Abstract
Emerging evidence suggests that the β2 integrin family of adhesion molecules have an important role in suppressing immune activation and inflammation. β2 integrins are important adhesion and signaling molecules that are exclusively expressed on leukocytes. The four β2 integrins (CD11a, CD11b, CD11c, and CD11d paired with the β2 chain CD18) play important roles in regulating three key aspects of immune cell function: recruitment to sites of inflammation; cell–cell contact formation; and downstream effects on cellular signaling. Through these three processes, β2 integrins both contribute to and regulate immune responses. This review explores the pro- and anti-inflammatory effects of β2 integrins in monocytes, macrophages, and dendritic cells and how they influence the outcome of immune responses. We furthermore discuss how imbalances in β2 integrin function can have far-reaching effects on mounting appropriate immune responses, potentially influencing the development and progression of autoimmune and inflammatory diseases. Therapeutic targeting of β2 integrins, therefore, holds enormous potential in exploring treatment options for a variety of inflammatory conditions.
Keywords: β2 integrins, CD11/CD18, dendritic cells monocytes and macrophages, immune regulation, autoimmunity
Introduction
The integrin family of proteins is comprised of 24 heterodimeric transmembrane adhesion receptors. Each integrin is formed through the non-covalent association of 1 α-subunit and 1 β-subunit; currently, 16 α-subunits and 8 β-subunits have been identified. Their expression on virtually all human cells and their complex signaling mechanisms explain their wide variety of biological roles, including blood clotting, cell adhesion, and migration.
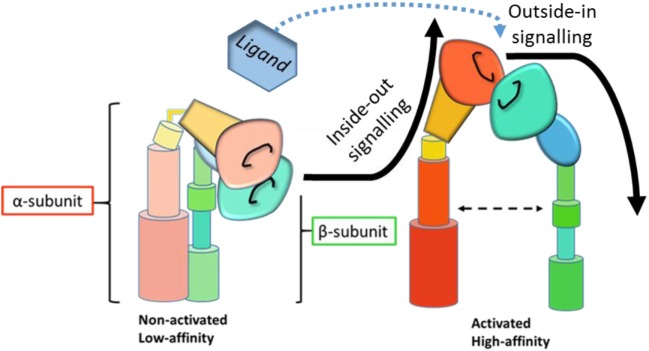
Due to their extensive importance in biological systems, elucidating integrin signaling and receptor function has been of great interest since their characterization as adhesion molecules over 30 years ago. Integrins are important signaling proteins that mediate interactions of the cell with extracellular matrix proteins and with other cells via cell-surface ligands. Integrins exist in a continuum between a folded inactive form with low affinity for their ligand and an extended high affinity conformation (1), although even bent integrins are able to bind ligand in rare instances (2). As immune cell adhesion and extravasation into lymph nodes and tissues forms part of initiating an effective immune response, β2 integrin conformation on the surface of leukocytes needs to be tightly regulated. β2 integrins on the surface of circulating leukocytes tend, therefore, to be largely inactive (2) until inside-out and outside-in signaling trigger integrin-mediated adhesion and extravasation into tissue (Figure 1).
Figure 1.
Schematic representation of integrin activation and signaling. Inside-out signaling induces a conformational change in the integrin to the active, high affinity state. Upon ligand binding, active integrins then transmit outside-in signals and downstream signaling cascades. [Adapted from Byron et al. (3), with permission from the Journal of Cell Science].
Inside-out signaling modifies how cells interact with their environment by facilitating receptor affinity and avidity (4) to allow binding to extracellular ligands. Outside-in signaling, on the other hand, mediates intracellular events in response to their environment by eliciting downstream signaling cascades in response to receptor occupation. The complex details of integrin signaling are reviewed elsewhere (5, 6) and are beyond the scope of this review. Briefly, inside-out signaling is mediated by talin (7) and kindlin (8, 9) binding to the intracellular domain of the β2 subunit, a process initiated by chemokine receptor or Toll-like receptor (TLR) engagement (10, 11), which results in a conformational change in the integrin from a low-affinity to a high-affinity state. Outside-in signaling is then initiated by ligand binding to high-affinity integrin receptors (Figure 1). Downstream signaling events mediate the formation of focal complexes and adhesions through rearrangement of the actin cytoskeleton. The relative importance of affinity and avidity on integrin signaling and function is heavily debated (12, 13), but dynamic interaction between these processes and both inside-out and outside-in signaling seems likely (14).
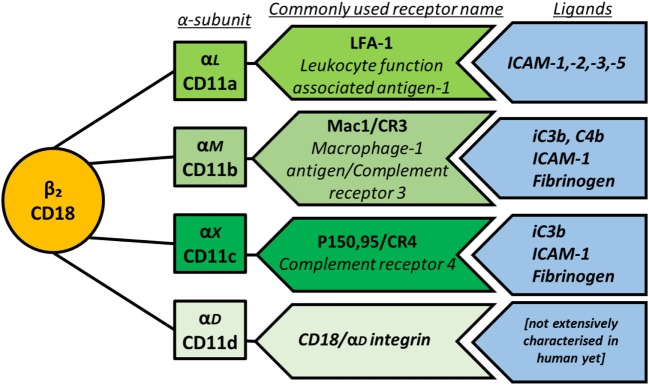
β2 integrins are the focus of this review, as they are exclusively found on leukocytes and therefore of particular importance for the immune system. They mediate cell recruitment into lymphoid organs and inflamed tissues by facilitating firm leukocyte arrest on endothelial cells and extravasation after cell rolling (15); cellular interactions between leukocytes including immunological synapse formation (16); and intracellular signaling cascades that influence cytoskeletal rearrangement, activation, proliferation and impact on cellular responses to TLRs. Importantly, through these three processes, β2 integrins can have either pro-inflammatory or anti-inflammatory outcomes. The β2 integrin subunit (CD18) can pair with one of four α-subunits (αL—CD11a, αM—CD11b, αX—CD11c, and αD—CD11d), forming leukocyte function-associated antigen-1, Mac1/CR3 (macrophage-1 antigen, complement receptor 3), P150,95/CR4 (complement receptor 4), and CD18/CD11d, respectively (Figure 2). For consistency, this review will utilize only the CD nomenclature. Both function and cell-specific expression of β2 integrins vary according to the α-subunit involved.
Figure 2.
Schematic representation of β2 integrin subunit pairing, depicting the β-subunit CD18 as the common subunit non-covalently associating with one of four α-subunits. The main ligands for each integrin are also shown.
The main ligands for the β2 integrin family members are outlined in Figure 2. Briefly, CD11a binds to intracellular adhesion molecule-1 (ICAM-1), -2, -3, and -5, which are expressed by a variety of cells including leukocytes and endothelial cells, thereby mediating leukocyte recruitment to lymph nodes and sites of inflammation as well as cell–cell adhesion. CD11b binds the complement proteins iC3b and C4b with high affinity, mediating phagocytosis of complement-coated particles but can also bind ICAM-1, fibrinogen, and more than 40 other ligands (17). The sequence of CD11c is very close to that of CD11b, and indeed CD11c binds several of the same ligands including iC3b, ICAM-1, and fibrinogen. Multi-ligand binding capacity of CD11d is proposed to largely overlap with CD11b and includes ECM-associated proteins fibronectin, fibrinogen, vitronectin, Cyr61, and plasminogen (18).
