Abstract
Soybean isoflavones have been one of the potential preventive candidates for antitumor research in recent years. In this paper, we first studied the transformation of soybean isoflavones with the homogenized slurry of Ganoderma lucidum. The resultant transformed products (TSI) contained (703.21±4.35) mg/g of genistein, with transformed rates of 96.63% and 87.82% of daidzein and genistein, respectively, and TSI also could enrich the bioactive metabolites of G. lucidum. The antitumor effects of TSI on human colorectal cancer cell line HTL-9, human breast cancer cell line MCF-7, and human immortalized gastric epithelial cell line GES-1 were also studied. The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay showed that TSI could dramatically reduce the viability rates of HTL-9 cells and MCF-7 cells without detectable cytotoxicity on GES-1 normal cells when the TSI concentration was lower than 100 μg/ml. With 100 μg/ml of TSI, HTL-9 cells were arrested in the G1 phase, and late-apoptosis was primarily induced, accompanied with partial early-apoptosis. TSI could induce primarily early-apoptosis by arresting cells in the G1 phase of MCF-7 cells. For HTL-9 cells, Western-blot and reverse-transcriptase polymerase chain reaction (RT-PCR) analysis showed that TSI (100 μg/ml) can up-regulate the expression of Bax, Caspase-3, Caspase-8, and cytochrome c (Cyto-c), indicating that TSI could induce cell apoptosis mainly through the mitochondrial pathway. In addition, the expression of p53 was up-regulated, while the expression of Survivin and nuclear factor κB (NF-κB) was down-regulated. All these results showed that TSI could induce apoptosis of HTL-9 cells by the regulation of multiple apoptosis-related genes.
Keywords: Soybean isoflavones, Ganoderma lucidum, Transformation, Antiproliferative activity, Apoptosis
1. Introduction
The GLOBOCAN 2012 shows that there are approximate 14.1 million new cancer cases and 8.2 million death cases each year worldwide. The most commonly diagnosed cancers are lung cancer, breast cancer, and colorectal cancer, and the colorectal cancer cases represent almost 1.4 million and account for 9.7% of the total cancer cases (Ferlay et al., 2015). Due to the rising incidence and mortality rate of cancers, more and more natural products are being used for cancer prevention and adjuvant treatment, such as polysaccharides, macrolide (Lim et al., 2012), phytoestrogen, peptides (Suarez-Jimenez et al., 2012), isoflavones (Szliszka et al., 2011), and anthranone (Gong et al., 2011).
Soybean isoflavones are the secondary metabolites formed during the period of soybean growth, and their main ingredients contain genistein, daidzein, and glycitein. Epidemiologic research has proved that soybean isoflavones have many pharmacological effects. They can enhance body immunity (Wei et al., 2012; Zhou et al., 2015) and improve learning ability and memory in menopausal women. Moreover, they can prevent and treat cardiovascular disease, osteoporosis (Srivastava et al., 2014; Baglia et al., 2015), breast cancer, prostate cancer (Li et al., 2012a), colon cancer (Tse and Eslick, 2016), and menopausal syndrome (Ollberding et al., 2012). The possible mechanisms of antitumor activity include an estrogen-like effect (Mense et al., 2008; Choi and Kim, 2013), the impact on the androgen receptor pathway by inhibiting the expression of prostate specific antigen (PSA) (Banerjee et al., 2012), the regulation of cell cycle and apoptosis induction (Prietsch et al., 2014; Tsuboy et al., 2014), and the regulation of mitogen activated protein kinase (MAPK) pathway (Cotrim et al., 2013; Chen et al., 2014).
In natural conditions, 97%–98% soybean isoflavones exist in the form of glycosides (Hati et al., 2015) which cannot be directly absorbed by the body. Isoflavones have to be hydrolyzed into aglycones for absorption and to exert their pharmacological activity (Andlauer et al., 2000; Handa et al., 2014). Therefore, more and more research focuses on the enzymatic hydrolysis of isoflavones by microorganisms (Yeo and Liong, 2010; Yeom et al., 2012; Maitan-Alfenas et al., 2014). Also there has been research on how to transform isoflavones by food microorganisms and then create transformed products as a health food of potential to be a new application of isoflavones (Ewe et al., 2012; Titiek et al., 2013; Yin et al., 2014).
Ganoderma lucidum (Leyss. ex. Fr.) Karst, a fungus in the group of Basidiomycetes, has increasingly been explored as a valuable traditional Chinese medicinal mushroom for over 2000 years. A lot of research has demonstrated that G. lucidum is extremely effective in prevention and treatment of various diseases, such as cancer (Zhao et al., 2011), hyperglycaemia (Guo et al., 2013), hepatitis (Kim et al., 2006), cardiovascular diseases (Lee and Rhee, 1990), human immunodeficiency virus 1 (HIV-1) (Kang et al., 2015). In addition, the polysaccharides and triterpenoids are the major bioactive metabolites and have some important medicinal properties (Keypour et al., 2010).
In this study, soybean isoflavones were transformed by the homogenized slurry of G. lucidum for the first time. The antitumor effects of the transformed products on HTL-9 cells and MCF-7 cells were tested, followed by the determination of cell cycle arrest and apoptosis induction. The apoptosis-related genes and proteins were analyzed to preliminarily clarify the antitumor mechanism.
2. Materials and methods
2.1. Liquid cultivation of Ganoderma lucidum
G. lucidum was grown in a 250-ml flask containing 100 ml seed culture at 28 °C for 8 d with shaking at 180 r/min, and was then inoculated at 10% (v/v) into the fermentation culture and cultivated at 28 °C for 7 d with shaking at 180 r/min.
