Abstract
Aim: The aim of this study was to investigate whether information on arterial stiffness can improve the value of single-photon emission computed tomography (SPECT) in the detection of obstructive coronary artery disease (CAD).
Methods: A total of 233 patients (age: 62.2 ± 10.8 years, 60.3% males) with detected ischemia on SPECT undergoing invasive coronary angiography (ICA) and brachial-ankle pulse wave velocity (baPWV) measurement within a month was retrospectively reviewed.
Results: Of the 233 patients, 190 (81.5%) had obstructive CAD (≥ 50% luminal stenosis). The difference in baPWV according to the presence of obstructive CAD was significant in patients in the mild ischemia group [summed stress score (SSS): 4–8] (1,770 ± 364 cm versus 1,490 ± 328 cm, p < 0.001) but not in the moderate (SSS: 9–13) or severe (SSS: ≥14) ischemia groups (p > 0.05 for each). Receiver operating characteristic curve analyses showed that the diagnostic value of baPWV for obstructive CAD was significant only in patients in the mild ischemia group (area under curve: 0.714; p = 0.001) but not in the moderate or severe ischemia groups (p > 0.05 for each). Adding information on baPWV to SPECT results and clinical parameters significantly increased diagnostic accuracy in the detection of obstructive CAD in patients with mild ischemia (integrated discrimination improvement p = 0.006) but not in those with moderate or severe ischemia on SPECT (p > 0.05 for each).
Conclusions: The results of this study suggest that baPWV may have additional value to SPECT for the detection of obstructive CAD, especially in patients with mild ischemia on SPECT.
Keywords: Arterial stiffness, Coronary artery disease, Pulse wave velocity, Single-photon emission computed tomography
Introduction
Coronary artery disease (CAD) is the most common cause of death worldwide. CAD is highly prevalent, and its social and medical expenses have been steadily rising1, 2). Therefore, early detection and intensive management of high-risk patients are important for improving CAD-related poor prognosis. Invasive coronary angiography (ICA) is the gold standard in the diagnosis of CAD; however, it usually carries potential risk and increases the medical costs of patients3). In order to overcome these limitations of ICA, various noninvasive tests have been developed to detect CAD and guide ICA. Among them, single-proton emission computed tomography (SPECT), as a conventional method, is one of the most widely used noninvasive imaging tests in clinical settings. SPECT provides not only diagnostic but also prognostic information in patients with known or suspected CAD4–10). It has been reported that diagnostic sensitivity of SPECT for the detection of obstructive CAD (≥ 50% luminal stenosis) is as high as above 80%, but the specificity of SPECT is as low as about 70%9). In addition, a substantial discrepancy between myocardial ischemia and coronary atherosclerosis has been demonstrated, suggesting that SPECT is imperfect as a single test in the detection of CAD11). For these reasons, there have been efforts to improve the diagnostic accuracy of SPECT, such as combined12) or sequential imaging studies13). However, the diagnostic yield of ICA is still low, and there is a concern regarding the role of noninvasive tests as gatekeepers of ICA14).
Arterial stiffness reflects aging, injury, and arteriosclerosis of the arterial walls15). Arterial stiffness can be estimated using several tools; among them, pulse wave velocity (PWV) is the most widely applied noninvasive method in the clinical and research fields16). There have been several reports showing a significant association between arterial stiffness measured by PWV and CAD17–20). It has been suggested that reduced diastolic coronary blood flow and shared common cardiovascular risk factors associated with increased arterial stiffness may be involved in the development and progression of coronary atherosclerosis20, 21).
Aim
Since both SPECT and PWV are useful in the diagnosis of CAD, it can be speculated that a combination of results from both tests is conceptually attractive in the diagnosis of CAD. We hypothesized that additional information on PWV can improve the diagnostic value of SPECT. Therefore, this study was performed to investigate the incremental diagnostic value of PWV to SPECT in patients undergoing ICA for the evaluation of CAD.