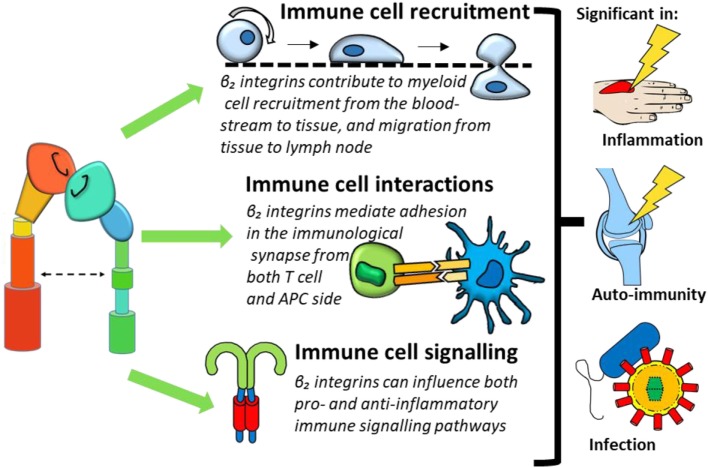
This review will provide an overview of β2 integrin expression on monocytes, macrophages and DCs, before exploring the paradoxical pro-inflammatory and regulatory roles of β2 integrins in immune regulation in three key aspects of immune function: recruitment and migration, cellular interactions, and downstream cell signaling (Figure 3). We will furthermore review how dysregulated integrin signaling could contribute to inflammatory and autoimmune conditions and introduce the therapeutic potential of targeting β2 integrins.
Figure 3.
β2 integrin involvement in immune cell function can be categorized into three processes: immune cell recruitment, immune cell interactions, and immune cell signaling. Dysregulation of these functions could contribute to conditions such as inflammation, immunity, and infection.
Expression of β2 Integrin Subunits by Dendritic Cells (DCs), Monocytes, and Macrophages
The expression of β2 integrin subunits varies in different leukocyte subsets and between mice and humans. In general terms, CD11a is expressed on all leukocytes at varying levels, while CD11b, CD11c, and CD11d are predominantly expressed by monocytes, macrophages and DCs. Specifically, in humans, monocytes express all four β2 integrin-associated alpha subunits (CD11a, CD11b, CD11c, and CD11d) with CD11a and CD11b expression greater than CD11c (19, 20); macrophages express CD11a and CD11b at lower levels than monocytes together with CD11c at similar levels to monocytes (21); while DCs mainly express CD11c together with CD11a, though some DC subsets also express CD11b (22). While CD11d has received less attention than the other β2 integrins due to the absence of commercially available human antibodies, Miyazaki and colleagues showed CD11d expression on monocyte-derived DCs and macrophages as well as most circulating monocytes (23). To complement the scarce available data, mRNA expression data for the CD11d subunit ITGAD were consulted. While Villani and colleagues (24) find monocytes to express highest levels of ITGAD mRNA, the Expression Atlas (25) reports highest expression in DCs, with ITGAD expression in monocytes remaining below detectable threshold. However, overall both RNAseq data sets show that CD11d mRNA expression is very low in monocytes, macrophages, and DCs. Table 1 provides the details of expression of all β2 integrin subunits in human and murine monocytes, macrophages, and DCs. Where available, expression analysis on DC subsets is given using the Guilliams nomenclature (26), which was recently confirmed and expanded by Villani and colleagues (24).
Table 1.
β2 integrin expression on dendritic cells (DCs), monocytes and macrophages—human and murine findings.
| Cell type | CD11a/CD18 (αL/β2) | CD11b/CD18 (αM/β2) | CD11c/CD18 (αX/β2) | CD11d/CD18 (αD/β2) |
|---|---|---|---|---|
| DCs | Human: high levels of CD11a on monocyte-derived DCs (22, 27–29); plasmacytoid DCs (pDCs) also express CD11a (30); reduced CD11a/CD18 levels upon DC activation (31) | Human: CD11b present on monocyte-derived DCs (22, 27, 28); detected in cDCs, but not in pDCs (31–33); higher on cDC2 than cDC1s (33, 34); reduced CD11b/CD18 levels upon DC activation (31) | Human: pDCs lack CD11c (31); expressed on mature DCs (31); CD11c expression is higher on cDC2 than cDC1s (33, 34); monocyte-derived DCs also express CD11c (32); reduced CD11c/CD18 levels upon DC activation (31) | Human: expressed on monocyte-derived DCs (23), single-cell mRNA data suggests low gene expression in DCs (25) |
| Mouse: expressed by cDCs, particularly the CD8+ subset, and by pDCs (35); also highly expressed by bone marrow-derived DCs | Mouse: expression of CD11b in mouse cDCs is subset-specific: higher on CD8− than CD8+ splenic DCs (35); expressed in sub-populations of gut DCs (36); absent from pDCs (37); expressed by bone marrow-derived DCs (38) | Mouse: CD11c highly expressed on cDCs and typically used as a DC marker (38); expressed by pDCs (39) and bone marrow-derived DCs (40) | Mouse: no protein expression data available, RNA-seq data suggest medium ITGAD gene expression in murine DCs (25) | |
| Monocytes | Human: expressed by circulating monocytes (21, 29, 41) | Human: highly expressed by circulating monocytes (21, 34, 41); differentially expressed on osteoclast precursors (42) | Human: expressed on circulating monocytes (21, 34) and classical, non-classical, and intermediate monocytes (31) | Human: expressed on majority of circulating monocytes, higher on CD16− cells compared to CD16+ cells (23) |
| Mouse: expressed by circulating monocytes (43) | Mouse: high expression of CD11b on murine monocytes (44) | Mouse: thought to be absent from most monocytes (45); though may be upregulated upon stimulation/maturation (44) | Mouse: lowly expressed by circulating monocytes, upregulated upon differentiation into macrophages (46), low ITGAD mRNA expression (25) | |
| Macrophages | Human: expressed by monocyte-derived macrophages (21, 43); reduced expression on monocyte-derived macrophages compared to blood monocytes (21) | Human: expressed on monocyte-derived macrophages (47–49); expressed on alveolar macrophages, though at lower levels compared to blood monocytes (21) | Human: lowly expressed by monocyte-derived macrophages (21, 48–50) | Human: expressed on monocyte-derived macrophages in vitro (23) |
| Mouse: expression dependent on tissue: present on pulmonary, but not on microglia, spleen or peritoneal macrophages (51) | Mouse: abundantly expressed by peritoneal macrophages (52, 53); highly expressed on dermal macrophages (54) | Mouse: expressed on alveolar macrophages (55); absent from bone marrow-derived macrophages and dermal macrophages (54) | Mouse: expressed by peritoneal macrophages (56) | |
Animal studies have been instrumental in elucidating integrin function in monocytes, macrophages, and DCs. β2 integrins are highly conserved across species, with mice, rats, and rabbits most commonly used as models. Importantly β2 integrin-deficient mice are considered an appropriate model of the human condition leukocyte adhesion deficiency (LAD) where β2 integrin expression or function is lost (57). However, while β2 integrin structure is largely similar between species, cellular expression levels can vary significantly. A common example is CD11c, which in mice is predominantly expressed by conventional (cDCs) and plasmacytoid DCs (pDCs), although can also be expressed on lymphocyte subsets. In humans, on the other hand, CD11c is expressed not only on DCs but also monocytes, macrophages, granulocytes, and natural killer cells (19, 38). Animal and human studies therefore have to be compared with great care, and validation of concepts conceived in animal models in human cells remains a priority in elucidating the functions of β2 integrins.
