Abstract
Influenza pneumonia remains a common and debilitating viral infection despite vaccination programs and antiviral agents developed for prophylaxis and treatment. The neuraminidase inhibitor oseltamivir is frequently prescribed for established influenza A virus infections, but the emergence of neuraminidase inhibitor resistant viruses, a brief therapeutic window and competing diagnoses complicate its use. PUL-042 is a clinical stage, aerosol drug comprised of synthetic ligands for Toll-like receptor (TLR) 2/6 and TLR 9. This host-targeted, innate immune stimulant broadly protects against bacterial, fungal and viral pneumonias, including those caused by influenza, when given prophylactically to animals. This study evaluated the therapeutic antiviral effects of PUL-042 against established influenza A pneumonia, when given alone or in combination with oseltamivir. Mice were treated with PUL-042 aerosol, oseltamivir or both at varying time points before or after challenge with influenza pneumonia. Treating established, otherwise lethal influenza A pneumonia (>1 LD100) with multiple inhaled doses of PUL-042 aerosol plus oral oseltamivir resulted in greater mouse survival than treatment with either drug alone. Single agent PUL-042 also protected mice against established infections following challenges with lower viral inocula (approximately 1 LD20). Aerosolized oseltamivir further enhanced survival when co-delivered with PUL-042 aerosol. The prophylactic and therapeutic benefits of PUL-042 were similar against multiple strains of influenza virus. In vitro influenza challenge of human HBEC3kt lung epithelial cells revealed PUL-042-induced protection against infection that was comparable to that observed in vivo. These studies offer new insights into means to protect susceptible populations against influenza A pneumonia.
Keywords: Innate immunity, Toll-like receptor, Influenza, Viral pneumonia, Oseltamivir
1. INTRODUCTION
Influenza viruses remain common causes of serious infection worldwide, despite large scale vaccination programs. In the United States, 20,000–40,000 cases of seasonal influenza occur annually, with attributable mortality as high as 7.9% (Russell 2016). This translates to estimated hospitalization costs of $10.4 billion and lost earnings of $16.3 billion dollars per year (Molinari 2007). Influenza causes disproportionate morbidity in certain populations, with individuals at the extremes of age (<2 years, >50 years) and those with comorbid or immunocompromising conditions the most susceptible to influenza pneumonia (Louie 2009, Jain 2009, Poehling 2006).
The available treatments for influenza infections are adamantane derivatives (rimantadine and amantadine) or neuraminidase inhibitors (oseltamivir, zanamivir, and peramivir) (Fiore 2011). Neuraminidase inhibitors are first line agents, due to their efficacy against influenza A and B viruses and the high prevalence of adamantane resistant influenza. Unfortunately, viruses resistant to neuraminidase inhibitors such as oseltamivir have been increasingly reported from both seasonal and pandemic H1N1 influenza isolates (Baz, 2009, Gubareva 2001, Stephenson 2009). Current guidelines recommend treating with neuraminidase inhibitors within 48 h of symptom development in the general population. However, resistance has been shown to emerge as early as 48 h after initiation of treatment (Inoue 2010), and transmission of resistant strains has been documented (Hatakeyama 2007, Le 2010).
We have previously reported that lung epithelial cells can be stimulated to protect mice against bacterial, fungal and viral pneumonia following treatment with PUL-042, a clinical stage, inhaled drug comprised of synthetic ligands for Toll-like receptor (TLR)2/6 (Pam2CSK4) and TLR9 (ODN M362), formulated at a 4:1 molar ratio of Pam2CSK4 to ODN M362. PUL-042 treatment of isolated lung epithelial cells in vitro or PUL-042 treatment of mice via nebulization results in robust enhancement of survival and reduction in pathogen burden following challenges with bacteria, fungi or viruses, including influenza A (Cleaver 2014, Duggan 2011, Leiva-Juarez 2016, Tuvim 2012). This epithelium-dependent effect persists despite leukocyte lineage depletion (Alfaro 2014, Cleaver 2014).
Neuraminidase inhibitors such as oseltamivir are approved for use as therapy for established influenza infections, as they act directly on the virus (Fiore 2011). Oseltamivir is also recommended for prophylaxis of influenza without evidence of prior infection. PUL-042 has principally been tested in prophylactic models, with its protective effect resulting from generation of an antimicrobial environment by the host (Cleaver 2014, Duggan 2011, Leiva-Juarez 2016, Tuvim 2012). The prophylactic benefit of PUL-042 persists for at least eight days after a single inhaled treatment (Alfaro 2014), and PUL-042 also confers a survival advantage when delivered to mice up to three days after influenza challenge (Duggan 2011).
Given the differing mechanisms of protection afforded by oseltamivir and PUL-042, we hypothesized that the two treatments might complement each other, enhancing antiviral benefits over that conferred by either treatment alone. Similarly, given the non-overlapping kinetics of the protection induced by the treatments, we theorized that combination treatment with oseltamivir and PUL-042 might extend the window of opportunity for successful intervention beyond that for either treatment alone.
