Abstract
Potato virus S (PVS) often causes significant losses in potato production in potato-growing countries. In this study, the ordinary strain of PVS (PVSO) was purified from PVS-infected potato plants and used as the immunogen to produce hybridomas secreting monoclonal antibodies (MAbs). Five highly specific and sensitive murine MAbs (1A3, 16C10, 18A9, 20B12, and 22H4) against PVS were prepared using conventional hybridoma technology. Using these MAbs, tissue print-enzyme-linked immunosorbent assay (ELISA), dot-ELISA, and double-antibody sandwich (DAS)-ELISA were developed for sensitive and specific detection of PVS infection in potato plants. The results of sensitivity assays revealed that PVS could be reliably detected in PVS-infected leaf crude extracts diluted at 1:10 240 and 1:163 840 (w/v, g/ml) in phosphate buffer saline (PBS) by dot-ELISA and DAS-ELISA, respectively. Twenty-two samples collected from potato fields in Yunnan Province, China were tested for PVS infection using the serological assays we had developed, and 14 of them were found to be positive. This indicates that PVS is now prevalent in potato fields in Yunnan Province.
Keywords: Potato virus S, Tissue print-enzyme-linked immunosorbent assay (ELISA), Dot-ELISA, Double-antibody sandwich (DAS)-ELISA
1. Introduction
Potato is the fourth most important food crop after wheat, rice, and corn in the world, and has high nutritional value. Viral diseases, including Potato virus S (PVS), often cause huge losses in potato production. PVS was first found in a potato field in the Netherlands in 1948 and is now in all potato-growing areas worldwide. It is a member of the genus Carlavirus in the family Betaflexiviridae (Lin et al., 2014). The PVS virion is filamentous with 610–710 nm in length and 10–15 nm in diameter, and its genome is single-stranded, positive-sense RNA approximately 8.4 to 8.5 kb long (Foster et al., 1992; Matousek et al., 2005) containing a 5' cap structure, a 3' poly(A) tail, and six open reading frames (ORFs). ORF1 encodes a 223-kDa protein containing NTP-binding helicase (HEL), methyltransferase (MTR), and RNA-dependent RNA polymerase (RdRp) domains. ORF2, ORF3, and ORF4 constitute the triple gene block (TGB). ORF2 encodes a protein of 25 kDa with an NTPase/helicase domain. ORF3 and ORF4 encode 12 and 7 kDa proteins, respectively, which is known to be involved in PVS intracellular movement (Mackenzie et al., 1989; Morozov and Solovyev, 2003). ORF5 encodes a 34-kDa coat protein (CP) and ORF6 encodes an 11-kDa nucleotide-binding protein (Lin et al., 2014).
PVS only infects members in the Solanaceae and Cheonopodiaceae families (Morelli and Vayda, 1996), with spread between crops being transmitted by aphids (Cerovska and Filigarova, 1995). Spread of the disease between distant regions is thought to be caused by trading of PVS-infected seed tubers. Most PVS-infected potato plants do not show obvious symptoms or only mild leaf rugosity, vein deepening and leaf bronzing (Yardimci et al., 2015), but a few highly susceptible cultivars, such as Pentland Javelin and Epicure, can show severe symptoms (Salari et al., 2011). In the field, PVS often co-infects plants along with other viruses such as Potato virus X (PVX) and Potato virus M (PVM), resulting in a 20%–30% reduction of potato yield (Wu et al., 2002).
The current PVS management strategy includes breeding disease-resistant varieties and planting virus-free seed tubers. As PVS does not cause clear symptoms in most infected potato plants, establishment of rapid and reliable detection methods is crucial for PVS-resistant potato breeding and PVS-free seed tuber production. Several PVS detection methods have been reported, including assays using indicator plants (Mao, 2009), a reverse transcription-polymerase chain reaction (RT-PCR) (Cheng et al., 2010), and serological detection using PVS-specific polyclonal (Yardimci et al., 2015) and monoclonal (Cerovska and Filigarova, 1995) antibodies (PAbs and MAbs). All, however, have drawbacks: detection using indicator plants takes multiple days to complete, needs greenhouse or growth chamber space, and is questionable in its ability to detect low levels of PVS; RT-PCR is a sensitive and specific assay, but is not practical for large-scale field studies; and serological assays are known to be rapid, cost-effective, and high throughput, but are less sensitive than RT-PCR (Gil et al., 2013). To overcome these issues, we prepared five highly specific and sensitive MAbs specific for PVS and developed three serological assays for PVS detection, which should be of use in large scale epidemiological studies and potato breeding.
