Abstract
This study was conducted to investigate the effects of fresh fermented soybean meal (FSM) on the growth performance of nursery piglets, nitrogen excretion in feces, and the concentrations of ammonia (NH3) and particulate matter (PM) in the piggery. A total of 472 nursery piglets (Landrace×Yorkshire, (16.3±0.36) kg body weight) were randomly allocated into two treatments with 236 pigs in each treatment. The pigs were fed the basal diet without fresh FSM (control) or diet containing 10% (100 g/kg) fresh FSM (FSM group), and the crude protein content of the two groups was consistent. The feeding trial lasted for 28 d. The results showed that the pigs fed fresh FSM had increased (P<0.05) average daily gain (ADG) compared with the control. There was no significant difference (P>0.05) in feed to gain ratio (F:G) between the two groups. During the whole experiment, the concentration of NH3 in the piggery decreased (P<0.05) by 19.0%, and the concentrations of PM (PM10 and PM2.5) in the piggery decreased (P<0.05) by 19.9% and 11.6%, respectively, in the FSM group, compared with the control. The ammonia nitrogen and nitrite content in feces increased (P<0.05) by 32.9% and 28.4%, respectively, in the FSM group. The fecal pH declined (P<0.05) significantly in the FSM group compared with the control. At the end of experiment, total protein (TP) concentration was increased (P<0.05) significantly and blood urea nitrogen (BUN) concentration was decreased (P<0.05) for pigs fed the diet with fresh FSM. The results indicated that dietary fresh FSM not only improved the growth performance of nursery piglets, but also reduced the NH3 concentration in the piggery due to nitrogen conversion, and decreased the concentrations of PM10 and PM2.5 in the piggery.
Keywords: Fresh fermented soybean meal, Ammonia, Particulate matter, Nitrogen conversion, Nursery piglet
1. Introduction
Livestock production is now one of the major contributors to environmental problems. Among these, greenhouse gas emission is one of the most serious problems (Ilea, 2009). Lots of poisonous and harmful gases are generated from the barn and in the process of animal fecal sewage treatment (Webb et al., 2014), endangering animals and human health. This also captures the attention of the pig-breeding industry. Among these gases, ammonia (NH3) is the foremost pollutant gas contributing to nitrogen eutrophication of ecosystems, acid rain, and soil acidification (Krupa, 2003). Ammonia also has a direct effect on animal health, inducing infectious disease (Banhazi et al., 2008). Reducing NH3 emissions of the pig-breeding industry contributes to promoting animal growth, improving economic benefits of farms, and lessening environmental pollution. Particulate matter (PM) is also an important environmental pollutant produced in animal husbandry, causing haze problems of concern to the world and endangering the health of animals and humans (Vogelzang et al., 2000). Factors that influence NH3 emissions and PM formation include the fecal sewage treatment system (Hoff et al., 2006), feeding technology, and the housing system (Zhou et al., 2015). The dietary composition is also one of the factors (Chiavegato et al., 2015).
Techniques such as treatment of sewage, biofiltration of ventilation air, and floor design for separating feces and urine have been developed to reduce emissions of NH3 and PM (Portejoie et al., 2003; Ye et al., 2007; Dumont et al., 2014), but these measures are often impractical or uneconomical for application in livestock production (Rigolot et al., 2010). Reducing NH3 emissions by regulating dietary composition is considered economical and feasible. Optimization of feed ingredients and formula could improve the utilization of nitrogen, and then reduce NH3 emissions. Fermented soybean meal (FSM) is one of the most concerned ingredients. The fermentation process increases the number of small-size peptides and the activity of the digestive enzymes compared with soybean meal (Mukherjee et al., 2016).
Fresh FSM was obtained from the fermentation production of soybean meal using a compound bacterium. Without drying, it had high humidity and rich probiotics, which may contribute to the nutrient to be digested and be easily absorbed by animals. We hypothesized that the fresh FSM could improve air quality in the piggery because of its special properties. Therefore, the objective of this study was to investigate the effects of fresh FSM on growth performance of nursery piglets, on nitrogen excretion in feces, and on the concentrations of NH3 and PM in the piggery.
2. Materials and methods
2.1. Fresh fermented soybean meal
The fresh FSM was provided by a commercial company (Zhejiang Chengyuan Biotechnology Co., Ltd., China). Dried soybean meals were soaked in distilled water at 4 °C overnight. Hydrated soybean meals were cooked in a steam tank at 60‒70 °C for 1 h. The cooked soybean meals were then allowed to cool to room temperature. Lactobacillus plantarum (CGMCC No. 1.1209), Bacillus subtilis MA 139 and Saccharomyces cerevisiae (CGMCC No. 2.0707) were stirred evenly at a ratio of 1:2:2 (volume ratio). Then soybean meals were incubated with L. plantarum, B. subtilis, and S. cerevisiae at 37 °C for 72 h. Sterile distilled water was added to achieve a certain initial moisture content. The total inoculum was 0.4% of the weight of the soybean meals. The initial temperature was controlled at 30 °C and the fermentation process lasted for 72 h. After fermentation, no drying process was required.