Methods
Study Population
This single-center study was performed at Boramae Medical Center (Seoul, Korea). Between January 2010 and December 2015, a total of 274 patients with ischemia detected on SPECT who underwent ICA within two weeks were retrospectively reviewed. Brachial-ankle pulse wave velocity (baPWV) was measured within 30 days of SPECT and/or ICA in all study patients. Forty one patients with the following conditions were excluded: (1) prior history of myocardial infarction and coronary revascularization with percutaneous coronary intervention (PCI) or coronary bypass surgery (n = 26), (2) ankle-brachial index < 0.9 or > 1.4 (n = 4), (3) non-sinus rhythm (n = 6), and (4) numerous missing variables (n = 5). Finally, 233 patients were analyzed in this study. This study complies with the Declaration of Helsinki, and the Institutional Review Board (IRB) of Boramae Medical Center approved this study protocol. Written informed consent was waived by the IRB due to the retrospective study design and routine nature of the information collected.
Data Collection
Body mass index (BMI) was calculated by dividing weight in kilograms by height squared in meters. Information on cardiovascular risk factors including hypertension, diabetes mellitus, dyslipidemia, and smoking status was obtained. Hypertension was defined as either repeated measurements of blood pressure ≥ 140/90 mmHg or currently using antihypertensive medications. Diabetes mellitus was defined as fasting serum glucose level ≥ 126 mg/dL at least twice, hemoglobin A1c (HbA1c) ≥ 6.5%, or currently using antihyperglycemic medications. Dyslipidemia was defined as either a history of dyslipidemia or currently using antidyslipidemic medications. Smoking was defined as a history of cigarette smoking during the past 12 months. The pretest probability of CAD was defined based on the patient's age, sex, and chest pain nature22) and classified as low (< 10%), intermediate (10%–90%), or high (> 90%). Venous blood samples were acquired in the morning after more than eight hours of fasting. Blood levels of hemoglobin, creatinine, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, C-reactive protein, and HbA1c were measured. Estimated glomerular filtration rate (eGFR) was calculated using the following formula: 175 × serum creatinine−1.154 × age−0.203 (× 0.742 if a woman).
SPECT Protocol
The SPECT protocol has previously been described and published elsewhere13, 23). Myocardial perfusion SPECT was performed with a one-day protocol using dual isotopes of 201TI and 99mTc-sestamibi. First, rest SPECT images were acquired five minutes after the intravenous administration of 201Tl chloride (111 MBq). For the pharmacological stress test, adenosine was continuously injected at a rate of 0.14 mg/kg/min for six minutes. Three minutes after adenosine infusion, 99mTc-sestamibi (555 MBq) was injected without the interruption of continued adenosine infusion, and then stress SPECT images were obtained four hours after 99mTc-sestamibi injection. SPECT images were acquired using a dual-headed camera (Infinia, Hawkeye 4, General Electric Co., USA), employing 64 projections over 180° (from right anterior oblique 45° to left posterior oblique 45°), low-energy high-resolution collimator, and step/shoot acquisition at 3° intervals and for 25 seconds per step. For dual isotopes, images were obtained with a 20% window centered over both 70 and 167 keV photopeaks for 201Tl chloride and a 20% window centered over the 140 keV photopeak for 99 mTc-sestamibi. SPECT images were reconstructed by the filtered back projection method using a Butterworth filter (cut-off frequency 0.49, order 5) and iterative reconstruction, and the reconstructed images were transferred to a designated workstation (Xeleris, General Electric Co., USA) for interpretation. The SPECT study was repeated when its image quality was suboptimal after scrutinization of the reconstructed images. SPECT images were interpreted by well-trained nuclear medicine physicians who were blinded to clinical information in accordance with the recommendation of the American Society of Nuclear Cardiology. Summed stress scores (SSS) were calculated to quantify myocardial ischemia by adding the scores of 17 myocardial segments24). Mild ischemia was defined as SSS 4–8, moderate ischemia as SSS 9–13, and severe ischemia as SSS ≥ 14. Visual assessment of perfusion defect was performed using a 5-point scoring system, and perfusion defect was classified as small (< 10% of myocardium), medium (10%–20% of myocardium), and large (≥ 20% of myocardium)25).
ICA
Techniques of the ICA and PCI procedures were based on the current guideline's recommendations3). PCI decision was made by the interventional cardiologist performing ICA according to ICA results. Obstructive CAD was defined as ≥ 50% luminal stenosis of one or more major epicardial coronary arteries in ICA.
