Abstract
Aim: Children exposed to parental smoking are at increased long-term risk of subclinical atherosclerosis in adulthood. However, it has not been quantified if exposure to parental smoking in childhood is associated with adult systemic inflammation. This study aimed to determine if childhood exposure to parental smoking was associated with high-sensitivity C-reactive protein (hsCRP) in adulthood.
Methods: This longitudinal analysis of 2,511 participants used data from the Cardiovascular Risk in Young Finns Study, a prospective cohort of Finnish children. In 1980 or 1983, parents self-reported their smoking status and serum hsCRP was collected up to 31 years later in adulthood.
Results: Compared with children with non-smoking parents, the relative risk of developing high hsCRP (> 3 mg/L) in adulthood increased among those with 1 or both parents who smoked [relative risk (RR), 1.3; 95%confidence interval (CI), 1.0–1.8] after adjustment for socioeconomic status, cardiovascular risk factors, and smoking status in childhood and adulthood. Moreover, children exposed to mother smoking [RR, 2.4; 95% CI, 1.3–4.2] had highest risk of developing high hsCRP in adulthood compared with those exposed to father smoking [RR, 1.6; 95% CI, 1.2–2.3] and both parents smoking [RR, 1.4; 95% CI, 0.9–2.0].
Conclusion: Our findings suggest that children exposed to parental smoking are at increased risk of having high hsCRP in adulthood. Limiting children's exposure to passive smoking may have long-term benefits on general low-grade inflammation.
Keywords: Child, Parental smoking, Atherosclerosis, Inflammation, C-reactive protein, Cohort
See editorial vol. 24: 1204–1205
Introduction
Exposure to environmental tobacco smoke (passive smoking) is a major modifiable risk factor for cardiovascular health1–4). Children appear particularly vulnerable to increased exposure to passive smoking and acute and long-term health burdens from exposure to passive smoking5–7). For example, studies have shown that exposure to parental smoking in utero or in childhood is associated with poorer markers of vascular structure and function in childhood and adulthood, indicating advanced preclinical atherosclerosis8–15). Furthermore, parents who smoked but did so hygienically (i.e. did not smoke in the vicinity of their child) could decrease their child's risk of developing atherosclerosis in young to middle adulthood12).
Low-grade inflammation, characterized by only minor high-sensitivity C-reactive protein (hsCRP) elevation, is considered one potential patho-physiological mechanism by which exposure to smoking might increase risk for coronary artery disease, endothelial dysfunction, and atherosclerotic plaque formation16, 17). Studies in adults have indicated that active smoking increases coronary artery disease risk possibly due to a significant increase of low-grade inflammation18–20). Epidemiological studies in adults have observed a direct association between exposure to passive smoking and low-grade inflammation, while the implementation of smoke-free legislation was shown to result in significant reduction of hsCRP and other inflammatory markers21–23). However, it has not been quantified if exposure to parental smoking in childhood is associated with low-grade inflammation related to atherosclerosis in their adult offspring.
Therefore, to better understand the long-term influence of exposure to parental smoking in childhood on later cardiovascular health, we aimed to determine if parental smoking in childhood was associated with hsCRP in adult offspring. To examine this aim, we used data from the Cardiovascular Risk in Young Finns Study - a prospective cohort of a representative sample of Finnish children that began in 1980 and who have up to 31 years of follow-up24).
Participants and Methods
Participants
Participants were from the Cardiovascular Risk in Young Finns Study, an ongoing population-based prospective cohort study designed to investigate the early-life risk factors of cardiovascular disease among children and young adults in Finland. In 1980, a total of 3,596 participants aged 3, 6, 9, 12, 15, and 18 years attended the first cross-sectional (baseline) survey, and of these, 2,991 attended the 3-year follow-up performed in 1983. Adult clinic follow-ups were performed in 2001, 2007, and 2011 when a total of 2,284, 2,204, and 2,063 participants attended (refer to Supplemental Fig. I). In this study, we excluded those participants with hsCRP levels more than 10 mg/L, chronic rheumatic disease, history of infection in the previous two weeks, type 1 diabetes, pregnant women, lactating women, and those women currently using oral contraceptives in 2011 (total n = 315), 2007(total n = 408) and 2001 (total n = 588), as we have shown these factors to influence hsCRP levels25). However, to maximize our sample size, we used the adult hsCRP level from the most recent available from the 2001, 2007, or 2011 adult follow-ups. For example, data from 2007 or 2001 was used if it was missing in 2011. This was also the case for those who met our exclusion criteria on one occasion but not another. Because of this approach, we had data from up to 2,511 participants for our analyses. Other covariates in adulthood were generated in the same way. Local ethics committees approved the study and all participants or their parents provided written informed consent. Full details of the Cardiovascular Risk in Young Finns Study have been described elsewhere24).
Supplemental Fig. 1.
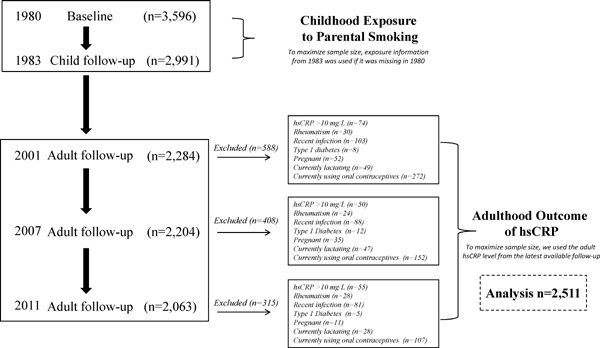
Flow chart showing participants in the Cardiovascular Risk in Young Finns Study and those eligible for this study
Measures
Exposure Measures
At 1980 and 1983, parental smoking status was self-reported by parents as part of a questionnaire that was sent to the participant's home. Parents were asked whether the mother or father had ever smoked daily for at least 1 year (responses could be “yes” or “no”). Accordingly, “regular smoker” was designated to those whom ever smoked daily for at least 1 year. Families were further classified into “none” or “1 or both parents smoking”12). In general, we used parental smoking status data from 1980. Where these data were missing in 1980, data from 1983 was used.