β2 Integrins as Regulators of Immune Function
Evidence for β2 Integrin Contribution to Immune Regulation
There is mounting evidence that puts β2 integrins at the center of the balance between immune priming and tolerance. Integrin-deficient humans and mouse models show that β2 integrins are important negative regulators of the immune system. LADs are genetic human disorders caused by the reduction or complete absence of β2-integrins (LAD-I) (58) or by mutations in the integrin-activating protein kindlin-3 (LAD-III) (59). These disorders are characterized by profound impairment of leukocyte recruitment to peripheral sites of infection. Patients with LAD suffer from increased susceptibility to infection and impaired inflammatory responses (60), resulting in markedly reduced lifespan if no therapeutic measures are taken. Paradoxically LAD patients also suffer from chronic inflammatory diseases. Examples of conditions prevalent in LAD patients include intestinal colitis (61) and periodontitis (62) suggest that β2 integrins have an important role in suppressing inflammation and promoting immune tolerance. Supporting this, the presence of functional β2 integrins improved symptoms in a model of skin inflammation by restricting DC-mediated T cell activation (63).
LAD pathology can be replicated in β2 integrin knockout (KO) mouse models, underlining the importance of β2 integrins for immune cell recruitment in both humans and murine models and the similarities between the species. From studies in KO mice and LAD patients, we know β2 integrins are essential in mediating T cell recruitment to lymph nodes and leukocyte, particularly neutrophil and T cell, recruitment to sites of inflammation. Here, we will further explore the roles of these integrins in monocytes, macrophages, and DCs.
β2 Integrins Regulate Recruitment and Migration of Mononuclear Phagocytes
Evidence suggests that leukocyte recruitment to tissues is dependent on β2 integrins, because of the requirement for these adhesion molecules in the firm adhesion to the endothelial layer under shear flow conditions and for subsequent transendothelial migration (64). However, leukocyte migration within tissues is thought to occur independently of β2 integrins, as cells use an actin-dependent flowing and squeezing mechanism of movement in three-dimensional environments (64).
Geissmann and colleagues showed that the adhesion of patrolling murine monocytes to blood vessel walls is significantly decreased when CD11a is blocked (45). Similarly, chemotactic migration of human monocytes in vitro is inhibited when CD18 function is blocked (65). However, murine monocyte recruitment to sites of inflammation was found to occur independently of CD11a and CD11b (66), suggesting that β2 integrins are primarily involved in the homeostatic migration of monocytes and that their role is redundant during inflammation. On the other hand, increased expression levels of CD11d on macrophages mediates their retention at inflammatory sites in mice (56).
The role of β2 integrins in DC and macrophage recruitment to secondary lymphoid organs and tissues seems to be dependent on the inflammatory state of the body. Bone marrow-derived DCs (BMDCs) from mice where all integrins, including β2, are knocked out, migrated from the site of injection (ear) to the draining lymph node in similar numbers to their wild-type counterparts when activated with lipopolysaccharide (LPS). This suggests that DC migration during inflammation is not dependent on integrins. However, under steady-state conditions, the absence of functional β2 integrins from murine BMDCs (using signaling-deficient β2 integrin knock-in BMDCs) was found to increase migration from tissue (footpad) to draining lymph node, leading to the hypothesis that β2 integrins function to restrict migration in the steady-state by anchoring DCs in the tissue site. As a consequence of increased DC migration to the draining lymph node, the same study showed an increase in Th1 cytokine production (67), further supporting a negative regulatory role for β2 integrins on DCs. In addition, a murine model of skin inflammation also showed an increase in migratory DCs in the draining lymph node of β2 integrin signaling-deficient mice, as well as at the site of inflammation, though whether this was dependent on the inflammation or not was not determined (63). Overall, the cellular environment seems to determine the requirement for functional β2 integrins in the migration of both monocytes and DCs in vivo: integrins play a role in monocyte recruitment and DC migration under steady-state conditions, but are dispensable during inflammation.
β2 Integrins Regulating DC–T Cell Interactions
In addition to their roles in leukocyte recruitment and migration, β2 integrins are also important mediators of cellular interactions. Functional β2 integrins are important in the formation of the immunological synapse between antigen-presenting cells (APCs) and T cells. The context and dynamics of this interaction determine whether T cells become activated or tolerized. β2 integrins, and their ligand, ICAM-1, are expressed by both the T cell and the APC and are vital in immune synapse formation. Importantly, it is becoming increasingly clear that β2 integrins expressed by the APC and T cell have opposing functions in the immune synapse, resulting in differential outcomes for the T cell response.
On the T cell side, CD11a clusters in the peripheral supramolecular activation cluster (P-SMAC) and binds to ICAM-1 on the APC (68). This molecular interaction stabilizes the connection made between T cell receptor and peptide:MHC on the APC in the central SMAC (16, 69), thereby enhancing TCR signal transduction (70). While T cell CD11a therefore has a largely pro-inflammatory effect, enhancing T cell activation, proliferation, and differentiation, a role for T cell integrins in regulation of activation, for example, in different T cell subsets, is not ruled out.
On the APC side of the immunological synapse, β2 integrins have also been shown to be involved, likely binding to ICAM-1 on the T cell. Importantly, the integrins on the APC regulate the outcome of the T cell response. For example, in murine models, active CD11b on DC surfaces inhibits the DC–T cell interaction (71). The reduced antigen-presenting capabilities of murine bone marrow-derived macrophages compared to BMDCs were therefore proposed to be due to their comparably larger surface expression of activated CD11b (71, 72). This suppressive role for DC CD11b has also been shown in human cells. When CD11b on human monocyte-derived DCs binds its ligand ICAM-1, both CD86 expression on DCs and DC-induced T cell proliferation were reduced (73). Interestingly, ligation of CD11b/CD18 decreases the ability of murine BMDCs to stimulate T cells and elicit a downstream response (74), CD11b/CD18 interactions can suppress Th17 cell differentiation (75), suggesting a strong role for this specific β2 integrin in immune regulation. This suggests that the activated conformation of CD11b/CD18 is extensively involved in regulating the immune system and has strong negative and positive regulatory functions depending on cell type they are expressed on.
Furthermore, the expression of activated β2 integrins on murine DC surfaces significantly reduces T cell activation (71) and further studies actually demonstrated an inverse relationship between forced activation of murine BMDC CD11a and T cell activation (72), suggesting a directly limiting effect of active β2 integrins on T cell activation by APCs.
Overall, the role of integrins as adhesion molecules carefully mediating and regulating cellular interactions is not to be underestimated for mounting an effective immune response.
β2 Integrins Regulate Immune Cell Signaling
In addition to their roles in leukocyte recruitment and interactions, several studies show that integrin outside-in signaling following ligand binding can directly affect cell function. Chinese Hamster Ovarian cells transfected with CD11c acquire the ability to bind both LPS and Gram-negative bacteria, as well as the ability to initiate downstream activation signals (76). In contrast to their anti-inflammatory roles on DCs, CD11b or CD11c receptor occupation on the surface of human monocytes stimulates cell-specific pro-inflammatory pathways (77), such as secretion of IL-8, MIP1α, and MIP1β.
