Abstract
Background
Although several studies have shown that blood type O is associated with increased risk of peptic ulcer, few studies have investigated these associations in Japan. We sought to investigate the association between the ABO blood group and risk of gastroduodenal ulcers (GDU) using combined analysis of both retrospective and prospective data from a large cohort study of Japanese women, the Japan Nurses’ Health Study (JNHS; n = 15,019).
Methods
The impact of the ABO blood group on GDU risk was examined using Cox regression analysis to estimate hazard ratios (HRs) and 95% confidence intervals (CI), with adjustment for potential confounders.
Results
Compared with women with non-O blood types (A, B, and AB), women with blood type O had a significantly increased risk of GDU from birth (multivariable-adjusted HR 1.18; 95% CI, 1.04–1.34). Moreover, the highest cumulative incidence of GDU was observed in women born pre-1956 with blood type O. In a subgroup analysis stratified by birth year (pre-1956 or post-1955), the multivariable-adjusted HR of women with blood type O was 1.22 (95% CI, 1.00–1.49) and 1.15 (95% CI, 0.98–1.35) in the pre-1956 and post-1955 groups, respectively.
Conclusion
In this large, combined, ambispective cohort study of Japanese women, older women with blood type O had a higher risk of developing GDU than those with other blood types.
Key words: ABO blood group, gastroduodenal ulcer, Japan Nurses’ Health Study
INTRODUCTION
The ABO gene on chromosome 9q34 encodes glycosyltransferases that catalyze the transfer of nucleotide donor sugars to the H antigen to form the ABO blood group antigens.1 Human ABO blood group antigens are expressed on the surface of red blood cells and a variety of human cells and tissues. Several studies have suggested that the blood type may influence carcinogenesis,2,3 and ABO blood types have been associated with risk of several malignancies.4 The association between ABO blood types and risk of certain diseases of the upper gastrointestinal tract has also been investigated in several studies.4,5 For example, the association between ABO and peptic ulcer was one of the first to be identified,6 and it was shown that individuals with blood type O had a higher susceptibility to peptic and duodenal ulcers compared with individuals with other blood types.7
We conducted an ambispective analysis of the ABO blood group and risk of gastroduodenal ulcers (GDU) in the Japan Nurses’ Health Study (JNHS; n = 15,019). Our study aimed to examine the association with self-reported serologic blood type among 13,420 women, including 1,336 incident cases of GDU from birth.
METHODS
Study population
The JNHS is a large prospective cohort study designed to investigate the effects of lifestyle and healthcare practices on the health of Japanese women.8 Participants were recruited from 2001 through 2007. A total of 49,927 women from all 47 prefectures in Japan responded to the baseline survey. Among them, 15,019 women agreed to be followed up and returned signed informed-consent sheets, together with their completed baseline questionnaires. The study population included women of at least 25 years of age that practiced as registered nurses, licensed practical nurses, public health nurses, and/or midwives and resided in Japan at the time of the baseline survey. The JNHS coordination and data center is located at the Epidemiological Research Office of the School of Health Sciences at Gunma University. The JNHS study was approved by the Ethics Committee of Gunma University, Japan.
In the first follow-up questionnaire (2nd-year questionnaire), we asked participants to report their blood type (A, AB, B, O, or unknown) and Rh factor (positive, negative, or unknown). We conducted a validation study by performing serologic testing (ABO serotyping) in a subsample of 38 subjects from the Gunma Nurses’ Health Study.9,10 The consistency of self-reported and serologically confirmed ABO blood type was 92%. For the serologically checked ABO blood types that showed inconsistency with the self-reported ABO blood types (probably caused by the low quality of those frozen blood samples), we further confirmed the self-reported ABO blood types by performing ABO genotyping. We examined three single nucleotide polymorphisms (SNPs) (loci) on the ABO gene, namely rs8176719, rs8176746, and rs505922, using DNA sequencing. These SNPs are responsible for ABO blood group phenotypes in the Japanese populations.11,12 The rs8176719 and rs505922 polymorphisms are markers of the O allele, while rs8176746 is used to distinguish the B allele from the A allele. The consistency of self-reported and ABO genotyping was 100%.
