill them (Janssen et al., 2009; Guo et al., 2013) and will further sensitize tumor cells to chemotherapy (Zaki et al., 2014). Therefore, we expect that, by inducing GIN in Colo205 cells, PE makes them more sensitive to MMC and cDDP. This activity is interesting and warrants further investigation.
Like many other herbal medicines, the multi-component and multi-target nature of PE hinders its in-depth investigation and translation. Therefore, it is important to identify the individual chemical compounds responsible for the ability of PE to protect against chemotherapeutic-induced GIN. However, the chemical nature of the compounds responsible for the protective effects of PE is difficult to identify, since a wide range of mechanisms could be operating in this complex mixture. Some of the components in PE may play critical roles in the chemoprotective effect of PE. For example, vitamin C and quercetin have been reported to reduce the frequency of MN induced by MMC or cDDP in bone marrow cells of mice (Giri et al., 1998; Attia, 2010; Mazumdar et al., 2011), ellagic acid (Aiyer et al., 2008) and quercetin (Min and Ebeler, 2009) have been shown to activate the DNA repair system, and geraniin has the potential to reproduce the effect of PE in protection against GIN in NCM460 cells (unpublished data). Despite these findings, we expect that the remarkable chemopreventive potential of PE is more likely to result from the combination of a wide range of bioactive compounds, with various intracellular targets and various additive or synergistic activity.
5. Conclusions
In conclusion, our current investigation provides evidence that under the condition of simultaneous treatment, PE potentiates the efficacy of MMC or cDDP against CRC cells, while having an important role in protecting the genome from instability and inhibiting the clonal expansion of unstable genome induced by MMC and cDDP in normal colon cells. These results suggest that PE holds great promise for circumventing the huge challenges associated with the chemotherapy of tumors. Further studies, mainly in vivo, are needed to clarify the biological importance of the observed effects. The challenges for the future are: (1) to define the precise molecular mechanisms through which PE prevents GIN and clonal expansion induced by chemotherapeutic drugs, and (2) to identify the active compounds that are responsible for these effects of PE. We expect that overcoming these challenges will lead to new discoveries in the biological and biomedical sciences.
Acknowledgments
This work was also supported by Yunnan Province New Academic Talent Award for Ph.D. 2016 (to Xi-han GUO).
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31260268 and 31560307)
Compliance with ethics guidelines: Xi-han GUO, Juan NI, Jing-lun XUE, and Xu WANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Aiyer HS, Vadhanam MV, Stoyanova R, et al. Dietary berries and ellagic acid prevent oxidative DNA damage and modulate expression of DNA repair genes. Int J Mol Sci. 2008;9(3):327–341. doi: 10.3390/ijms9030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson JK. Meyler’s Side Effects of Drugs Used in Cancer & Immunology. Elsevier, Amsterdam; 2010. [Google Scholar]
- 3.Attia SM. The impact of quercetin on cisplatin induced clastogenesis and apoptosis in murine marrow cells. Mutagenesis. 2010;25(3):281–288. doi: 10.1093/mutage/geq004. [DOI] [PubMed] [Google Scholar]
- 4.Banu SM, Selvendiran K, Singh JPV, et al. Protective effect of Emblica officinalis ethanolic extract against 7,12-dimethylbenz(a)anthracene (DMBA) induced genotoxicity in Swiss albino mice. Hum Exp Toxicol. 2004;23(11):527–531. doi: 10.1191/0960327104ht484oa. [DOI] [PubMed] [Google Scholar]
- 5.Brüsehafer K, Rees BJ, Manshian BB, et al. Chromosome breakage induced by the genotoxic agents mitomycin C and cytosine arabinoside is concentration and p53 dependent. Toxicol Sci. 2014;140(1):94–102. doi: 10.1093/toxsci/kfu058. [DOI] [PubMed] [Google Scholar]
- 6.Cheung-Ong K, Giaever G, Nislow C. DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol. 2013;20(5):648–659. doi: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 7.De A, De A, Papasian C, et al. Emblica officinalis extract induces autophagy and inhibits human ovarian cancer cell proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS ONE. 2013;8(8):e72748. doi: 10.1371/journal.pone.0072748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11(7):467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dertinger SD, Avlasevich SL, Torous DK, et al. Persistence of cisplatin-induced mutagenicity in hematopoietic stem cells: implications for secondary cancer risk following chemotherapy. Toxicol Sci. 2014;140(2):307–314. doi: 10.1093/toxsci/kfu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eng C. Are herbal medicines ripe for the cancer clinic? Sci Transl Med. 2010;2(45):45ps41. doi: 10.1126/scitranslmed.3001517. [DOI] [PubMed] [Google Scholar]
- 11.Fenech M. Cytokinesis-block micronucleus assay evolves into a “cytome” assay of chromosomal instability, mitotic dysfunction and cell death. Mutat Res Fundam Mol Mech Mutagen. 2006;600(1):58–66. doi: 10.1016/j.mrfmmm.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2(5):1084–1104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 13.Fenech M, Chang WP, Kirsch-Volders M, et al. HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res Genet Toxicol Environ Mutagen. 2003;534(1-2):65–75. doi: 10.1016/S1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 14.Fenech M, Kirsch-Volders M, Natarajan A, et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26(1):125–132. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 15.Giri A, Khynriam D, Prasad SB. Vitamin C mediated protection on cisplatin induced mutagenicity in mice. Mutat Res. 1998;421(2):139–148. doi: 10.1016/S0027-5107(98)00158-4. [DOI] [PubMed] [Google Scholar]
