Abstract
Cross-sectional studies have shown that pericardial fat is associated with atherosclerotic burden above and beyond generalized and central adiposity. Whether pericardial fat is longitudinally associated with coronary artery calcium (CAC) has not been firmly established. We examined the associations between cardiac ectopic fat including pericardial and intrathoracic fat with CAC progression and incidence in a community-based study setting. Study participants were from the Framingham Heart Study Offspring and Third Generation Cohorts who underwent multi-detector computed tomography at 2 consecutive examinations (2002–2005 and 2008–2011) for the assessment of CAC. Multivariable-adjusted regression models were used to evaluate the associations between cardiac ectopic fat with CAC. Non-linear associations were also examined. We included 1,732 participants (49.6% women, mean age 49.9 years). Of 1,024 participants with a CAC score=0 at baseline, 197 individuals developed a CAC score>0 (19.2%) during 6.1 years of follow-up. The remaining 708 participants with a CAC score>0 at baseline were eligible for CAC progression analysis. We identified non-linear association between pericardial fat and CAC progression. Higher pericardial fat was associated with higher CAC progression only for those participants with pericardial fat higher than the median value (β=56.0, p=0.04). Intrathoracic fat was linearly associated with CAC progression (β=23.0, p=0.02). However, all of these associations did not persist after additional adjustment for body mass index, abdominal visceral adipose tissue or waist circumference (all p≥0.14). Neither pericardial nor intrathoracic fat were associated with CAC incidence (all p≥0.34). Overall, both of the cardiac ectopic fat measures were longitudinally associated with CAC progression.
Keywords: Pericardial fat, intrathoracic fat, coronary artery calcium, epidemiology
Introduction
The primary aim of this study was to determine whether pericardial and intrathoracic fat are longitudinally associated with either the progression of coronary artery calcium (CAC) or the development of CAC during follow-up in the Framingham Heart Study. Because of possible local effects on the cardiac tissue, we hypothesized that pericardial fat is more closely associated with CAC progression and incidence, as compared to intrathoracic fat. Additionally, we hypothesized that these associations would persist after accounting for multiple confounders and baseline levels of generalized adiposity [body mass index (BMI)], central adiposity (waist circumference) or abdominal visceral adipose tissue.
Methods
The Framingham Heart Study is a prospective cohort study that commenced in 1948 to investigate the risk factors of cardiovascular disease in the community.1,2 Our study sample was derived from individuals in the Framingham Heart Study Offspring and Third Generation Cohorts who participated in the multi-detector computed tomography (MDCT) sub-studies from 2002 to 2005 (baseline) and from 2008 to 2011 (follow-up). The MDCT sub-study was open to participants who resided in the greater New England area who were women ≥40 years old and not pregnant or men ≥35 years old with a body weight <159 kg due to the weight limit of the scanner. We identified 1,815 participants who had CAC measurement available at both examinations with no missing pericardial fat, intrathoracic fat, and covariates at the baseline examination. Of these, 83 individuals with cardiovascular disease at baseline and/or follow-up were excluded from the study; resulting in a total of 1,732 participants for the analysis. The institutional review boards of Boston University and Massachusetts General Hospital approved the study protocol, and all participants provided written informed consent.
MDCT images of the chest and abdomen were obtained with the participants in a supine position via an 8-slice MDCT scanner (LightSpeed Ultra, General Electric, Milwaukee, WI), using a standard protocol described in detail previously.3,4 For the assessment of CAC, intrathoracic fat, and pericardial fat, the radiographic assessment of the heart was completed on 48 contiguous 2.5 mm thickness of the MDCT scans based on a prospective electrocardiographic triggering scanning protocol (tube voltage of 120 kVp, tube current of 320 mA for body weight of <100 kg and 400 mA for body weight of >100 kg, temporal resolution of 330 ms, and gantry rotation time of 500 ms). For the assessment of abdominal visceral adipose tissue, images of the abdomen encompassing 125 mm above the level of S1 were captured with 25 contiguous 5 mm thickness of the MDCT slices with scanner setting of tube voltage of 120 kVp, tube current 320 mA for body weight of <100 kg and 400 mA for body weight of >100 kg, gantry rotation time of 500 ms, and table feed of 3:1.