Measurement of baPWV
Patients were examined in the supine position after five minutes of rest. baPWV was measured using a volume-plethysmographic apparatus (VP-1000; Colin Co. Ltd., Komaki, Japan) in accordance with the manufacturer's recommendations23, 26). Cuffs were wrapped on both upper arms and ankles. The baPWV values were calculated by measuring the time for the pulse wave to travel between the brachial and posterior tibial arteries. Caffeine consumption or cigarette smoking were not allowed before the examination. To measure blood pressure and semiconductor pulse wave, the measurement cuffs were placed around bilateral antecubital areas and ankles. Pulse volume waveform, phonogram, blood pressure, and heart rate were recorded simultaneously. The average value of the left and right baPWV values was used in the analysis. All values were measured by the same experienced operator who was blinded to the patients' information. The coefficient of variance for inter-observer reliability of baPWV was 5.1% in our laboratory23).
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are expressed as percentages. Univariate comparisons between patients with and without CAD were made using Student's t-test for continuous variables and the chi-square test for dichotomous variables. Trend of the incidence of obstructive CAD according to the severity of ischemia on SPECT was estimated by the linear-by-linear chi-square test. Receiver operating characteristic (ROC) curve analyses were used to evaluate the additional value of baPWV to SPECT. The integrated discrimination improvement index (IDI) was used to compare areas under curve of ROC27). A p value of < 0.05 was considered statistically significant. All analyses were two-sided. All data were analyzed using SPSS for Windows 18.0 (IBM, Armonk, NY, USA). R version 3.2.1 (http://www.r-projec-t.org) was used to calculate IDI.
Results
Clinical Characteristics of the Study Patients
The clinical characteristics of the study subjects are shown in Table 1. For the study patients (n = 233), the mean age was 67.3 ± 10.8 years, and there were 140 male patients (60.1%). Most patients (n = 190, 81.5%) had obstructive CAD in ICA. Patients with CAD were older (68.0 ± 10.5 years versus 63.8 ± 11.9 years, p = 0.020) and predominantly male (64.7% versus 39.5%, p = 0.002) compared with those without CAD. There was no difference in BMI according to the presence of obstructive CAD (p = 0.435). All traditional risk factors including hypertension, diabetes mellitus, dyslipidemia, and smoking tended to be more prevalent in patients with CAD than in those without (p < 0.10); however, only diabetes mellitus showed a significant difference between these two groups (18.6% versus 43.7%, p = 0.002). Most study patients (83.7%) had intermediate pretest probability, which was not significantly different between patients with and without CAD (p = 0.362). In the laboratory test results, the HDL cholesterol level was lower and the triglyceride and HbA1c levels were higher in patients with CAD compared with those without CAD (p < 0.05 for each).
Table 1. Clinical characteristics of the study subjects.
| Characteristic | Total | CAD (−) | CAD (+) | p value |
|---|---|---|---|---|
| (n = 233) | (n = 43) | (n = 190) | ||
| Age, years | 67.3 ± 10.8 | 63.8 ± 11.9 | 68.0 ± 10.5 | 0.020 |
| Male, n (%) | 140 (60.1) | 17.0 (39.5) | 123 (64.7) | 0.002 |
| Body mass index (kg/m2) | 24.5 ± 4.1 | 24.9 ± 4.9 | 24.4 ± 3.9 | 0.435 |
| Risk factors, n (%) | ||||
| Hypertension | 148 (63.5) | 22 (51.2) | 126 (66.3) | 0.062 |
| Diabetes mellitus | 91 (39.1) | 8 (18.6) | 83 (43.7) | 0.002 |
| Dyslipidemia | 75 (32.2) | 9 (20.9) | 66 (34.7) | 0.080 |
| Smoking | 53 (22.7) | 5 (11.6) | 48 (25.3) | 0.054 |
| Pretest probability, n (%) | ||||
| Low (< 10%) | 10 (4.3) | 3 (7.0) | 7 (3.7) | |
| Intermediate (10%–89%) | 195 (83.7) | 37 (86.0) | 158 (83.2) | 0.362 |
| High (> 90%) | 28 (12.0) | 3 (7.0) | 25 (13.2) | |
| Laboratory findings | ||||
| Hemoglobin, g/dl | 13.5 ± 7.3 | 13.1 ± 1.6 | 13.6 ± 8.0 | 0.670 |
| Estimated GFR, mL/min/1.73 m2 | 74.7 ± 26.1 | 78.0 ± 26.1 | 73.9 ± 26.1 | 0.361 |
| Total cholesterol, mg/dl | 158 ± 46 | 163 ± 39 | 157 ± 47 | 0.446 |
| LDL cholesterol, mg/dl | 99.0 ± 50.6 | 97.1 ± 34.9 | 99.5 ± 53.6 | 0.786 |
| HDL cholesterol, mg/dl | 42.7 ± 12.3 | 47.3 ± 13.7 | 41.6 ± 11.8 | 0.008 |
| Triglyceride, mg/dl | 131 ± 86 | 108 ± 58 | 136 ± 90 | 0.014 |
| C-reactive protein, mg/dl | 0.73 ± 1.89 | 0.49 ± 1.34 | 0.78 ± 1.99 | 0.377 |
| HbA1c, % | 6.87 ± 1.72 | 6.26 ± 0.99 | 7.0 ± 1.81 | 0.007 |
CAD, coronary artery disease; GFR, glomerular filtration rate; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
SSS values of SPECT (11.6 ± 6.5 versus 8.1 ± 4.3, p < 0.001) and baPWV values (1,730 ± 461 cm/s versus 1,573 ± 361 cm/s, p = 0.037) were significantly higher in patients with CAD than in those without (Fig. 1).