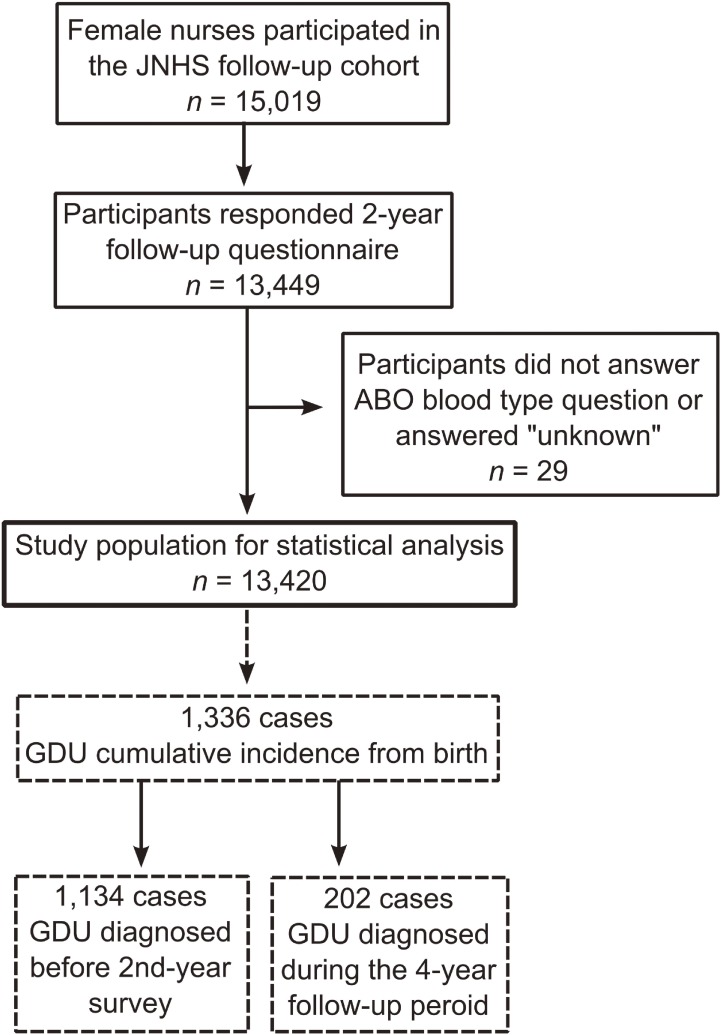
In the 2nd-year follow up questionnaire, we asked the women if they had received a diagnosis of GDU from a physician during the past 2 years. As for the 4th- and 6th-year follow-up questionnaires, we asked the women if they had ever received a diagnosis of GDU from a physician. Out of the 13,420 women, 1,336 women reported a history of physician-diagnosed GDU from birth. After excluding cases of GDU diagnosed before the 2nd-year survey, we identified 202 incident GDU cases during the 4-year follow-up period (from the 2nd-year survey to the 6th-year survey) (Figure 1). Information on lifestyle factors, such as smoking, alcohol consumption, use of aspirin/nonsteroidal anti-inflammatory drugs (NSAIDs), and dietary factors, was collected through the baseline and follow-up self-administered questionnaires.
Figure 1. Data collection flow chart by ABO blood types information and gastroduodenal ulcer (GDU) diagnosis in the Japan Nurses’ Health Study (JNHS).
Statistical analysis
Differences in characteristics between ABO blood types were assessed using the chi-squared test. The effects of ABO blood types on the risk of GDU were examined using Cox regression analysis, with adjustment for potential confounders to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). Potential confounders considered in the multivariable analysis were birth year (pre-1956 or post-1955), smoking status (non-smoker or current smoker), alcohol consumption (≤2 days/week or ≥3 days/week), use of NSAIDs (no or yes), and consumption of green tea (≤2 days/week or ≥3 days/week), coffee (≤2 days/week or ≥3 days/week), miso soup (≤3 days/week or ≥4 days/week), and breakfast (bread or rice) at the 2nd-year survey. Additionally, we conducted subgroup analyses stratified by birth year (pre-1956 or post-1955) because the prevalence of Helicobacter pylori (H. pylori) infection was quite different between these two age groups.29 Using Kaplan-Meier analysis, we plotted the cumulative incidence of GDU by blood group in women born pre-1956 and post-1955 and tested for differences using the log-rank test and the generalized Wilcoxon test.