The seed culture contained (g/L): potato extract 10, glucose 20, peptone 18, KH2PO4 3, MgSO4 1.5, and vitamin B1 (VB1) 0.05 (pH 5.5). The fermentation culture contained (g/L): wort 41, peptone 18.9, KH2PO4 3, MgSO4 1.5, and VB1 0.05 (pH 5.4) (Cui et al., 2015).
Wort was made from barley by means of official analysis methods of the European Brewery Convention (EBC) (Munck et al., 1989), and was assessed by its total sugar contents.
2.2. Transformation of soybean isoflavones by Ganoderma lucidum
After fermentation, the broth and mycelia of G. lucidum were homogenized and the activity of β-glucosidase was determined. A volume of 100 ml slurry with β-glucosidase of 1.0 U/ml was made as a reaction solution, and 5 g soybean isoflavones were added and transformed for 48 h at 60 °C with pH 5.0. The transformed products were freeze-dried and collected. The ingredients of the transformed products were extracted with 50% (v/v) ethanol at 60 °C for 1 h, and then the supernatant was collected, concentrated, and made as a stock solution by passing through a 0.22-μm filter (Millipore, USA). This solution was called TSI and assessed by its total isoflavone content.
The analysis of isoflavone ingredients and the determination of transformed rates of daidzein (R d) and genistein (R g) were made using an Agilent series 1100 high-performance liquid chromatography (HPLC) instrument (Agilent, Germany) and R d and R g were calculated by
R d=C d/C dd×100%,
R g=C g/C gg×100%,
where C d and C g are the contents of daidzein and genistein after transformation, respectively; while C dd and C gg are the contents of “daidzin+daidzein” and “genistin+genistein” before transformation, respectively. The sample was separated on a ZORBAX SB-C18 column (5 μm, 4.6 mm×250 mm; Agilent). The mobile phase consisted of 0.2% (v/v) aqueous acetic acid (A) and acetonitrile (B) with a linear gradient program of B including: 10%–63% in 0–25 min. The flow rate was 1 ml/min with a column temperature of 30 °C. The diode array detector (DAD) detector was monitored at 254 nm ultra violet (UV) spectra and 3D-plots were recorded between 200 and 400 nm.
2.3. Cell lines and cell culture
Human colorectal cancer cell line HTL-9, human breast cancer cell line MCF-7, and human immortalized gastric epithelial cell line GES-1 were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Science (Shanghai, China). These three cell lines were routinely suspended in RPMI-1640 medium (HyClone, USA) containing 10% (v/v) fetal bovine serum (FBS) and 100 U/ml streptomycin-penicillin, and incubated in a humidified atmosphere at 37 °C with 5% CO2.
2.4. MTT assay of cell viability
The effects of TSI on the viability of these three cell lines were determined using a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay. These cells were harvested and seeded at a density of 1×105–5×105 cells/ml in 96-well plates. After incubation for 24 h, the serial dilutions of TSI (0, 20, 40, 60, 80, 100, 120 μg/ml) were added to each well and incubated for 24 h. The viability was determined by adding 20 μl of MTT solution (5 mg/ml in phosphate buffered saline (PBS)). Living cells reduce the yellow MTT to a blue formazan product. After 3 h of incubation at 37 °C, the formazan product was dissolved in 150 μl dimethyl sulfoxide (DMSO) from each well, and the plates were read at 490 nm using the Multiskan GO. The percentage of cell viability was expressed as a ratio versus control (van Meerloo et al., 2011).
2.5. Assay of cell apoptosis and cell cycle by flow cytometry
All these cells were seeded in 6-well plates at a density of 1×106 cells/ml and incubated for 24 h, and then treated with the suitable concentration of TSI for 24 h (media alone as control). At the end of the incubation period, the cells were collected and washed twice by cold PBS (200g, 5 min). For apoptosis assay, the cells (1×106 cells/ml) were resuspended in 500 μl binding buffer, and were stained with Annexin V-fluorescein isothiocyanate (FITC) (5 μl) and propidium iodide (PI) (10 μl) in the dark for 5–15 min at 2–8 °C. The treated cells were analyzed by the flow cytometry method (FCM) within 1 h (Priyadarsini et al., 2010). For cell cycle assay, the cells (1×106 cells/ml) were fixed with 70% cold ethanol overnight at −20 °C, and then washed twice by cold PBS (200g, 5 min), followed by treating with RNaseA (100 μl) for 30 min at 37 °C and PI (400 μl) staining in the dark for 30 min at 4 °C. Finally, these treated cells were analyzed by FCM within 1 h.
2.6. RT-PCR analysis of apoptosis-related genes
The expression of apoptosis-related genes was measured by reverse transcription-polymerase chain reaction (RT-PCR), with the expression of human 18S rRNA as internal control. HTL-9 cells were seeded in 6-well plates with a density of 1×106 cells/ml and incubated for 24 h, followed by treating with a suitable concentration of TSI for 24 h (media alone as control). After treatment, the total RNA of cells was isolated using TRIzol reagent (Sangon Biotech, China) according to the manufacturer’s protocol, and RNA quality was determined by NanoDrop-1000 (Thermo, USA). Reverse transcription was performed using the Roche Transcriptor complementary DNA (cDNA) Synth. Kit (Roche, Switzerland) and the primers of target genes are shown in Table 1. PCR reaction was performed using a Roche FastStart Universal SYBR Green Master (ROX) kit (Roche, Switzerland).
Table 1.