Clinic Characteristics and Risk Factors
At baseline and adult follow-ups, height and weight were measured and body mass index (BMI) calculated as weight in kilograms divided by height in meters squared. There were two different kinds of physical activity questionnaires for the younger (3–6 years old) and older (9–18 years old) children. The calculated physical activity indices were age-standardized to allow comparison across age groups26). At baseline, parents of participants self-reported total fruit and vegetable consumption of their child. Participants aged 12–18 years in 1980 reported their own smoking status without their parents present (for the purpose of our analyses, we assumed children aged < 12 years were non-smokers). At adult follow-ups, questionnaires gathered data on participant's own active and passive smoking status. In childhood, parents' total years of schooling was considered an indicator of socio-economic status, whereas in adulthood, the participant's total years of schooling was used in the analyses26). Blood pressure was measured at baseline from the brachial artery using a standard mercury sphygmomanometer for those aged 6 years and older, and using an ultrasound device for those aged 3 years. Blood pressure for the adult follow-ups was measured using a random-zero sphygmomanometer. At baseline and follow-ups, serum total cholesterol, triglyceride and high-density lipoprotein (HDL)-cholesterol concentrations were determined using standard enzymatic methods and corrected for changes in methods and reagents across study years. Low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald equation. Full details of serum lipids have been published previously27). Childhood serum samples were taken in 1980 and stored at −20°C. During the storage, the samples were not thawed or refrozen. Childhood hsCRP level in 1980 was assayed from stored serum samples that were analyzed in 2005, with values shown to be stable despite storage25).
Outcome Measures
hsCRP levels were determined on an automated analyzer (Olympus AU400) using a turbidimetric immunoassay kit (1980 and 2001, CRP-UL reagent, detection limit = 0.02 mg/L, interassay CV < 5%; 2007 and 2011, CRP Latex reagent)25, 27). In this study, participant's adult hsCRP status was categorized as either low (≤ 3 mg/L) or high (> 3 mg/L) based on recommendations issued by the American Heart Association and a number of published papers28–32).
Statistical Analyses
Continuous variables were displayed as mean (SD) or, if skewed distributions, as median (25th–75th percentiles), and categorical variables as proportions. Comparisons of baseline and adult characteristics according to the adult hsCRP status (≤ 3 vs. > 3 mg/L) were examined using t-tests or One-way ANOVA. The association between childhood exposure to parental smoking and serum hsCRP in adulthood was examined using multivariable log binomial regression adjusting for a series of potential confounders and mediators. Model 1 adjusted for child age and sex. Model 2 additionally adjusted for child BMI, physical activity index, fruit and vegetable consumption, and parental school years. Model 3 additionally adjusted for child risk factors (systolic blood pressure, serum lipids). Model 4 additionally adjusted for adult BMI, own smoking, school years. Model 5 additionally adjusted for adult risk factors (systolic blood pressure, serum lipids). As a separate analysis to determine if the effect of maternal vs. paternal smoking on offspring hsCRP differed, we stratified our exposure into none vs. maternal only vs. paternal only vs. both parents smoking. All models adjusted for the covariates listed for model 5 (above), but the sample size was somewhat reduced owing to restriction to two-parent families (n = 2010). To supplement the dichotomous hsCRP outcome, we also repeated the above models using continuous hsCRP in adulthood as the outcome. Continuous hsCRP in adulthood was log-transformed because of a right-skewed distribution. The association between childhood exposure to parental smoking and log-hsCRP in adulthood was examined using multivariable linear regression. In sensitivity analyses, we further adjusted for child hsCRP. This was not included in the above models as only a reduced sample size (n = 2060/2511) had child hsCRP data available. We also performed a sensitivity analysis that additionally adjusted for adult exposure to passive smoking. Self report exposure to passive smoking in the home, workplace, and elsewhere was collected in 2001, 2007, and 2011. Where data on adult exposure to passive smoking were missing, we assumed these participants were not passive smoking. The proportion of participants exposed to passive smoking in adulthood was 6.9% (n = 174/2511). As we observed no significant multiplicative sex* or age*exposure interactions, the data were not stratified by these covariates. Statistical analyses were performed with STATA version 13 (Stata Corp, TX). All probability values were two-tailed and all confidence intervals were estimated at the 95% level.
Results
Child and adult characteristics of participants according to adult hsCRP status are shown in Table 1. High hsCRP (> 3 mg/L) was present in 271 (10.9%) participants. There were age and sex differences between hsCRP groups with the low hsCRP group younger on average and less likely to be females than those with high hsCRP levels.