2.2. Experimental design, animals, and diets
A total of 472 nursery piglets (Landrace×Yorkshire, 50% male and 50% female) with an average body weight of (16.3±0.36) kg were randomly allocated to two treatments with 236 piglets in each treatment group. Each treatment group in one piggery consisted of 12 pens, and 19 to 20 piglets per pen. A pen served as the experimental unit. Each pen was equipped with a nipple bowl drinker and a long cement trough. The pigs had free access to feed and water during the experiment. The pig room temperature was maintained at 20 to 22 °C and the relative humidity was 50% to 70%. This feeding trial lasted for 28 d. The present study followed the laws and regulations that control experiments and procedures in live animals in China, under the supervision of the Chinese Animal Research Authority.
The pigs in the control group were fed diet without fresh FSM as the basal diet. The pigs in the FSM group were fed the basal diet with 10% (100 g/kg) fresh FSM. The composition and nutrient contents of the experimental diets are shown in Table 1. Experimental diets were formulated to have similar nutrient contents to meet nutritional requirements for nursery pigs recommended by the National Research Council (NRC, 2012).
Table 1.
Ingredients and nutrient composition of the experimental diets1
| Item | Control | FSM |
| Ingredient | ||
| Corn (%) | 58.5 | 56.5 |
| Soybean meal (%) | 21.0 | 13.0 |
| Puffed soybean (%) | 6.0 | 6.0 |
| Fresh FSM (%) | 10.0 | |
| Fish meal (%) | 4.0 | 4.0 |
| Whey powder (%) | 5.0 | 5.0 |
| Soybean oil (%) | 2.0 | 2.0 |
| Salt (%) | 0.3 | 0.3 |
| Stone powder (%) | 1.2 | 1.2 |
| Calcium hydrogen phosphate (%) | 1.0 | 1.0 |
| Vitamin premix2 (%) | 0.2 | 0.2 |
| Trace mineral premix3 (%) | 0.5 | 0.5 |
| L-Lysine (%) | 0.20 | 0.18 |
| DL-Methionine (%) | 0.05 | 0.07 |
| L-Threonine (%) | 0.05 | 0.05 |
| Analyzed nutrient level4 | ||
| Metabolizable energy (MJ/kg)5 | 13.85 | 13.83 |
| Crude protein (%) | 17.50±0.85 | 17.47±0.70 |
| Crude fat (%) | 4.22±0.32 | 4.16±0.24 |
| Ash (%) | 6.47±0.40 | 6.59±0.42 |
| Calcium (%) | 0.77±0.06 | 0.76±0.03 |
| Phosphorus, total (%) | 0.69±0.05 | 0.69±0.04 |
| Lysine (%) | 1.24±0.06 | 1.23±0.05 |
| Methionine (%) | 0.35±0.02 | 0.35±0.03 |
| Threonine (%) | 0.68±0.02 | 0.71±0.02 |
1 Experimental diets were: dietary inclusion of 100 g/kg fresh fermented soybean meal (FSM) in the FSM group, and no fresh FSM in diet as the control.
Provided per kilogram of diet: vitamin A, 10000 IU; vitamin D3, 1500 IU; vitamin E, 60 mg; vitamin K3, 0.5 mg; vitamin B1, 5 mg; vitamin B2, 20 mg; pantothenate acid, 12 mg; niacin, 20 mg; folic acid, 1.25 mg.
Provided per kilogram of diet: Fe, 150 mg as iron sulfate; Cu, 100 mg as copper sulfate; Zn, 140 mg as zinc oxide; Mn, 35 mg as manganese sulfate; I, 0.5 mg as potassium iodate; Se, 0.3 mg as sodium selenite.
Data are expressed as mean±standard deviation (SD), n=3.
Calculated values. All percentages are mass fractions
2.3. Measurements and sample collection
The diets were analyzed for crude protein (N×6.25; method 988.03), crude fat (method 920.39A), ash (method 942.05), calcium (method 968.08), and total phosphorus (method 946.06) according to Association of Official Analytical Chemists (AOAC, 2006) Official Methods. Individual amino acid was measured using an amino acid automatic analyzer (Hitachi L8900, Japan). These analytical processes include analysis of crude protein and amino acid in the soybean products. The contents of glycinin and trypsin inhibitor in the soybean products were measured by quantitative detection kit (purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd., China). On 0 and 28 d of the experiment, pigs were weighed, and the feeds provided and remaining were recorded on a pen basis to calculate average daily gain (ADG) and average daily feed intake (ADFI). Feed conversion rate was calculated as kilograms of feed intake per gram of body weight gain and it was expressed as feed to gain ratio (F:G).
Blood samples were collected from two pigs randomly selected per pen. Blood samples (5 ml) were collected on Day 28 via jugular vein puncture into vacutainer tubes. Blood samples were centrifuged at 4 °C for 10 min at 3000g, and serum was obtained. The samples were packed in Eppendorf tubes, snap frozen in liquid nitrogen, and stored at −70 °C until measuring total protein (TP) and blood urea nitrogen (BUN) concentrations.
Fresh feces of two pigs with the same diet from each pen were collected randomly between 09:00 and 12:00 on Days 25 to 27. The feces were frozen in liquid nitrogen immediately, and then temporarily stored in the −20 °C refrigerator until analyzing the ammonia nitrogen and nitrite in feces and the fecal pH.