RT-PCR primers of target genes
| Gene sequence number | Gene name | Primer sequence |
| NR_003286 | HOMO-18S-F | CAGCCACCCGAGATTGAGCA |
| HOMO-18S-R | TAGTAGCGACGGGCGGTGTG | |
| NM_002046 | HOMO-P53/TP53-F | GAAGATGGTGATGGGATTTC |
| HOMO-P53/TP53-R | GAAGGTGAAGGTCGGAGTC | |
| NM_000546.4 | HOMO-AMPKa2/PRKAA2-F | AGGCCTTGGAACTCAAGGAT |
| HOMO-AMPKa2/PRKAA2-R | CCCTTTTTGGACTTCAGGTG | |
| NM_006252.3 | HOMO-Bax-F | CTGAGAAGCAGAAGCACGAC |
| HOMO-Bax-R | ACAACATCTAAACTGCGAAT | |
| NM_001291428.1 | HOMO-Bcl2-F | CTCAGGATGCGTCCACCAA |
| HOMO-Bcl2-R | CCTCTGCAGCTCCATGTTACTGT | |
| NM_138578.1 | HOMO-Caspase-3/CASP3-F | CCTGACGGGCATGACTGTGG |
| HOMO-Caspase-3/CASP3-R | TGGACGGAGGATGTGGTGGA | |
| NM_032991.2 | HOMO-Caspase-8/CASP8-F | TGGTTCATCCAGTCGCTTTG |
| HOMO-Caspase-8/CASP8-R | AATTCTGTTGCCACCTTTCG | |
| NM_001228.4 | HOMO-Caspase-9/CASP9-F | AGGAGCTGCTCTTCCGAATT |
| HOMO-Caspase-9/CASP9-R | GTGTCTGGCACTGGCTGTTT | |
| NM_032996.2 | HOMO-Survivin/BIRC5-F | AAGCCCAAGCTCTTTTTCATC |
| HOMO-Survivin/BIRC5-R | ACTCGTCTTCAGGGGAAGTG | |
| NM_001168.2 | HOMO-GAPDH-F | TGGGAAGGGTTGTGAATGAG |
| HOMO-GAPDH-R | CAGTTTGGCTTGCTGGTCTC | |
| NM_019887.5 | HOMO-Smac/DIABLO-F | GCAGCGTAACTTCATTCTTC |
| HOMO-Smac/DIABLO-R | CAAAGCCAATCGTCACAG |
2.7. Western-blot analysis
HTL-9 cells were seeded into 6-well plates at a density of 1×106 cells/ml and incubated for 24 h, followed by treating with a suitable concentration of TSI for 24 h (media alone as control). After treatment, the cells were collected and washed twice by PBS. Lysis buffer (0.2–0.5 ml) mixed with phosphatase inhibitors (10 μl), protease inhibitors (1 μl), and 100 mmol/L phenylmethanesulfonyl fluoride (PMSF) (5 μl) was added to extract cell protein. After quantitative detection, the cell protein was separated by 5% (0.05 g/ml) sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). The protein expression of cytochrome c (Cyto-c), nuclear factor κB (NF-κB), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (internal control) was detected by Western-blot using protein-selective antibodies (KeyGen Biotech, China). Antibodies were incubated overnight at 4 °C and then incubated with goat anti-Rb IgG-HRP with shaking for 1–2 h at room temperature. The immunolabeled proteins were detected by G:BOX ChemiXR5 (Syngene, UK) using an enhanced chemiluminescence (ECL) reagent kit.
2.8. Statistical analysis
All experiments were performed in triplicate and the results were presented as the mean±standard deviation (SD) using SPSS 17.0. Comparisons among all groups were performed with the one-way analysis of variance (ANOVA) test. P<0.05 was considered statistically significant.
3. Results
3.1. Transformation of soybean isoflavones by Ganoderma lucidum
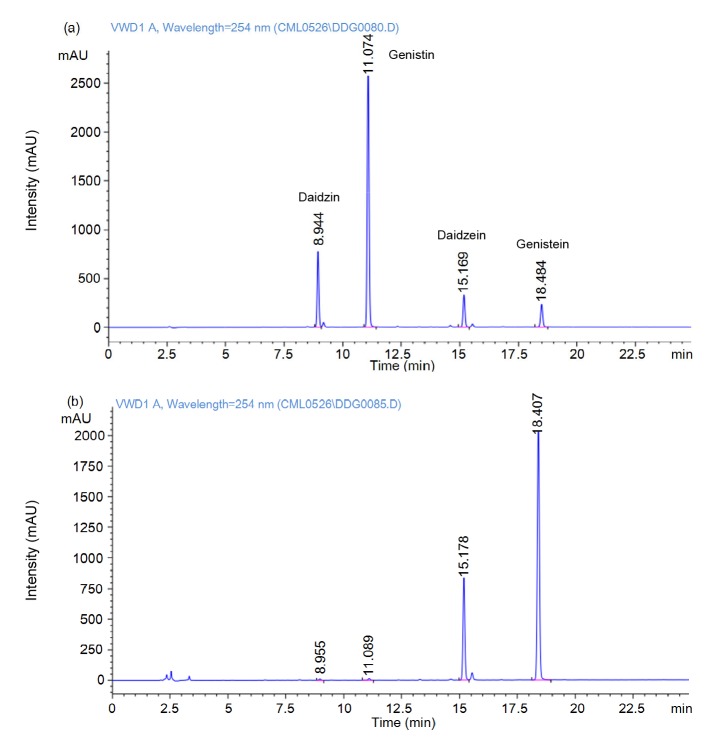
The contents of daidzein and genistein in soybean isoflavones extract were (85.45±1.72) and (31.48±1.04) mg/g, respectively. After transformation, the genistein content increased to (703.21±4.35) mg/g, and the transformed rates of daidzein and genistein could reach 96.63% and 87.82%, respectively (Fig. 1). More importantly, this is the first time soybean isoflavones have been transformed with the homogenate of G. lucidum, including (1.87±0.02) g of mycelia, (93.21±0.79) mg of intracellular triterpenoid, and (45.63±0.36) mg of intracellular polysaccharides. These could eventually be collected in the transformed products. In brief, the transformed products contained not only glycosides, but also bioactive metabolites of G. lucidum, and these could provide a new direction for the development of health products based on soybean isoflavones.
Fig. 1.