2. Materials and methods
2.1. Viruses and field potato samples
Potato leafroll virus (PLRV)-infected, ordinary strain of PVS (PVSO)-infected, and virus-free potato seedlings were kindly supplied by the Institute of Biotechnology and Germplasm Resources, Yunnan Provincial Academy of Agricultural Sciences (Kunming, China). Potato virus A (PVA), Potato virus Y (PVY) and PVX were previously characterized and maintained in potato plants. Field potato samples showing virus-like symptoms were collected from Yunnan Province, China. Virus in the PVSO-infected potato plants was purified according to a previously described method (Zhou et al., 1994) and was used as the immunogen for anti-PVS MAb preparation.
2.2. MAb preparation
Purified PVS virion was used to immunize five BALB/c female mice as described previously by Shang et al. (2011). Hybridoma cells secreting anti-PVS MAbs and ascitic fluids containing MAbs were prepared as described by Wu et al. (2013a). Crude extract from PVS-inoculated plants was used as the coating antigen during an indirect-enzyme-linked immunosorbent assay (ELISA) to determine the titers of the resulting ascitic fluids. The isotypes of MAbs were discriminated with a mouse MAb isotyping kit as described (Sigma-Aldrich, St. Louis, MO, USA). The specificity and sensitivity of the MAbs were determined by Western blot and a plate-trapped antigen (PTA)-ELISA as described previously (Shang et al., 2011; Wu et al., 2013b).
2.3. DAS-ELISA
IgG was purified from ascitic fluids containing MAbs with a protein A-agarose column as instructed by the manufacturer (GE Healthcare, Bjokgatan, Uppsala, Sweden). Individual purified MAbs were conjugated with alkaline phosphatase (AP) to produce MAb/AP conjugate, and the best combination of a MAb and its MAb/AP conjugate was determined as previously described (Liu et al., 2016). The double-antibody sandwich (DAS)-ELISA procedure was the same as described previously (Liu et al., 2016) with the following specific modifications. ELISA 96-well plates were first coated with a capture MAb (100 µl/well) diluted in a 0.01 mol/L phosphate buffer saline (PBS, pH 7.4) at 4 °C for overnight. After three washes with PBS containing 0.05% Tween 20 (PBST), the wells were blocked with PBST containing 5% skimmed milk powder for 30 min. PVS-infected potato leaf crude extract diluted at 1:20 (w/v, g/ml) in PBS, was added to each well, and the plates were incubated at 37 °C for 1 h. After three washes with PBST, the corresponding MAb/AP conjugate was added to the wells and the plates were incubated at 37 °C for 1 h. After four washes with PBST, a p-nitrophenylphosphate solution (Sigma-Aldrich) was added to each well. After 30 min of incubation in the dark and at 37 °C, the optical density value at 405 nm (OD405) of each well was measured with a Microplate Reader Model 680 (BIO-RAD, Hercules, CA, USA). A sample was considered to be positive if its absorbance value was at least three times greater than that shown by the negative controls which were crude extracts from healthy potato plants.
2.4. Dot-ELISA and tissue print-ELISA
PVS in crude extracts from PVS-infected plants was detected using the dot-ELISA and tissue print-ELISA described previously by Shang et al. (2011) and Wu et al. (2013b).
2.5. RT-PCR and sequence analyses
RT-PCR was conducted to confirm the detection results using the serological assays described above. After alignment with the known PVS genomic sequences from the GenBank, the forward primer (5'-CACCTTTAGGTTCACAGG-3', corresponding to PVS nucleotides 7164–7181) and the reverse primer (5'-CGATTTAGGCTCCCAGACACTT-3', corresponding to nucleotides 8422–8446) were designed. RT-PCR was performed as described by Li et al. (2015). The resulting PCR products were sequenced and analyzed using the Clustal W method in DNAStar (Version 7.0, DNAStar Inc., Madison, WI, USA).
3. Results
3.1. Virus purification
PVS virion was purified from PVS-infected potato leaves through differential centrifugation. Flexuous filamentous virion with 610–710 nm long and 10–15 nm in diameter was observed in the purified preparation by transmission electron microscopy (Fig. 1).
Fig. 1.