Table 1. Baseline and adult characteristics of participants according to circulating hsCRP level in adulthood (n = 2,511).
| Adult hsCRP |
|||
|---|---|---|---|
| Low (≤ 3 mg/L) | High (< 3 mg/L) | P value | |
| (n = 2,238) | (n = 273) | ||
| Baseline | |||
| Female sex, % | 49 | 60 | 0.001 |
| Age, y | 10.6 (5.0) | 11.4 (5.1) | 0.013 |
| Parental school years, y* | 9 (8, 11.5) | 9 (8, 10.5) | 0.006 |
| Smoking prevalence, %† | 6.2 | 8.0 | 0.258 |
| BMI, kg/m2 | 17.8 (2.9) | 19.0 (3.8) | < 0.001 |
| Systolic BP, mm Hg | 112 (12) | 115 (13) | < 0.001 |
| HDL cholesterol, mmol/L | 1.56 (0.31) | 1.54 (0.31) | 0.329 |
| LDL cholesterol, mmol/L | 3.45 (0.82) | 3.40 (0.78) | 0.322 |
| TG, mmol/L | 0.59 (0.45, 0.79) | 0.64 (0.47, 0.89) | 0.007 |
| hsCRP, mg/L | 0.21 (0.11 ,0.52) | 0.44 (0.2, 0.84) | < 0.001 |
| Fruit consumption, frequency/wk# | 6.3 (6.3, 9.5) | 6.3 (6.3, 9.5) | 0.327 |
| Vegetable consumption, frequency/wk# | 6.3 (3.0, 9.5) | 6.3 (3.0, 9.5) | 0.037 |
| standardized PAI‡ | 0.02 (1.01) | −0.05 (0.94) | 0.326 |
| Adulthood | |||
| Age, y | 41.6 (5.0) | 42.4 (5.1) | 0.013 |
| Own school years, y* | 15 (12, 17) | 14 (12, 17) | 0.007 |
| Own smoking prevalence, % | 19 | 22 | 0.159 |
| Passive smoking prevalence, % | 7 | 9 | 0.194 |
| BMI, kg/m2 | 25.8 (4.3) | 31.1 (6.6) | < 0.001 |
| Systolic BP, mm Hg | 119 (14) | 124 (15) | < 0.001 |
| HDL cholesterol, mmol/L | 1.32 (0.32) | 1.22 (0.31) | < 0.001 |
| LDL cholesterol, mmol/L | 3.27 (0.83) | 3.29 (0.84) | 0.770 |
| TG, mmol/L | 1.05 (0.75, 1.5) | 1.36 (0.95, 1.9) | < 0.001 |
Data are mean (SD) or median (25th, 75th percentile) for continuous variables and percentages for categorical variables. Participants with hsCRP levels < 10 mg/L, type 1 diabetes, chronic rheumatic disease, history of recent infection, and pregnant women, lactating women, and those using oral contraceptives were excluded from the analysis. Abbreviations: confidence interval, CI; high-sensitivity C-reactive protein, hsCRP; body mass index, BMI; physical activity index, PAI; blood pressure, BP; high-density lipoprotein, HDL; low-density lipoprotein, LDL; triglycerides, TG.
In childhood, parent school years was considered an indicator of socioeconomic status. In adulthood, the participant's own school years was used.
Data on childhood smoking status was collected only on those aged12 to 18 years in 1980, to maintain the sample size for these analyses, we assigned all children aged 3 to 9 years as nonsmokers.
There were two different physical activity questionnaires used for younger (3-6 years old) and older (9-18 years old) children. The calculated physical activity indices were age-standardized to allow comparison across age groups.
When examined as mean (SD), fruit consumption [6.87 (2.79) vs. 6.66 (2.93), P value = 0.254] and vegetable consumption [6.33 (2.83) vs. 5.90 (3.05), P value = 0.021] by low vs. high hsCRP levels.
Risk of high hsCRP in offspring according to parental smoking is shown in Table 2. Compared with those with non-smoking parents, exposure to parental smoking in childhood was associated with an increased risk of high hsCRP in adulthood when adjusted for age and sex [relative risk (RR), 1.5; 95% confidence interval (CI), 1.2–2.0]. After adjustment for cardiovascular risk factors and participants' own smoking status in both childhood and adulthood, the risk of developing high hsCRP in adulthood reduced but remained statistically significant [RR, 1.3; 95% CI, 1.0–1.8]. Parental smoking in childhood was associated with higher levels of adult hsCRP when examined as a continuous outcome (Table I in the online-only supplement). But the effect was attenuated after adjustment for cardiovascular risk factors in adulthood (model 4 and 5).
Table 2. Relative risk (RR) and 95% confidence interval (CI) of high hsCRP in adult offspring according to parental smoking status in offspring's childhood.
| Models | Parental Regular Smoking (None vs. 1 or Both) |
||
|---|---|---|---|
| RR | 95% CI | P value | |
| 1 = adjusted with child age, sex (n = 2,511) | 1.5 | 1.2, 2.0 | 0.002 |
| 2 = model 1 plus child BMI, own smoking, PAI, parental school years, fruit and vegetable consumption (n = 2,369) | 1.4 | 1.0, 1.8 | 0.025 |
| 3 = model 2 plus child systolic BP, HDL, LDL, TG (n = 2,344) | 1.4 | 1.0, 1.9 | 0.019 |
| 4 = model 3 plus adult BMI, adult own smoking, adult own school years (n = 2,314) | 1.3 | 1.0, 1.8 | 0.033 |
| 5 = model 4 plus adult systolic BP, HDL, LDL, TG (n = 2,295) | 1.3 | 1.0, 1.8 | 0.035 |
See Table 1 footnote for abbreviations.
Because we observed a significant interaction (P = 0.002) between mother vs. father smoking, risks of high hsCRP in offspring stratified by exposure to mother or father smoking are displayed in Fig. 1. After adjustment for potential confounding and mediating factors, the effect of having high hsCRP levels as adults was strongest for exposure to mother only smoking in childhood [RR, 2.4; 95% CI, 1.3–4.2], followed by father only smoking [RR, 1.6; 95% CI, 1.2–2.3], and both parents smoking [RR, 1.4; 95% CI, 0.9–2.0]. When hsCRP was examined as a continuous outcome, we again observed the strongest effect for mother only smoking [β, 0.22 mg/L; 95% CI, 0.03–0.42] compared with father only smoking [β, 0.08 mg/L; 95% CI, −0.02–0.18] and both parents smoking [β, 0.07 mg/L; 95% CI, −0.04–0.19].