HPLC analysis for soybean isoflavones
(a) Non-transformed soybean isoflavones; (b) Isoflavones of transformed products (transformation conditions: soybean isoflavones 5 g, 60 °C, 48 h). In non-transformed soybean isoflavones, the contents of daidzein and genistein were (85.45±1.72) and (31.48±1.04) mg/g, respectively. After transformation, the contents of genistein could reach (703.21±4.35) mg/g, and their transformed rates could reach 96.63% and 87.82%, respectively
3.2. Antiproliferative activity in vitro
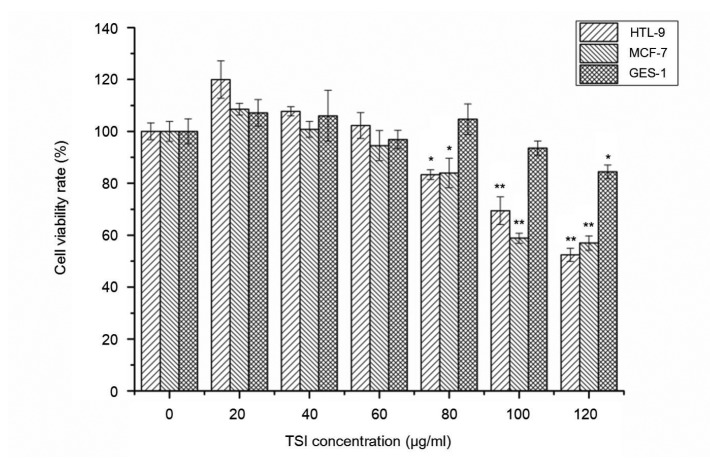
Fig. 2 showed the viability of HTL9 cells and MCF-7 cells in the presence of different concentrations of TSI. The proliferation of cancer cells was dramatically reduced with TSI concentration affected. When the concentration of TSI was 80 μg/ml, the cell viability rates of HTL-9 cells and MCF-7 cells were decreased to (83.32±0.08)% (P<0.05) and (83.96±0.08)% (P<0.05), respectively, and when the concentration reached 100 μg/ml, the cell viability rate fell to (69.38±0.02)% (P<0.01) and (58.85±0.11)% (P<0.01), respectively. In addition, the viability of these two cancer cell lines was more seriously decreased as TSI concentration continuously increased. For GES-1 cells, the viability of cells was not significantly affected when the TSI concentration was lower than 100 μg/ml. However, the GES-1 cell viability rate decreased to (84.42±0.02)% (P<0.05) when the TSI concentration reached 120 μg/ml, which showed that this concentration was cytotoxic to GES-1. Therefore, TSI (≤100 μg/ml) had effectively antiproliferative activity on cancer cells in vitro, and TSI of 100 μg/ml was chosen for the following experiments.
Fig. 2.
Effect of TSI on viability of HTL-9 cells, MCF-7 cells, and GES-1 cells assayed by MTT
Values were expressed as the average of triple determination with ±SD. * P<0.05, ** P<0.01 (values of test groups compared with that of non-treatment). TSI could dramatically reduce viability of HTL-9 cells and MCF-7 cells as TSI concentration increased, whereas, the viability of normal GES-1 cells was not significantly affected when the TSI concentration was lower than 100 μg/ml
3.3. Apoptosis-inducing and cell cycle arrest analyses
The apoptosis-inducing analysis of TSI on these cells was carried out by FCM with Annexin V-FITC and PI staining. As shown in Fig. 3, cells were gated into groups B1, B2, B3, and B4, which represented dead cells (Annexin V-FITC−/PI+), late-apoptotic cells (Annexin V-FITC+/PI+), viable cells (Annexin V-FITC−/PI−), and early-apoptotic cells (Annexin V-FITC+/PI−), respectively. After treatment with TSI (100 μg/ml), for HTL-9 cells, the late-apoptotic cells increased from 8.27% to 40.13%, and the early-apoptotic cells also increased from 0.95% to 9.05%, while the viable cells were decreased from 90.55% to 50.11%. This showed TSI could induce HTL-9 cells apoptosis primarily by means of late-apoptosis, accompanied with partial early-apoptosis. For MCF-7 cells, the late-apoptotic cells increased from 9.62% to 10.33%, and the early-apoptotic cells increased from 1.31% to 5.40%. This showed that TSI could induce MCF-7 cells apoptosis by early-apoptosis.
Fig. 3.
Apoptosis induction by TSI on HTL9 cells and MCF-7 cells
(-1) Non-treatment; (-2) TSI (100 μg/ml). Proportions in different groups were as following: for HTL-9, (H-1) B2, 8.27%, B4, 0.95%, (H-2) B2, 40.13%, B4, 9.05%; for MCF-7, (M-1) B2, 9.62%, B4, 1.31%, (M-2) B2, 10.33%, B4, 5.40%, which showed that TSI (100 μg/ml) could effectively induce cell apoptosis of HTL-9 and MCF-7 cancer cells
HTL-9 cells and MCF-7 cells were treated with 100 μg/ml of TSI for 24 h and analyzed by FCM with PI staining for cell cycle arrest analysis. It is evident from the data shown in Fig. 4 that TSI (100 μg/ml) produced a significant arrest of growth of the cells in the G1 phase (47.73% to 75.24%) with a decrease in the S phase (37.11% to 19.01%) and the G2 phase (15.15% to 5.74%) of HTL-9 cancer cells compared with the untreated control. An increase in the G1 phase (68.98% to 73.54%) and the G2 phase (0.86% to 6.51%) with a decrease in S phase (30.15% to 19.94%) was observed in MCF-7 cells. It can be seen that treatment with TSI (100 μg/ml) can arrest cells in the G1 phase and prevent cell DNA synthesis and further division of HTL-9 cells and MCF-7 cells, and these eventually induce cell apoptosis.