Fig. 1.
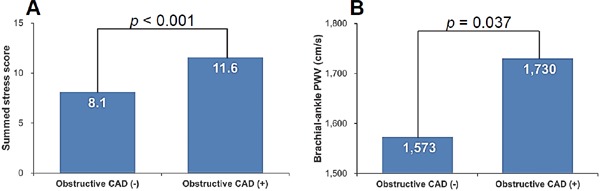
Values of SSS (A) and brachial-ankle PWV (B) according to the presence of obstructive CAD CAD, coronary artery disease.
The Association between the SPECT Results and the Incidence of Obstructive CAD
About half of the patients (n = 111, 47.6%) had mild ischemia, 55 patients (23.7%) had moderate ischemia, and 67 patients (28.7%) had severe ischemia on SPECT. The incidence of obstructive CAD increased proportionally according to ischemia severity on SPECT (73.9% in the mild ischemia group, 83.6% in the moderate ischemia group, and 92.6% in the severe ischemia group; p for trend = 0.002) (Fig. 2).
Fig. 2.

Incidence of obstructive CAD according to the degree of ischemia on SPECT
CAD, coronary artery disease; SPECT, single-photon emission computed tomography.
Changes in baPWV Values According to the Degree of Ischemia on SPECT
In the mild ischemia group, the baPWV values were significantly higher in patients with CAD than in those without (1,770 ± 364 cm versus 1,490 ± 328 cm, p < 0.001). However, there were no significant differences in the baPWV values between patients with and without CAD in the moderate or severe ischemia groups (p > 0.05 for each) (Fig. 3).
Fig. 3.
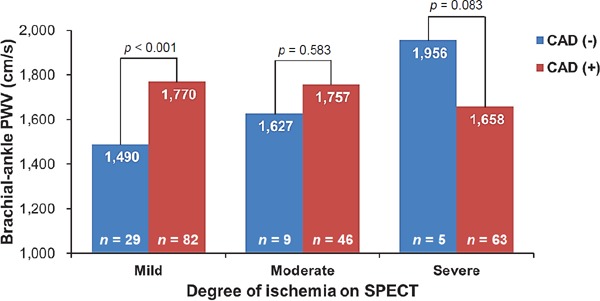
Values of brachial-ankle PWV according to groups based on the degree of ischemia on SPECT PWV, pulse wave velocity; SPECT, single-photon emission computed tomography.
Incremental Prognostic Value of baPWV Predicting Obstructive CAD
ROC curve analyses showed that the diagnostic value of baPWV for obstructive CAD was significant only in the mild ischemia group (cut-off value: 1,494 cm/s; sensitivity: 81.7%; specificity: 58.6%; area under curve: 0.714; p = 0.001) but not in the moderate or severe ischemia groups (p > 0.05 for each) (Table 2). Adding information on baPWV to SPECT results and clinical parameters significantly increased diagnostic accuracy for the detection of obstructive CAD in patients with mild ischemia (IDI p = 0.006) (Fig. 4) but not in those with moderate or severe ischemia on SPECT. When we used the data on visual assessment of perfusion defect instead of SSS, ROC curve analysis showed that diagnostic value of baPWV predicting obstructive CAD was significant only in patients with small perfusion defect (area under curve: 0.830; p = 0.001) but not in those with medium or large perfusion defects (p > 0.05 for each) on SPECT (Table 3).