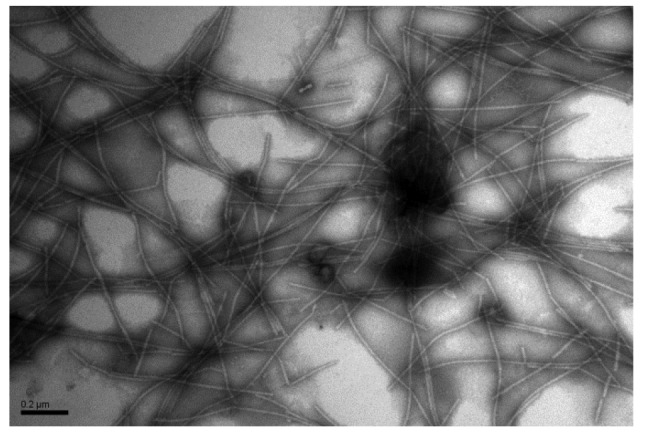
Electron micrograph of negatively stained PVS virion in a purified preparation
Purified PVS virion was loaded on a formvar-coated grid, negatively stained with 2% (0.02 g/ml) phosphotungstic acid, and photographed under a transmission electron microscope. Bar=0.2 µm
3.2. Production and characterization of MAbs against PVS
Five BALB/c mice were immunized with purified PVS virion. After the fourth immunization, two of them were found to produce high titer antibodies against PVS. Spleen cells of these two mice were used to prepare hybridomas. In two cell fusion experiments, each well in a cell culture plate contained at least one hybridoma clone. Five hybridoma cell lines (1A3, 16C10, 18A9, 20B12, and 22H4) were found to secrete anti-PVS specific MAbs through selections in a hypoxanthine-aminopterin-thymidine medium, antibody detection, and cell cloning. The resulting hybridomas were injected intraperitoneally into pristine-primed BALB/c mice to produce ascitic fluids. Isotypes and subclasses of the five MAbs were found to be IgG1, κ light chain (Table 1). The yield of IgG in ascitic fluids ranged from 6.76 to 8.54 mg/ml, and the titers of MAbs ranged from 10−6 to 10−7 according to results determined using an indirect-ELISA (Table 1).
Table 1.
Properties of the monoclonal antibodies
| MAb | Isotype and subclass | Titer | Yield of IgG in ascites (mg/ml) |
| 1A3 | IgG1, κ chain | 10–7 | 7.44 |
| 16C10 | IgG1, κ chain | 10–7 | 6.76 |
| 18A9 | IgG1, κ chain | 10–7 | 8.54 |
| 20B12 | IgG1, κ chain | 10–6 | 7.32 |
| 22H4 | IgG1, κ chain | 10–7 | 8.22 |
Specificity of the MAbs was initially tested by PTA-ELISA. The results of the assays indicated that all five MAbs reacted strongly and specifically with the PVSO strain, but not with the crude extracts from healthy potato leaves or from the leaves infected with other common potato viruses, including PVA, PVY, PVX, and PLRV (Fig. 2a), indicating that the prepared MAbs were specific for PVS. The specificity of the MAbs was then tested by Western blot using crude leaf extracts. The results showed that the five MAbs reacted with a protein of approximately 34 kDa in the PVS-infected potato leaf extract. No positive detection signal was observed in lanes loaded with an extract from healthy potato leaves (Fig. 2b). Based on the estimated molecular weight of the protein band, we concluded that all five MAbs are specific for the PVS coat protein.
Fig. 2.
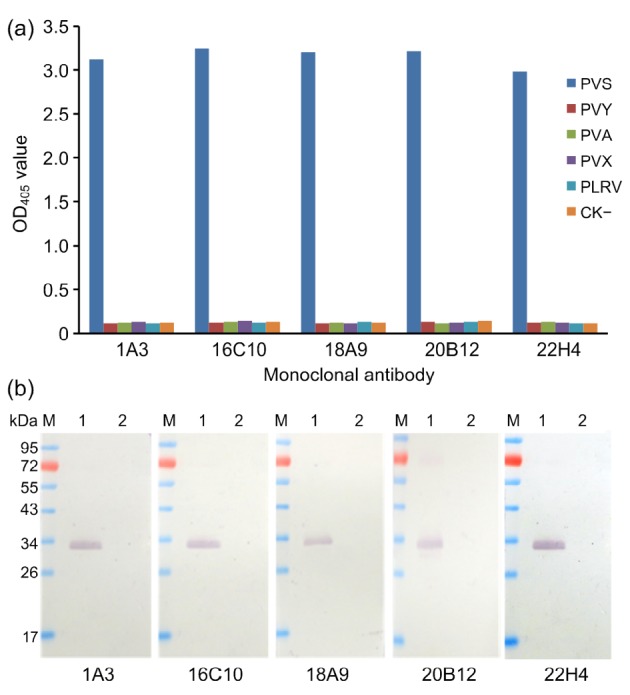
Specificity analyses of anti-PVS monoclonal antibodies using PTA-ELISA
(a) and Western blot (b) (a) PVSO-, PLRV-, PVA-, PVY-, or PVX-infected potato leaf tissues were used for the analyses. Healthy potato leaf tissues (negative control, CK−) were used as a negative control. (b) Lane M is a protein molecular marker. Lanes 1 and 2 were PVSO-infected and healthy potato leaf extracts, respectively. All the MAbs were diluted at 1:5000 (v/v) in PBS prior to use. Goat anti-mouse IgG/AP conjugate was used as the second antibody during the assay
PTA-ELISA was used to analyze the sensitivities of MAbs. The results demonstrated that all five MAbs could detect viruses in crude extracts of infected leaf tissue diluted up to 1:40 960 (w/v, g/ml), indicating that the MAbs were very sensitive for PVS detection (Fig. 3)
Fig. 3.