Regions of interest were quantified by using 3-dimensional workstation tool in a semiautomatic manner (Aquarius 3D Workstation, TeraRecon Inc., San Mateo, CA). A predefined image display window width of −195 to −45 Hounsfield units (HU) and a window center of −120 HU were applied to identify radiographic pixels that correspond to the adipose tissue property. Total thoracic fat was defined as the fat located in the thorax (i.e., from the level of the right pulmonary artery to the diaphragm, vertically; and from the chest wall to descending aorta, horizontally) was quantified. An experienced reader manually traced the pericardium to quantify the fat accumulated within the pericardial sac, which was defined as pericardial fat. Intrathoracic fat was computed by subtracting the pericardial fat from total thoracic fat.
For the assessment of abdominal visceral adipose tissue, the abdominal muscular wall was manually outlined by a trained reader to differential subcutaneous and visceral adipose tissue from the total abdominal fat. Volumetric fat content detected within the abdominal muscular wall was defined as abdominal visceral adipose tissue. High reproducibility of these fat measures were previously reported with intra- and inter-observer reproducibility of 0.97 and 0.95 for pericardial fat;4 0.99 and 0.98 for intrathoracic fat;4 and 0.99 and 0.99 for abdominal visceral adipose tissue, respectively.5 The extent of coronary artery calcium was assessed by a dedicated off-line workstation tool (Aquarius 3D Workstation, TeraRecon Inc., San Mateo, CA) based on the Agatston method.6 Briefly, calcified lesions were counted if the lesions met the pre-specified area of ≥1 mm2, attenuation range of ≥130 HU, and ≥3 connected pixels by applying 3-dimensional connectivity criteria. Agatston score was automatically calculated by multiplying the pixel area of each lesion in mm2 with weighted attenuation according to the maximum HU of 1 for 130–199 HU; 2 for 200–299 HU, 3 for 300–399 HU, and 4 for ≥400 HU.
All covariates were assessed at the baseline examination. As a measure of generalized adiposity, BMI was calculated based on the equation of weight in kilograms divided by height in square meters. Waist circumference as a measure of central adiposity was assessed at the level of the umbilicus to the nearest 0.5 cm using a measuring tape. Systolic and diastolic blood pressures were measured while participants were seated and rested for a minimum of 5 minutes. Fasting plasma glucose, total cholesterol, and high-density lipoprotein (HDL) cholesterol were assessed on 8-hour fasting blood samples in the morning. Diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or use of diabetes treatment. Information regarding use of antihypertensive medication, lipid lowering treatment, hormone replacement therapy, smoking status, alcohol use, and postmenopausal status were collected via physician-administered questionnaires during the regular clinic visit at the Framingham Heart Study. Current smoking was defined as smoking at least 1 cigarette per day in the year preceding the study visit. Alcohol use was defined as >7 drinks/week for women and >14 drinks/week for men.