Generally, the interplay between TLR4- and β2 integrin-mediated signaling is controversial. On the one hand, it has been shown that CD11b positively regulates TLR4 signaling (78), especially in murine BMDCs. Several studies report β2 integrins act in synergy with LPS (79–81), therefore suggesting a potential pro-inflammatory role for CD11b. By contrast, other studies report that β2 integrins negatively affect TLR signaling. Complete absence of β2 integrins in mice (CD18 KO) was shown to result in a strong increase of TLR signaling (82) and the absence of CD11b specifically from murine macrophages causes exacerbated TLR-mediated inflammatory responses, resulting in increased susceptibility to endotoxin shock and Escherichia coli sepsis (83). Mechanistically, CD11b signaling has been shown to induce degradation of the key TLR signaling components, MyD88 and TRIF, directly dampening TLR responses in macrophages (83). Moreover, activation of CD11b on human inflammatory arthritis synovial macrophages via binding to its ligand ICAM was shown to indirectly inhibit TLR signaling (84) by inducing expression of IL-10 and the inhibitory factors SOCS3, ABIN-3, and A20. Integrins furthermore restrict TLR signaling on both murine macrophages and DCs (63). The role of β2 integrins in modulating TLR signaling is, therefore, complex, although one could tentatively propose that CD11b specifically seems to have opposing TLR4-mediated roles in inflammation, depending on the APC surface it is expressed on. However, while this could hold true for TLR4 signaling, this might not be the case for all TLRs. CD11b deficiency in murine BMDCs, while negatively affecting TLR4-mediated pathways, actually leads to an increase in DC cross-priming of cytotoxic T cells, a process mediated by the microRNA-146a (85). β2 integrin regulation of TLR-mediated responses therefore remains incompletely understood, with future studies hopefully elucidating the complex and intricate nature of these receptor interactions.
A variety of studies available suggest a significant immunoregulatory role for β2 integrins, not only by their mediation of adhesive and migratory processes, but also by immunological signaling. However, other studies suggest that, given the right cellular environment or cell type, β2 integrins can also have a strong pro-inflammatory effect (see Table 2 for comparison). When considering these opposing functions of integrins, it seems likely that even slight disturbances in integrin expression, signaling or activation could result in significant immunological effects, thus potentially contributing to a variety of autoimmune, inflammatory, and infectious conditions.
Table 2.
Summary of the roles for β2 integrins in monocytes, macrophages, and dendritic cells (DCs).
| Cell type | Recruitment and migration | Interactions with T cells | Signaling |
|---|---|---|---|
| Monocytes | β2 integrins mediate recruitment of monocytes under homeostatic conditions (45, 65), but dispensable for recruitment during inflammation (66) | Yet to be determined | Yet to be determined |
| Pro-inflammatory | Unknown | Unknown | |
| Macrophages | β2 integrins reported to mediate macrophage retention at inflammatory sites (56, 86) | Yet to be determined | β2 integrin signaling dampens macrophage responses to Toll-like receptor (TLR) stimulation (82, 83) |
| Pro-inflammatory | Unknown | Regulatory | |
| DCs | Under homeostatic conditions β2 integrins restrict DC migration from tissue to lymph nodes (67); Migration from tissue site to draining lymph nodes during inflammation occurs independently of integrins (64) | DC integrins contribute to contact formation with T cells—this role inhibits full T cell activation (71, 72, 74) | β2 integrin signaling functions to restrict DC activation both in response to TLR stimulation and under homeostatic conditions (67) |
| Regulatory | Regulatory | Regulatory | |
β2 Integrins in Inflammation, Infection, and Autoimmunity
Evidence for the role of β2 integrins in contributing to the development and progression of inflammatory and autoimmune conditions is accumulating. Considering that β2 integrin signaling can have opposing functions depending on subunit pairing and the immune cell type it is expressed on, it is not surprising that these receptors play important roles in both contributing to as well as negatively regulating inflammatory processes.
Human genetic studies point to a role of β2 integrins in inflammation and autoimmunity. A polymorphism of ITGAM, the CD11b subunit, increases the risk for the autoimmune disease systemic lupus erythematosus (87) (SLE), which shares genetic risk factors with rheumatoid arthritis (RA) (88). Disease risk for inflammatory bowel disease, similarly characterized by dysregulation of immune function specifically in the intestine, increases with amplified expression of alleles for both ITGAL, encoding CD11a, and the β2 integrin ligand ICAM1 (89). Gene expression of CD11d in humans and mice was found to be increased in white adipose tissue in obesity, a condition characterized by an increase in systemic inflammation (90). Furthermore, CD11d activation led to increased IL-1β expression (23), which when overproduced can contribute to a variety of autoinflammatory conditions (91). While dysregulation of β2 integrin signaling seems likely to be involved in a variety of autoimmune diseases and inflammatory conditions, exact mechanisms are still unclear, and further investigation of both signaling pathways and genetic basis will be needed to fully elucidate their complex roles.
Recent studies have focused on β2 integrin involvement in RA, which serves as an excellent example of the opposing roles β2 integrins can take in disease. Expression of CD11a is increased in inflamed synovial tissue, where it is hypothesized to contribute to cell activation and on-going joint destruction (92, 93), but not in peripheral blood of RA patients. However, as CD11a is also involved in facilitating immune cell migration to sites of inflammation, clear-cut cause and effect of the presence of activated β2 integrins in the synovium is difficult to establish. Blocking all β2 integrins reduced inflammation in a rabbit RA model (94), while absence of CD11a led to complete resistance to disease induction in a KB × N serum transfer mouse model of arthritis (95). Furthermore, both a small molecule antagonist against CD11a and a CD11a-monoclonal antibody (mAb) proved to be similarly successful in reducing both inflammatory-mediated bone destruction and cytokine mRNA levels within the murine joint (96, 97). Mice with mutations in the β2 integrin ligand ICAM-1 also show reduced susceptibility to the collagen-induced arthritis (CIA) model (98). Clearly, CD11a–ICAM-1 interactions are essential for leukocyte recruitment to the inflamed joint.
However, evidence is emerging that other β2 integrins may function to control inflammation in arthritis. CD11b KO mice, for example, show exacerbated joint pathology in the KB x N serum transfer model of arthritis, underlining the starkly opposite roles different β2 integrins can play (95). A recent study replicated these results in a CIA model and, furthermore, showed that exacerbated joint pathology resulted from elevated IL-6 levels and an increase in Th17 cell priming, which could be rescued by introducing a CD11b-expressing DC cell line (99). On the other hand, blocking CD11b immediately before onset of disease significantly reduced disease burden in two different models of arthritis (CIA and a DBA/1 to severe combined immunodeficiency transfer model of arthritis) (100), suggesting that the role of CD11b in inflammatory arthritis may differ depending on the cell type involved and the disease stage.
When considering the importance, as well as the obvious complexity, of β2 integrin function in autoimmune diseases such as RA, therapeutically targeting β2 integrins will have to be carefully balanced but also holds great promise to offer novel treatment options.
Applicability of Integrin-Targeting Therapies
Modulating integrin function to improve mal-adaptation or excessive activation of the immune system is of great interest in a variety of autoimmune and inflammatory conditions. However, achieving efficacy without immunocompromising side effects might prove challenging. Here, we discuss the progress and failures in developing integrin-targeted therapies and speculate on the routes forward for success.