Because GDU is not life-threatening and because ABO information is not time-dependent, the ambispective analysis combining retrospective and prospective data plays an appropriate role, as does the separate prospective analysis. We restrictively analyzed the prospective data as a sensitivity analysis, despite 4 years being a relatively short follow-up period. All statistical analyses were carried out using Statistical Analysis Software SAS version 9.4 (SAS Institute, Cary, NC, USA). All P values were two-sided. A P value <0.05 was considered statistically significant.
RESULTS
The 13,449 participants responded to the 2-year questionnaire, including questions of ABO blood type and Rh factor type. Most (99.8%) of the respondents provided their blood type, and 98.5% of these women also provided their Rh type. We then excluded participants who did not report their blood type (n = 29), which resulted in an ultimate sample of 13,420 women (Figure 1).
Characteristics of the study subjects by ABO blood type in JNHS are shown in Table 1. There were no differences in the baseline characteristics of study participants by blood type. Of the women in our study population, 29.4% reported blood type O, 38.6% type A, 22.4% type B, and 9.6% type AB.
Table 1. Characteristics of 13,420 women by ABO blood type at the 2nd-year follow-up survey of the Japan Nurses’ Health Study, 2003–2009.
| ABO blood type | |||||||||
| O (n) | % | A (n) | % | B (n) | % | AB (n) | % | P valuea | |
| Number of women | 3,943 | 5,173 | 3,012 | 1,292 | |||||
| Birth year | |||||||||
| Pre-1956 | 1,137 | 28.8 | 1,489 | 28.8 | 903 | 30.0 | 388 | 30.0 | 0.57 |
| Post-1955 | 2,806 | 71.2 | 3,684 | 71.2 | 2,109 | 70.0 | 904 | 70.0 | |
| Smoking status | |||||||||
| Non-smoker | 3,425 | 86.9 | 4,521 | 87.4 | 2,638 | 87.7 | 1,115 | 86.3 | 0.57 |
| Current smoker | 515 | 13.1 | 651 | 12.6 | 371 | 12.3 | 177 | 13.7 | |
| Missing | 3 | 1 | 3 | 0 | |||||
| Alcohol consumption | |||||||||
| ≤2 days/week | 3,009 | 76.9 | 3,974 | 77.2 | 2,329 | 77.6 | 962 | 74.7 | 0.20 |
| ≥3 days/week | 906 | 23.1 | 1,171 | 22.8 | 674 | 22.4 | 326 | 25.3 | |
| Missing | 28 | 28 | 9 | 4 | |||||
| Rh factor | |||||||||
| Positive | 3,639 | 93.5 | 4,780 | 93.8 | 2,768 | 93.2 | 1,186 | 92.6 | 0.39 |
| Negative | 253 | 6.5 | 315 | 6.2 | 202 | 6.8 | 95 | 7.4 | |
| Missing | 51 | 78 | 42 | 11 | |||||
| NSAID user | |||||||||