Fig. 4.
Cell cycle analysis of HTL-9 cells and MCF-7 cells by FCM
(-1) Non-treatment; (-2) TSI (100 μg/ml). For HTL-9 cells: TSI (100 μg/ml) produced a significant arrest of growth of the cells in G1 phase (47.73% to 75.24%) with a decrease in S phase (37.11% to 19.01%) and G2 phase (15.15% to 5.74%). For MCF-7 cells: an increase in G1 phase (68.98% to 73.54%) and G2 phase (0.86% to 6.51%) with a decrease in S phase (30.15% to 19.94%)
From the cell apoptosis and cell cycle analyses, TSI (100 μg/ml) is more effective in inducing cell apoptosis in HTL-9 cancer cells than in MCF-7 cells, so HTL-9 cells were chosen for further study to explore the apoptosis mechanism with TSI treatment.
3.4. Apoptosis-related protein and gene expression of HTL-9 cells with TSI treatment
The protein expression of Cyto-c and NF-κB (P65) was detected using Western-blot analysis. As shown in Fig. 5, TSI (100 μg/ml) can induce a significant increase in the protein expression of Cyto-c, while an obvious decrease occurred in the expression of NF-κB. The relative protein expression level of Cyto-c was approximately 5.5 times higher than that in non-treated cells, while the NF-κB expression was approximately 0.53 times that of control.
Fig. 5.
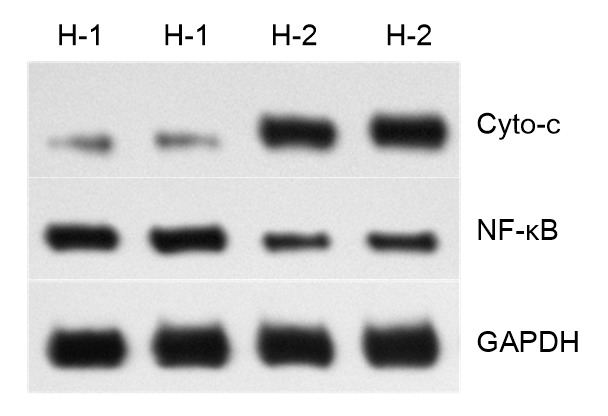
Expression of Cyto-c and NF-κB of HTL-9 cells with TSI treatment
(-1) Non-treatment; (-2) Treatment with TSI (100 μg/ml). GAPDH was used to normalize protein loading. TSI could induce a significant increase (5.5 times higher) in the protein expression of Cyto-c, while an obvious decrease (0.53 times) occurred in expression of NF-κB
RT-PCR was performed for the expression of apoptosis-related genes of HTL-9 cells with TSI (100 μg/ml) treatment. The method of 2−∆∆ C T was used to calculate the variation of gene expression levels, and 2∆ C T×106 was used for gene relative expression levels. Compared with the untreated cells, the relative mRNA expression of p53, Bax, Caspase-3, Caspase-8, and Survivin showed significant changes in HTL-9 cells with TSI treatment. As shown in Table 2, the mRNA expression of p53, Bax, Caspase-3, Caspase-8 was significantly increased, while the mRNA expression level of the anti-apoptosis factor Survivin was significantly decreased to 1.156±0.140 while the expression in the control was 3.450±0.300.
Table 2.
Relative expression of the target genes in HTL-9 cells measured by RT-PCR
| Gene | Expression level (2∆
C
T×106) |
|
| Control | TSI (100 μg/ml) | |
| p53 | 0.943±0.050 | 1.587±0.050** |
| Smac | 1.093±0.090 | 1.160±0.140 |
| AMPKa2 | 1.006±0.080 | 1.090±0.010 |
| Bax | 1.016±0.040 | 1.446±0.130** |
| Bcl-2 | 1.000±0.180 | 1.179±0.180 |
| Caspase-3 | 1.000±0.020 | 1.330±0.020** |
| Caspase-8 | 1.083±0.070 | 1.438±0.130** |
| Caspase-9 | 0.923±0.090 | 0.917±0.040 |
| Survivin | 3.450±0.300 | 1.156±0.140** |
The results were expressed as the average of triple determination with ±SD. * P<0.05,
P<0.01 (values of test groups compared with that of non-treatment)
From the comprehensive results of Western-blot and RT-PCR analyses with TSI treatment, it is shown that the relative mRNA expression of Bax significantly increased, the ratio of Bcl-2/Bax mRNA expression was significantly decreased, and the protein expression of Cyto-c was also significantly increased; simultaneously, Caspase-3 and Caspase-8 were activated and their expression was both significantly increased. All these indicated that TSI treatment could induce cell apoptosis mainly through the mitochondrial pathway. Moreover, the mRNA expression of pro-apoptosis factor p53 was significantly increased, and the expression of anti-apoptosis factor Survivin and NF-κB was significantly decreased. In conclusion, TSI could induce HTL-9 cells apoptosis by the regulation of multiple apoptosis-related genes.
4. Discussion
Increasing lines of evidences have showed that both G. lucidum (Tang et al., 2006; Loganathan et al., 2014; Hsin et al., 2015) and soybean isoflavones (Kurahashi et al., 2007; Li et al., 2012b) have antitumor activity. In this study, for the first time, the transformation of soybean isoflavones was performed using the homogenate of G. lucidum, and the genistein contents could reach (703.21±4.35) mg/g, with transformed rates of 96.63% and 87.82% of daidzein and genistein, respectively. The transformed products also contained the bioactive metabolites of G. lucidum, including (1.87±0.02) g of mycelia, (93.21±0.79) mg of intracellular triterpenoids, and (45.63±0.36) mg of intracellular polysaccharides.