To date, targeting integrins therapeutically has had mixed success in the clinic. The only mAb targeting β2 integrins, Efalizumab, which targets CD11a, was originally developed as a treatment for psoriasis (101). However, several patients presented with the potentially fatal disease progressive multifocal leukoencephalopathy (PML), caused by reactivation of the JC virus, which results in a white matter disorder of the brain (102). Although the mechanism of PML development in Efalizumab-treated patients was not investigated, we speculate that viral reactivation was likely either due to the loss of immune cell recruitment to the brain to control the virus (103) or due to the mAb itself crossing the blood–brain barrier (104). Due to the occurrence of PML, Efalizumab was withdrawn from European and American markets due to its associated safety issues in 2009.
Although targeting β2 integrins has so far failed in the clinic, targeting other integrins for the treatment of colitis and Crohn’s disease has proved successful. The mAb against the α4 integrin, Natalizumab, was developed for the treatment of multiple sclerosis and Crohn’s disease (105, 106). This mAb binds to α4β1 and α4β7. However, PML also occurs in some Natalizumab-treated patients (integrin α4β1 is also involved in leukocyte recruitment to the brain) and so is no longer used widely (107). More recently, a specific α4β7 targeting mAb Vedolizumab has shown success in safety efficacy in Crohn’s disease and ulcerative colitis. This success story underlines the potential of targeting integrins for therapeutic purposes.
In order to realize the potential of targeting β2 integrins therapeutically, it will be necessary to improve the strategy. As indicated by the success of Vedolizumab over Natalizumab, one way to do this is to target the right integrin subunit(s) in order to reduce the likelihood of side effects. Targeting CD11a, in the form of Efalizumab, proved unsuccessful in the clinic. As CD11a is expressed by almost all leukocytes, has vital roles in leukocyte recruitment and has immunoregulatory effects in mononuclear phagocytes, the resulting serious side effects from targeting this molecule therapeutically are, perhaps, not surprising. Targeting other CD11 subunits might be a more effective strategy. For example, CD11b, CD11c, and CD11d have a more restricted pattern of expression in leukocytes (predominantly on monocytes, macrophages, and DCs), which may make these molecules more suitable targets. Importantly, it is vital that we consider the pro- and anti-inflammatory functions of β2 integrin subunits and design drugs to target them appropriately. CD11b, for example, has clear regulatory roles in macrophages and DCs, meaning that we could potentially exploit this immuno-suppressive pathway by activating, rather than blocking, this integrin subunit. Such a strategy may have less risk of serious side effects. It is, therefore, essential that we fully understand the specific functions of individual integrin subunits in different leukocyte populations in order to target β2 integrin subunits effectively in the clinic.
Another option to explore is blocking not the β2 integrin itself, but the ligand of interest. Targeting the CD11a and CD11b ligand, ICAM-1, has shown beneficial results especially in early RA (108), although immunogenicity of the mAb in question restricts clinical use (109) and problems caused by impaired leukocyte recruitment prevail.
Further potential difficulties in developing integrin-targeting therapy include the close signaling relationships that exist in some integrins, potentially leading to complex downstream effects mediated even by an activating mAb highly specific for a β2 integrin (110). Carefully elucidating downstream signaling pathways and further increasing drug specificity is therefore essential to bring more integrin therapeutics into the clinic.
Innovative avenues to explore include computationally designed integrin proteins with constitutively activated or inactivated subunits, which could find applications in both pharmacological testing and therapy (111). Furthermore, developing small molecular drugs targeting β2 integrins viable for oral use remains a priority, as it could offer an alternative way to yield the same beneficial results without the dangerous side effects of mAbs. An example is the small molecule CD11b agonist, Leukadherin-1, which previous studies found to reduce monocyte-mediated TNF-release by mimicking natural ligand binding. When NK cells and monocytes were pre-treated with Leukadherin-1, innate inflammatory signaling in human ex vivo studies was suppressed (112). While the study noted some caveats, for example, the differences of CD11b function on different cell types (78), the drug is still being explored for the treatment of SLE. Another small molecule currently in development is the CD11a antagonist BMS-587101, which acts by reducing CD11a-mediated adhesion and to a lesser effect T cell proliferation. It significantly improved both murine models of lung inflammation and transplant viability (113).
Continuous effort to increase drug specificity and further understand their complex delicate signaling networks will be needed to bring β2 integrin-targeting drugs into the clinic. But while the use of integrin-targeting drugs has been contentious in the past, their potential in treating a wide variety of immune diseases is enormous and should not be neglected.
Conclusion
This review explored the opposing nature of β2 integrin pro- and anti-inflammatory functions in three main immune functions, making them prime candidates to be both important mediators and regulators of the immune system. The first is migration, which allows for targeted immune cell recruitment to sites of infection and tissue damage. The second is adhesion, not only preceding immune cell extravasation at sites of inflammation, but also an important factor in initiating the adaptive immune response by facilitating cellular interactions. Finally, immune cell signaling, which allows for fine-tuned cooperation between a wide variety of immune cells. Considering the fact that β2 integrins play a complex role in three important areas of the immune system and their differential expression on monocytes, macrophages and DCs, it becomes clear that the variety of studies presented in this review is by no means exhaustive. The common message is evident: β2 integrins are involved in complex immunoregulatory signaling pathways. However, in addition to their well-established pro-inflammatory roles in recruitment and activation, β2 integrins also have essential immunoregulatory functions. Dysregulated integrin signaling, expression and surface activation is therefore likely to contribute to a variety of inflammatory and autoimmune conditions. Elucidating the function of β2 integrins further therefore promises to provide novel therapeutic targets for various disorders, RA being just one example.
Author Contributions
CH and VM designed the structure of the review. LS wrote the first draft. CH and VM revised the manuscript. LS composed the figures. All authors have seen and agreed on the finally submitted version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a shared affiliation, though no other collaboration, with the authors LS and CH.
Acknowledgments
The authors are supported by Arthritis Research UK (grant number 20848 to VM) and the Arthritis Research UK Rheumatoid Arthritis Pathogenesis Centre of Excellence (RACE) (grant number 20298). They thank Dr. Megan MacLeod for critically reading the manuscript.