2. Materials and methods
2.1 In vitro treatment and infection
Immortalized human bronchial epithelial (HBEC3kt) cells were kindly provided by John Minna at the University of Texas Southwestern Medical Center. Cells were cultured in supplemented keratinocyte serum-free media (KSFM) (Thermo Fisher Scientific, Waltham, MA) until 100% confluence was reached in 24-well plates. Cells were treated with 9.3μM of Pam2CSK4 and 2.2μM ODN362 (InvivoGen, San Diego, CA), 2.25 μM oseltamivir carboxylate (Toronto Research Chemicals, Toronto, ON), or both in KSFM for 24 h, then infected with influenza A/HK/8/68 (H3N2) at an MOI of 0.1 in pre-conditioned media. 24 h after infection, cells were lysed and RNA was extracted using Qiagen RNeasy kit (Qiagen, Valencia, CA). 500 ng of total RNA was reversed transcribed to cDNA using iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). Viral and reference transcripts were quantified by qPCR using SYBR green PCR master mix (Applied Biosystems, Life Technologies) and measured on a ABI ViiA 7 Real Time PCR system. Viral gene expression was normalized to 18s transcript levels. Primer sequences used for qPCR were: 18S (5’-GTAACCCGTTGAACCCCATT-3’) (5’-CCATCCAATCGGTAGTAGCG-3’) and influenza nucleoprotein NP (5’-CTCATCCTTTATGACAAAGAAG-3’) (5’-AGATCATCATGTGAGTCAGAC-3’).
2.2 Influenza virus source and preparation
Clinical isolates of influenza A [Hong Kong/8/68 (H3N2), California/04/2009 (H1N1), Puerto Rico/8/34 (H1N1)] and B (Lee/40) were obtained and prepared for nebulization as shown in Supplemental Table 1.
2.3 Animals
Six to eight week old NIH Swiss mice of approximately 20 g (Charles River, Wilmington, MA) were used for all experiments. 15 mice were used for each treatment condition. Due to the large number of animals required per experiment, female mice were used in these studies to allow maximally efficient housing. However, pilot studies and prior publications demonstrate no differences in protection for male mice by PUL-042. All mice were handled in accordance with the policies of the Baylor College of Medicine Institutional Animal Care and Use Committee, full details of the study were approved by that body (approval AN-2307), and any mice that exhibited signs of distress were humanely euthanized.
2.4 Synthetic TLR ligand and oseltamivir treatment preparation and administration in vivo
Pam2CSK4 acetate (Peptides International, Louisville, KY) and ODN362 sodium (phosphorothioate backbone) (TriLink Biotechnologies, San Diego, CA) dry powder drug substances were stored at −20ºC and thawed prior to each experiment. Taking into account purity (Pam2CSK4 and ODNM362) and peptide content (Pam2CSK4), the drug substances were diluted to a concentration of 8 μM of Pam2CSK4 and 2 μM of ODNM362, giving the required 4:1 molar ratio in a total volume of 6 ml sterile water. PUL-042 was always administered as an aerosol. For LD50 experiments, the GMP produced clinical trial material formulation of PUL-042 was used at 8 μM Pam2CSK4 and 2μM ODN M362. Oseltamivir phosphate was obtained from contents of Tamiflu® tablets (mean 167 mg powder/capsule; 45% oseltamivir free base), suspended in 1 ml of sterile water to a 75 mg/ml concentration, vortexed, and sonicated in a water bath at room temperature for 1–5 min. For oral gavage, oseltamivir was diluted in sterile water to a total dose of 4 mg/kg/day administered in a total volume of 100 μl. For nebulized treatments, oseltamivir carboxylate was suspended in sterile water to an 18.8 mg/ml concentration. For experiments in Fig. 3B–C, powder from 9 capsules of oseltamivir carboxylate (167 mg/capsule of 45% oseltamivir carboxylate equivalent) were suspended in 9 ml of sterile water or PUL-042 to a total concentration of 75 mg/ml (5.1 mg/kg), 18.8 mg/ml (1.3 mg/kg), and 4.7 mg/ml (0.32 mg/kg).