| No | 3,273 | 83.8 | 4,265 | 83.1 | 2,471 | 82.8 | 1,045 | 82.1 | 0.46 |
| Yes | 632 | 16.2 | 870 | 16.9 | 514 | 17.2 | 228 | 17.9 | |
| Missing | 38 | 38 | 27 | 19 | |||||
| Green tea consumption | |||||||||
| ≤2 days/week | 853 | 21.7 | 1,140 | 22.1 | 639 | 21.3 | 276 | 21.4 | 0.83 |
| ≥3 days/week | 3,081 | 78.3 | 4,021 | 77.9 | 2,366 | 78.7 | 1,015 | 78.6 | |
| Missing | 9 | 12 | 7 | 1 | |||||
| Coffee consumption | |||||||||
| ≤2 days/week | 728 | 18.5 | 1,035 | 20.1 | 593 | 19.7 | 242 | 18.8 | 0.26 |
| ≥3 days/week | 3,206 | 81.5 | 4,126 | 79.9 | 2,412 | 80.3 | 1,049 | 81.2 | |
| Missing | 9 | 12 | 7 | 1 | |||||
| Miso soup consumption | |||||||||
| ≤3 days/week | 1,961 | 49.9 | 2,535 | 49.1 | 1,471 | 49.0 | 658 | 51.2 | 0.51 |
| ≥4 days/week | 1,971 | 50.1 | 2,629 | 50.9 | 1,533 | 51.0 | 628 | 48.8 | |
| Missing | 11 | 9 | 8 | 6 | |||||
| Breakfast | |||||||||
| Bread | 1,507 | 41.6 | 1,984 | 41.8 | 1,143 | 41.2 | 450 | 38.0 | 0.13 |
| Rice | 2,116 | 58.4 | 2,767 | 58.2 | 1,631 | 58.8 | 733 | 62.0 | |
| Missing | 320 | 422 | 238 | 109 | |||||
NSAID, nonsteroidal anti-inflammatory drug; Rh, Rhesus.
aChi-squared test.
The mean age at study entry was 41.9 years (median, 41; interquartile range, 13). Compared with women born post-1955, women born pre-1956 were at significantly higher risk of GDU. The cumulative incidence of GDU from birth was 13.8% (95% CI, 12.8–15.0%) and 8.4% (95% CI, 7.8–8.9%) in women born pre-1956 and post-1955, respectively.
The cumulative incidence of GDU (n = 1336) from birth was 10.9% (95% CI, 9.9–11.9%), 9.7% (95% CI, 8.9–10.5%), 9.1% (95% CI, 8.1–10.2%), and 10.3% (95% CI, 8.7–12.1%) in women with blood types O, A, B, and AB, respectively. The 4-year cumulative incidence of GDU (n = 202) was 1.5% (95% CI, 1.1–1.9%), 1.7% (95% CI, 1.3–2.1%), 1.1% (95% CI, 0.8–1.6%), and 1.9% (95% CI, 1.2–2.8%) in women with blood types O, A, B, and AB, respectively (Table 2). Additionally, we examined the cumulative incidence of GDU by birth-year group. The cumulative incidence of GDU from birth was 15.5% (95% CI, 13.4–17.7%), 13.2% (95% CI, 11.6–15.1%), 12.7% (95% CI, 10.6–15.1%), and 13.9% (95% CI, 10.6–17.8%) in women born pre-1956 with blood types O, A, B, and AB, respectively (Table 2).
Table 2. Cumulative incidence of ulcer from birth and during the 4-year follow-up period, Japan Nurses’ Health Study, 2001–2013.