2.4. Determination of ammonia and particulate matter concentrations in piggery
An ammonia meter (Drager X-am 7000 portable gas monitor) was used to determine NH3 concentration in piggery three times daily, namely at 06:00‒ 07:00, 12:00‒13:00, 19:00‒20:00, and by choosing three points in the axis of the barn at 50, 70, and 90 cm from the ground. Doors and windows of the piggery were closed at 1 h before the determination, and the monitors were placed at the three points until the data were stable to record and the mean values were taken.
This study measured the mass concentrations of particulate air pollutants, PM10 and PM2.5, which are classified according to particle size, representing PM smaller than 10 and 2.5 μm aerodynamic diameters, respectively. A PM indexer (Hinaway CW-HAT200 Handheld Air Quality Tester, China) was used to determine directly the PM10 and PM2.5 numerically within 0.5 h after morning feeding. Three points on the axis of the barn were chosen, 80 cm from the ground. The air quality testers were settled in the air until the data were stable to record and the mean values were taken.
2.5. Chemical analysis
TP and BUN concentrations in serum were measured using the auto-biochemical analyzer (RX Daytona™). Ammonia nitrogen in feces was measured by Nessler’s reagent colorimetric method (Leonard, 1963). Nitrite in feces was determined by Griess reaction (Griess, 1879). The fecal pH was measured by pH meter according to the method described by Canh et al. (1998).
2.6. Statistical analysis
A completely randomized design was used in this experiment. All the data were analyzed statistically using the independent sample T-test procedure of SPSS 20.0 statistical analysis to conduct variance analysis. The pen was the experimental unit. A probability level of P<0.05 was considered statistically significant.
3. Results
3.1. Nutrient composition of fresh fermented soybean meal
The crude protein content of fresh FSM was higher than that of soybean meal, and the same was the case for the concentrations of analyzed amino acids. In contrast to this, the contents of glycinin and trypsin inhibitor decreased in the fresh FSM. The nutrient composition of the soybean meal pre-and post-fermentation is shown in Table 2.
Table 2.
Nutrient composition of soybean meal and fresh fermented soybean meal1
| Nutrient level (dry matter basis) | Soybean meal | FSM |
| CP2 (%) | 46.2±1.43b | 58.5±1.67a |
| Lysine (%) | 2.48±0.07b | 3.22±0.08a |
| Threonine (%) | 1.84±0.07b | 2.34±0.05a |
| Methionine+cysteine (%) | 1.32±0.09b | 1.91±0.06a |
| Glycinin (%) | 14.06±0.39a | 3.77±0.06b |
| β-Conglycinin (%) | 13.13±0.16a | 3.21±0.03b |
| Trypsin inhibitor (mg/g) | 11.21±0.21a | 0.01±0.001b |
1 Data are expressed as mean±SEM, n=3.
CP: crude protein. a, b Means in the same row followed by different superscript letters differ significantly at P<0.05. All percentages are mass fractions
3.2. Effect of fresh fermented soybean meal on growth performance of nursery piglets
Piglets were healthy with a mortality of 1.69% and 1.27% in the control and FSM groups, respectively, throughout the experiment, which was not associated with dietary treatment. There was no bacterial or viral infection during the experiment.
Pigs fed diet containing fresh FSM had increased (P<0.05) ADG and ADFI. The ADG and ADFI in the FSM group increased by 13.6% and 8.7%, respectively, compared with the control group. The feed to gain ratio (F:G) decreased in the FSM group, but there was no significant difference (P>0.05) between experimental groups. The effect of fresh FSM on the growth performance of nursery piglets is shown in Table 3.
Table 3.
Effect of fresh fermented soybean meal on the growth performance of nursery piglets1
| Group | IW (kg) | FW (kg) | ADG (g/d) | ADFI (g/d) | F:G |
| Control | 16.3 | 33.0 | 599b | 1205b | 2.05 |
| FSM | 16.3 | 35.4 | 680a | 1310a | 1.93 |
|
| |||||
| SEM | 1.03 | 1.79 | 30.1 | 28.1 | 0.06 |
| P-value | 0.95 | 0.22 | 0.02 | 0.004 | 0.19 |
1 Experimental diets were: dietary inclusion of 100 g/kg fresh fermented soybean meal (FSM) in the FSM group, and no fresh FSM in diet as the control. IW: initial weight; FW: final weight; ADG: average daily gain; ADFI: average daily feed intake; F:G: feed to gain ratio; SEM: pooled standard error of the mean of two groups. a, b Means in the same column followed by different superscript letters differ significantly at P<0.05
3.3. Effect of fresh fermented soybean meal on ammonia concentration in piggery
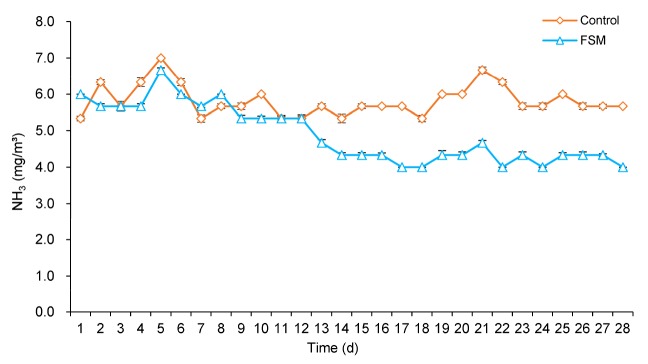
The concentration of NH3 in the piggery was reduced significantly in the FSM group. During the first two weeks of the experiment, the concentration of NH3 in the FSM group was reduced (P>0.05) by 4.7% compared with the control group. During the third to fourth week of the experiment, the concentration of NH3 in the FSM group declined (P<0.05) by 37.6% compared with the control group. During the whole experiment, the concentration of NH3 in the FSM group was decreased (P<0.05) by 19.0% compared with the control group. The effect of fresh FSM on the concentration of NH3 in the piggery is shown in Fig. 1.