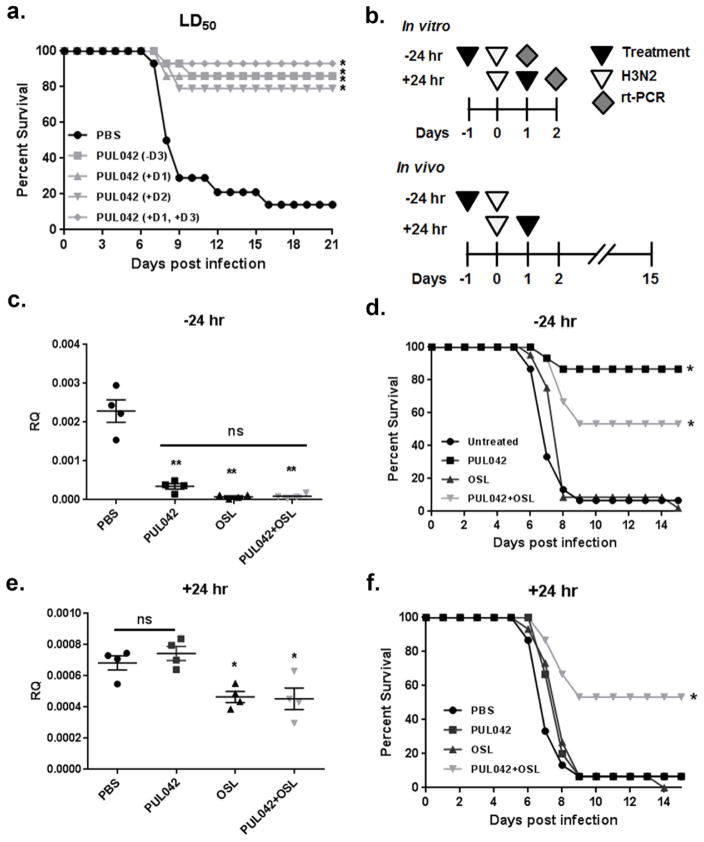
Fig. 3. Aerosolized PUL-042 and oseltamivir are effective treatments against established influenza A virus infection.
Survival in mice treated with PUL-042 or oseltamivir after challenge with influenza A/HK virus, (A) Aerosol treatment with PUL-042 and/or aerosolized oseltamivir (B) single dose aerosol co-delivery of PUL-042 with oseltamivir or aerosolized oseltamivir alone at 48 h post-infection and (C) single dose aerosol co-delivery of PUL-042 with oseltamivir or aerosolized oseltamivir alone at 96 h post-infection. OSL: oseltamivir, PBS: phosphate buffered saline (control). Figs. 3A and B are separated displays of the same experiment using the same control (PBS) group. *P<0.05 by a Kaplan-Meier log-rank test with a post-hoc Mantel-Cox compared to the PBS control.
Mice received nebulized drugs by whole-body exposure. Mice were placed in a plastic lidded container modified with inlet and exit ports for flow-through of the aerosol. A filter to collect drug was mounted on the exit port and the apparatus was placed in a chemical safety hood during the nebulization period. 6 ml of each treatment were placed in a nebulizer reservoir and aerosolized for 15 min in an Aerotech II nebulizer driven by 10 L/min of room air generated by an Aridyne 2000.
2.5 Influenza A virus challenge in vivo
Influenza frozen stocks from pooled mouse lungs were thawed and diluted 1:500 in 0.05% gelatin-MEM and suspended in a total of 10ml of MEM. The virus suspension was transported in ice and mice were infected by whole-body exposure (described above) to aerosolized virus for 20 min using an Aerotech II nebulizer flowing at 10 L/min of room air generated from by an Aridyne 2000 compressor. Titers of the influenza in the nebulization reservoirs pre- and post-nebulization were determined by hemagglutination assay of infected MDCK cells (LD100 ~ 105 TCID50/ml, LD50 ~ 44 TCID50/mouse). If mice showed signs of distress, they were humanely euthanized.
2.6 Statistical analysis
Continuous variables were compared using a one-way analysis of variance test with a post-hoc Tukey’s test. Survival was plotted as percent survival from the starting cohort and compared using Kaplan-Meier log-rank test with a post-hoc Mantel-Cox test. Post-hoc direct comparisons of survival rates between treatment groups were evaluated by Chi-square. A P<0.05 was considered statistically significant. All statistical analysis were performed using GraphPad Prism version 6.0.
3. RESULTS
3.1 Pre-emptive and prophylactic therapy with PUL-042 protects against H3N2 influenza in vitro and against pneumonia in vivo, with or without oseltamivir
We have previously shown that nebulization of PUL-042 protects mice against influenza infection, particularly when delivered prior to challenge (Cleaver 2014, Duggan 2011, Tuvim 2012). Fig. 1A demonstrates that PUL-042 also robustly protects mice when delivered after infection with influenza A virus. Even a single treatment of PUL-042 given in the first two days after infection was sufficient to protect mice against an influenza/A/HK/8/68 virus challenge of approximately 1 LD50.
Fig. 1. Treatment or prophylaxis with PUL-042 aerosol protects against H3N2 infection in vivo and in vitro combinations with oseltamivir are effective.
(A) In vivo infection with standard H3N2 influenza virus challenge. (B) Schematic of treatment protocols used for panels C–F, (C and E) HBEC3kt cells were pretreated with either PBS, PUL-042, oseltamivir, or PUL-042 with oseltamivir 24 h prior to infection and NP gene expression was measured at 24 h post infection by qRT-PCR, in vivo H3N2 infection in NIH Swiss mice with prophylactic (D) or therapeutic PUL-042 aerosol (F) or oseltamivir treatment (at −24 and +24 h, respectively) OSL: oseltamivir, ns: non-significant. Figs. 1D and 1F are separated displays of the same experiment using the same control (PBS) group. (n=14 mice/group). In c and e, *P<0.05, **P<0.001 by a one-way ANOVA with a post-hoc Tukey’s test. In A, D, and F *P<0.05 by a Kaplan-Meier log-rank test with a post-hoc Mantel-Cox compared to the PBS control.