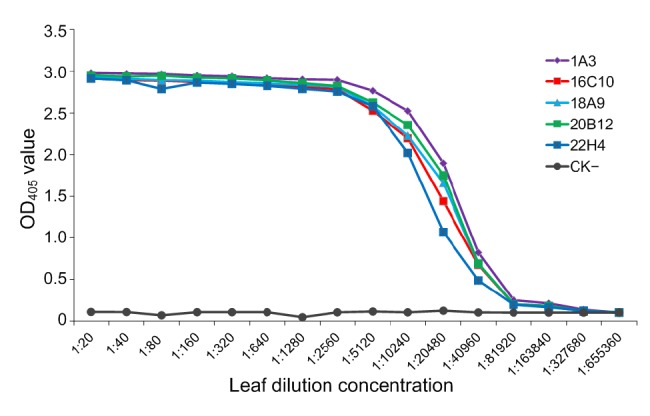
Sensitivity analyses of MAbs by PTA-ELISA
PVS-infected potato leaf crude extracts and healthy potato leaf crude extracts (negative control, CK−) were serially two-fold diluted in PBS from 1:40 to 1:655 360 (w/v, g/ml) and probed with MAbs followed by AP-labeled goat anti-mouse IgG. Each OD405 value was obtained at 30 min after the addition of the substrate at room temperature
3.3. DAS-ELISA for PVS detection
Three replicated phalanx tests were first performed to determine the optimal MAb pairing and the working dilutions of the capture MAb and their corresponding MAb/AP conjugates for the serological assay. The results indicated that using 1:5000 diluted MAb 1A3 as the capture antibody and 1:8000 diluted MAb 16C10/AP conjugate gave the best detection results during DAS-ELISA. To determine the specificity of the DAS-ELISA, crude extracts from PLRV-, PVY-, PVA-, or PVX-infected potato leaf tissues were tested. Crude extract from healthy potato leaf tissues was used as a negative control (CK−). The result of the assay showed that the DAS-ELISA only reacted positively with the crude extract from PVS-infected potato leaf tissues. Like the negative control sample, all crude extracts from the PVA-, PVY-, PVX-, or PLRV-infected leaf tissues gave negative reactions (Fig. 4), indicating that this DAS-ELISA is highly specific for PVS.
Fig. 4.
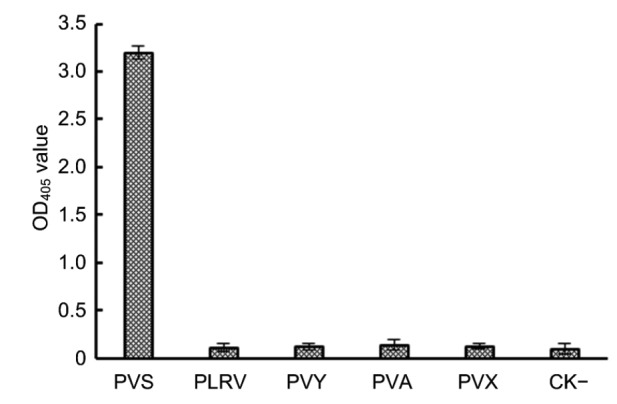
Specificity of the DAS-ELISA Crude extracts from PVS-, PLRV-, PVY-, PVA-, or PVX-infected potato plants were used in the assay.
Crude extract from a healthy potato plant was used as a negative control (CK−). ELISA plate wells were first coated with 1:5000 (v/v) diluted MAb 1A3. The test samples were individually diluted at 1:20 (w/v, g/ml) in PBS and loaded into individual wells. The MAb 16C10/AP conjugate was diluted at 1:8000 (v/v) prior to use. The absorbance values (OD405) were the mean values±standard deviation (SD) from three independent assays
To determine the sensitivity of the DAS-ELISA, crude extracts from the PVS-infected or healthy potato leaf tissues were serially two-fold diluted from 1:320 to 1:655 360 (w/v, g/ml) in 0.01 mol/L PBS. The results showed that the DAS-ELISA was sensitive enough to detect PVS in 1:163 840 diluted PVS-infected crude extract (Fig. 5), indicating that it is highly sensitive for PVS detection.