Fig. 1.
Effect of fresh fermented soybean meal (FSM) on the concentration of ammonia (NH3) in piggery during the experiment
Experimental diets were: dietary inclusion of 100 g/kg fresh FSM in the FSM group, and no fresh FSM in diet as the control. Data are expressed as mean±SEM, n=3
3.4. Effect of fresh fermented soybean meal on particulate matter concentration in piggery
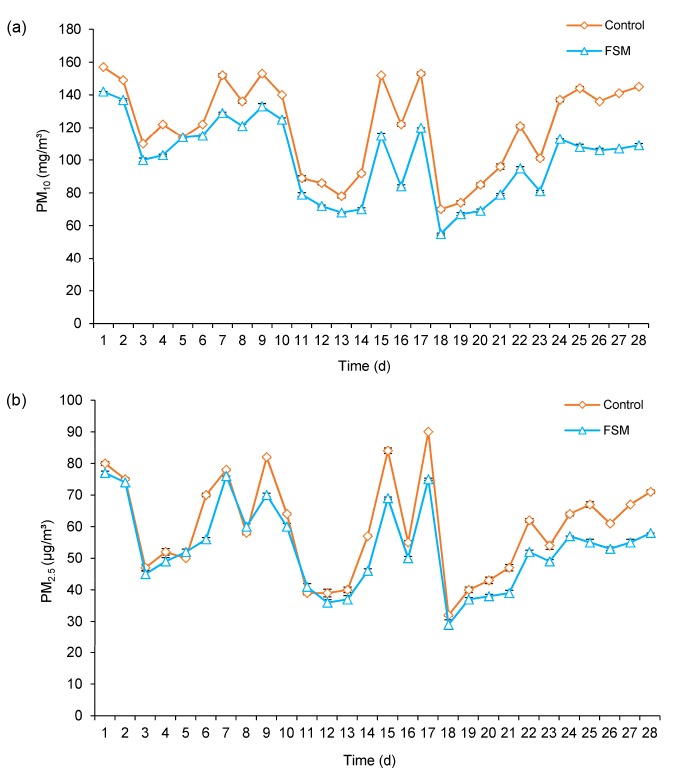
From the Fig. 2, it is clear that the concentrations of PM10 and PM2.5 in piggery were reduced significantly in the FSM group. During the first two weeks of the experiment, the concentration of PM10 reduced (P<0.05) by 12.7% in the FSM group compared with the control group. During the third to fourth week of the experiment, the concentration of PM10 declined (P<0.05) by 28.2% in the FSM group compared with the control group. During the whole experiment, the concentration of PM10 decreased (P<0.05) by 19.9% in the FSM group compared with the control group. During the first two weeks of the experiment, the concentration of PM2.5 reduced (P>0.05) by 6.7% in the FSM group compared with the control group. During the third to fourth week of the experiment, the concentration of PM2.5 declined (P<0.05) by 16.9% in the FSM group compared with the control group. During the whole experiment, the concentration of PM2.5 decreased (P<0.05) by 11.6% in the FSM group compared with the control group. The effects of fresh FSM on the concentrations of PM10 and PM2.5 in the piggery are shown in Figs. 2a and 2b, respectively.
Fig. 2.
Effects of fresh fermented soybean meal (FSM) on the concentrations of PM10 (a) and PM2.5 (b) in piggery during the experiment
PM10: particulate matter smaller than 10 μm aerodynamic diameters; PM2.5: particulate matter smaller than 2.5 μm aerodynamic diameters. Experimental diets were: dietary inclusion of 100 g/kg fresh FSM in the FSM group, and no fresh FSM in diet as the control. Data are expressed as mean±SEM, n=3
3.5. Effects of fresh fermented soybean meal on fecal and blood indicators in nursery piglets
The ammonia nitrogen and nitrite content in feces increased (P<0.05) by 32.9% and 28.4%, respectively in the FSM group, compared with the control group (Table 4). Fresh FSM contained in diet reduced the fecal pH significantly. The fecal pH in the FSM group decreased (P<0.05) by 5.9% compared with the control group (Table 4). Fresh FSM in diet had a significant effect on some serum biochemical parameters. The TP concentration increased (P<0.05) by 23.6% and the BUN concentration decreased (P<0.05) by 10.8% in the FSM group compared with the control group (Table 4).
Table 4.