To further establish the efficacy of PUL-042 in protecting against viral pneumonia and assess the potential benefit of combination therapies, we evaluated the in vitro and in vivo effects of treating with PUL-042 and/or oral oseltamivir before and after influenza challenge (Fig. 1B). In immortalized human bronchial epithelial cells (HBEC3kt), pretreatment with PUL-042 24 h before infection led to a statistically significant (P<0.001) decrease in viral nucleoprotein (NP) gene expression at 24 h post infection (Fig. 1C). Oseltamivir administered 24 h before infection similarly reduced the viral burden 24 h after infection. The combination of PUL-042 aerosol and oral oseltamivir administered prophylactically did not result in an identified further decrease in viral gene expression over either individual treatment, though it is notable that the two treatments were so efficacious individually that further reductions may be below the limit of detection. Conversely, when the in vitro treatments were applied 24 h after infection, only oseltamivir-containing treatments (alone or in combination with PUL-042) resulted in a detectable reduction in viral burden 48 h after infection (p=0.002) (Fig. 1E) No statistically significant difference from oseltamivir alone was obtained by the addition of PUL-042.
Having established the strong in vivo protection afforded by PUL-042 alone in a standard challenge, studies were performed with a virus challenge that produced 100% lethality (>1 LD100). Prophylactic nebulized PUL-042 delivered 24 h before infection significantly increased survival over sham treated mice in this influenza challenge model (Fig. 1D). Prophylactic delivery of oral oseltamivir resulted in no appreciable impact on survival. Unexpectedly, mice that received both nebulized PUL-042 and oral oseltamivir were not as strongly protected as mice that received PUL-042 alone, and this result was confirmed in a second experiment (presented below). However, when delivered to mice 24 h after infection, the combination of PUL-042 aerosol and oral oseltamivir resulted in strikingly greater protection than either individual treatment alone against this strong challenge dose (Fig. 1F).
3.2 Multiple combined treatments with aerosolized PUL-042 and oral oseltamivir protects mice against established influenza A virus infection
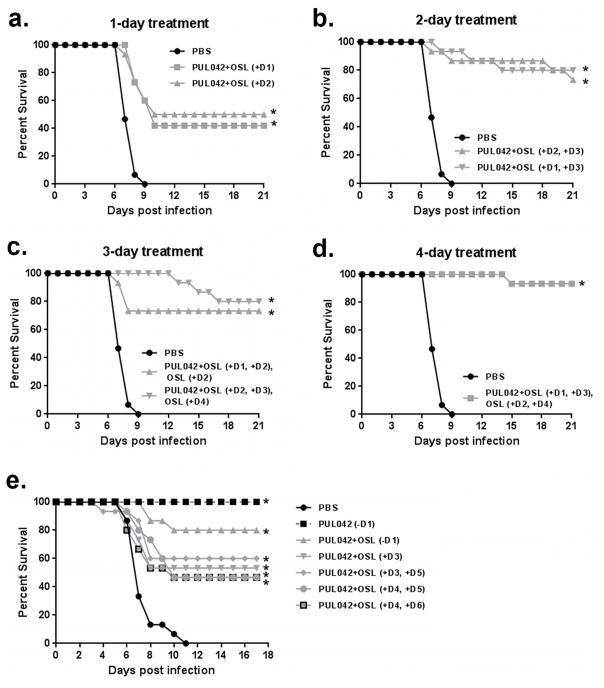
To identify optimized strategies for protecting against established influenza infections, a variety of aerosol PUL-042 -oral oseltamivir combination treatment regimens were tested. A single treatment given on day 1 or 2 after infection conferred moderately increased survival compared to controls (Fig. 2A). The survival advantage was greater following combined treatments given on two days (D1 and D3 or D2 and D3, Fig. 2B). Adding one extra dose of oseltamivir to the regimens shown in Fig. 2B did not appreciably influence survival, whether given between or after the combination treatments (Fig. 2C). The additional survival benefit of adding two extra doses of oseltamivir alone to the two combination treatments was not statistically significant (n = 15 per group, Chi-square p = 0.282745) (Fig. 2D). These data show that PUL-042 aerosol with oral oseltamivir protects mice against active, otherwise 100% lethal, influenza A virus challenge, with outcomes dependent on the number of treatments and timing of administration. Interestingly, we found in subsequent experiments exploring optimized treatment regimens that the addition of prophylactic (D -1) oseltamivir actually reduced the protective effect of prophylactic (D -1) PUL-042 (Fig. 2E). This same series of investigations also revealed that the 2-day combination treatments could be deferred as late as 4 days after infection, the day of maximal viral proliferation in this model, and still improve survival rate to 46% from 0%. (Fig. 2E)
Fig. 2. Multiple combined treatments with PUL-042 aerosol and oral oseltamivir protect mice against established influenza A virus infection.