Table 2. ROC curve analyses showing value of baPWV predicting obstructive CAD.
| SSS value | Area under curve | 95% confidence interval | p |
|---|---|---|---|
| 4–8 (mild ischemia) | 0.714 | 0.60–0.82 | 0.001 |
| 9–13 (moderate ischemia) | 0.510 | 0.33–0.68 | 0.927 |
| ≥ 14 (severe ischemia) | 0.302 | 0–0.61 | 0.142 |
ROC, receiver operating characteristic; baPWV, brachial-ankle pulse wave velocity; CAD, coronary artery disease; SSS, summed stress score.
Fig. 4.
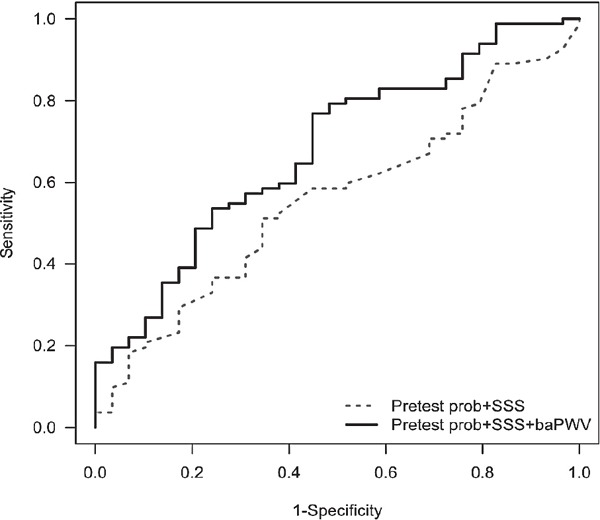
ROC curve analysis showing additional value of baPWV to SSS in the diagnosis of obstructive CAD
prob, probability; SSS, summed stress score; baPWV, brachial-ankle pulse wave velocity.
Table 3. ROC curve analyses showing value of baPWV predicting obstructive CAD.
| Visual assessment of perfusion defect on SPECT | Area under curve | 95% confidence interval | p |
|---|---|---|---|
| Small | 0.83 | 0.68–0.97 | 0.001 |
| Medium | 0.448 | 0.30–0.59 | 0.534 |
| Large | 0.696 | 0.48–0.91 | 0.087 |
ROC, receiver operating characteristic; baPWV, brachial-ankle pulse wave velocity; CAD, coronary artery disease; SPECT, single-photon emission computed tomography.
Discussion
The main finding of our study was that baPWV had incremental value to SPECT for the diagnosis of CAD in patients with a mild degree of myocardial ischemia but not in those with moderate or severe ischemia on SPECT. Our patients with mild ischemia on SPECT showed the followings: (1) the baPWV values were significantly higher in patients with angiographic obstructive CAD than in those without, (2) prediction of CAD by baPWV was evident in ROC curve analysis, and (3) baPWV provided additional diagnostic information to clinical parameters and SPECT results for the detection of obstructive CAD. However, these diagnostic values of baPWV were not observed in patients with moderate or severe ischemia on SPECT. This is the first study to provide evidence that baPWV is useful for detecting CAD, especially in patients with mild ischemia on SPECT.
Traditionally, stress tests, including the exercise treadmill test and SPECT, have played a main role in the noninvasive assessment of CAD. However, the diagnostic value of these tests for coronary obstruction is still modest28), and there is a concern regarding their roles as gatekeepers for invasive cardiac catheterization14). With technical advances, emerging coronary computed tomographic angiography (CCTA) has shown reliable accuracy in the diagnosis of obstructive CAD, with particularly high negative predictive value for excluding obstructive CAD29, 30). However, the use of CCTA is limited by relatively higher false positive rates and lack of information on myocardial ischemia31). In this context, combined information from two different noninvasive imaging tests has been used to make up for the shortcomings of each other and improve diagnostic accuracy. In particular, many reports have shown the improved diagnostic accuracy of integrated anatomical and functional imaging results for obstructive CAD compared with that of either result of a single test12, 32). Although combined imaging seemed promising, medical costs and radiation hazards hinder their wide use in clinical practice32). New modalities that can give complementary information and improve diagnostic power for CAD are needed. baPWV is noninvasive and a very simple measuring method, and it can be useful for mass screening33). In addition, there have been numerous data about the reliability and utility of baPWV in clinical practice20, 26, 34–36). In this context, our study results are valuable and deserve attention because we first showed the incremental diagnostic value of baPWV for obstructive CAD, especially in patients with mild ischemia on SPECT. Of course, we do not think that baPWV can replace the role played by noninvasive tests; however, baPWV can help determine whether patients should undergo invasive testing, especially when combined with other tests.