Fig. 5.
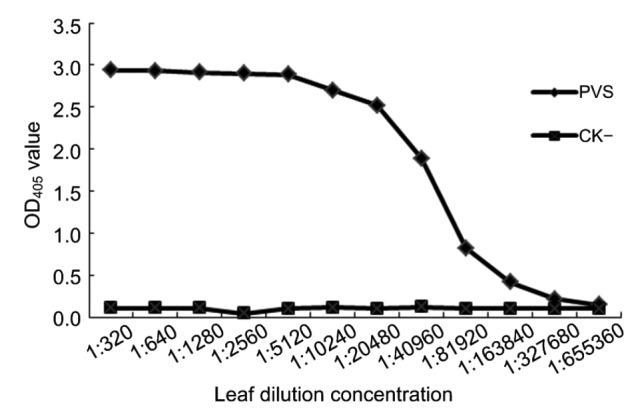
Sensitivity of the DAS-ELISA
PVS-infected (PVS) or healthy (CK−) potato leaf crude extract were serially two-fold diluted from 1:320 to 1:655 360 (w/v, g/ml) in a 0.01 mol/L PBS, and 100 μl of the diluted extract was loaded into a well pre-coated with the capture MAb 1A3. MAb 16C10/AP was diluted at 1:8 000 (v/v) in PBS prior to use. Each OD405 value represents the mean of three independent assays obtained at 30 min after the addition of the substrate at room temperature
3.4. Dot-ELISA and tissue print-ELISA for PVS detection
Dot-ELISA and tissue print-ELISA were used to detect PVS infection in potato samples blotted on nitrocellulose membranes. Phalanx tests were first performed to decide the optimal working dilutions of MAb 1A3 and goat anti-mouse IgG/AP conjugate. Results of three independent phalanx tests showed that the optimal working dilutions of MAb 1A3 and goat anti-mouse IgG/AP conjugate were 1:5000 and 1:8000 (v/v), respectively. Crude extracts from PVS-, PVY-, PVA-, PVX-, or PLRV-infected potato plants were used in the dot-ELISA. Crude extract from a healthy potato plant was used as a negative control (CK−). The results of the assays indicated that only the spots made using the crude extract from the PVS-infected potato plant showed a dark purple positive reaction. Spots made using crude extracts from PVY-, PVA-, PVX-, or PLRV-infected, or healthy potato plant tissues (Fig. 6a) remained light or very light green in color. This dot-ELISA was capable of detecting PVS in dots made using 1:10 240 diluted PVS-infected crude extract (Fig. 6b). For the tissue print-ELISA, young stems of PVS-, PLRV-, PVY-, PVA-, or PVX-infected, or healthy potato plants were cut and printed individually on nitrocellulose membranes. After addition of the nitroblue tetrazolium/5'-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) substrate, the stem prints made using PVS-infected potato plants turned purple. Stem prints made using all other plant stems remained green (Fig. 6c). These results demonstrated that both dot-ELISA and tissue print-ELISA were highly reliable for PVS detection.
Fig. 6.