Treatment schematic and survival post infection in mice infected with influenza A/HK/8/68 virus and treated with PUL-042 aerosol and oral oseltamivir combined for (A) one day, (B) two days (Groups 1 and 2), (C) PUL-042 and oral oseltamivir combined for two days and one additional day of oral oseltamivir alone (Groups 3 and 4), or (D) four days, consisting of two doses of aerosol PUL-042 and oral oseltamivir, with an additional two days of oral oseltamivir, (E) survival post infection in mice infected with A/HK/8/68 virus and treated with PUL-042 aerosol or a combination of PUL-042 aerosol with oral oseltamivir; prophylaxis with PUL-042 alone or PUL-042 aerosol with oral oseltamivir were included as comparators. OSL: oseltamivir gavage. Figs. 2A–2D are separated displays of the same experiment using the same control (PBS) group. *P<0.05 by a Kaplan-Meier log-rank test with a post-hoc Mantel-Cox compared to the PBS control.
3.3 PUL-042 and oseltamivir aerosol is an effective treatment for established influenza A virus infection
Oseltamivir systemic treatment may be associated with significant side effects such as nausea, abdominal pain, headache, and dizziness (Strong 2010). This can become particularly problematic in patients with other comorbidities, potentially leading to incomplete treatment courses and development of resistant strains. PUL-042 is being developed as an aerosolized drug, to directly stimulate antiviral responses from lung cells. If the oseltamivir and PUL-042 combination were to be used clinically for severe influenza for the hospitalized patient, it would be ideal to co-administer them via the same aerosol route. We investigated the efficacy of the two drugs combined in a single aerosol, versus established influenza A virus infections, beginning at 48 h post-infection. Survival rates were compared between PUL-042 aerosol alone, oseltamivir aerosol alone, PUL-042 and oseltamivir combination aerosol, and PUL-042 aerosol combined with oral oseltamivir. We found that oseltamivir alone conferred significantly improved survival when given in multiple doses by aerosol (Fig. 3A). A single dose of the combined aerosol was equally beneficial. (Fig. 3A). We followed this experiment with an evaluation of dose response to oseltamivir in the combined aerosol compared to aerosol oseltamivir alone, with single aerosol treatments administered at either 48 or 96 h post-infection. Fig. 3B shows that a single treatment with aerosolized oseltamivir at the highest dose was effectively equivalent to the combined aerosol treatment with PUL-042. A statistically significant benefit of combining PUL-042 with oseltamivir as an aerosol was seen when lower doses of oseltamivir were used. In that same experiment (Fig. 3C), when treatment was delayed to 96 h post-infection the high dose of oseltamivir in the aerosol was no longer clearly the most efficacious; when using 1.3 mg/kg oseltamivir aerosol the combination with PUL-042 showed statistically significant improvement over oseltamivir aerosol alone (Chi-square, p = 0.03).
3.4 Prophylaxis with PUL-042 or treatment with PUL-042 and oral oseltamivir are effective against different strains of influenza virus
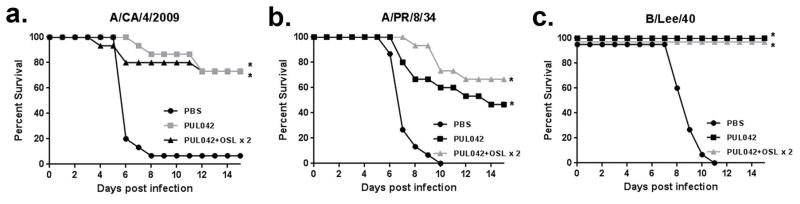
The pathogenicity of influenza viruses is highly correlated with rearrangements in their genome (Iwasaki 2014.) To determine the generalizability of the foregoing experiments in different viral challenge models, the efficacy of prophylactic PUL-042 and therapeutic PUL-042-oseltamivir combination were tested in multiple influenza A and B virus strains. As shown in Fig. 4, pretreatment with aerosolized PUL-042 similarly protected against both influenza A and B strains. Similarly, combination with two post-exposure doses of aerosolized PUL-042 and oral oseltamivir combination therapy significantly enhanced survival of influenza challenge in all three models. This suggests that PUL-042 is protective against different strains of influenza viruses and is effective both as a prophylactic agent and treatment in established infection if used with oseltamivir.
Fig. 4. Prophylaxis with PUL-042 or treatment with PUL-042 and oral oseltamivir are effective against different strains of influenza virus.
Survival in mice treated with either prophylactic PUL-042 24 h prior to infection or the combination during 2 days of established infection and infected with influenza A/CA/4/2009 (H1N1) (A), A/PR/8/34 (H1N1) (B) or B/Lee/40 (C) virus strains. *P<0.05 by a Kaplan-Meier log-rank test with a post-hoc Mantel-Cox compared to the PBS control.