Fig. 1.
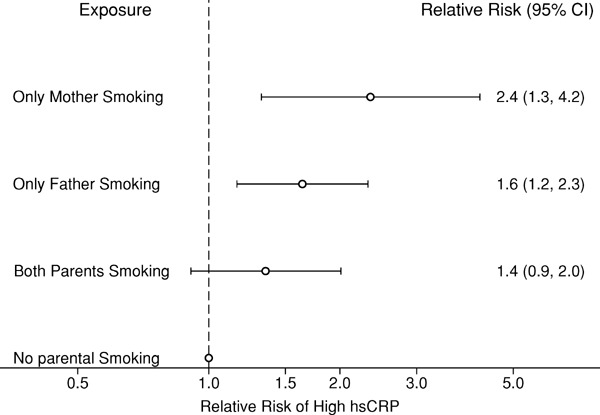
Relative risks and 95% confidence intervals (CI) of high hsCRP in adult offspring stratified by parent smoking status in the offspring's childhood.
In sensitivity analyses, we further adjusted with child hsCRP and adult exposure to passive smoking in addition to the covariates included in model 5 (Table 2). Child hsCRP was not included as a covariate in our models as a number of participants were missing these data (n = 451 for child hsCRP). Nevertheless, the risks of developing high hsCRP in adulthood remained essentially similar in this reduced sample to the estimates shown in Table 2 for the full sample [RR, 1.4; 95% CI, 1.0–1.9; and 1.3; 95% CI, 1.0–1.8]. Adjustment for adult physical activity did not modify the observed effect for model 5 in Table 2 (effect of parental smoking on high hsCRP was RR = 1.3, 95% CI = 1.0–1.8 when adult physical activity was included vs. RR = 1.3, 95% CI = 1.0–1.8 when adult physical activity was not included).
Discussion
In this study, we found that offspring exposed to parental smoking in childhood or adolescence had a greater risk of high hsCRP in adulthood compared with those with non-smoking parents. Elevated hsCRP is associated with an increased risk of atherosclerosis and coronary events in patients with CVD as well as in healthy adults30, 31, 33). The American Heart Association recommends that hsCRP is a marker for atherosclerosis, with the measurement of hsCRP in adults used as an adjunct to other established risk factors to predict cardiovascular risk28). These data add further evidence that exposure to parental smoking early in life has a long-term impact on cardiovascular health in adulthood; consistent with our previous findings regarding parental smoking in childhood with impaired adult endothelial function, increased carotid artery intima-media thickness, and presence of atherosclerotic plaque9, 11, 12).
In this study, we observed a stronger risk for high hsCRP among adult offspring for those exposed to maternal smoking compared with those exposed to paternal smoking. Similarly, in terms of other cardiovascular risk factors, it has been reported that there was a stronger effect for maternal vs. paternal smoking on metabolic syndrome, waist circumference, and carotid artery intima-media thickness in offspring8, 34). Also, we previously observed stronger risk for developing atherosclerotic plaque in adult offspring for those exposed to maternal compared with paternal smoking12). The stronger effect for maternal smoking might be explained by the fact that mothers spend, on average, more time around the child compared with fathers, thus offspring could reasonably have more exposure to maternal smoking. This assumption, however, presumes the effects of passive smoking are direct, i.e. due to the cells exposure to the chemicals in tobacco smoke. An alternative explanation is that these effects are indirect due to epigenetic inheritance. For example, a transgenerational influence of prenatal cigarette smoking by the grandmother on the grandchild's respiratory health and anthropometry has been reported35, 36). As there were no substantial differences in baseline characteristics between groups, it is unclear as to the underlying reason for why we observed a stronger effect for mother only smoking compared with both parents smoking. The difference may be related to unmeasured sociodemographic factors or differences in smoking hygiene between the groups.
The link between exposure to tobacco smoke, both active and passive, in adults and low-grade systemic inflammation related to coronary artery disease has previously been reported. The Emerging Risk Factors Collaboration reported that hsCRP levels were 37% (95% CI, 31–44%) higher in adult smokers compared with non-smokers20). The Multi-Ethnic Study of Atherosclerosis found that the risk of high hsCRP was almost two-fold higher for smokers (odds ratio, 1.7; 95% CI, 1.5–2.1) compared with nonsmokers in adults37). It has been shown that systemic inflammation induced by passive smoking is nearly as substantial as those from long-term active smoking in adults. For example, Panagiotakos et al. observed that higher hsCRP levels (0.08 mg/dL; P = 0.03) and higher white blood cell counts among adults who self-reported exposure to passive smoking more than three days per week compared with those not exposed to secondhand smoke21). Data from cross-sectional and longitudinal studies that used more objective markers of passive smoke exposure (salivary cotinine) provided further confirmation of the Panagiotakos et al. study22, 38). In contrast, cross-sectional studies using objectively measured exposure by serum cotinine showed passive smoking in adults was not associated with CRP or white blood cell counts. However, the authors pointed out this might be because the study did not use high-sensitivity laboratory methods for CRP23, 39). The data reported in our study are the first linking childhood exposure to passive smoking with adult hsCRP levels.