Progression and incidence of CAC were defined using the methods previously used by the study from the Multi-Ethnic Study of Atherosclerosis.7 Progression of CAC was calculated as the absolute difference in CAC score between baseline and follow-up for those with a CAC >0 at baseline. For participants with a CAC=0 at baseline, we defined incident CAC as having a follow-up CAC score >0. For the continuous outcome of CAC progression, multivariable-adjusted linear regression models were constructed to determine the associations between pericardial or intrathoracic fat with progression of CAC. For the dichotomous outcome variable of CAC incidence, multivariable-adjusted logistic regression models were used to examine the associations between pericardial or intrathoracic fat with incident CAC. A separate analysis was conducted for each independent variable. For both CAC progression and CAC incidence, 2 models were constructed: 1) age and sex-adjusted model, and 2) multivariable model adjusted for age, sex, systolic blood pressure, antihypertensive treatment, diabetes mellitus, total cholesterol, HDL cholesterol, lipid-lowering treatment, current smoking, alcohol use, postmenopausal status, and hormone replacement therapy. The CAC progression multivariable models were additionally adjusted for baseline CAC score. Additional multivariable models were adjusted for BMI, waist circumference or abdominal visceral adipose tissue (separately). Tests for sex-interaction were conducted based on the multivariable model. Potential non-linear association was assessed by adding the squared term of cardiac ectopic fat to the multivariable models, and further evaluated by the smoothing spline method. A 2-tailed p<0.05 was considered statistically significant. Finally, we stratified our study sample according to the tertiles of pericardial and intrathoracic fat volume and explored incident CAC for each tertile group. Tertile 3 corresponded to a higher fat, as compared to tertile 1. All analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics of the participants are shown in Table 1. A total of 1,732 participants (49.6% women, 37.5% Offspring and 62.5% Third Generation) were included in the study. Among 708 participants (273 women and 435 men) who had a CAC score >0 at baseline, the median (25th–75th percentile) baseline CAC score was 43.8 (7.5–166.2); change in CAC score was 75.6 (17.7–208.5); and the CAC score at follow-up was 142.7 (36.6–403.1). Among 1,024 participants (586 women and 438 men) who had a CAC score of 0 at baseline, 197 individuals (95 women and 102 men) developed incident CAC after the follow-up (19.2%). Pearson correlations between cardiac ectopic fat with generalized and central adiposity are shown in Supplemental Table 1. When we tested the possible non-linear association between ectopic fat and CAC progression by additionally adding squared term of the ectopic fat in the multivariable model, we found significant non-linear association with pericardial fat (p=0.02), but not with intrathoracic fat (p=0.18). We observed that the direction of the association changed around the median value of pericardial fat (116.0 cm3, Figure 1). For those participants with pericardial fat lower than the median value, lower pericardial fat was associated with higher CAC progression. Conversely, for those participants with pericardial fat higher than the median value, higher pericardial fat was associated with higher CAC progression (Figure 1). To assess the trend of the non-linear association, we further stratified the participants into low and high pericardial fat groups based on the median value of pericardial fat. In the multivariable model, higher pericardial fat was associated with greater CAC progression only for the high pericardial fat group [Table 2, β=56.0, 95% confidence interval (CI)=3.5, 108.5, p=0.04]. This significant association was attenuated after additionally adjusting for BMI, waist circumference or visceral adipose tissue (all p≥0.14). Table 3 shows the multivariable-adjusted linear regression analysis for the association between intrathoracic fat with CAC progression. In the multivariable-adjusted models, each additional 50 cm3 increment in intrathoracic fat was associated with a 23.0-unit increase in CAC score (all p=0.02). Intrathoracic fat was not associated with CAC progression after further accounting for adiposity measures (all p≥0.34).
Table 1.
Baseline Characteristics of Study Participants
| Parameters | Overall Participants (n=1,732) |
|---|---|
| Age (years) | 49.9 ± 9.4 |
| Body mass index (kg/m2) | 27.4 ± 5.0 |
| Obesity* | 422 (24.4%) |
| Waist Circumference (cm) | 96.1 ± 13.7 |
| Pericardial Fat (cm3) | 108.5 ± 40.3 |
| Intrathoracic Fat (cm3) | 93.8 ± 58.9 |
| Subcutaneous Adipose Tissue (cm3) | 2845 ± 1375 |
| Visceral Adipose Tissue (cm3) | 1705 ± 951 |
| Systolic Blood Pressure (mmHg) | 121.1 ± 16.0 |
| Diastolic Blood Pressure (mmHg) | 76.0 ± 9.2 |
| Fasting Plasma Glucose (mg/dL) | 97.6 ± 18.1 |
| Total Cholesterol (mg/dL) | 198.1 ± 35.0 |
| High-Density Lipoprotein Cholesterol (mg/dL) | 54.2 ± 16.5 |
| Current Smoking | 165 (9.5%) |
| Moderate to Heavy Alcohol Use | 259 (15.0%) |
Data are shown as means ± standard deviations for continuous variables or counts (%) for dichotomous variables.