Our study demonstrated that additional diagnostic value of baPWV to SPECT was found only in patients with mild ischemia but not in those with moderate or severe ischemia on SPECT. The underlying pathophysiology explaining why baPWV has value only in mild ischemia cases is unknown. However, a hypothesis can be suggested. Patients with moderate or severe ischemia on SPECT have a higher likelihood of having obstructive CAD, as shown in our results (83.6% in moderate ischemia and 92.6% in severe ischemia on SPECT), and thus, there may be very little space for diagnostic value to be improved by baPWV. However, the diagnostic accuracy of SPECT was relatively low (73.9% of patients had obstructive CAD), and baPWV could increase the diagnostic value of SPECT in the mild ischemia group. The result of the present study is in line with that of a previous study showing the usefulness of additional information of CCTA in those with mild ischemia but not with moderate or severe ischemia on SPECT13).
Diagnosis of CAD is important for risk stratification and proper management. Although ICA is the gold standard for the detection of coronary stenosis, its invasive nature and cost limit routine use in clinical practice. Several noninvasive tools have been developed and widely used in clinical fields; however, they have shown limited diagnostic value as a single test14). Recent studies have shown an independent association between baPWV and CAD, suggesting that baPWV can be applied in CAD diagnosis34, 36). The results of the present study demonstrated that baPWV improved the diagnostic value of SPECT, especially in patients with mild ischemia on SPECT. In practice, it is more difficult for physicians to decide whether they should perform ICA in patients with mild ischemia as compared with those with moderate or severe ischemia on SPECT. Therefore, such patients more frequently need further diagnostic tests. Based on its noninvasiveness, simplicity, and reliability32), baPWV is useful in the diagnosis of CAD in patients with mild ischemia on SPECT. ICA can be considered in patients with high baPWV even though they have mild degree ischemia on SPECT. However, other tests or medical follow-up, rather than directly performing ICA, can be other options in patients with low baPWV and mild ischemia on SPECT.
Contrary to our expectation, mean value of baPWV was numerically higher in patients without CAD than those with CAD in patients with severe ischemia on SPECT (Fig. 3). Although underlying pathophysiology was not revealed in our study, there may be a possibility that coronary microvascular dysfunction existed in patients without CAD but with severe ischemia on SPECT37). This can partially explain the increased baPWV in patients with severe ischemia in spite of the absence of obstructive CAD. Further researches are warranted to prove our hypothesis.
Besides its retrospective study design, our study has several limitations. Firstly, our cross-sectional analysis did not confirm a causal relationship between arterial stiffness and CAD. Secondly, there was a time delay among the three tests, including baPWV, SPECT, and ICA. Thirdly, this study did not include patients who did not receive ICA, even though they had ischemia on SPECT; thus, there is a possibility of selection bias. Fourthly, some parameters did not reach statistical significance due to the relatively small number of study subjects. Lastly, this study did not provide information on the extent/severity of CAD or the location of the culprit lesion in ICA.
In conclusion, baPWV may provide additional value to SPECT for the detection of obstructive CAD, especially in patients with mild ischemia on SPECT. As a simple measurement, baPWV can be useful for CAD diagnosis. Further studies with a larger sample size are needed to confirm our findings.
Acknowledgments
None.
Conflict of Interest
The authors declare that there is no conflict of interest associated with this manuscript.