Tobacco smoke promotes atherosclerosis, mainly through a disorder of cholesterol proteins, accelerating lipid peroxidation, as well as platelet aggregation and clotting, leading to endothelial dysfunction. Moreover, tobacco smoke exposure in adults, both active and passive, enhances the low-grade systemic inflammation related to atherosclerosis. In this study, we adjusted for several conventional cardiovascular risk factors as potential mediating factors, such as child and adult BMI, systolic blood pressure, HDL-cholesterol, LDL-cholesterol, triglycerides, but also included child and adult own smoking status as potential confounding factors. Further adjustment of our models for child hsCRP did not modify the associations, suggesting that parental smoking might act on later risk of high hsCRP through other mechanisms than tracking of hsCRP from childhood to adulthood25).
Normally, acute inflammation induced by traumatic injury or infection leads to a marked release of CRP into the circulation, while only minor CRP elevation (concentrations between 3 and 10 mg/L) has been generally regarded as a marker of low-grade inflammation related to chronic inflammatory environment due to autoimmune diseases and metabolic disorders, such as rheumatic disease, diabetes, hypertension and obesity. In early stage atherosclerosis following exposure to risk factors such as tobacco smoke, circulating inflammatory and immune cells migrate to the developing lesion in response to lipid peroxidation. Here they interact with the arterial wall and release pro-inflammatory cytokines, interleukin-1, tumor necrosis factor, that further induce release of interleukin-6 into the circulation. Interleukin-6, in turn, stimulates the production of large amounts of acute-phase reactants such as CRP, especially in the liver and is released into circulation16, 17).
It has previously been reported that stricter public smoking restrictions might have displaced smokers into the home, resulting in increased passive smoking exposure to their children40). Although the fetal and neonatal effects of parental smoking in offspring have been recognized, particularly for respiratory health and normal growth and development, our findings highlight the importance of reducing passive smoke exposure on children and adolescents in an effort to improve long-term cardiovascular health in adulthood. In this study, our data suggest that those children and adolescents exposed to passive smoking at home are more likely to have low-grade, systemic inflammation than their counterparts who were not exposed. Given minor CRP elevation was reported with increased risk of atherosclerosis, ischemic stroke, overweight/obesity and lipid disorders41–43), limiting children's exposure to passive smoking may have long-term benefits on later cardiovascular health. Future research should determine the utility of retrospective reports of parent smoking to cardiovascular risk screening.
This study had limitations. Although it has been shown that intrauterine exposure to maternal and paternal tobacco smoke has a long-term influence on the offspring's cardiovascular health44, 45), data on parental smoking during pregnancy was not measured in this study. As such, it is possible that exposure to parental smoking in childhood is a marker of those exposed to passive smoking in utero and it may be this exposure that impacts on systemic inflammation. Another limitation concerns residual confounding that may have resulted from storage of child hsCRP for 25 years before measurement. However, the samples were not thawed or refrozen during storage and we have previously shown high agreement between hsCRP levels from the same participants measured at the time of blood sampling and after storage for 5 years25). A further limitation is the reliance on a self-report exposure measure. However, in a subset of participants in this study we have shown that participants were significantly less likely to have no detectable serum cotinine as the level of reported parental smoking increased12). Additionally, the lack of follow-up data on antiinflammatory medication, such as non-steroidal antiinflammatory drugs and steroids, is a limitation. Finally, differential loss to follow-up of the initial representative cohort is possible. However, we have previously found participants are largely representative of the original sample24). The major strengths of this study include the very long-term follow-up with exposure measurements in childhood, measures of important potential confounders and mediators, and outcome data from a follow-up of over 30 years. Moreover, we were able to adjust for child hsCRP levels to determine the independent association of our exposure, which is unique to child to adult cohorts of this age and length to follow-up.
Conclusion
Exposure to parental smoking in childhood has a long-term association with risk of high hsCRP in adulthood. Limiting children's exposure to passive smoking may have long-term benefits on general low-grade inflammation.
Acknowledgements
We thank the clinic and administrative staff for their contribution to data collection. We wish to thank Noora Kartiosuo, MSSc, from the Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, for assistance in compiling these data. Above all, we thank the participants of the Cardiovascular Risk in Young Finns Study.
Conflict of Interest
There is no financial relationship with a biotechnology manufacturer, a pharmaceutical company or any other commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
Financial Supports
The Young Finns Study has been financially supported by the Academy of Finland: grants 134309 (Eye), 126925, 121584 124282, 129378 (Salve), 117787 (Gendi), and 41071 (Skidi); the Social Insurance Institution of Finland; Kuopio, Tampere, and Turku University Hospital Medical Funds; Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation of Cardiovascular Research and Finnish Cultural Foundations; Tampere Tuberculosis Foundation; and Emil Aaltonen Foundation. Dr. Di Wang is supported by the National Natural Science Foundation of China (No.81503080), the Anhui Provincial Natural Science Foundation (No.1608085 QH210), and the visiting scholar fellowship from the Chinese Society of Anesthesiology. Dr. Costan Magnussen is supported by a National Heart Foundation of Australia Future Leader Fellowship (100849).