Defined as body mass index ≥ 30 kg/m2.
Figure 1.
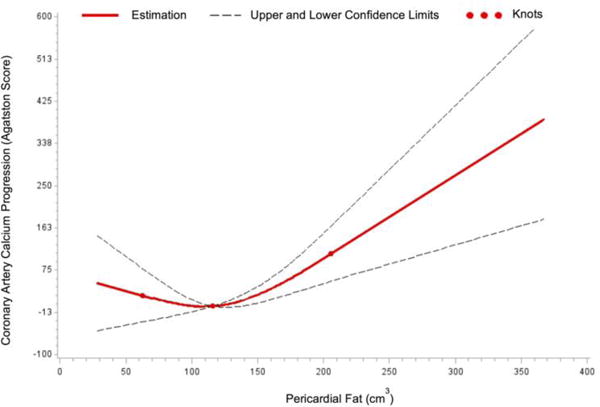
Non-linear association between pericardial fat and coronary artery calcium progression.
Table 2.
Multivariable-adjusted linear regression models for the associations between pericardial fat and coronary artery calcium progression. Due to the significant non-linear association, analysis was stratified by low and high pericardial fat groups determined by the median pericardial fat value.
| Low Pericardial Fat Group | High Pericardial Fat Group | |||
|---|---|---|---|---|
|
|
|
|||
| Model | β (95% Confidence Interval) | p-Value | β (95% Confidence Interval) | p-Value |
|
|
|
|||
| Age + Sex | −28.9 (−83.5, 25.8) | 0.30 | 90.7 (32.0, 149.4) | 0.003 |
| MV | −28.7 (−82.7, 25.2) | 0.30 | 56.0 (3.5, 108.5) | 0.04 |
| MV + Body Mass Index | −16.8 (−72.4, 38.8) | 0.55 | 40.0 (−13.4, 93.3) | 0.14 |
| MV + Waist Circumference | −18.5 (−74.7, 37.7) | 0.52 | 40.8 (−12.8, 94.4) | 0.14 |
| MV + Visceral Adipose Tissue | −9.4 (−68.3, 49.4) | 0.75 | 42.7 (−14.6, 100.1) | 0.15 |
Data are shown as β coefficients (95% confidence interval) of coronary artery calcium progression based on each additional 50 cm3 increment in the pericardial fat measure.
Multivariable (MV) model was adjusted for baseline coronary artery calcium score, systolic blood pressure, antihypertensive treatment, diabetes mellitus, total cholesterol, high-density lipoprotein cholesterol, lipid-lowering treatment, current smoking, alcohol use, postmenopausal status (women only), and hormone replacement therapy (women only).
Table 3.
Multivariable-adjusted linear regression models for the association between intrathoracic fat and coronary artery calcium progression.
| Model | β (95% Confidence Interval) | p-Value |
|---|---|---|
| Age + Sex | 33.0 (12.1, 53.8) | 0.002 |
| MV | 23.0 (3.3, 42.7) | 0.02 |
| MV + Body Mass Index | 10.7 (−11.9, 33.4) | 0.35 |
| MV + Waist Circumference | 10.6 (−12.2, 33.4) | 0.36 |
| MV + Visceral Adipose Tissue | 13.9 (−14.7, 42.4) | 0.34 |
Data are shown as β coefficients (95% confidence interval) of coronary artery calcium progression based on each additional 50 cm3 increment in the intrathoracic fat measure.