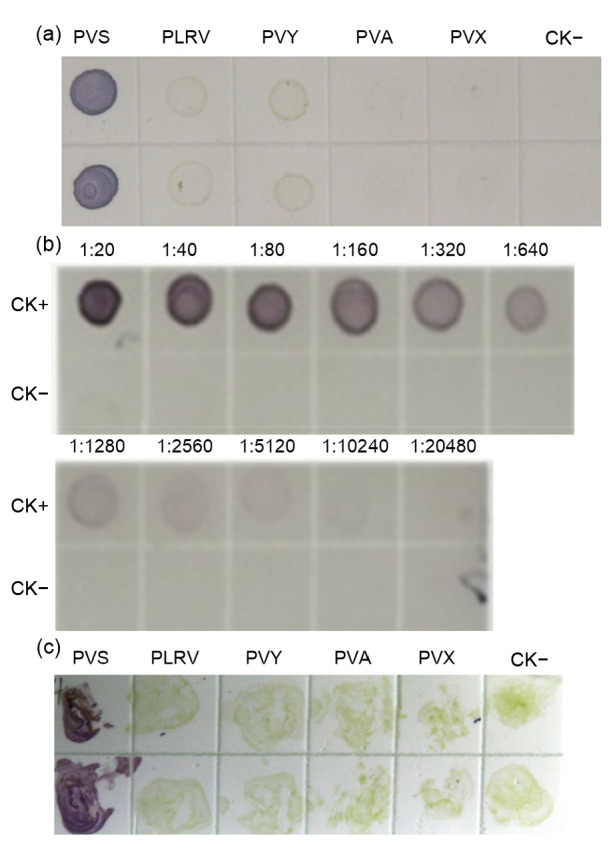
Specificity and sensitivity of the dot-ELISA and tissue print-ELISA
(a) Crude extracts from PVSO-(PVS), PLRV-(PLRV), PVY-(PVY), PVA-(PVA), or PVX-infected (PVX) potato plants were used in the assay. Crude extract from a healthy potato plant was used as a negative control (CK−). Each sample had two upper and lower dots and was analyzed by the dot-ELISA. (b) Crude extracts from PVSO-infected (positive control, CK+) or healthy (CK−) potato plants were serially two-fold diluted from 1:20 to 1:20 480 (w/v, g/ml) in 0.01 mol/L PBS and used in the dot-ELISA. (c) Stem prints made using PVSO-, PLRV-, PVY-, PVA-, or PVX-infected potato plants, or healthy potato plants were used for the tissue print-ELISA. Purple colored spots or stem prints indicated a positive reaction, while green or colorless spots or stem prints indicated a negative reaction
3.5. RT-PCR for PVS detection
To confirm that these results were accurate, we tested the above samples by RT-PCR using PVS-specific primers. The results of the assay showed that a specific product of approximately 1.3 kb was amplified only from a PVS-infected potato sample. No gene product was obtained from the healthy, or PVY-, PVA-, PVX-, or PLRV-infected potato samples (Fig. 7).
Fig. 7.
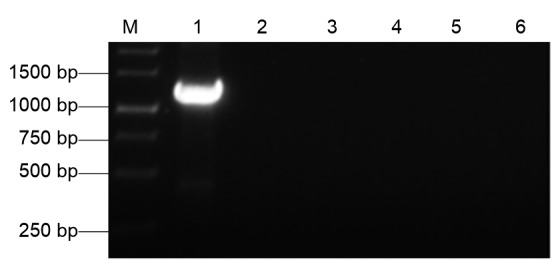
Detection of PVS infection in potato samples by RT-PCR
Lane M is a 1-kb DNA marker. Lanes 1–5 were RT-PCR products from potato plants infected with PVS, PLRV, PVY, PVA, or PVX. Lane 6 was the RT-PCR product from a healthy potato plant (a negative control)
3.6. Detection of PVS infection in field samples using the above described assays
To demonstrate that the serological assays described above were specific and sensitive for PVS detection, 22 potato plants showing virus-like symptoms were collected from fields in Yunnan Province, China, and tested for PVS infection by the four methods described above. Results from the three serological assays indicated that 14 of the 22 field samples were infected with PVS (Fig. 8). The results from RT-PCR followed by DNA sequencing agreed with those from the three serological assays. The DNA sequencing results also indicated that the RT-PCR products had shared 95%–98% nucleotide identities with the PVS genomic sequences (JQ 183955.1, JQ647830.1, JX183956.1, JX683388.1) deposited in the GenBank.
Fig. 8.