- 16.Glen CD, Dubrova YE. Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc Natl Acad Sci USA. 2012;109(8):2984–2988. doi: 10.1073/pnas.1119396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Wang X. Phyllanthus emblica fruit extract activates spindle assembly checkpoint, prevents mitotic aberrations and genomic instability in human colon epithelial NCM460 cells. Int J Mol Sci. 2016;17(9):1437. doi: 10.3390/ijms17091437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Ni J, Liu X, et al. Phyllanthus emblica L. fruit extract induces chromosomal instability and suppresses necrosis in human colon cancer cells. Int J Vitam Nutr Res. 2013;83(5):271–280. doi: 10.1024/0300-9831/a000169. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Ni J, Xue J, et al. Extract of bulbus Fritillaria cirrhosa perturbs spindle assembly checkpoint, induces mitotic aberrations and genomic instability in human colon epithelial cell line. Exp Toxicol Pathol. 2017;69(3):163–171. doi: 10.1016/j.etp.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Heng HH, Bremer SW, Stevens JB, et al. Chromosomal instability (CIN): what it is and why it is crucial to cancer evolution. Cancer Metast Rev. 2013;32(3-4):325–340. doi: 10.1007/s10555-013-9427-7. [DOI] [PubMed] [Google Scholar]
- 22.Iamsaard S, Arun S, Burawat J, et al. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2014;15(4):405–408. doi: 10.1631/jzus.B1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen A, Kops GJ, Medema RH. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci USA. 2009;106(45):19108–19113. doi: 10.1073/pnas.0904343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempf SR, Ivankovic S. Carcinogenic effect of cisplatin (cis-diammine-dichloroplatinum (II), CDDP) in BD IX rats. J Cancer Res Clin Oncol. 1986;111(2):133–136. doi: 10.1007/BF00400751. [DOI] [PubMed] [Google Scholar]
- 25.Lam W, Bussom S, Guan F, et al. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2(45):45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 26.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- 27.Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2009;10(3):219–229. doi: 10.1631/jzus.B0820273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X, Yang G, Li X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS ONE. 2013;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo W, Zhao M, Yang B, et al. Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit. Food Chem. 2011;125(2):277–282. doi: 10.1016/j.foodchem.2010.09.027. [DOI] [Google Scholar]
- 30.Maley CC, Galipeau PC, Li X, et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64(20):7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 31.Mathai RT, Tonse R, Kalekhan F, et al. Amla in the prevention of aging: scientific validation of the ethnomedicinal claims. In: Watson RR, editor. Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults. Academic Press, Waltham; 2015. pp. 29–35. [Google Scholar]
- 32.Mazumdar M, Giri S, Giri A. Role of quercetin on mitomycin C induced genotoxicity: analysis of micronucleus and chromosome aberrations in vivo . Mutat Res Genet Toxicol Environ Mutagen. 2011;721(2):147–152. doi: 10.1016/j.mrgentox.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the childhood cancer survivor study cohort. J Clin Oncol. 2009;27(14):2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min K, Ebeler SE. Quercetin inhibits hydrogen peroxide-induced DNA damage and enhances DNA repair in Caco-2 cells. Food Chem Toxicol. 2009;47(11):2716–2722. doi: 10.1016/j.fct.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Mirunalini S, Krishnaveni M. Therapeutic potential of Phyllanthus emblica (AMLA): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21(1):93–105. doi: 10.1515/JBCPP.2010.21.1.93. [DOI] [PubMed] [Google Scholar]
- 36.Moyer MP, Manzano LA, Merriman RL, et al. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32(6):315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 37.Pan SS, Andrews PA, Glover CJ, et al. Reductive activation of mitomycin C and mitomycin C metabolites catalyzed by NADPH-cytochrome P-450 reductase and xanthine oxidase. J Biol Chem. 1984;259(2):959–966. [PubMed] [Google Scholar]
- 38.Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, et al. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol. 2008;14(10):1491–1497. doi: 10.3748/wjg.14.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruan WJ, Lai MD, Zhou JG. Anticancer effects of Chinese herbal medicine, science or myth? J Zhejiang Univ-Sci B. 2006;7(12):1006–1014. doi: 10.1631/jzus.2006.B1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma N, Trikha P, Athar M, et al. Inhibitory effect of Emblica officinals on the in vivo clastogenicity of benzo alpyrene and acyclophosphamide in mice. Hum Exp Toxicol. 2000;19(6):377–384. doi: 10.1191/096032700678815945. [DOI] [PubMed] [Google Scholar]
- 41.Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121(23):3859–3866. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]
- 42.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 43.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. New Eng J Med. 1999;340(5):351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 44.van den Belt-Dusebout AW, Wit RD, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25(28):4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 45.Waters MD, Brady AL, Stack HF, et al. Antimutagenicity profiles for some model compounds. Mutat Res Rev Genet Toxicol. 1990;238(1):57–85. doi: 10.1016/0165-1110(90)90039-E. [DOI] [PubMed] [Google Scholar]
- 46.Zaki BI, Suriawinata AA, Eastman AR, et al. Chromosomal instability portends superior response of rectal adenocarcinoma to chemoradiation therapy. Cancer. 2014;120(11):1733–1742. doi: 10.1002/cncr.28656. [DOI] [PubMed] [Google Scholar]