We evaluated the viability and apoptotic effects of TSI on human colorectal cancer cells HTL-9. The results showed that TSI can dramatically reduce cell viability of HTL-9 cells as TSI concentration increased, and 100 μg/ml of TSI can induce HTL-9 cells apoptosis primarily by means of late-apoptosis by arresting cells in the G1 phase. These findings were consistent with previous reports that isoflavones inhibited the proliferation of human colorectal cancer cells (Yan et al., 2010; Budhathoki et al., 2011). Therefore, it is possible to make TSI as a competitive candidate for antitumor research.
Cell apoptosis is regulated by complicated apoptosis-related genes. In the mitochondria apoptotic signaling pathway, when cells are at mitochondrial dysfunction, Cyto-c accesses into cytoplasm from mitochondria membrane (Mayola et al., 2011), binding with apoptotic protease activating factor-1 (APAF-1). In the presence of deoxyadenosine triphosphate (dATP), the caspase family is activated and triggers apoptotic cascade reactions (Indran et al., 2011; Miranda et al., 2014). Of these, the Bcl-2 family genes play a vital role in the regulation of apoptosis, and contain anti-apoptotic genes (Bcl-2, Bcl-xL, etc.) and pro-apoptotic genes (Bax, Bid, etc.) (Thomas et al., 2013). Proliferation of cancer cells will increase with overexpression of Bcl-2, while the accumulation of apoptotic cells will occur with overexpression of Bax, with the determination of cell’s susceptibility for apoptosis by the ratio of Bcl-2/Bax expression (Xu et al., 2013; Al-Fatlawi et al., 2014). In this study, to further understand the apoptosis mechanism, apoptosis-related genes and proteins were detected by Western-blot and RT-PCR analyses. The results showed that TSI induced the expression of Bax, Caspase-3, Caspase-8, and Cyto-c while reducing the ratio of Bcl-2/Bax, indicating that TSI could induce cell apoptosis mainly through the mitochondrial pathway.
In cell apoptosis, NF-κB mainly acts as an anti-apoptosis factor and its expression can significantly increase in malignant tumors (Liu et al., 2012; Varfolomeev et al., 2015). Because of gene mutation in cancer cells, p53 is dysfunctional and cannot inhibit generation of glucose transporters (GLUTs), and this can result in significant accumulation of the level of glucose metabolism. Therefore, activation of p53 can induce cell apoptosis by down-regulating the glucose metabolism level (Liu et al., 2015). In addition, Survivin is a newly discovered member of inhibitors of apoptosis proteins (IAPs) family, and can inhibit cell apoptosis mainly through inhibition of the activity of the caspase family (Selent et al., 2013). In this study, with TSI treatment, the relative expression of NF-κB was approximately 0.53 times that of the control, the mRNA expression of p53 was significantly increased, while the expression of Survivin was significantly decreased. In summary, TSI can induce cell apoptosis mainly through the mitochondrial pathway, accompanied by the regulation of other apoptosis-related genes.
From the above experiment, we can obtain antitumor products with multiple-factors from the transformation, and these have a good effect on apoptosis induction as demonstrated in the cell experiment.
In addition, 5 g soybean isoflavones were added for the transformation, and then the ingredients of the transformed products were extracted with 50% (v/v) ethanol for 1 h. Because of the difference of polarity and extracted rates between bioactive compounds in G. lucidum and soybean isoflavones, we can see that the content and extracted rate of aglycones were the highest in the extracted supernatant. The extracted rates were not only affected by the solvent selection, but also by their own contents to a large extent. Therefore, it was speculated that the contribution of aglycones was much greater in the observed apoptosis induction.
5. Conclusions
Soybean isoflavones were transformed using the homogenized slurry of the mycelia and broth of G. lucidum. The transformed products (TSI) can significantly reduce viability of HTL-9 and MCF-7 cells in vitro, arrest HTL-9 cells in the G1 phase, and primarily induce cell late-apoptosis by means of the mitochondrial pathway, along with the regulation of other apoptosis-related genes. Our results suggested the potential use of TSI as functional food or nutraceutical ingredients for chemotherapy. Further studies should be carried out to detail the interreaction and optimal proportion between aglycones, polysaccharides, and triterpenoids for apoptosis induction, as well as the mechanism underlying TSI-induced apoptosis.