Multivariable (MV) model is adjusted for baseline coronary artery calcium score, systolic blood pressure, antihypertensive treatment, diabetes mellitus, total cholesterol, high-density lipoprotein cholesterol, lipid-lowering treatment, current smoking, alcohol use, postmenopausal status (women only), and hormone replacement therapy (women only).
Tertile-specific analyses showed an increase in the incidence of CAC for higher ectopic fat depots (Figure 2). For example, from the lowest to the highest tertile of cardiac fat, the incident CAC increased from 15% to 26% for pericardial fat; and from 11% to 26% for intrathoracic fat. The multivariable-adjusted logistic regression analysis for the associations of cardiac ectopic fat with CAC incidence is shown in Table 4. We did not find non-linear associations between cardiac ectopic fat and incident CAC (p>0.05). Neither of the cardiac ectopic fat depots were associated with incident CAC regardless of the type of covariate adjustments (all p≥0.08). One sex interaction was found (p for sex-interaction=0.02), with the relationship between pericardial fat and CAC progression becoming only significant in men, but not in women based on multivariable-adjusted models (men, β=54.9, 95% CI=17.9, 92.0; women β=−9.6, 95% CI=−35.2, 16.1). Due to the significant non-linear associations between pericardial fat and CAC progression, we further assessed the evidence of sex interaction by stratifying women and men into low and high pericardial fat groups according to the sex specific median value of 105.4 cm3 for women and 122.3 cm3 for men. Based on the multivariable model, higher pericardial fat was associated with higher CAC progression only for the higher pericardial fat group in men, β=82.6, 95% CI=3.4, 161.8, p=0.04). Additionally adjusting the models for follow-up time did not change our primacy results.
Figure 2.
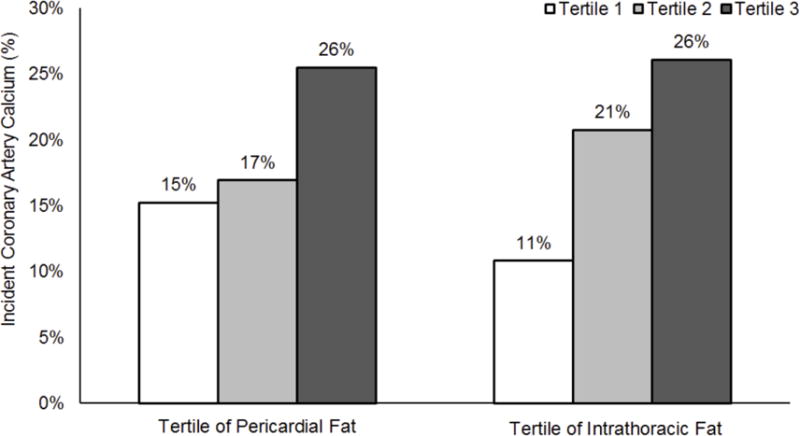
Incidence of coronary artery calcium according to the tertiles of pericardial and intrathoracic fat volume in overall participants. Tertile 3 corresponds to a larger cardiac fat volume, as compared to tertile 1. Tests for linear trend resulted in p-values of 0.0007 for pericardial fat and p<0.0001 for intrathoracic fat.
Table 4.
Multivariable-adjusted logistic regression models for the associations between cardiac ectopic fat depots and coronary artery calcium incidence.