4. DISCUSSION
Viral pneumonia is a common infection and a severe public health burden. Although vaccination programs have successfully decreased the incidence of influenza pneumonia, emergent endemic and pandemic strains are frequently lethal in immunocompromised individuals. In addition, resistance to current antivirals mandates the need for alternative therapies. We have previously shown that lung innate immune stimulation with a combination of inhaled ligands for TLR 2/6 and TLR9 induces an antimicrobial response that is protective against influenza pneumonia (Tuvim 2012). The protection is associated with enrichment of diverse antimicrobial peptides and reactive oxygen species (Cleaver 2014, Tuvim 2012). Here we evaluated human bronchial epithelial cell stimulation with PUL-042 relative to the effects of oseltamivir on prophylaxis and treatment of established influenza infections.
Prophylactic dosing with PUL-042 was as effective as prophylaxis with oseltamivir or the combination in cultured human epithelial cells. This pattern was not seen in vivo, where mice were not protected with prophylactic oseltamivir. Our in vivo model system was not designed to evaluate prophylaxis by oseltamivir alone, which has been demonstrated by others (Ilyushina 2008, Smee 2012). Lack of oseltamivir prophylaxis in our studies may be due to the high viral inoculum used in our model, which is titrated to 100% death in the control groups, compared to the 50% lethal dose used in many other murine influenza models (Galabov 2015, Marathe 2016), and may also reflect partial resistance to oseltamivir. It is notable that PUL-042 does not appreciably reduce viral burden in vitro when given 24 h after influenza challenge, yet it provides significant benefit when given to mice more than 24 h after in vivo challenge. The explanation for this differential effect is likely multifold, but most notably relates to progression patterns of the infection. Whereas, by 24 h after influenza challenge in vitro, the entire well effectively infected, by 24 h after influenza challenge in vivo the infection is still spreading in a patchy, step-wise manner. Thus, there are more opportunities for PUL-042 to act on uninfected or recently infected cells in vivo, even at later time points. We have previously reported that statistically significant reductions in viral titer are achieved by PUL-042 in vitro at earlier post-challenge time points (4 h after challenge), so there may be some reduction at 24 h after challenge, as well, though it may be below the level of detection. Further, while the in vitro studies exclusively investigate the therapeutic manipulation of direct antiviral effects from lung epithelial cells, the aggregate in vivo effect of PUL-042 also includes the influences of epithelial responses on leukocytes and, possibly, direct stimulation of leukocyte responses. Moreover, while we observe pathogen reductions (here, of virus burden) to be associated with protection in every tested model, it is possible that some of the protection is due to reduced infection induced immunopathology. Additionally, in viral models such as those tested here, we cannot exclude the possibility that some of the survival benefit conferred by PUL-042 may relate to protection against secondary bacterial infections, as PUL-042 protects against a quite broad array of pathogens.
In mouse studies using a 100% lethal dose of virus, the benefit of a single dose of aerosolized PUL-042 was greater when used as a prophylactic (24 h prior to influenza virus challenge) than as a therapeutic agent (24 h after infection). The modest therapeutic benefit was enhanced with repetitive dosing, and likely reflects the high viral titer used in our challenge model, given that PUL-042 aerosol was efficacious when tested against the standard LD50 often used in the literature. A single extra dose of aerosolized PUL-042 and oseltamivir dramatically increased the survival of mice infected with influenza (Fig. 2), and it was enhanced with further doses of oseltamivir or the combination.
Protection with prophylactic PUL-042 or therapeutic PUL-042-oseltamivir combination was demonstrated against both influenza A and B viruses, allowing for broader potential generalization of this strategy. Currently, there are not established clinical guidelines for pre-exposure prophylaxis against influenza, but these studies suggest that this may be a feasible strategy to protect at-risk populations. Studies by other investigators have shown protection of prophylactic or pre-emptive neuraminidase inhibitors in the setting of institutionalized patients (Lee 2000, Peters 2001, Schilling 1998), community-dwelling high-risk patients (LaForce 2007), or stem cell transplant units (Yue 2016).
We could reduce both the drug concentration and number of dosages of oseltamivir below standard efficacious doses in the mouse model and still produce a beneficial effect in conjunction with PUL- 042. Interestingly, when attempting to optimize delivery strategies, nebulization of both compounds together proved to be a highly efficacious approach. The combined aerosol was beneficial even as a single dose administered as late as 96 h after infection. There is a suggestion in these results that at later timepoints, when viral proliferation is highest, the highest dose of oseltamivir aerosol could have a detrimental effect, as lower doses were more effective than higher doses at this timepoint. Our results are congruent with other reports showing that oral oseltamivir delivery is less efficacious than aerosolized oseltamivir. Notably, Leyva-Grado and colleagues compared several routes of administration of oseltamivir, and found that aerosolized oseltamivir given 24 h after infection confers protection against several strains of influenza, while oral oseltamivir protection was strain-specific and achieved at high doses only (Leyva-Grado 2017). Other reports indicate that oral oseltamivir may be efficacious at lower viral titers (Marjuki 2014) for extended treatment periods (Byrn 2015, Smee 2016,). This is notable, since oseltamivir is currently approved only for oral administration. Our system of aerosol administration uses whole body exposure and total oseltamivir dose is received both by inhalation and orally. The experiments reported by Leyva-Grado and colleagues used a nose-only exposure system. Future dose-response studies of the combined PUL-042+ oseltamivir aerosol could be performed using nose only exposure to establish the separate contributions of oral and aerosol oseltamivir. Further studies in co-delivery of PUL-042 and the approved aerosolized antiviral zanamivir may also prove this benefit.