References
- 1). Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2010; 129: 1493-1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2015 update: A report from the American Heart Association. Circulation. 2015; 131: e29-322 [DOI] [PubMed] [Google Scholar]
- 3). Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011; 58: e44-122 [DOI] [PubMed] [Google Scholar]
- 4). Iskander S, Iskandrian AE. Risk assessment using single-photon emission computed tomographic technetium-99m sestamibi imaging. J Am Coll Cardiol. 1998; 32: 57-62 [DOI] [PubMed] [Google Scholar]
- 5). Stratmann HG, Williams GA, Wittry MD, Chaitman BR, Miller DD. Exercise technetium-99m sestamibi tomography for cardiac risk stratification of patients with stable chest pain. Circulation. 1994; 89: 615-622 [DOI] [PubMed] [Google Scholar]
- 6). Udelson JE, Beshansky JR, Ballin DS, Feldman JA, Griffith JL, Handler J, Heller GV, Hendel RC, Pope JH, Ruthazer R, Spiegler EJ, Woolard RH, Selker HP. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: A randomized controlled trial. JAMA. 2002; 288: 2693-2700 [DOI] [PubMed] [Google Scholar]
- 7). Bodenheimer MM, Wackers FJ, Schwartz RG, Brown M. Prognostic significance of a fixed thallium defect one to six months after onset of acute myocardial infarction or unstable angina. Multicenter Myocardial Ischemia Research Group. Am J Cardiol. 1994; 74: 1196-1200 [DOI] [PubMed] [Google Scholar]
- 8). Miller DD, Stratmann HG, Shaw L, Tamesis BR, Wittry MD, Younis LT, Chaitman BR. Dipyridamole technetium 99m sestamibi myocardial tomography as an independent predictor of cardiac event-free survival after acute ischemic events. J Nuc Cardiol. 1994; 1: 72-82 [DOI] [PubMed] [Google Scholar]
- 9). Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O'Gara PT, Carabello BA, Russell RO, Jr., Cerqueira MD, St John Sutton MG, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Jr., Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO, ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging-executive summary : A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASNC committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging). Circulation. 2003; 108: 1404-1418 [DOI] [PubMed] [Google Scholar]
- 10). Underwood SR, Anagnostopoulos C, Cerqueira M, Ell PJ, Flint EJ, Harbinson M, Kelion AD, Al-Mohammad A, Prvulovich EM, Shaw LJ, Tweddel AC. Myocardial perfusion scintigraphy: The evidence. Eur J Nucl Med Mol Imaging. 2004; 31: 261-291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos A, Lamb HJ, Stokkel MP, Dibbets-Schneider P, Decramer I, De Bondt P, van der Wall EE, Vanhoenacker PK, Bax JJ. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006; 48: 2508-2514 [DOI] [PubMed] [Google Scholar]
- 12). Kajander S, Joutsiniemi E, Saraste M, Pietila M, Ukkonen H, Saraste A, Sipila HT, Teras M, Maki M, Airaksinen J, Hartiala J, Knuuti J. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010; 122: 603-613 [DOI] [PubMed] [Google Scholar]
- 13). Kim HL, Kim YJ, Lee SP, Park EA, Paeng JC, Kim HK, Lee W, Cho GY, Zo JH, Choi DJ, Sohn DW. Incremental prognostic value of sequential imaging of single-photon emission computed tomography and coronary computed tomography angiography in patients with suspected coronary artery disease. Eur Heart J Cardiovasc Imag. 2014; 15: 878-885 [DOI] [PubMed] [Google Scholar]
- 14). Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010; 362: 886-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Lee HY, Oh BH. Aging and arterial stiffness. Circ J. 2010; 74: 2257-2262 [DOI] [PubMed] [Google Scholar]
- 16). Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: Current understanding and future directions. J Am Coll Cardiol. 2011; 57: 1511-1522 [DOI] [PubMed] [Google Scholar]
- 17). Hope SA, Antonis P, Adam D, Cameron JD, Meredith IT. Arterial pulse wave velocity but not augmentation index is associated with coronary artery disease extent and severity: Implications for arterial transfer function applicability. J Hypertens. 2007; 25: 2105-2109 [DOI] [PubMed] [Google Scholar]
- 18). Venkitachalam L, Mackey RH, Sutton-Tyrrell K, Patel AS, Boraz MA, Simkin-Silverman LR, Kuller LH. Elevated pulse wave velocity increases the odds of coronary calcification in overweight postmenopausal women. Am J Hypertens. 2007; 20: 469-475 [DOI] [PubMed] [Google Scholar]