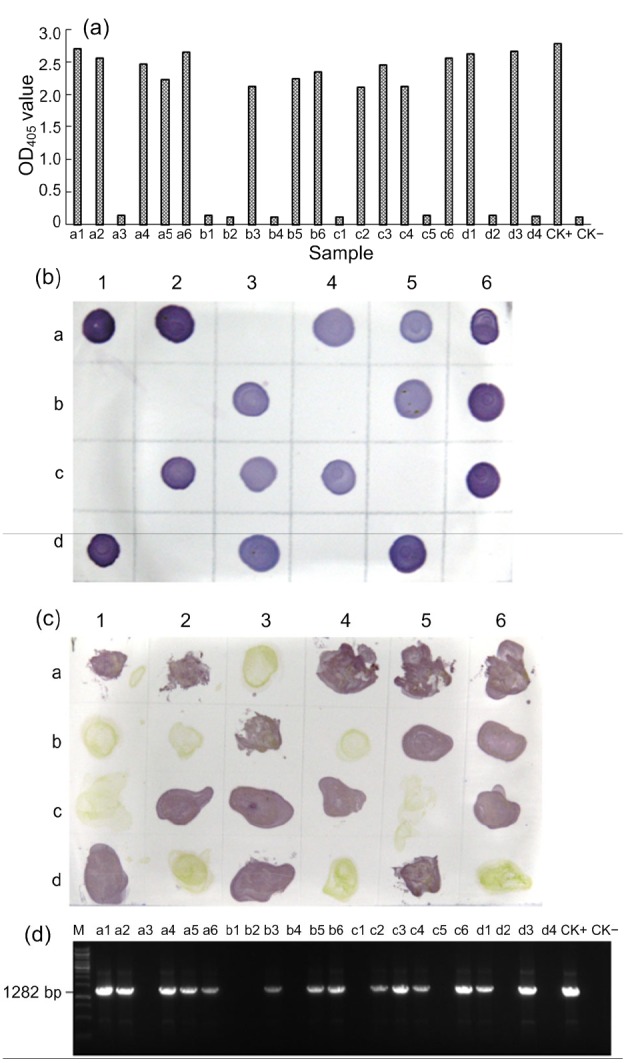
Detection of PVS infection in field-collected samples using DAS-ELISA (a), dot-ELISA (b), tissue print-ELISA (c), and RT-PCR (d)
Twenty-two samples i.e. a1–a6, b1–b6, c1–c6, and d1–d4 were collected from potato fields. Samples d5 and d6 (b and c) or CK+and CK−(a and d) were from a PVS-infected and a healthy potato plant, respectively, and were used as the positive and negative controls in the assays, respectively. Lane M was a 1-kb DNA marker
4. Discussion
China accounts for nearly a quarter of the total potato-growing area in the world, but the average yield in China is relatively low due mainly to damage caused by several potato viruses. Six viruses i.e. PVY, PVX, PVS, PVA, PLRV, and PVM are known to be prevalent and economically important in China (Mao, 2009), and potato plants are often infected with multiple viruses. To prevent and control these viral diseases, high throughput and accurate virus detection methods are required.
PVS remained unknown until the 1950s because its symptoms are inconspicuous (Yardimci et al., 2015). Electron microscopy has been used by many laboratories to study the morphology of PVS, but it requires expensive microscopes and skilled personnel, and cannot distinguish PVS from other viruses with similar virion morphology. RT-PCR is an accurate virus detection method, but it is time-consuming and is applied mostly in laboratories due to high cost. Serological assays are fast and easy-to-use, and have been widely used in field studies (Shang et al., 2011; Wu et al., 2013a; 2013b; Li et al., 2015; Liu et al., 2016). Using a PAb-based DAS-ELISA, Yardimci et al. (2015) reported that the most common viruses in the potato-growing areas in Turkey were PVS and PVY. In Iran, 44 of the 240 potato samples tested by a PAb-based DAS-ELISA were found to be infected with PVS (Salari et al., 2011). To our knowledge, the current serological assays for PVS detection mostly rely on anti-PVS PAbs. However, PAbs have some shortcomings, such as limited output, cross reaction with other viruses or host proteins, and high background. Cerovska and Filigarova (1995) reported four anti-PVS MAbs and showed that they could react with the Andean strain of PVS (PVSA), but not with PVSO. Using these specific MAbs, they were able to develop a MAb-based ELISA to distinguish PVSA from PVSO. In our study, we prepared five highly sensitive and specific MAbs against the PVSO strain, which is known to be the most prevalent PVS strain in potato fields in China. Because PVSA is not available in the laboratory, the usefulness of these five MAbs for PVSA detection remains unknown.
We have developed highly specific and sensitive tissue print-ELISA, dot-ELISA, and DAS-ELISA for PVS detection. The dot-ELISA and DAS-ELISA we have described can be used to detect PVS in infected tissue crude extracts diluted at 1:10 240 and 1:163 840 (w/v, g/ml), respectively. To our knowledge, these two serological methods are the most sensitive and accurate methods for PVS detection in plant samples. This conclusion is supported by the detection results of the field collected potato samples, and by the results from RT-PCR followed by DNA sequencing. The methods developed in this study should benefit PVS epidemiological studies, the selection of virus-free seed potatoes, and breeding for PVS-resistant potato cultivars.
Acknowledgments
We are grateful to Dr. Xin-shun DING (Samuel Roberts Noble Foundation, Ardmore, USA) for his valuable comments and manuscript edits.