Footnotes
Compliance with ethics guidelines: Mei-lin CUI, Huan-yi YANG, and Guo-qing HE declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Al-Fatlawi AA, Abbas A, Zafaryab M, et al. Rhein induced cell death and apoptosis through caspase dependent and associated with modulation of p53, Bcl-2/Bax ratio in human cell lines. Int J Pharm Pharmaceut Sci. 2014;6(2):515–519. [Google Scholar]
- 2.Andlauer W, Kolb J, Stehle P, et al. Absorption and metabolism of genistein in isolated rat small intestine. J Nutr. 2000;130(4):843–846. doi: 10.1093/jn/130.4.843. [DOI] [PubMed] [Google Scholar]
- 3.Baglia ML, Gu K, Zhang X, et al. Soy isoflavone intake and bone mineral density in breast cancer survivors. Cancer Causes Control. 2015;26(4):571–580. doi: 10.1007/s10552-015-0534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee S, Ali S, Azmi A, et al. Abstract 2698: improved therapeutic activity of isoflavone-G2535 and docetaxel combination in hormone refractory prostate cancer. Cancer Res. 2012;72(8 Suppl.):2698. doi: 10.1158/1538-7445.AM2012-2698. [DOI] [Google Scholar]
- 5.Budhathoki S, Joshi AM, Ohnaka K, et al. Soy food and isoflavone intake and colorectal cancer risk: the fukuoka colorectal cancer study. Scand J Gastroenterol. 2011;46(2):165–172. doi: 10.3109/00365521.2010.522720. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Hou R, Zhang X, et al. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS ONE. 2014;9(3):e91245. doi: 10.1371/journal.pone.0091245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi EJ, Kim GH. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Mol Med Rep. 2013;7(3):781–784. doi: 10.3892/mmr.2013.1283. [DOI] [PubMed] [Google Scholar]
- 8.Cotrim CZ, Fabris V, Doria ML, et al. Estrogen receptor β growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32(19):2390–2402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 9.Cui ML, Yang HY, He GQ. Submerged fermentation production and characterization of intracellular triterpenoids from Ganoderma lucidum using HPLC-ESI-MS. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2015;16(12):998–1010. doi: 10.1631/jzus.B1500147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewe JA, Wan-Abdullah WN, Alias AK, et al. Enhanced growth of lactobacilli and bioconversion of isoflavones in biotin-supplemented soymilk by electroporation. Int J Food Sci Nutr. 2012;63(5):580–596. doi: 10.3109/09637486.2011.641940. [DOI] [PubMed] [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Li Y, Lu Y, et al. Bioactive tanshinones in Salvia miltiorrhiza inhibit the growth of prostate cancer cells in vitro and in mice. Int J Cancer. 2011;129(5):1042–1052. doi: 10.1002/ijc.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo XY, Liu D, Ye M, et al. Structural characterization of minor metabolites and pharmacokinetics of ganoderic acid C2 in rat plasma by HPLC coupled with electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2013;75:64–73. doi: 10.1016/j.jpba.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Handa CL, Couto UR, Vicensoti AH, et al. Optimisation of soy flour fermentation parameters to produce beta-glucosidase for bioconversion into aglycones. Food Chem. 2014;152:56–65. doi: 10.1016/j.foodchem.2013.11.101. [DOI] [PubMed] [Google Scholar]
- 15.Hati S, Vij S, Singh BP, et al. β-Glucosidase activity and bioconversion of isoflavones during fermentation of soymilk. J Sci Food Agric. 2015;95(1):216–220. doi: 10.1002/jsfa.6743. [DOI] [PubMed] [Google Scholar]
- 16.Hsin IL, Ou CC, Wu MF, et al. GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells. Mol Pharm. 2015;12(5):1534–1543. doi: 10.1021/mp500840z. [DOI] [PubMed] [Google Scholar]
- 17.Indran IR, Tufo G, Pervaiz S, et al. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. BBA-Bioenergetics. 2011;1807(6):735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Kang D, Mutakin M, Levita J. Computational study of triterpenoids of Ganoderma lucidum with aspartic protease enzymes for discovering HIV-1 and plasmepsin inhibitors. Int J Chem. 2015;7(1):62–68. doi: 10.5539/ijc.v7n1p62. [DOI] [Google Scholar]
- 19.Keypour S, Rafati H, Riahi H, et al. Qualitative analysis of ganoderic acids in Ganoderma lucidum from Iran and China by RP-HPLC and electrospray ionization-mass spectrometry (ESI-MS) Food Chem. 2010;119(4):1704–1708. doi: 10.1016/j.foodchem.2009.09.058. [DOI] [Google Scholar]
- 20.Kim HM, Paik SY, Ra KS, et al. Enhanced production of exopolysaccharides by fed-batch culture of Ganoderma resinaceum DG-6556. J Microbiol. 2006;44(2):233–242. [PubMed] [Google Scholar]
- 21.Kurahashi N, Iwasaki M, Sasazuki S, et al. Soy product and isoflavone consumption in relation to prostate cancer in Japanese men. Cancer Epidemiol Biomarkers Prev. 2007;16(3):538–545. doi: 10.1158/1055-9965.EPI-06-0517. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Rhee HM. Cardiovascular effects of mycelium extract of Ganoderma lucidum: inhibition of sympathetic outflow as a mechanism of its hypotensive action. Chem Pharm Bull. 1990;38(5):1359–1364. doi: 10.1248/cpb.38.1359. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Kong D, Ahmad A, et al. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics. 2012;7(8):940–949. doi: 10.4161/epi.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Kong D, Ahmad A, et al. Targeting bone remodeling by isoflavone and 3,3’-diindolylmethane in the context of prostate cancer bone metastasis. PLoS ONE. 2012;7(3):e33011. doi: 10.1371/journal.pone.0033011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim JCW, Chan TK, Ng DS, et al. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol. 2012;39(3):300–310. doi: 10.1111/j.1440-1681.2011.05633.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Bardhan K, Yang D, et al. NF-κB directly regulates Fas transcription to modulate Fas-mediated apoptosis and tumor suppression. J Biol Chem. 2012;287(30):25530–25540. doi: 10.1074/jbc.M112.356279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Zhang C, Hu W, et al. Tumor suppressor p53 and its mutants in cancer metabolism. Cancer Lett. 2015;356(2):197–203. doi: 10.1016/j.canlet.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loganathan J, Jiang J, Smith A, et al. The mushroom Ganoderma lucidum suppresses breast-to-lung cancer metastasis through the inhibition of pro-invasive genes. Int J Oncol. 2014;44(6):2009–2015. doi: 10.3892/ijo.2014.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitan-Alfenas GP, Lorena GA, de Almeida MN, et al. Hydrolysis of soybean isoflavones by Debaryomyces hansenii UFV-1 immobilised cells and free β-glucosidase. Food Chem. 2014;146:429–436. doi: 10.1016/j.foodchem.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 30.Mayola E, Gallerne C, Esposti DD, et al. Withaferin a induces apoptosis in human melanoma cells through generation of reactive oxygen species and down-regulation of Bcl-2. Apoptosis. 2011;16(10):1014–1027. doi: 10.1007/s10495-011-0625-x. [DOI] [PubMed] [Google Scholar]