Effects of fresh fermented soybean meal on fecal and blood indicators in nursery piglets1
| Group | Fecal ammonia nitrogen (g/kg) | Fecal nitrite (mg/kg) | Fecal pH | Blood TP (g/L) | BUN (mmol/L) |
| Control | 5.93b | 6.83b | 6.93a | 55.2b | 5.34a |
| FSM | 7.88a | 8.77a | 6.52b | 68.3a | 4.82b |
|
| |||||
| SEM | 0.32 | 0.44 | 0.04 | 1.68 | 0.14 |
| P-value | <0.001 | 0.001 | <0.001 | <0.001 | 0.003 |
1 Experimental diets were: dietary inclusion of 100 g/kg fresh fermented soybean meal (FSM) in the FSM group, and no fresh FSM in diet as the control. Determination of fecal ammonia nitrogen and nitrite content was based on dry matter. TP: total protein; BUN: blood urea nitrogen; SEM: pooled standard error of the mean of two groups. a, b Means in the same column followed by different superscript letters differ significantly at P<0.05
4. Discussion
In this study, dietary fresh FSM significantly improved the growth performance of nursery pigs. The present results showed that pigs fed the diet containing fresh FSM had increased ADG and improved feed utilization (reduced F:G) compared with pigs in the control group. However, the higher growth rates were, at least in part, a result of a higher feed consumption, rendering the feed efficiency to be only numerically different between groups. The results showed the improved growth performance, consistent with other studies (Jones et al., 2010). Fermentation improved the bioavailability of nutrients, because of the increase in soluble protein and small peptide content, and the effective removal of anti-nutritional factors in soybean meal after fermentation (Mukherjee et al., 2016). Canibe and Jensen (2003) reported that fermented liquid feed could reduce the quantity of gastrointestinal pathogens in growing pigs, maintain intestinal health, and then improve growth performance of pigs compared with dry feed.
In the present study, the diet containing fresh FSM reduced NH3 concentration in the piggery. Ammonia emissions in the piggery are mainly generated from surplus nitrogen. Therefore, reducing dietary crude protein, which provides the basic requirement for TP intake, and supplementing with synthetic amino acids will significantly decrease NH3 emissions (Hayes et al., 2004; Cho et al., 2008; Wu et al., 2015). Modulating dietary composition to reduce NH3 emission is economic and feasible, and the most important thing is that it is performed at source. In the present study, the reduction of NH3 emissions was due to fresh fermentation of soybean meal. Mukherjee et al. (2016) have shown that fresh FSM contained a high proportion of small peptides and digestive enzymes, improving nutritional quality of soybean meal, increasing the utilization of dietary nitrogen, and thereby reducing the emissions of nitrogenous substances in livestock. Hobbs et al. (1997) reported that feeding wet material could promote protein digestion and absorption, and then reduce nitrogen emissions. The fresh FSM used in this study resulted in a decrease of nitrogen excretion, and then reduction in the concentrations of NH3 and PM in the piggery.
Ammonia emissions in piggery are mainly generated from urea in the urine, because approximately 70% of nitrogen intake was excreted in the form of fecal nitrogen (about 20%) and urinary nitrogen (about 50%), and 80% of fecal nitrogen was in the form of organic nitrogen, with 97% of urinary nitrogen in the form of urea. Urea is easily converted to NH3 by fecal enzyme urease, and conversely, most of the nitrogen in feces is not readily degradable (van Faassen and van Dijk, 1987). Therefore, in addition to reducing nitrogen excretion by reducing dietary protein, transferring nitrogen from the urine to the feces and then converting fecal nitrogen into bacterial protein would be an effective way to reduce nitrogen excretion, and further reducing NH3 emissions. In the pig-breeding industry, more than 20 years ago, the method of adding microorganisms to enhance conversion of ammonia to bacterial protein was developed (Groenestein et al., 1993). Aarnink and Verstegen (2007) suggested that reducing crude protein intake supplemented with fermentable carbohydrates into the diet could lessen total nitrogen excretion and transfer nitrogen from urine to feces, which could decrease NH3 emissions in the production of growing-finishing pigs. It had been observed previously that diet supplemented with fiber reduced fecal NH3 emissions from pig barns, because it led to the transfer of nitrogen excretion from urine to feces (Lynch et al., 2008; Patráš et al., 2012). Indigestible sugar had also been shown to increase transport of BUN into the large intestine for bacterial protein synthesis, and to enhance nitrogen utilization, which resulted in a significant increase in bacterial protein in the feces, with a decrease in urinary nitrogen excretion (Shriver et al., 2003; Min et al., 2014). Thus, it is feasible that more nitrogen being excreted via the feces in the form of bacterial protein and less from the urine as urea could reduce NH3 emissions. In this study, there may be a higher transfer of nitrogen or urea into the gastrointestinal tract in the FSM group, which may lead to an increased fixation of nitrogen within microbial protein. This is also consistent with the results of ammonia nitrogen and nitrite content in feces as well as the blood urea content in this experiment.