Stimulation of lung epithelial cells with PUL-042, a clinical stage therapeutic consisting of two TLR ligands, is a potent means of inducing antiviral protection and enhancing survival against experimental viral challenges in a validated animal model. This effect is evident whether used as a single dose prophylaxis or as therapy. This is true when tested against multiple strains of influenza. Addition of oseltamivir to a multiple dose treatment with PUL-042 provides maximal post-infection benefit. Our results suggest that treatment with PUL-042 aerosol in addition to oral oseltamivir could be of significant benefit in cases of severe influenza (pneumonia) and when initiation of influenza treatment is delayed beyond the current 48 h window for oral oseltamivir.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) grant 1R43HL118926-01A1 to B.S., R01 HL117976 to S.E.E., NIH NHLBI Office of the Director grant DP2 HL123229 to S.E.E., and NIH National Cancer Institute (NCI) support grant P30 CA016672 to the MD Anderson Cancer Center. M.J.T., B.F.D., and S.E.E. are authors on US patent 8 883 174 entitled “Stimulation of Innate Resistance of the Lungs to Infection with Synthetic Ligands.” M.J.T., B.F.D., S.E.E. and B.S. own stock in Pulmotect, Inc., which holds the commercial options on these patent disclosures. B.S. and D.M. are employees of Pulmotect, Inc.
References
- Alfaro VY, Goldblatt DL, Valverde GR, Munsell MF, Quinton LJ, Walker AK, Dantzer R, Varadhachary A, Scott BL, Evans SE, Tuvim MJ, Dickey BF. Safety, tolerability, and biomarkers of the treatment of mice with aerosolized Toll-like receptor ligands. Front Pharmacol. 2014;5:8. doi: 10.3389/fphar.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baz M, Abed Y, Papenburg J, Bouhy X, Hamelin ME, Boivin G. Emergence of oseltamivir-resistant pandemic H1N1 virus during prophylaxis. N Engl J Med. 2009;361(23):2296–2297. doi: 10.1056/NEJMc0910060. [DOI] [PubMed] [Google Scholar]
- Byrn RA, Jones SM, Bennett HB, Bral C, Clark MP, Jacobs MD, Kwong AD, Ledeboer MW, Leeman JR, McNeil CF, Murcko MA, Nezami A, Perola E, Rijnbrand R, Saxena K, Tsai AW, Zhou Y, Charifson PS. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Chemother. 2015;59(3):1569–1582. doi: 10.1128/AAC.04623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver JO, You D, Michaud DR, Pruneda FA, Juarez MM, Zhang J, Weill PM, Adachi R, Gong L, Moghaddam SJ, Poynter ME, Tuvim MJ, Evans SE. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 2014;7(1):78–88. doi: 10.1038/mi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan JM, You D, Cleaver JO, Larson DT, Garza RJ, Guzmán Pruneda FA, Tuvim MJ, Zhang J, Dickey BF, Evans SE. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J Immunol. 2011;186(10):5916–5926. doi: 10.4049/jimmunol.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM (CDC) CfDCaP. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60(1):1–24. [PubMed] [Google Scholar]
- Galabov AS, Mileva M, Simeonova L, Gegova G. Combination activity of neuraminidase inhibitor oseltamivir and α-tocopherol in influenza virus A (H3N2) infection in mice. Antivir Chem Chemother. 2015;24(3–4):83–91. doi: 10.1177/2040206616656263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Kaiser L, Matrosovich MN, Soo-Hoo Y, Hayden FG. Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir. J Infect Dis. 2001;183(4):523–531. doi: 10.1086/318537. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Sugaya N, Ito M, Yamazaki M, Ichikawa M, Kimura K, Kiso M, Shimizu H, Kawakami C, Koike K, Mitamura K, Kawaoka Y. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA. 2007;297(13):1435–1442. doi: 10.1001/jama.297.13.1435. [DOI] [PubMed] [Google Scholar]
- Ilyushina NA, Hay A, Yilmaz N, Boon ACM, Webster RG, Govorkova EA. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008;52(11):3898–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Barkham T, Leo YS, Chan KP, Chow A, Wong CW, Tze Chuen Lee R, Maurer-Stroh S, Lin R, Lin C. Emergence of oseltamivir-resistant pandemic (H1N1) 2009 virus within 48 hours. Emerg Infect Dis. 2010;16(10):1633–1636. doi: 10.3201/eid1610.100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L Team PIAHNVHI. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- LaForce C, Man CY, Henderson FW, McElhaney JE, Hampel FC, Bettis R, Kudule L, Harris J, Yates P, Tisdale M, Webster A. Efficacy and safety of inhaled zanamivir in the prevention of influenza in community-dwelling, high-risk adult and adolescent subjects: a 28-day, multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2007;29(8):1579–1590. doi: 10.1016/j.clinthera.2007.08.023. discussion 1577–1578. [DOI] [PubMed] [Google Scholar]