| ABO blood type | From birth (n = 1,336) | Four-year follow-up (n = 202) | ||||
| Number of Cases |
Cumulative Incidence % |
95% CI | Number of Cases |
Cumulative Incidence % |
95% CI | |
| O | 428 | 10.9 | 9.9–11.9 | 58 | 1.5 | 1.1–1.9 |
| Non-O | 908 | 9.6 | 9.0–10.2 | 144 | 1.5 | 1.3–1.8 |
| A | 500 | 9.7 | 8.9–10.5 | 86 | 1.7 | 1.3–2.1 |
| B | 275 | 9.1 | 8.1–10.2 | 34 | 1.1 | 0.8–1.6 |
| AB | 133 | 10.3 | 8.7–12.1 | 24 | 1.9 | 1.2–2.8 |
| By birth year | ||||||
| Pre-1956 | ||||||
| O | 176 | 15.5 | 13.4–17.7 | 19 | 1.7 | 1.0–2.6 |
| Non-O | 366 | 13.2 | 11.9–14.5 | 47 | 1.7 | 1.2–2.2 |
| A | 197 | 13.2 | 11.6–15.1 | 29 | 2.0 | 1.3–2.8 |
| B | 115 | 12.7 | 10.6–15.1 | 12 | 1.3 | 0.7–2.3 |
| AB | 54 | 13.9 | 10.6–17.8 | 6 | 1.6 | 0.6–3.3 |
| Post-1955 | ||||||
| O | 252 | 9.0 | 8.0–10.1 | 39 | 1.4 | 1.0–1.9 |
| Non-O | 542 | 8.1 | 7.5–8.8 | 97 | 1.5 | 1.1–1.8 |
| A | 303 | 8.2 | 7.4–9.2 | 57 | 1.6 | 1.2–2.0 |
| B | 160 | 7.6 | 6.5–8.8 | 22 | 1.0 | 0.7–1.6 |
| AB | 79 | 8.7 | 7.0–10.8 | 18 | 2.0 | 1.2–3.1 |
CI, confidence interval; Non-O, A, B, and AB blood groups.
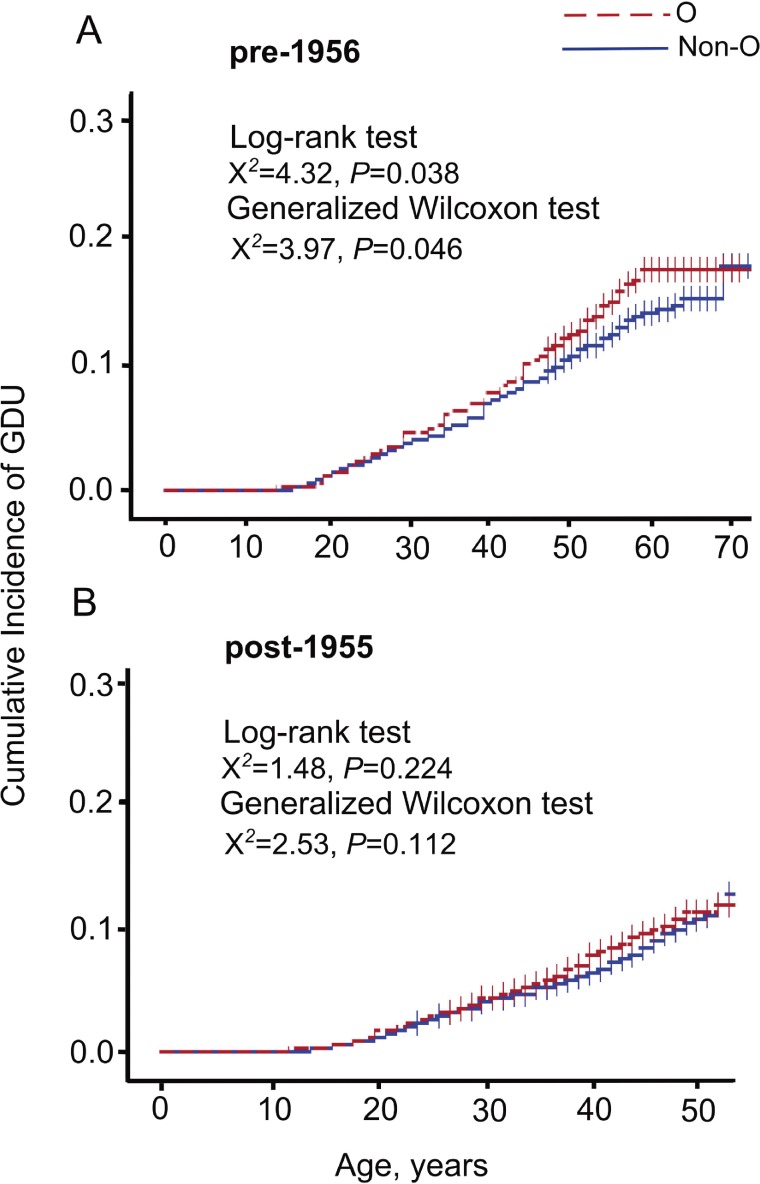
We examined the risk of GDU by comparing women with blood type O with women with non-O (A, B, and AB) blood types. Compared with women reporting non-O blood types, those with blood type O had a significantly higher risk of GDU (multivariable-adjusted HR 1.18; 95% CI, 1.04–1.34) (Table 3). We did not observe any significant difference of GDU risk in women with Rh-negative factor as compared with women with Rh-positive factor (multivariable-adjusted HR 0.98; 95% CI, 0.78–1.23). In the subgroup analyses stratified by birth year, the cumulative GDU incidence from birth in women with blood type O in the pre-1956 subgroup was significantly higher than it was in those with non-O blood types (log-rank test, P = 0.038; generalized Wilcoxon test, P = 0.046). However, in the post-1955 subgroup, the increased risk of GDU was not statistically significant (log-rank test, P = 0.224; generalized Wilcoxon test, P = 0.112) (Figure 2A and Figure 2B). The multivariable-adjusted HRs of women with blood type O were 1.22 (95% CI, 1.00–1.49) and 1.15 (95% CI, 0.98–1.35) in the pre-1956 subgroup and the post-1955 subgroup, respectively (Table 3).
Table 3. Multivariable-adjusted HR for incident gastroduodenal ulcer among 13 420 women according to ABO blood type, Rh factor, and lifestyle factors in the Japan Nurses’ Health Study (from birth).
| (a) All participants | |||
| Multivariable-adjusted HR | 95% CI | P value | |
| Birth year | |||
| Post-1955 | 1.00 | Referent | |
| Pre-1956 | 1.04 | 0.91–1.19 | 0.59 |