Supplemental Table 1. Regression (β) coefficient and 95% confidence interval (CI) of log-hsCRP in adult offspring according to parental smoking status in offspring's childhood.
| Models | Parental Regular Smoking (None vs. 1 or Both) |
||
|---|---|---|---|
| β coefficient | 95%CI | P value | |
| 1 = adjusted with child age, sex | 0.16 | 0.07, 0.25 | 0.001 |
| 2 = model 1 plus child BMI, own smoking, PAI, parental school years, fruit and vegetable consumption | 0.12 | 0.03, 0.22 | 0.011 |
| 3 = model 2 plus child systolic BP, HDL, LDL, TG | 0.13 | 0.04, 0.23 | 0.006 |
| 4 = model 3 plus adult BMI, adult own smoking, adult own school years | 0.05 | −0.04, 0.13 | 0.264 |
| 5 = model 4 plus adult systolic BP, HDL, LDL, TG | 0.04 | −0.04, 0.13 | 0.299 |
Abbreviations: confidence interval, CI; high-sensitivity C-reactive protein, hsCRP; body mass index, BMI; physical activity index, PAI; blood pressure, BP; high-density lipoprotein, HDL; low-density lipoprotein, LDL; triglycerides, TG.
Supplemental Table 2. Baseline and adult characteristics of participants according to exposure to parental regular smoking in childhood (n = 2,511).
| Parental smoking (None vs. 1 or Both) |
|||
|---|---|---|---|
| None | 1 or Both | P value | |
| (n = 768) | (n = 1,743) | ||
| Baseline | |||
| Female sex, % | 51 | 50 | 0.757 |
| Age, y | 10.9 (5.2) | 10.6 (4.9) | 0.182 |
| Parental school years, y* | 9 (8, 12) | 9 (8, 11) | 0.037 |
| Smoking prevalence, %† | 6.3 | 6.4 | 0.941 |
| BMI, kg/m2 | 17.8 (3.0) | 17.9 (3.1) | 0.408 |
| Systolic BP, mm Hg | 113 (12) | 113 (12) | 0.169 |
| HDL cholesterol, mmol/L | 1.56 (0.30) | 1.56 (0.31) | 0.767 |
| LDL cholesterol, mmol/L | 3.49 (0.83) | 3.42 (0.81) | 0.083 |
| TG, mmol/L | 0.61 (0.46, 0.80) | 0.59 (0.45, 0.79) | 0.262 |
| hsCRP, mg/L | 0.20 (0.11, 0.48) | 0.23 (0.11, 0.61) | 0.115 |
| Fruit consumption, frequency/wk¶ | 6.3 (6.3, 9.5) | 6.3 (6.3, 9.5) | 0.016 |
| Vegetable consumption, frequency/wk¶ | 6.3 (6.3, 9.5) | 6.3 (3.0, 9.5) | < 0.001 |
| standardized PAI‡ | 0.002 (1.019) | 0.015 (0.997) | 0.768 |
| Adulthood | |||
| Age, y | 41.9 (5.2) | 41.6 (4.9) | 0.182 |
| Own school years, y* | 16 (13, 18) | 15 (12, 17) | < 0.001 |
| Own smoking prevalence, % | 12.4 | 21.9 | < 0.001 |
| Passive smoking prevalence, % | 5.2 | 7.7 | 0.024 |
| BMI, kg/m2 | 25.9 (4.8) | 26.5 (5.0) | 0.006 |
| Systolic BP, mm Hg | 120 (14) | 120 (14) | 0.938 |
| HDL cholesterol, mmol/L | 1.31 (0.33) | 1.31 (0.32) | 0.937 |
| LDL cholesterol, mmol/L | 3.33 (0.86) | 3.25 (0.82) | 0.046 |
| TG, mmol/L | 1.05 (0.75, 1.56) | 1.05 (0.75, 1.56) | 0.445 |
| hsCRP, mg/L | 0.67 (0.31, 1.42) | 0.77 (0.34, 1.72) | 0.002 |
Data are mean (SD) or median (25th, 75th percentile) for continuous variables and percentages for categorical variables.
Abbreviations: confidence interval, CI; high-sensitivity C-reactive protein, hsCRP; body mass index, BMI; physical activity index, PAI; blood pressure, BP; high-density lipoprotein, HDL; low-density lipoprotein, LDL; triglycerides, TG.
In childhood, parent school years was considered an indicator of socioeconomic status. In adulthood, the participant's own school years was used.
Data on childhood smoking status was collected only on those aged12 to 18 years in 1980, to maintain the sample size for these analyses, we assigned all children aged 3 to 9 years as nonsmokers.
There were two different physical activity questionnaires used for younger (3–6 years old) and older (9–18 years old) children. The calculated physical activity indices were age-standardized to allow comparison across age groups.
When examined as mean (SD), fruit consumption [7.05 (2.74) vs. 6.76 (2.84), P value = 0.016] and vegetable consumption [6.60 (2.78) vs. 6.14 (2.88), P value < 0.001] by low vs. high hsCRP levels.