| Fat Measure | Model | Odds Ratios (95% Confidence Interval) | p-Value |
|---|---|---|---|
| Pericardial Fat | Age + Sex | 1.21 (0.96, 1.53) | 0.10 |
| MV | 0.94 (0.72, 1.21) | 0.62 | |
| MV + Body Mass Index | 0.92 (0.70, 1.23) | 0.58 | |
| MV + Visceral Adipose Tissue | 1.05 (0.76, 1.44) | 0.77 | |
| MV + Waist Circumference | 1.01 (0.75, 1.35) | 0.96 | |
| Intrathoracic Fat | Age + Sex | 1.17 (0.98, 1.39) | 0.08 |
| MV | 0.98 (0.80, 1.19) | 0.82 | |
| MV + Body Mass Index | 0.97 (0.78, 1.21) | 0.79 | |
| MV + Visceral Adipose Tissue | 1.15 (0.87, 1.51) | 0.33 | |
| MV + Waist Circumference | 1.06 (0.84, 1.32) | 0.64 |
Data are shown as odds ratios (95% confidence interval) of incident coronary artery calcium based on each additional 50 cm3 increment in the fat measure.
Multivariable (MV) model is adjusted for systolic blood pressure, antihypertensive treatment, diabetes mellitus, total cholesterol, high-density lipoprotein cholesterol, lipid-lowering treatment, current smoking, moderate to heavy alcohol use, postmenopausal status (women only), and hormone replacement therapy (women only).
Discussion
Our findings are 3-fold. First, pericardial and intrathoracic fat were associated with progression of CAC over an average of 6.1 years of follow-up. Second, these associations did not persist after further accounting for measures of generalized or central adiposity. Third, cardiac ectopic fat was not associated with incidence of CAC after approximately 6 years in any of the models evaluated.
An extensive body of literature has described the cross-sectional associations between higher pericardial fat with increased burden of CAC.8–12 Of interest, we previously reported the cross-sectionally associations between pericardial and intrathoracic fat with CAC in an age- and sex adjusted model. Only the association with pericardial fat, but not with intrathoracic fat, remained significant after further accounting for multiple potential covariates, as well as BMI, waist circumference or abdominal visceral adipose tissue.10 Findings of our prior study supported that pericardial fat may impose a local toxic effect on the adjacent coronary arteries, which potentially led to greater accumulation of CAC.10 Prospective studies evaluating the association of pericardial fat with CAC progression are needed to help reduce residual confounding from baseline factors which may be present in cross-sectional studies.
Our present investigation builds upon our prior study to evaluate the association between cardiac ectopic fat at baseline and the progression and incidence of CAC after 6.1 years of follow-up. In the present study, both of the cardiac ectopic fat measures were longitudinally associated with CAC progression only in a multivariable-adjusted model. These associations did not persist after additionally accounting for other fat measures. A prior study of 600 multiethnic participants of whites, Filipinos and African-Americans, reported similar findings where the associations between pericardial and intrathoracic fat with CAC were cross-sectionally, but not longitudinally, significant after 4 years of follow-up.11 Our study expands the existing literature by using continuous and dichotomous measures of CAC precisely assessed by MDCT in a larger study sample of 1,732 participants over an extended follow-up period. Our study further expands the literature by reporting the significant non-linear association between pericardial fat and CAC progression where the positive and significant association between pericardial fat and CAC progression was observed only among the participants with pericardial fat above the median value. Our findings were not consistent with the hypothesis that cardiac ectopic fat directly causes or promotes progression or incidence of CAC independent of generalized or central adiposity, though confirmatory studies are necessary.
Several potential mechanisms may further explain our findings. The significant prospective associations between volumetric measures of pericardial and intrathoracic fat with CAC progression in a multivariable-adjusted model suggest that higher cardiac ectopic fat is temporally associated with the greater burden of calcification in the coronary arteries after accounting for multiple confounders. Pericardial fat has been postulated as an active endocrine fat depot that contributes to the pathogenesis of coronary atherogenesis attributed to the close anatomical proximity with coronary arteries via several mechanisms, including paracrine effects.13,14 The pathogenic properties of pericardial fat include local secretion and expression of pro-inflammatory and oxidative stress biomarkers,15–17 endothelial dysfunction,13 promotion of vasoconstriction,14 and local proliferation of vascular smooth muscle cell18 that potentially accelerate the atherosclerotic process. All of these factors may subsequently manifest the development of coronary heart disease.19 However, the attenuation of the associations between cardiac ectopic fat depots and CAC progression after adjustment of BMI, waist circumference or visceral adipose tissue may suggest that 1) the effect of generalized and central adiposity may outweigh the local toxic pathogenicity of cardiac ectopic fat on coronary arteries; or 2) the collinearity between cardiac ectopic fat and generalized/central adiposity may have affected the multivariate models by increasing the confidence intervals of the estimation and limiting the statistical power of the study.