PM in the piggery mainly comes from dust in the air, feed particle dust, feces and aerosol particles produced by porcine respiration and pig skin shedding. Renard et al. (2004) showed that bacteria and allergens could be carried into the air by dust particles that cause infection in animals and humans. Hamscher et al. (2003) suggested that antibiotics in dust originating from the farm may be a new source of human health hazards. The main factors influencing the concentration of PM in a piggery include feed type, temperature, humidity, ventilation, animal behavior, etc. (Pedersen et al., 2000). Pedersen et al. (2000) reported on applying oil to the floors to decrease the concentration of inhalable dust. However, this method is impractical and uneconomical. In this study, the concentrations of PM10 and PM2.5 in the piggery were decreased by partially replacing soybean meal with non-dried high-humidity fresh FSM. The reasons may be that the substitution of high-humidity fresh FSM increases the moisture content of feed and then reduces the production of feed dust during the feeding process. In addition, the diet containing fresh FSM had a mellow flavor, which improved the feed palatability, to a certain extent, lessening feed fight and play behaviors, while reducing the feed residue, thereby decreasing the production of feed dust in the air. Renard et al. (2004) reported that NH3 could react with acid molecules in the atmosphere to form particles (by secondary conversion) that contribute to the load of PM significantly. Therefore, reduction of NH3 emissions also contributes to the decrease of PM concentration. Several steep peaks appeared in Figs. 2a and 2b, due to the haze in Zhejiang Province, China, which was quite severe on Days 1, 7, 9, 15, and 17 during the experiment, leading to sharp increase of PM concentrations in the piggery.
In this study, the TP concentration increased and the BUN concentration decreased in the FSM group. The TP and BUN can reflect the overall metabolism of the body protein. Within a certain range, the higher the TP concentration, the greater the ability of the body to synthesize and utilize the protein. The BUN concentration is negatively related with nitrogen deposition rate and protein utilization rate, namely, the urinary nitrogen excretion is correlated positively and linearly with BUN concentration, and the urinary nitrogen excretion could be predicted by BUN concentration (Brown and Cline, 1974; Kohn et al., 2005). In this study, the decrease of BUN concentration and the increase of TP concentration suggested that the pigs fed diets with fresh FSM could utilize dietary nitrogen efficiently, as well as leading to a reduction in urinary nitrogen excretion.
In the present study, the significant increase of ammonia nitrogen and nitrite content in feces and the significant decrease of fecal pH were observed in the FSM group. The correlation between ammonia nitrogen content in feces and NH3 emissions from feces is affected particularly by pH (Le et al., 2008). The total ammonia nitrogen is the sum of NH3 and NH4 +, and the amount of NH3 is determined by the acid dissociation constant (K a) for NH3 and the pH (Loehr et al., 1973). The lower pH inhibited conversion of NH4 + to NH3, resulting in the ammonia nitrogen content in feces being relatively high. Overall, the lower pH increased ammonia nitrogen content in feces and decreased NH3 emissions from the feces. Nitrite is the intermediate product of ammonia conversion to nitrate. The increase of nitrite content in feces indicated that more nitrogen was retained in the feces. The increase of nitrite content was also associated with a decline of fecal pH (Murray et al., 1975). The reason why the fecal pH was reduced may be that lots of beneficial bacteria such as Lactobacillus and Bacillus and large amounts of lactic acids were produced (Canibe and Jensen, 2003; Suiryanrayna and Ramana, 2015) and that these decreased the fecal pH during the fermentation. It can therefore be said that the fecal nitrogen was retained in the form of ammonia nitrogen and nitrite. In this study, the increase of ammonia nitrogen and nitrite content in feces and the decrease of BUN concentration might be caused by the conversion of urinary nitrogen to fecal nitrogen resulting from fresh FSM, thereby reducing NH3 emissions. The increase of ammonia nitrogen and nitrite content in feces and the decrease of fecal pH and BUN concentration were consistent with the reduction of NH3 concentration in the piggery.
5. Conclusions
The results of this study suggested that dietary fresh FSM not only improved the growth performance of nursery piglets, but also reduced the concentrations of NH3, PM10 and PM2.5 in the piggery by an increase in microbial N-fixation and a rerouting of urea from the blood to the feces instead of the urine. Therefore, it is suggested that fresh FSM could be used to partially replace soybean meal in diets, which would help to lower the contribution of livestock production to harmful gases and substances in the environment.
Acknowledgments
We are grateful to the Ningbo Funing Breeding Production Farm, Zhejiang, China for allowing the use of their pigs and facilities during this study.