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P Team VHNI. 200 A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med. 2010;362(1):86–87. doi: 10.1056/NEJMc0910448. [DOI] [PubMed] [Google Scholar]
- Lee C, Loeb M, Phillips A, Nesbitt J, Smith K, Fearon M, McArthur MA, Mazzulli T, Li Y, McGeer A. Zanamivir use during transmission of amantadine-resistant influenza A in a nursing home. Infect Control Hosp Epidemiol. 2000;21(11):700–704. doi: 10.1086/501727. [DOI] [PubMed] [Google Scholar]
- Leiva-Juárez MM, Ware HH, Kulkarni VV, Zweidler-McKay PA, Tuvim MJ, Evans SE. Inducible epithelial resistance protects mice against leukemia-associated pneumonia. Blood. 2016;128(7):982–992. doi: 10.1182/blood-2016-03-708511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Grado VH, Palese P. Aerosol administration increases the efficacy of oseltamivir for the treatment of mice infected with influenza viruses. Antiviral Res. 2017;142:12–15. doi: 10.1016/j.antiviral.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, Vugia D, Harriman K, Matyas B, Glaser CA, Samuel MC, Rosenberg J, Talarico J, Hatch D Group CPHNW. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- Marathe BM, Wong SS, Vogel P, Garcia-Alcalde F, Webster RG, Webby RJ, Najera I, Govorkova EA. Combinations of Oseltamivir and T-705 Extend the Treatment Window for Highly Pathogenic Influenza A(H5N1) Virus Infection in Mice. Sci Rep. 2016;6:26742. doi: 10.1038/srep26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjuki H, Mishin VP, Chesnokov AP, De La Cruz JA, Fry AM, Villanueva J, Gubareva LV. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. J Infect Dis. 2014;2210(3):435–440. doi: 10.1093/infdis/jiu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Peters PH, Gravenstein S, Norwood P, De Bock V, Van Couter A, Gibbens M, von Planta TA, Ward P. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc. 2001;49(8):1025–1031. doi: 10.1046/j.1532-5415.2001.49204.x. [DOI] [PubMed] [Google Scholar]
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, Herrera G, Erdman D, Hall CB, Seither R, Griffin MR, Network NVS. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355(1):31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- Russell K, Blanton L, Kniss K, Mustaquim D, Smith S, Cohen J, Garg S, Flannery B, Fry AM, Grohskopf LA, Bresee J, Wallis T, Sessions W, Garten R, Xu X, Elal AI, Gubareva L, Barnes J, Wentworth DE, Burns E, Katz J, Jernigan D, Brammer L. Update: Influenza Activity--United States, October 4, 2015–February 6, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(6):146–153. doi: 10.15585/mmwr.mm6506a3. [DOI] [PubMed] [Google Scholar]
- Schilling M, Povinelli L, Krause P, Gravenstein M, Ambrozaitis A, Jones HH, Drinka P, Shult P, Powers D, Gravenstein S. Efficacy of zanamivir for chemoprophylaxis of nursing home influenza outbreaks. Vaccine. 1998;16(18):1771–1774. doi: 10.1016/s0264-410x(98)00141-8. [DOI] [PubMed] [Google Scholar]
- Smee DF, Julander JG, Tarbet EB, Gross M, Nguyen J. Treatment of oseltamivir-resistant influenza A (H1N1) virus infections in mice with antiviral agents. Antiviral Res. 2012;96(1):13–20. doi: 10.1016/j.antiviral.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Barnard DL, Jones SM. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antiviral Res. 2016;136:45–50. doi: 10.1016/j.antiviral.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Democratis J, Lackenby A, McNally T, Smith J, Pareek M, Ellis J, Bermingham A, Nicholson K, Zambon M. Neuraminidase inhibitor resistance after oseltamivir treatment of acute influenza A and B in children. Clin Infect Dis. 2009;48(4):389–396. doi: 10.1086/596311. [DOI] [PubMed] [Google Scholar]
- Strong M, Burrows J, Stedman E, Redgrave P. Adverse drug effects following oseltamivir mass treatment and prophylaxis in a school outbreak of 2009 pandemic influenza A(H1N1) in June 2009, Sheffield, United Kingdom. Euro Surveill. 2010;15(19) pii/19565. [PubMed] [Google Scholar]
- Tuvim MJ, Gilbert BE, Dickey BF, Evans SE. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS One. 2012;7(1):e30596. doi: 10.1371/journal.pone.0030596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue MC, Collins JT, Subramoniapillai E, Kennedy GA. Successful use of oseltamivir prophylaxis in managing a nosocomial outbreak of influenza A in a hematology and allogeneic stem cell transplant unit. Asia Pac J Clin Oncol. 2017;13(1):37–43. doi: 10.1111/ajco.12565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.