Footnotes
Project supported by the National Key Research and Development Project of China (No. 2017YFD0201604) and the Fund for Agro-scientific Research in the Public Interest (No. 201303028), China
Compliance with ethics guidelines: Ge SONG, Jia-yu WU, Yan XIE, Yong LIU, Ya-juan QIAN, Xue-ping ZHOU, and Jian-xiang WU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Cerovska N, Filigarova M. Specific detection of the Andean strain of potato virus S by monoclonal antibodies. Ann Appl Biol. 1995;127(1):87–93. doi: 10.1111/j.1744-7348.1995.tb06653.x. [DOI] [Google Scholar]
- 2.Cheng Q, Zhu YF, Shen YF, et al. Study on detection of potato virus by RT-PCR. Amino Acids Biotic Resour. 2010;32(4):8–11. doi: 10.1016/S0923-2516(06)80089-0. (in Chinese) [DOI] [Google Scholar]
- 3.Foster GD. The structure and expression of the genome of carlaviruses. Res Virol. 1992;143:103–112. doi: 10.1016/S0923-2516(06)80089-0. [DOI] [PubMed] [Google Scholar]
- 4.Gil JF, Cotes JM, Marin M. Detection and molecular characterization of Potato virus S (PVS, Carlavirus) from Colombia. Rev Biol Trop. 2013;61(2):565–575. doi: 10.15517/rbt.v61i2.11149. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Chen Z, Liu Y, et al. Development of monoclonal antibodies and serological assays specific for Barley yellow dwarf virus GAV strain. Virol J. 2015;12(1):136. doi: 10.1186/s12985-015-0367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YH, Abad JA, Maroon-Lango CJ, et al. Molecular characterization of domestic and exotic potato virus S isolates and a global analysis of genomic sequences. Arch Virol. 2014;159(8):2115–2122. doi: 10.1007/s00705-014-2022-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Chen Z, Hong J, et al. Monoclonal antibody-based serological methods for detecting Citrus tristeza virus in citrus groves. Virol Sin. 2016;31(4):324–330. doi: 10.1007/s12250-016-3718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie DJ, Tremaine JH, Stace-Smith R. Organization and interviral homologies of the 3'-terminal portion of potato virus S RNA. J Gen Virol. 1989;70(5):1053–1063. doi: 10.1099/0022-1317-70-5-1053. [DOI] [PubMed] [Google Scholar]
- 9.Mao YZ. Survey of potato virus detection technique. China Vegetables. 2009;12:1–6. (in Chinese) [Google Scholar]
- 10.Matousek J, Schubert J, Ptacek J, et al. Complete nucleotide sequence and molecular probing of potato virus S genome. Acta Virol. 2005;49(3):195–205. [PubMed] [Google Scholar]
- 11.Morelli JK, Vayda ME. Mechanical wounding of potato tubers induces replication of potato virus S. Physiol Mol Plant Pathol. 1996;49(1):33–47. doi: 10.1006/pmpp.1996.0037. [DOI] [Google Scholar]
- 12.Morozov SY, Solovyev AG. Triple gene block: modular design of a multifunctional machine for plant virus movement. J Gent Virol. 2003;84(6):1351–1366. doi: 10.1099/vir.0.18922-0. [DOI] [PubMed] [Google Scholar]
- 13.Salari K, Massumi H, Heydamejad J, et al. Analysis of Iranian potato virus S isolates. Virus Genes. 2011;43(2):281–288. doi: 10.1007/s11262-011-0619-3. [DOI] [PubMed] [Google Scholar]
- 14.Shang HL, Xie Y, Zhou XP, et al. Monoclonal antibody-based serological methods for detection of Cucumber green mottle mosaic virus. Virol J. 2011;8(1):228. doi: 10.1186/1743-422X-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu JX, Wang Q, Liu H, et al. Monoclonal antibody-based serological methods for maize chlorotic mottle virus detection in China. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013;14(7):555–562. doi: 10.1631/jzus.B1200275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JX, Ni YQ, Liu H, et al. Monoclonal antibody-based serological assays and immunocapture-RT-PCR for detecting Rice dwarf virus in field rice plants and leafhopper vectors. J Virol Methods. 2013;195(1):134–140. doi: 10.1016/j.jviromet.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Wu XQ, Wu ZJ, Xie LH, et al. The clone and expression of the coat protein gene of potato virus S in E. coli . Virol Sin. 2002;17(3):248–251. (in Chinese) [Google Scholar]
- 18.Yardimci N, Culal KH, Demir Y. Detection of PVY, PVX, PVS, PVA, and PLRV on different potato varieties in Turkey using DAS-ELISA. J Agric Sci Technol. 2015;17(3):757–764. [Google Scholar]
- 19.Zhou XP, Chen JS, Li DB. A method for high yield purification of potyviruses. Microbiology. 1994;21(3):184–186. (in Chinese) [Google Scholar]