References
- 1.Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr Opin Cell Biol (2004) 16(5):544–51. 10.1016/j.ceb.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Adair BD, Xiong JP, Maddock C, Goodman SL, Arnaout MA, Yeager M. Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J Cell Biol (2005) 168(7):1109–18. 10.1083/jcb.200410068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. Anti-integrin monoclonal antibodies. J Cell Sci (2009) 122(22):4009–11. 10.1242/jcs.056770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem (1996) 61(4):554–61. [DOI] [PubMed] [Google Scholar]
- 5.Hogg N, Patzak I, Willenbrock F. The insider’s guide to leukocyte integrin signalling and function. Nat Rev Immunol (2011) 6:416–26. 10.1038/nri2986 [DOI] [PubMed] [Google Scholar]
- 6.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci (2009) 122(2):159–63. 10.1242/jcs.018093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, et al. Talin binding to integrin ß tails: a final common step in integrin activation. Science (2003) 302(5642):103–6. 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- 8.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fässler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med (2008) 14(3):325–30. 10.1038/nm1722 [DOI] [PubMed] [Google Scholar]
- 9.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, et al. Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med (2009) 15(3):300–5. 10.1038/nm.1921 [DOI] [PubMed] [Google Scholar]
- 10.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity (2000) 13(6):759–69. 10.1016/S1074-7613(00)00074-1 [DOI] [PubMed] [Google Scholar]
- 11.Chung KJ, Mitroulis I, Wiessner JR, Zheng YY, Siegert G, Sperandio M, et al. A novel pathway of rapid TLR-triggered activation of integrin-dependent leukocyte adhesion that requires Rap1 GTPase. Mol Biol Cell (2014) 25(19):2948–55. 10.1091/mbc.E14-04-0867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci (1998) 23(1):30–4. 10.1016/S0968-0004(97)01141-9 [DOI] [PubMed] [Google Scholar]
- 13.Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol (2003) 15(5):547–56. 10.1016/j.ceb.2003.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol (2015) 36:41–7. 10.1016/j.ceb.2015.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors KE, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A (1991) 88(17):7538–42. 10.1073/pnas.88.17.7538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monks CRF, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature (1998) 395(6697):82–6. 10.1038/25764 [DOI] [PubMed] [Google Scholar]
- 17.Podolnikova NP, Podolnikov AV, Haas TA, Lishko VK, Ugarova TP. Ligand recognition specificity of leukocyte integrin αMβ2 (Mac-1, CD11b/CD18) and its functional consequences. Biochemistry (2015) 54(6):1408–20. 10.1021/bi5013782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yakubenko VP, Yadav SP, Ugarova TP. Integrin α D β 2, an adhesion receptor up-regulated on macrophage foam cells, exhibits multiligand-binding properties. Blood (2006) 107(4):1643–50. 10.1182/blood-2005-06-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sándor N, Lukácsi S, Ungai-Salánki R, Orgován N, Szabó B, Horváth R, et al. CD11c/CD18 dominates adhesion of human monocytes, macrophages and dendritic cells over CD11b/CD18. PLoS One (2016) 11(9):e0163120. 10.1371/journal.pone.0163120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erdei A, Lukácsi S, Mácsik-Valent B, Nagy-Baló Z, Kurucz I, Bajtay Z. Non-identical twins: different faces of CR3 and CR4 in myeloid and lymphoid cells of mice and men. Semin Cell Dev Biol (2017). 10.1016/j.semcdb.2017.11.025 [DOI] [PubMed] [Google Scholar]
- 21.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol (1994) 156(1):191–211. 10.1006/cimm.1994.1164 [DOI] [PubMed] [Google Scholar]
- 22.Freudenthal PS, Steinman RM. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A (1990) 87(19):7698–702. 10.1073/pnas.87.19.7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyazaki Y, Vieira-de-Abreu A, Harris ES, Shah AM, Weyrich AS, Castro-Faria-Neto HC, et al. Integrin α D β 2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS One (2014) 9(11):e112770. 10.1371/journal.pone.0112770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science (2017) 356(6335):eaah4573. 10.1126/science.aah4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EMBL-EBI Expression Atlas Development Team, github.com/gxa/atlas/graphs/contributors. Expression atlas. EMBL-EBI Gene Expression Atlas. European Molecular Biology Laboratory - European Bioinformatics Institute; (2017). Available from: http://www.ebi.ac.uk/gxa/home [Google Scholar]
- 26.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol (2014) 14(8):571–8. 10.1038/nri3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor. J Exp Med (1994) 179(4):1109–18. 10.1084/jem.179.4.1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell (2000) 100(5):575–85. 10.1016/S0092-8674(00)80693-5 [DOI] [PubMed] [Google Scholar]
- 29.Ammon C, Meyer SP, Schwarzfischer L, Krause SW, Andreesen R, Kreutz M. Comparative analysis of integrin expression on monocyte-derived macrophages and monocyte-derived dendritic cells. Immunology (2000) 100(3):364–9. 10.1046/j.1365-2567.2000.00056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Rosa G, Longo N, Rodríguez-Fernández JL, Puig-Kroger A, Pineda A, Corbí AL, et al. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol (2003) 73(5):639–49. 10.1189/jlb.1002516 [DOI] [PubMed] [Google Scholar]
- 31.Rieckmann JC, Geiger R, Hornburg D, Wolf T, Kveler K, Jarrossay D, et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat Immunol (2017) 18(5):583–93. 10.1038/ni.3693 [DOI] [PubMed] [Google Scholar]
- 32.Li K, Fazekasova H, Wang N, Sagoo P, Peng Q, Khamri W, et al. Expression of complement components, receptors and regulators by human dendritic cells. Mol Immunol (2011) 48(9):1121–7. 10.1016/j.molimm.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, et al. Human tissues contain CD141 hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity (2012) 37(1):60–73. 10.1016/j.immuni.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robbins SH, Walzer T, Dembélé D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol (2008) 9(1):R17. 10.1186/gb-2008-9-1-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segura E, Guérin C, Hogg N, Amigorena S, Théry C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol (2007) 179(3):1489–96. 10.4049/jimmunol.179.3.1489 [DOI] [PubMed] [Google Scholar]
- 36.Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol (2014) 35(6):270–7. 10.1016/j.it.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 37.Nakano H, Yanagita M, Gunn MD. CD11c+ B220+ Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med (2001) 194(8):1171–8. 10.1084/jem.194.8.1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol (2002) 2(3):151–61. 10.1038/nri746 [DOI] [PubMed] [Google Scholar]
- 39.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol (2012) 13(9):888–99. 10.1038/ni.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helft J, Böttcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c+ MHCII+ macrophages and dendritic cells. Immunity (2015) 42(6):1197–211. 10.1016/j.immuni.2015.05.018 [DOI] [PubMed] [Google Scholar]