- 31.Mense SM, Hei TK, Ganju RK, et al. Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect. 2008;116(4):426–433. doi: 10.1289/ehp.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda CF, Morales-Cruz M, Suarez B, et al. Effect of cytochrome c modification with co-polymer on its apoptotic activity for cancer treatment (LB248) FASEB J. 2014;28(1 Suppl.):248. [Google Scholar]
- 33.Munck L, Jorgensen KG, Ruud-Hansen J, et al. The EBC methods for determination of high molecular weight β-glucan in barley, malt, wort and beer. J Inst Brewing. 1989;95(2):79–82. doi: 10.1002/j.2050-0416.1989.tb04612.x. [DOI] [Google Scholar]
- 34.Ollberding NJ, Lim U, Wilkens LR, et al. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst. 2012;104(1):67–76. doi: 10.1093/jnci/djr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prietsch RF, Monte LG, da Silva FA, et al. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol Cell Biochem. 2014;390(1-2):235–242. doi: 10.1007/s11010-014-1974-x. [DOI] [PubMed] [Google Scholar]
- 36.Priyadarsini RV, Murugan RS, Maitreyi S, et al. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649(1-3):84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Selent J, Kaczor AA, Guixa-Gonzalez R, et al. Rational design of the survivin/CDK4 complex by combining protein-protein docking and molecular dynamics simulations. J Mol Model. 2013;19(4):1507–1514. doi: 10.1007/s00894-012-1705-8. [DOI] [PubMed] [Google Scholar]
- 38.Srivastava K, Singh AK, Khan K, et al. Assessment of enhancement of peak bone gain by isoflavone enriched standardized soy extract in female rats. J Funct Foods. 2014;7:314–321. doi: 10.1016/j.jff.2014.01.029. [DOI] [Google Scholar]
- 39.Suarez-Jimenez GM, Burgos-Hernandez A, Ezquerra-Brauer JM. Bioactive peptides and depsipeptides with anticancer potential: sources from marine animals. Marine Drugs. 2012;10(12):963–986. doi: 10.3390/md10050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szliszka E, Czuba ZP, Sędek L, et al. Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia . Pharm Rep. 2011;63(1):139–148. doi: 10.1016/S1734-1140(11)70408-X. [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Liu JW, Zhao WM, et al. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80(3):205–211. doi: 10.1016/j.lfs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Thomas S, Quinn BA, Das SK, et al. Targeting the Bcl-2 family for cancer therapy. Exp Opin Therapeut Tar. 2013;17(1):61–75. doi: 10.1517/14728222.2013.733001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Titiek F, Umar S, Cahyanto M, et al. Effect of indigenous lactic acid bacteria fermentation on enrichment of isoflavone and antioxidant properties of kerandang (Canavalia virosa) extract. Int Food Res J. 2013;20(5):2945–2950. [Google Scholar]
- 44.Tse G, Eslick GD. Soy and isoflavone consumption and risk of gastrointestinal cancer: a systematic review and meta-analysis. Eur J Nutr. 2016;55(1):63–73. doi: 10.1007/s00394-014-0824-7. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboy MS, Marcarini JC, de Souza AO, et al. Genistein at maximal physiologic serum levels induces G0/G1 arrest in MCF-7 and HB4a cells, but not apoptosis. J Med Food. 2014;17(2):218–225. doi: 10.1089/jmf.2013.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. In: Cree I, editor. Cancer Cell Culture. Methods in Molecular Biology (Methods and Protocols), Vol. 731. Humana Press; 2011. pp. 237–245. [DOI] [PubMed] [Google Scholar]
- 47.Varfolomeev E, Goncharov T, Vucic D. Roles of c-IAP proteins in TNF receptor family activation of NF-κB signaling. In: May M, editor. NF-κ B. Methods in Molecular Biology, Vol. 1280. Humana Press, New York; 2015. pp. 269–282. [DOI] [PubMed] [Google Scholar]
- 48.Wei J, Bhatt S, Chang LM, et al. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS ONE. 2012;7(10):e47979. doi: 10.1371/journal.pone.0047979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu CF, Wu AR, Zhu H, et al. Melatonin is involved in the apoptosis and necrosis of pancreatic cancer cell line SW-1990 via modulating of Bcl-2/Bax balance. Biomed Pharmacother. 2013;67(2):133–139. doi: 10.1016/j.biopha.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 50.Yan L, Spitznagel EL, Bosland MC. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidem Biomar Prev. 2010;19(1):148–158. doi: 10.1158/1055-9965.EPI-09-0856. [DOI] [PubMed] [Google Scholar]
- 51.Yeo SK, Liong MT. Angiotensin I-converting enzyme inhibitory activity and bioconversion of isoflavones by probiotics in soymilk supplemented with prebiotics. Int J Food Sci Nutr. 2010;61(2):161–181. doi: 10.3109/09637480903348122. [DOI] [PubMed] [Google Scholar]
- 52.Yeom SJ, Kim BN, Kim YS, et al. Hydrolysis of isoflavone glycosides by a thermostable beta-glucosidase from Pyrococcus furiosus . J Agric Food Chem. 2012;260(6):1535–1541. doi: 10.1021/jf204432g. [DOI] [PubMed] [Google Scholar]
- 53.Yin LJ, Tai HM, Lee HH, et al. Proteolysis and lactobacillus fermentation effects on the isoflavones transformation and removal of anti-nutritional factors of soy bean. J Mar Sci Technol. 2014;22(4):525–530. [Google Scholar]
- 54.Zhao W, Xu JW, Zhong JJ. Enhanced production of ganoderic acids in static liquid culture of Ganoderma lucidum under nitrogen-limiting conditions. Bioresour Technol. 2011;102(17):8185–8190. doi: 10.1016/j.biortech.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 55.Zhou C, Lin H, Ge X, et al. The effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus . Fish Shellfish Immunol. 2015;43(1):158–166. doi: 10.1016/j.fsi.2014.12.014. [DOI] [PubMed] [Google Scholar]