- 19). Alarhabi AY, Mohamed MS, Ibrahim S, Hun TM, Musa KI, Yusof Z. Pulse wave velocity as a marker of severity of coronary artery disease. J Clin Hypertens. 2009; 11: 17-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kim HL, Jin KN, Seo JB, Choi YH, Chung WY, Kim SH, Kim MA, Zo JH. The association of brachial-ankle pulse wave velocity with coronary artery disease evaluated by coronary computed tomography angiography. PLoS One. 2015; 10: e0123164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Leung MC, Meredith IT, Cameron JD. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am J Physiol Heart Circ Physiol. 2006; 290: H624-630 [DOI] [PubMed] [Google Scholar]
- 22). Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O'Rourke RA, Schafer WP, Williams SV, ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: executive summary and recommendations A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients with Chronic Stable Angina). Circulation. 1999. 99: 2829-2848 [DOI] [PubMed] [Google Scholar]
- 23). Lee HS, Kim HL, Kim H, Hwang D, Choi HM, Oh SW, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Incremental prognostic value of brachial-ankle pulse wave velocity to single-photon emission computed tomography in patients with suspected coronary artery disease. J Atheroscl Thromb. 2015; 22: 1040-1050 [DOI] [PubMed] [Google Scholar]
- 24). Berman DS, Abidov A, Kang X, Hayes SW, Friedman JD, Sciammarella MG, Cohen I, Gerlach J, Waechter PB, Germano G, Hachamovitch R. Prognostic validation of a 17-segment score derived from a 20-segment score for myocardial perfusion SPECT interpretation. J Nuc Cardiol. 2004; 11: 414-423 [DOI] [PubMed] [Google Scholar]
- 25). Berman DS, Kang X, Gransar H, Gerlach J, Friedman JD, Hayes SW, Thomson LE, Hachamovitch R, Shaw LJ, Slomka PJ, Yang LD, Germano G. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nuc Cardiol. 2009; 16: 45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Kim HL, Im MS, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. The association between arterial stiffness and left ventricular filling pressure in an apparently healthy korean population. Cardiovasc Ultrasound. 2013; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kerr KF, McClelland RL, Brown ER, Lumley T. (2011) Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011; 174: 364-374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Arbab-Zadeh A. Stress testing and non-invasive coronary angiography in patients with suspected coronary artery disease: Time for a new paradigm. Heart Int. 2012; 7: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008; 359: 2324-2336 [DOI] [PubMed] [Google Scholar]
- 30). Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE. Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the american heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation. 2006; 114: 1761-1791 [DOI] [PubMed] [Google Scholar]
- 31). Nissen SE. Limitations of computed tomography coronary angiography. J Am Coll Cardiol. 2008; 52: 2145-2147 [DOI] [PubMed] [Google Scholar]
- 32). Rispler S, Keidar Z, Ghersin E, Roguin A, Soil A, Dragu R, Litmanovich D, Frenkel A, Aronson D, Engel A, Beyar R, Israel O. Integrated single-photon emission computed tomography and computed tomography coronary angiography for the assessment of hemodynamically significant coronary artery lesions. J Am Coll Cardiol. 2007; 49: 1059-1067 [DOI] [PubMed] [Google Scholar]
- 33). Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr Hypertens Rev. 2014; 10: 49-57 [DOI] [PubMed] [Google Scholar]
- 34). Kim HL, Seo JB, Chung WY, Kim SH, Zo JH, Kim MA. The association between ambulatory blood pressure profile and brachial-ankle pulse wave velocity in untreated hypertensive subjects. Blood Press. 2015; 24: 139-146 [DOI] [PubMed] [Google Scholar]
- 35). Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012; 60: 556-562 [DOI] [PubMed] [Google Scholar]
- 36). Xiong Z, Zhu C, Zheng Z, Wang M, Wu Z, Chen L, Chen Y. Relationship between arterial stiffness assessed by brachial-ankle pulse wave velocity and coronary artery disease severity assessed by the SYNTAX score. J Atheroscl Thromb. 2012; 19: 970-976 [DOI] [PubMed] [Google Scholar]
- 37). Arroyo-Espliguero R, Mollichelli N, Avanzas P, Zouridakis E, Newey VR, Nassiri DK, Kaski JC. Chronic inflammation and increased arterial stiffness in patients with cardiac syndrome X. Eur Heart J. 2003; 24: 2006-2011 [DOI] [PubMed] [Google Scholar]