- 41.Bohuslav J, Horejsi V, Hansmann C, Stöckl J, Weidle UH, Majdic O, et al. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med (1995) 181(4):1381–90. 10.1084/jem.181.4.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprangers S, Schoenmaker T, Cao Y, Everts V, de Vries TJ. Integrin αMβ2 is differently expressed by subsets of human osteoclast precursors and mediates adhesion of classical monocytes to bone. Exp Cell Res (2017) 350(1):161–8. 10.1016/j.yexcr.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 43.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science (2007) 317(5838):666–70. 10.1126/science.1142883 [DOI] [PubMed] [Google Scholar]
- 44.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol (2004) 172(7):4410–7. 10.4049/jimmunol.172.7.4410 [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity (2003) 19(1):71–82. 10.1016/S1074-7613(03)00174-2 [DOI] [PubMed] [Google Scholar]
- 46.Noti JD. Expression of the myeloid-specific leukocyte integrin gene CD11d during macrophage foam cell differentiation and exposure to lipoproteins. Int J Mol Med (2002) 10:721–8. 10.3892/ijmm.10.6.721 [DOI] [PubMed] [Google Scholar]
- 47.Wright SD, Jong MT. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med (1986) 164(6):1876–88. 10.1084/jem.164.6.1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med (1998) 188(12):2313–20. 10.1084/jem.188.12.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlesinger LS, Horwitz MA. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol (1991) 147(6):1983–94. [PubMed] [Google Scholar]
- 50.Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog (2008) 4(8):e1000115. 10.1371/journal.ppat.1000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol (2012) 13(11):1118–28. 10.1038/ni.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med (1982) 156(4):1000–9. 10.1084/jem.156.4.1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drevets DA, Leenen PJ, Campbell PA. Complement receptor type 3 (CD11b/CD18) involvement is essential for killing of Listeria monocytogenes by mouse macrophages. J Immunol (1993) 151(10):5431–9. [PubMed] [Google Scholar]
- 54.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity (2013) 39(5):925–38. 10.1016/j.immuni.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 55.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A (2004) 61(2):170–7. 10.1002/cyto.a.20064 [DOI] [PubMed] [Google Scholar]
- 56.Yakubenko VP, Belevych N, Mishchuk D, Schurin A, Lam SC, Ugarova TP. The role of integrin α D β 2 (CD11d/CD18) in monocyte/macrophage migration. Exp Cell Res (2008) 314(14):2569–78. 10.1016/j.yexcr.2008.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Munoz F, Grabbe S, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med (1998) 188(1):119–31. 10.1084/jem.188.1.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris ES, Weyrich AS, Zimmerman GA. Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr Opin Hematol (2013) 20(1):16. 10.1097/MOH.0b013e32835a0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert P, Canault M, Farnarier C, Nurden A, Grosdidier C, Barlogis V, et al. A novel leukocyte adhesion deficiency III variant: kindlin-3 deficiency results in integrin- and nonintegrin-related defects in different steps of leukocyte adhesion. J Immunol (2011) 186(9):5273–83. 10.4049/jimmunol.1003141 [DOI] [PubMed] [Google Scholar]
- 60.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the β subunit common to the LFA-1, Mac-1, and p150, 95 glycoproteins cause leukocyte adhesion deficiency. Cell (1987) 50(2):193–202. 10.1016/0092-8674(87)90215-7 [DOI] [PubMed] [Google Scholar]
- 61.D’agata ID, Paradis K, Chad Z, Bonny Y, Seidman E. Leucocyte adhesion deficiency presenting as a chronic ileocolitis. Gut (1996) 39(4):605–8. 10.1136/gut.39.4.605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hajishengallis G, Moutsopoulos NM. Role of bacteria in leukocyte adhesion deficiency-associated periodontitis. Microb Pathog (2016) 94:21–6. 10.1016/j.micpath.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savinko TS, Morrison VL, Uotila LM, Wolff CHJ, Alenius HT, Fagerholm SC. Functional beta2-integrins restrict skin inflammation in vivo. J Invest Dermatol (2015) 135(9):2249–57. 10.1038/jid.2015.164 [DOI] [PubMed] [Google Scholar]
- 64.Lämmermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Söldner R, Hirsch K, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature (2008) 453(7191):51–5. 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- 65.Chuluyan HE, Issekutz AC. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest (1993) 92(6):2768. 10.1172/JCI116895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood (2003) 102(1):328–35. 10.1182/blood-2002-10-3228 [DOI] [PubMed] [Google Scholar]
- 67.Morrison VL, James MJ, Grzes K, Cook P, Glass DG, Savinko T, et al. Loss of beta2-integrin-mediated cytoskeletal linkage reprograms dendritic cells to a mature migratory phenotype. Nat Commun (2014) 5:5359. 10.1038/ncomms6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benvenuti F. The dendritic cell synapse: a life dedicated to T cell activation. Front Immunol (2016) 7:70. 10.3389/fimmu.2016.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science (1999) 285(5425):221–7. 10.1126/science.285.5425.221 [DOI] [PubMed] [Google Scholar]
- 70.Van Seventer GA, Shimizu Y, Horgan KJ, Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol (1990) 144(12):4579–86. [PubMed] [Google Scholar]
- 71.Varga G, Balkow S, Wild MK, Stadtbaeumer A, Krummen M, Rothoeft T, et al. Active MAC-1 (CD11b/CD18) on DCs inhibits full T-cell activation. Blood (2007) 109(2):661–9. 10.1182/blood-2005-12-023044 [DOI] [PubMed] [Google Scholar]
- 72.Balkow S, Heinz S, Schmidbauer P, Kolanus W, Holzmann B, Grabbe S, et al. LFA-1 activity state on dendritic cells regulates contact duration with T cells and promotes T-cell priming. Blood (2010) 116(11):1885–94. 10.1182/blood-2009-05-224428 [DOI] [PubMed] [Google Scholar]
- 73.Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol (2009) 183(3):1767–79. 10.4049/jimmunol.0802167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Behrens EM, Sriram U, Shivers DK, Gallucci M, Ma Z, Finkel TH, et al. Complement receptor 3 ligation of dendritic cells suppresses their stimulatory capacity. J Immunol (2007) 178(10):6268–79. 10.4049/jimmunol.178.10.6268 [DOI] [PubMed] [Google Scholar]
- 75.Ehirchiou D, Xiong Y, Xu G, Chen W, Shi Y, Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med (2007) 204(7):1519–24. 10.1084/jem.20062292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ingalls RR, Golenbock DT. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med (1995) 181(4):1473–9. 10.1084/jem.181.4.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c β2 integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1α (MIP-1α) and MIP-1β production in primary human monocytes through a pathway dependent on nuclear factor-κB. Blood (2001) 97(10):2932–40. 10.1182/blood.V97.10.2932 [DOI] [PubMed] [Google Scholar]
- 78.Ling GS, Bennett J, Woollard KJ, Szajna M, Fossati-Jimack L, Taylor PR, et al. Integrin CD11b positively regulates TLR4-induced signalling pathways in dendritic cells but not in macrophages. Nat Commun (2014) 5:3039. 10.1038/ncomms4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan ST, Edgington TS. Coupling of the adhesive receptor CD11b/CD18 to functional enhancement of effector macrophage tissue factor response. J Clin Invest (1991) 87(1):50. 10.1172/JCI115000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ingalls RR, Arnaout MA, Golenbock DT. Outside-in signaling by lipopolysaccharide through a tailless integrin. J Immunol (1997) 159(1):433–8. [PubMed] [Google Scholar]