Footnotes
Project supported by the Key Agricultural Projects of Ningbo Science and Technology Bureau of Zhejiang Province (No. 2013C11008), China
Compliance with ethics guidelines: Sai-sai CHENG, Yuan LI, Shi-jie GENG, Luan-sha HU, Xiong-feng FU, and Xin-yan HAN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Aarnink AJA, Verstegen MWA. Nutrition, key factor to reduce environmental load from pig production. Livest Sci. 2007;109(1-3):194–203. doi: 10.1016/j.livsci.2007.01.112. [DOI] [Google Scholar]
- 2.AOAC . Official Methods of Analysis, 18th Ed. Arlington, VA, USA: Association of Official Analytical Chemists; 2006. [Google Scholar]
- 3.Banhazi TM, Seedorf J, Rutley DL, et al. Identification of risk factors for sub-optimal housing conditions in Australian piggeries: Part 2. Airborne pollutants. J Agric Saf Health. 2008;14(1):21–39. doi: 10.13031/2013.24122. [DOI] [PubMed] [Google Scholar]
- 4.Brown JA, Cline TR. Urea excretion in the pig: an indicator of protein quality and amino acid requirements. J Nutr. 1974;104(5):542–545. doi: 10.1093/jn/104.5.542. [DOI] [PubMed] [Google Scholar]
- 5.Canh TT, Sutton AL, Aarnink AJ, et al. Dietary carbohydrates alter the fecal composition and pH and the ammonia emission from slurry of growing pigs. J Anim Sci. 1998;76(7):1887–1895. doi: 10.2527/1998.7671887x. [DOI] [PubMed] [Google Scholar]
- 6.Canibe N, Jensen BB. Fermented and nonfermented liquid feed to growing pigs: effect on aspects of gastrointestinal ecology and growth performance. J Anim Sci. 2003;81(8):2019–2031. doi: 10.2527/2003.8182019x. [DOI] [PubMed] [Google Scholar]
- 7.Chiavegato MB, Powers W, Palumbo N. Ammonia and greenhouse gas emissions from housed Holstein steers fed different levels of diet crude protein. J Anim Sci. 2015;93(1):395–404. doi: 10.2527/jas.2014-8167. [DOI] [PubMed] [Google Scholar]
- 8.Cho JH, Chen YJ, Min BJ, et al. Effects of reducing dietary crude protein on growth performance, odor gas emission from manure and blood urea nitrogen and IGF-1 concentrations of serum in nursery pigs. Anim Sci J. 2008;79(4):453–459. doi: 10.1111/j.1740-0929.2008.00549.x. [DOI] [Google Scholar]
- 9.Dumont E, Hamon L, Lagadec S, et al. NH3 biofiltration of piggery air. J Environ Manage. 2014;140:26–32. doi: 10.1016/j.jenvman.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Griess P. Griess reagent: a solution of sulphanilic acid and α-naphthylamine in acetic acid which gives a pink colour on reaction with the solution obtained after decomposition of nitrosyl complexes. Chem Ber. 1879;12:427. (in German) [Google Scholar]
- 11.Groenestein CM, Oosthoek J, van Faassen HG. Microbial Processes in Deep-Litter Systems for Fattening Pigs and Emission of Ammonia, Nitrous Oxide and Nitric Oxide. EAAP Publication, the Netherlands.1993. [Google Scholar]
- 12.Hamscher G, Pawelzick HT, Sczesny S, et al. Antibiotics in dust originating from a pig-fattening farm: a new source of health hazard for farmers? Environ Health Perspect. 2003;111(13):1590–1594. doi: 10.1289/ehp.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes ET, Leek ABG, Curran TP, et al. The influence of diet crude protein level on odour and ammonia emissions from finishing pig houses. Bioresour Technol. 2004;91(3):309–315. doi: 10.1016/S0960-8524(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 14.Hobbs PJ, Misselbrook TH, Pain BF. Characterisation of odorous compounds and emissions from slurries produced from weaner pigs fed dry feed and liquid diets. J Sci Food Agric. 1997;73(4):437–445. doi: 10.1002/(SICI)1097-0010(199704)73:4<437::AID-JSFA748>3.0.CO;2-7. [DOI] [Google Scholar]
- 15.Hoff SJ, Bundy DS, Nelson MA, et al. Emissions of ammonia, hydrogen sulfide, and odor before, during, and after slurry removal from a deep-pit swine finisher. J Air Waste Manag Assoc. 2006;56(5):581–590. doi: 10.1080/10473289.2006.10464472. [DOI] [PubMed] [Google Scholar]
- 16.Ilea RC. Intensive livestock farming: global trends, increased environmental concerns, and ethical solutions. J Agric Environ Ethics. 2009;22(2):153–167. doi: 10.1007/s10806-008-9136-3. [DOI] [Google Scholar]
- 17.Jones CK, DeRouchey JM, Nelssen JL, et al. Effects of fermented soybean meal and specialty animal protein sources on nursery pig performance. J Anim Sci. 2010;88(5):1725–1732. doi: 10.2527/jas.2009-2110. [DOI] [PubMed] [Google Scholar]
- 18.Kohn RA, Dinneen MM, Russek-Cohen E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs and rats. J Anim Sci. 2005;83(4):879–889. doi: 10.2527/2005.834879x. [DOI] [PubMed] [Google Scholar]
- 19.Krupa SV. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: a review. Environ Pollut. 2003;124(2):179–221. doi: 10.1016/S0269-7491(02)00434-7. [DOI] [PubMed] [Google Scholar]
- 20.Le PD, Aarnink AJA, Jongbloed AW, et al. Interactive effects of dietary crude protein and fermentable carbohydrate levels on odour from pig manure. Livest Sci. 2008;114(1):48–61. doi: 10.1016/j.livsci.2007.04.009. [DOI] [Google Scholar]