The discordant findings between significant cross-sectional associations but non-significant longitudinal relationship between pericardial fat with CAC after adjusting for various adiposity measures may be due to borderline power to detect the longitudinal associations and also due to insufficient follow-up period. In particular, the follow-up duration of this study was an average of 6.1 years, which may have not been a sufficient time to detect differences in CAC progression and incidence given that our participants are predominantly middle-aged adults. A study of 5,756 participants previously noted that age and duration of follow-up time are significant risk factors for both progression and incidence of CAC.20 Thus, further studies with prolonged follow-up period would be essential to confirm our findings.
This study has several strengths. We incorporated highly reproducible volumetric measures of fat depots and arterial calcification assessed by MDCT in a large, well-characterized, community-based sample of women and men. Repeated measures of arterial calcium at baseline and follow-up examinations based on standardized protocols for CAC assessment allowed the examination of the prospective associations between CAC with cardiac ectopic fat. Some limitations require comment. First, our findings are not applicable to individuals with different racial and ethnic backgrounds as our sample predominantly consists of whites. Second, the observational study setting precludes inferences of causality. Third, a 6.1-year follow-up period may not have been sufficiently long enough to capture changes in CAC and prospective associations with cardiac ectopic fat depots. Finally, our sample consists of community-dwelling participants implying that our results may not be generalized to cohorts of patients with more advanced disease.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract N01-HC-25195; HHSN268201500001I).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Alison Pedley is an employee of Merck & Company, Inc. There is nothing to disclose for any author other than Alison Pedley.
NHLBI Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- 1.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Sr, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 2.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 3.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham Heart Study. Circ Cardiovasc Imaging. 2010;3:559–566. doi: 10.1161/CIRCIMAGING.110.956706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102:1136–1141. doi: 10.1016/j.amjcard.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey AK, Blaha MJ, Sharma K, Rivera J, Budoff MJ, Blankstein R, Al-Mallah M, Wong ND, Shaw L, Carr J, O’Leary D, Lima JA, Szklo M, Blumenthal RS, Nasir K. Family history of coronary heart disease and the incidence and progression of coronary artery calcification: Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2014;232:369–376. doi: 10.1016/j.atherosclerosis.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, Ramesh A, Kakadiaris I, Germano G, Slomka PJ. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and metabolic syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadi N, Nabavi V, Yang E, Hajsadeghi F, Lakis M, Flores F, Zeb I, Bevinal M, Ebrahimi R, Budoff M. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad Radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 11.Wassel CL, Laughlin GA, Araneta MR, Kang E, Morgan CM, Barrett-Connor E, Allison MA. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity (Silver Spring) 2013;21:1704–1712. doi: 10.1002/oby.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miao C, Chen S, Ding J, Liu K, Li D, Macedo R, Lai S, Vogel-Claussen J, Brown ER, Lima JA, Bluemke DA. The association of pericardial fat with coronary artery plaque index at MR imaging: The Multi-Ethnic Study of Atherosclerosis (MESA) Radiology. 2011;261:109–115. doi: 10.1148/radiol.11110346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao Y-J, Takemori K, Su L-Y, An W-S, Lu C, Sharma AM, Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–445. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 17.Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG, Keaney JF, Lipinska I, Meigs JB, Kathiresan S. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity. 2010;18:1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2012;165:643–658. doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