- 81.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, et al. CD11b/CD18 acts in concert with CD14 and toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J Immunol (2001) 166(1):574–81. 10.4049/jimmunol.166.1.574 [DOI] [PubMed] [Google Scholar]
- 82.Yee NK, Hamerman JA. β2 integrins inhibit TLR responses by regulating NF-κB pathway and p38 MAPK activation. Eur J Immunol (2013) 43(3):779–92. 10.1002/eji.201242550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol (2010) 11(8):734–42. 10.1038/ni.1908 [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, et al. Indirect inhibition of toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity (2010) 32(4):518–30. 10.1016/j.immuni.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bai Y, Qian C, Qian L, Ma F, Hou J, Chen Y, et al. Integrin CD11b negatively regulates TLR9-triggered dendritic cell cross-priming by upregulating microRNA-146a. J Immunol (2012) 188(11):5293–302. 10.4049/jimmunol.1102371 [DOI] [PubMed] [Google Scholar]
- 86.Kushchayeva Y, Mishchuk D, Ugarova T. The role of beta 2 integrins in macrophage migration during resolution of inflammation. Blood (2009) 114:3600. [Google Scholar]
- 87.Nath SK, Han S, Kim-Howard X, Kelly JA, Viswanathan P, Gilkeson GS, et al. A nonsynonymous functional variant in integrin-αM (encoded by ITGAM) is associated with systemic lupus erythematosus. Nat Genet (2008) 40(2):152–4. 10.1038/ng.71 [DOI] [PubMed] [Google Scholar]
- 88.Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med (2007) 357(10):977–86. 10.1056/NEJMoa073003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet (2017) 49(2):256–61. 10.1038/ng.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas AP, Dunn TN, Oort PJ, Grino M, Adams SH. Inflammatory phenotyping identifies CD11d as a gene markedly induced in white adipose tissue in obese rodents and women. J Nutr (2011) 141(6):1172–80. 10.3945/jn.110.127068 [DOI] [PubMed] [Google Scholar]
- 91.Dinarello CA. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J Intern Med (2011) 269(1):16–28. 10.1111/j.1365-2796.2010.02313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cush JJ, Lipsky PE. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum (1988) 31(10):1230–8. 10.1002/art.1780311003 [DOI] [PubMed] [Google Scholar]
- 93.Takahashi H, Söderström K, Nilsson E, Kiessling R, Patarroyo M. Integrins and other adhesion molecules on lymphocytes from synovial fluid and peripheral blood of rheumatoid arthritis patients. Eur J Immunol (1992) 22(11):2879–85. 10.1002/eji.1830221119 [DOI] [PubMed] [Google Scholar]
- 94.Jasin HE, Lightfoot E, Davis LS, Rothlein R, Faanes RB, Lipsky PE. Amelioration of antigen-induced arthritis in rabbits treated with monoclonal antibodies to leukocyte adhesion molecules. Arthritis Rheum (1992) 35(5):541–9. 10.1002/art.1780350508 [DOI] [PubMed] [Google Scholar]
- 95.Watts GM, Beurskens FJ, Martin-Padura I, Ballantyne CM, Klickstein LB, Brenner MB, et al. Manifestations of inflammatory arthritis are critically dependent on LFA-1. J Immunol (2005) 174(6):3668–75. 10.4049/jimmunol.174.6.3668 [DOI] [PubMed] [Google Scholar]
- 96.Suchard SJ, Stetsko DK, Davis PM, Skala S, Potin D, Launay M, et al. An LFA-1 (αLβ2) small-molecule antagonist reduces inflammation and joint destruction in murine models of arthritis. J Immunol (2010) 184(7):3917–26. 10.4049/jimmunol.0901095 [DOI] [PubMed] [Google Scholar]
- 97.Kakimoto K, Nakamura T, Ishii K, Takashi T, Iigou H, Yagita H, et al. The effect of anti-adhesion molecule antibody on the development of collagen-induced arthritis. Cell Immunol (1992) 142(2):326–37. 10.1016/0008-8749(92)90294-Y [DOI] [PubMed] [Google Scholar]
- 98.Bullard DC, Hurley LA, Lorenzo I, Sly LM, Beaudet AL, Staite ND. Reduced susceptibility to collagen-induced arthritis in mice deficient in intercellular adhesion molecule-1. J Immunol (1996) 157(7):3153–8. [PubMed] [Google Scholar]
- 99.Stevanin M, Busso N, Chobaz V, Pigni M, Ghassem-Zadeh S, Zhang L, et al. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur J Immunol (2017) 47(4):637–45. 10.1002/eji.201646565 [DOI] [PubMed] [Google Scholar]
- 100.Taylor PC, Chu CQ, Plater-Zyberk C, Maini RN. Transfer of type II collagen-induced arthritis from DBA/1 to severe combined immunodeficiency mice can be prevented by blockade of Mac-1. Immunology (1996) 88(2):315–21. 10.1111/j.1365-2567.1996.tb00021.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leonardi CL, Papp KA, Gordon KB, Menter A, Feldman SR, Caro I, et al. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol (2005) 52(3):425–33. 10.1016/j.jaad.2004.09.029 [DOI] [PubMed] [Google Scholar]
- 102.Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med (2010) 61:35–47. 10.1146/annurev.med.080708.082655 [DOI] [PubMed] [Google Scholar]
- 103.Schwab N, Ulzheimer JC, Fox RJ, Schneider-Hohendorf T, Kieseier BC, Monoranu CM, et al. Fatal PML associated with efalizumab therapy insights into integrin αLβ2 in JC virus control. Neurology (2012) 78(7):458–67. 10.1212/WNL.0b013e3182478d4b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M, Progressive Multifocal Leukeoncephalopathy Consortium . Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord (2015) 8(6):255–73. 10.1177/1756285615602832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med (2006) 354(9):899–910. 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 106.Ghosh S, Goldin E, Gordon FH, Malchow HA, Rask-Madsen J, Rutgeerts P, et al. Natalizumab for active Crohn’s disease. N Engl J Med (2003) 348(1):24–32. 10.1056/NEJMoa020732 [DOI] [PubMed] [Google Scholar]
- 107.Berger JR, Fox RJ. Reassessing the risk of natalizumab-associated PML. J Neurovirol (2016) 22(4):533–5. 10.1007/s13365-016-0427-6 [DOI] [PubMed] [Google Scholar]
- 108.Kavanaugh AF, Davis LS, Jain RI, Nichols LA, Norris SH, Lipsky PE. A phase I/II open label study of the safety and efficacy of an anti-ICAM-1 (intercellular adhesion molecule-1; CD54) monoclonal antibody in early rheumatoid arthritis. J Rheumatol (1996) 23(8):1338–44. [PubMed] [Google Scholar]
- 109.Vuorte J, Lindsberg PJ, Kaste M, Meri S, Jansson SE, Rothlein R, et al. Anti-ICAM-1 monoclonal antibody R6.5 (enlimomab) promotes activation of neutrophils in whole blood. J Immunol (1999) 162(4):2353–7. [PubMed] [Google Scholar]
- 110.Grönholm M, Jahan F, Bryushkova EA, Madhavan S, Aglialoro F, Soto Hinojosa L, et al. LFA-1 integrin antibodies inhibit leukocyte α4β1-mediated adhesion by intracellular signaling. Blood (2016) 128(9):1270–81. 10.1182/blood-2016-03-705160 [DOI] [PubMed] [Google Scholar]
- 111.Shimaoka M, Shifman JM, Jing H, Takagi J, Mayo SL, Springer TA. Computational design of an integrin I domain stabilized in the open high affinity conformation. Nat Struct Mol Biol (2000) 7(8):674–8. 10.1038/77978 [DOI] [PubMed] [Google Scholar]
- 112.Roberts AL, Fürnrohr BG, Vyse TJ, Rhodes B. The complement receptor 3 (CD11b/CD18) agonist leukadherin-1 suppresses human innate inflammatory signalling. Clin Exp Immunol (2016) 185(3):361–71. 10.1111/cei.12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Potin D, Launay M, Monatlik F, Malabre P, Fabreguettes M, Fouquet A, et al. Discovery and development of 5-[(5 S, 9 R)-9-(4-cyanophenyl)-3-(3, 5-dichlorophenyl)-1-methyl-2, 4-dioxo-1, 3, 7-triazaspiro [4.4] non-7-yl-methyl]-3-thiophenecarboxylic acid (BMS-587101) a small molecule antagonist of leukocyte function associated antigen-1. J Med Chem (2006) 49(24):6946–9. 10.1021/jm0610806 [DOI] [PubMed] [Google Scholar]