| ABO blood group | |||
| Non-O | 1.00 | Referent | |
| O | 1.18 | 1.04–1.34 | <0.01 |
| O | 1.00 | Referent | |
| A | 0.88a | 0.78–1.01 | 0.06 |
| B | 0.83a | 0.72–0.97 | 0.02 |
| AB | 0.93a | 0.77–1.14 | 0.49 |
| Rh factor | |||
| Positive | 1.00 | Referent | |
| Negative | 0.98 | 0.78–1.23 | 0.85 |
| Smoking status | |||
| Non-smoker | 1.00 | Referent | |
| Current smoker | 1.74 | 1.48–2.03 | <0.01 |
| Alcohol consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.96 | 0.84–1.11 | 0.57 |
| NSAID users | |||
| No | 1.00 | Referent | |
| Yes | 1.35 | 1.16–1.57 | <0.01 |
| Green tea consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 1.00 | 0.85–1.17 | 0.98 |
| Coffee consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.81 | 0.70–0.93 | <0.01 |
| Miso soup consumption | |||
| ≤3 days/week | 1.00 | Referent | |
| ≥4 days/week | 0.93 | 0.83–1.06 | 0.28 |
| Breakfast | |||
| Rice | 1.00 | Referent | |
| Bread | 0.87 | 0.77–0.99 | 0.04 |
| (b) Participants born pre-1956 | |||
| Multivariable-adjusted HR | 95% CI | P value | |
| ABO blood group | |||
| Non-O | 1.00 | Referent | |
| O | 1.22 | 1.00–1.49 | 0.047 |
| O | 1.00 | Referent | |
| A | 0.83a | 0.68–1.02 | 0.07 |
| B | 0.80a | 0.63–1.01 | 0.06 |
| AB | 0.87a | 0.64–1.18 | 0.37 |
| Rh factor | |||
| Positive | 1.00 | Referent | |
| Negative | 1.01 | 0.73–1.38 | 0.98 |
| Smoking status | |||
| Non-smoker | 1.00 | Referent | |
| Current smoker | 1.89 | 1.45–2.48 | <0.01 |
| Alcohol consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.82 | 0.65–1.03 | 0.09 |
| NSAID users | |||
| No | 1.00 | Referent | |
| Yes | 1.20 | 0.89–1.63 | 0.23 |
| Green tea consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.87 | 0.66–1.17 | 0.36 |
| Coffee consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.86 | 0.68–1.09 | 0.21 |
| Miso soup consumption | |||
| ≤3 days/week | 1.00 | Referent | |
| ≥4 days/week | 1.04 | 0.85–1.27 | 0.70 |
| Breakfast | |||
| Rice | 1.00 | Referent | |
| Bread | 0.98 | 0.79–1.20 | 0.82 |
| (c) Participants born post-1955 | |||
| Multivariable-adjusted HR | 95% CI | P value | |
| ABO blood group | |||
| Non-O | 1.00 | Referent | |
| O | 1.15 | 0.98–1.35 | 0.09 |
| O | 1.00 | Referent | |
| A | 0.92a | 0.78–1.09 | 0.36 |
| B | 0.86a | 0.70–1.05 | 0.13 |
| AB | 0.98a | 0.76–1.27 | 0.88 |
| Rh factor | |||
| Positive | 1.00 | Referent | |
| Negative | 0.94 | 0.67–1.32 | 0.71 |
| Smoking status | |||
| Non-smoker | 1.00 | Referent | |
| Current smoker | 1.65 | 1.37–2.00 | <0.01 |
| Alcohol consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 1.06 | 0.89–1.27 | 0.50 |