References
- 1). Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005; 11: 2684-2698 [DOI] [PubMed] [Google Scholar]
- 2). Raupach T, Schäfer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J. 2006; 27: 386-392 [DOI] [PubMed] [Google Scholar]
- 3). Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012; 126: 2177-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Hurt RD, Weston SA, Ebbert JO, McNallan SM, Croghan IT, Schroeder DR, Roger VL. Myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, before and after smoke-free workplace laws. Arch Intern Med. 2012; 172: 1635-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Juonala M, Magnussen CG, Raitakari OT. Parental smoking produces long-term damage to vascular function in their children. Curr Opin Cardiol. 2013; 28: 569-574 [DOI] [PubMed] [Google Scholar]
- 6). Ayer JG, Belousova E, Harmer JA, David C, Marks GB, Celermajer DS. Maternal cigarette smoking is associated with reduced high-density lipoprotein cholesterol in healthy 8-year-old children. Eur Heart J. 2011; 32: 2446-2453 [DOI] [PubMed] [Google Scholar]
- 7). Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermajer D, Ward KD, de Ferranti SD, Zachariah J, American Heart Association Committee on Atherosclerosis, Hypertension, and Obesity in the Young of the Council on Cardiovascular Disease in the Young; Behavior Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; and Stroke Council Cardiovascular Consequences of Childhood Secondhand Tobacco Smoke Exposure: Prevailing Evidence, Burden, and Racial and Socioeconomic Disparities: A Scientific Statement From the American Heart Association. Circulation. 2016; 134: e336-e359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Geerts CC, Bots ML, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in young adult offspring: is early life exposure critical? The atherosclerosis risk in young adults study. Arterioscler Thromb Vasc Biol. 2008; 28: 2296-2302 [DOI] [PubMed] [Google Scholar]
- 9). Juonala M, Magnussen CG, Venn A, Gall S, Kähönen M, Laitinen T, Taittonen L, Lehtimäki T, Jokinen E, Sun C, Viikari JS, Dwyer T, Raitakari OT. Parental smoking in childhood and brachial artery flow mediated dilatation in young adults: the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Arterioscler Thromb Vasc Biol. 2012; 32: 1024-1031 [DOI] [PubMed] [Google Scholar]
- 10). Geerts CC, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CS. Parental smoking and vascular damage in their 5-year-old children. Pediatrics. 2012; 129: 45-54 [DOI] [PubMed] [Google Scholar]
- 11). Gall S, Huynh QL, Magnussen CG, Juonala M, Viikari JS, Kähönen M, Dwyer T, Raitakari OT, Venn A. Exposure to parental smoking in childhood or adolescence is associated with increased carotid intima-media thickness in young adults: evidence from the Cardiovascular Risk in Young Finns study and the Childhood Determinants of Adult Health study. Eur Heart J. 2014; 35: 2484-2491 [DOI] [PubMed] [Google Scholar]
- 12). West HW, Juonala M, Gall SL, Kähönen M, Laitinen T, Taittonen L, Viikari JS, Raitakari OT, Magnussen CG. Exposure to parental smoking in childhood is associated with increased risk of carotid atherosclerotic plaque in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2015; 131: 1239-1246 [DOI] [PubMed] [Google Scholar]
- 13). Chen W, Yun M, Fernandez C, Li S, Sun D, Lai CC, Hua Y, Wang F, Zhang T, Srinivasan SR, Johnson CC, Berenson GS. Secondhand smoke exposure is associated with increased carotid artery intima-media thickness: the Bogalusa Heart Study. Atherosclerosis. 2015; 240: 374-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Kallio K, Jokinen E, Hämäläinen M, Saarinen M, Volanen I, Kaitosaari T, Viikari J, Rönnemaa T, Simell O, Raitakari OT. Decreased aortic elasticity in healthy 11-year-old children exposed to tobacco smoke. Pediatrics. 2009; 123: e267-e273 [DOI] [PubMed] [Google Scholar]
- 15). Kallio K, Jokinen E, Saarinen M, Hämäläinen M, Volanen I, Kaitosaari T, Rönnemaa T, Viikari J, Raitakari OT, Simell O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ Cardiovasc Qual Outcomes. 2010; 3: 196-203 [DOI] [PubMed] [Google Scholar]
- 16). Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352: 1685-1695 [DOI] [PubMed] [Google Scholar]
- 17). Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014; 35: 1782-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005; 26: 1765-1773 [DOI] [PubMed] [Google Scholar]
- 19). Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med. 2005; 2: e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010; 375: 132-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Panagiotakos DB, Pitsavos C, Chrysohoou C, Skoumas J, Masoura C, Toutouzas P, Stefanadis C, ATTICA study Effect of exposure to secondhand smoke on markers of inflammation: the ATTICA study. Am J Med. 2004; 116: 145-150 [DOI] [PubMed] [Google Scholar]
- 22). Hamer M, Stamatakis E, Kivimaki M, Lowe GD, Batty GD. Objectively measured secondhand smoke exposure and risk of cardiovascular disease: what is the mediating role of inflammatory and hemostatic factors? J Am Coll Cardiol. 2010; 56: 18-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Venn A, Britton J. Exposure to secondhand smoke and biomarkers of cardiovascular disease risk in never-smoking adults. Circulation. 2007; 115: 990-995 [DOI] [PubMed] [Google Scholar]
- 24). Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, Hutri-Kähönen N, Taittonen L, Jokinen E, Marniemi J, Jula A, Telama R, Kähönen M, Lehtimäki T, Akerblom HK, Viikari JS. Cohort profile: the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008; 37: 1220-1226 [DOI] [PubMed] [Google Scholar]