- 21.Leonard RH. Quantitative range of Nessler’s reaction with ammonia. Clin Chem. 1963;9(4):417–422. [PubMed] [Google Scholar]
- 22.Loehr RC, Prakasam TBS, Srinath EG, et al. Development and Demonstration of Nutrient Removal from Animal Wastes. Office of Research and Monitoring. Environmental Protection Agency. US Government Printing Office, Washington, DC; 1973. [Google Scholar]
- 23.Lynch MB, O'Shea CJ, Sweeney T, et al. Effect of crude protein concentration and sugar-beet pulp on nutrient digestibility, nitrogen excretion, intestinal fermentation and manure ammonia and odour emissions from finisher pigs. Animal. 2008;2(3):425–434. doi: 10.1017/S1751731107001267. [DOI] [PubMed] [Google Scholar]
- 24.Min X, Xiao J, Kawasaki K, et al. Transfer of blood urea nitrogen to cecal microbes and nitrogen retention in mature rabbits are increased by dietary fructooligosaccharides. Anim Sci J. 2014;85(6):671–677. doi: 10.1111/asj.12205. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee R, Chakraborty R, Dutta A. Role of fermentation in improving nutritional quality of soybean meal–a review. Asian-Aust J Anim Sci. 2016;29(11):1523–1529. doi: 10.5713/ajas.15.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray I, Parsons JW, Robinson K. Inter-relationships between nitrogen balance, pH and dissolved oxygen in an oxidation ditch treating farm animal waste. Water Res. 1975;9(1):25–30. doi: 10.1016/0043-1354(75)90148-7. [DOI] [Google Scholar]
- 27.NRC (National Research Council) Nutrient Requirements of Swine, 11th Ed. Washington, DC: Natl. Acad. Press; 2012. [Google Scholar]
- 28.Patráš P, Nitrayová S, Brestenský M, et al. Effect of dietary fiber and crude protein content in feed on nitrogen retention in pigs. J Anim Sci. 2012;90(Suppl. 4):158–160. doi: 10.2527/jas.53837. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen S, Nonnenmann M, Rautiainen R, et al. Dust in pig buildings. J Agric Saf Health. 2000;6(4):261–274. doi: 10.13031/2013.1909. [DOI] [PubMed] [Google Scholar]
- 30.Portejoie S, Martinez J, Guiziou F, et al. Effect of covering pig slurry stores on the ammonia emission processes. Bioresour Technol. 2003;87(3):199–207. doi: 10.1016/S0960-8524(02)00260-2. [DOI] [PubMed] [Google Scholar]
- 31.Renard JJ, Calidonna SE, Henley MV. Fate of ammonia in the atmosphere–a review for applicability to hazardous releases. J Hazard Mater. 2004;108(1-2):29–60. doi: 10.1016/j.jhazmat.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Rigolot C, Espagnol S, Robin P, et al. Modelling of manure production by pigs and NH3, N2O and CH4 emissions. Part II: effect of animal housing, manure storage and treatment practices. Animal. 2010;4(08):1413–1424. doi: 10.1017/S1751731110000509. [DOI] [PubMed] [Google Scholar]
- 33.Shriver JA, Carter SD, Sutton AL, et al. Effects of adding fiber sources to reduced-crude protein, amino acid-supplemented diets on nitrogen excretion, growth performance, and carcass traits of finishing pigs. J Anim Sci. 2003;81(2):492–502. doi: 10.2527/2003.812492x. [DOI] [PubMed] [Google Scholar]
- 34.Suiryanrayna MV, Ramana JV. A review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. 2015;6(1):45. doi: 10.1186/s40104-015-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Faassen HG, van Dijk H. Manure as a source of nitrogen and phosphorus in soils. In: van der Meer HG, Unwin RJ, van Dijk TA, editors. Animal Manure on Grassland and Fodder Crops.Fertilizer or Waste? Springer Netherlands; 1987. pp. 27–45. [DOI] [Google Scholar]
- 36.Vogelzang PF, van der Gulden JW, Folgering H, et al. Longitudinal changes in bronchial responsiveness associated with swine confinement dust exposure. Chest. 2000;117(5):1488–1495. doi: 10.1378/chest.117.5.1488. [DOI] [PubMed] [Google Scholar]
- 37.Webb J, Thorman RE, Fernanda-Aller M, et al. Emission factors for ammonia and nitrous oxide emissions following immediate manure incorporation on two contrasting soil types. Atmos Environ. 2014;82:280–287. doi: 10.1016/j.atmosenv.2013.10.043. [DOI] [Google Scholar]
- 38.Wu L, He L, Cui Z, et al. Effects of reducing dietary protein on the expression of nutrition sensing genes (amino acid transporters) in weaned piglets. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(6):496–502. doi: 10.1631/jzus.B1400259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye Z, Li B, Cheng B, et al. A concrete slatted floor system for separation of faeces and urine in pig houses. Biosyst Eng. 2007;98(2):206–214. doi: 10.1016/j.biosystemseng.2007.07.007. [DOI] [Google Scholar]
- 40.Zhou C, Hu J, Zhang B, et al. Gaseous emissions, growth performance and pork quality of pigs housed in deep-litter system compared to concrete-floor system. Anim Sci J. 2015;86(4):422–427. doi: 10.1111/asj.12311. [DOI] [PubMed] [Google Scholar]