- 25). Juonala M, Viikari JS, Rönnemaa T, Taittonen L, Marniemi J, Raitakari OT. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2006; 26: 1883-1888 [DOI] [PubMed] [Google Scholar]
- 26). Juonala M, Voipio A, Pahkala K, Viikari JS, Mikkilä V, Kähönen M, Hutri-Kähönen N, Jula A, Burgner D, Sabin MA, Marniemi J, Loo BM, Laitinen T, Jokinen E, Taittonen L, Magnussen CG, Raitakari OT. Childhood 25-OH vitamin D levels and carotid intima-media thickness in adulthood: the cardiovascular risk in young Finns study. J Clin Endocrinol Metab. 2015; 100: 1469-1476 [DOI] [PubMed] [Google Scholar]
- 27). Mattsson N, Magnussen CG, Rönnemaa T, Mallat Z, Benessiano J, Jula A, Taittonen L, Kähönen M, Juonala M, Viikari JS, Raitakari OT. Metabolic syndrome and carotid intima-media thickness in young adults: roles of apolipoprotein B, apolipoprotein A-I, C-reactive protein, and secretory phospholipase A2: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol. 2010; 30: 1861-1866 [DOI] [PubMed] [Google Scholar]
- 28). Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F, Centers for Disease Control and Prevention; American Heart Association Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003; 107: 499-511 [DOI] [PubMed] [Google Scholar]
- 29). Oda E, Oohara K, Abe A, Veeraveedu PT, Watanabe K, Kato K, Aizawa Y. The optimal cut-off point of C-reactive protein as an optional component of metabolic syndrome in Japan. Circ J. 2006; 70: 384-388 [DOI] [PubMed] [Google Scholar]
- 30). Adukauskienė D, Čiginskienė A, Adukauskaitė A, Pentiokinienė D, Šlapikas R, Čeponienė I. Clinical relevance of high sensitivity C-reactive protein in cardiology. Medicina (Kaunas). 2016; 52: 1-10 [DOI] [PubMed] [Google Scholar]
- 31). Halcox JP, Roy C, Tubach F, Banegas JR, Dallongeville J, De Backer G, Guallar E, Sazova O, Medina J, Perk J, Steg PG, Rodríguez-Artalejo F, Borghi C. C-reactive protein levels in patients at cardiovascular risk: EURIKA study. BMC Cardiovasc Disord. 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Cortez AF, Muxfeldt ES, Cardoso CR, Salles GF. Prognostic Value of C-Reactive Protein in Resistant Hypertension. Am J Hypertens. 2016; 29: 992-1000 [DOI] [PubMed] [Google Scholar]
- 33). Iso H, Noda H, Ikeda A, Yamagishi K, Inoue M, Iwasaki M, Tsugane S. The impact of C-reactive protein on risk of stroke, stroke subtypes, and ischemic heart disease in middle-aged Japanese: the Japan public health center-based study. J Atheroscler Thromb. 2012; 19: 756-766 [PubMed] [Google Scholar]
- 34). Han TS, Hart CL, Haig C, Logue J, Upton MN, Watt GC, Lean ME. Contributions of maternal and paternal adiposity and smoking to adult offspring adiposity and cardiovascular risk: the Midspan Family Study. BMJ Open. 2015; 5: e007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Magnus MC, Håberg SE, Karlstad Ø, Nafstad P, London SJ, Nystad W. Grandmother's smoking when pregnant with the mother and asthma in the grandchild: the Norwegian Mother and Child Cohort Study. Thorax. 2015; 70: 237-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Golding J, Northstone K, Gregory S, Miller LL, Pembrey M. The anthropometry of children and adolescents may be influenced by the prenatal smoking habits of their grandmothers: a longitudinal cohort study. Am J Hum Biol. 2014; 26: 731-739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas-Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015; 35: 1002-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Lu L, Mackay DF, Newby DE, Pell JP. Association between salivary cotinine and cardiovascular biomarkers among nonsmokers and current smokers: cross-sectional study of 10,081 participants. Eur J Vasc Endovasc Surg. 2014; 48: 703-710 [DOI] [PubMed] [Google Scholar]
- 39). Clark JD, 3rd, Wilkinson JD, LeBlanc WG, Dietz NA, Arheart KL, Fleming LE, Lee DJ. Inflammatory markers and secondhand tobacco smoke exposure among U.S. workers. Am J Ind Med. 2008; 51: 626-632 [DOI] [PubMed] [Google Scholar]
- 40). Ho SY, Wang MP, Lo WS, Mak KK, Lai HK, Thomas GN, Lam TH. Comprehensive smoke-free legislation and displacement of smoking into the homes of young children in Hong Kong. Tob Control. 2010; 19: 129-133 [DOI] [PubMed] [Google Scholar]
- 41). Nagasawa SY, Ohkubo T, Masaki K, Barinas-Mitchell E, Miura K, Seto T, El-Saed A, Kadowaki T, Willcox BJ, Edmundowicz D, Kadota A, Evans RW, Kadowaki S, Fujiyoshi A, Hisamatsu T, Bertolet MH, Nakamura Y, Kuller LH, Ueshima H, Sekikawa A, ERA-JUNP Study Group Associations between Inflammatory Markers and Subclinical Atherosclerosis in Middle-aged White, Japanese-American and Japanese Men: The ERA-JUMP Study. J Atheroscler Thromb. 2015; 22: 590-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M, J-STARS Investigators Reduction in High-Sensitivity C-Reactive Protein Levels in Patients with Ischemic Stroke by Statin Treatment: Hs-CRP Sub-Study in J-STARS. J Atheroscler Thromb. 2017. March 7. 10.5551/jat.39354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Orenes-Piñero E, Pineda J, Roldán V, Hernández-Romero D, Marco P, Tello-Montoliu A, Sogorb F, Valdés M, Lip GY, Marín F. Effects of Body Mass Index on the Lipid Profile and Biomarkers of Inflammation and a Fibrinolytic and Prothrombotic State. J Atheroscler Thromb. 2015; 22: 610-617 [DOI] [PubMed] [Google Scholar]
- 44). Jaddoe VW, de Ridder MA, van den Elzen AP, Hofman A, Uiterwaal CS, Witteman JC. Maternal smoking in pregnancy is associated with cholesterol development in the offspring: A 27-years follow-up study. Atherosclerosis. 2008; 196: 42-48 [DOI] [PubMed] [Google Scholar]
- 45). Ayer JG, Belousova E, Harmer JA, David C, Marks GB, Celermajer DS. Maternal cigarette smoking is associated with reduced high-density lipoprotein cholesterol in healthy 8-year-old children. Eur Heart J. 2011; 32: 2446-2453 [DOI] [PubMed] [Google Scholar]