| NSAID users | |||
| No | 1.00 | Referent | |
| Yes | 1.41 | 1.19–1.68 | <0.01 |
| Green tea consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 1.05 | 0.87–1.26 | 0.60 |
| Coffee consumption | |||
| ≤2 days/week | 1.00 | Referent | |
| ≥3 days/week | 0.77 | 0.64–0.92 | <0.01 |
| Miso soup consumption | |||
| ≤3 days/week | 1.00 | Referent | |
| ≥4 days/week | 0.88 | 0.75–1.03 | 0.10 |
| Breakfast | |||
| Rice | 1.00 | Referent | |
| Bread | 0.82 | 0.70–0.97 | 0.02 |
CI, confidence interval; Non-O, A, B, and AB blood groups; NSAID, nonsteroidal anti-inflammatory drug; HR, hazard ratio; Rh, Rhesus.
aBirth-year-group adjusted HR.
Figure 2. Kaplan-Meier Curves of the cumulative incidence of gastroduodenal ulcer (GDU) in women with O and non-O blood types in the Japan Nurses’ Health Study (JNHS) A: women born pre-1956; B: women born post-1955.
We then conducted a sensitivity analysis by examining the above observed association using incident GDU cases during the 4-year prospective observation. We did not find any statistically significant increased risk of GDU in women with blood type O compared with those with non-O blood types for all participants (multivariable-adjusted HR 0.93; 95% CI, 0.66–1.32), in women born pre-1956 (HR 1.06; 95% CI, 0.58–1.94), or in women born post-1955 (HR 0.88; 95% CI, 0.58–1.35).
Although it was not a primary purpose of the study, we explored whether there was any association between GDU incident cases from birth and our already confirmed cases of gastric cancer from birth (n = 45) using logistic regression analysis. We found that women with GDU were at significantly higher risk of developing gastric cancer (birth-year-group adjusted odds ratio 2.28; 95% CI, 1.06–4.89). We further explored the association between gastric cancer and ABO blood types, and we found a non-significant increased risk of gastric cancer among women with blood type O (odds ratio adjusted for birth-year-group and GDU 1.04; 95% CI, 0.51–2.13).
DISCUSSION
In this prospective cohort of Japanese women, blood type A (38.6%) was more frequent than other blood types (type O, 29.4%; type B, 22.4%; and type AB, 9.6%). This frequency distribution is similar to that reported by Fujita et al,13 who studied the distribution of the ABO blood groups in Japan (n = 4,464,349 Japanese individuals). We found that the risk of GDU was significantly higher among those with blood type O than those with non-O blood types. The association between the ABO blood group and GDU was not significantly modified by other known risk factors for GDU, including smoking, alcohol consumption, NSAID use, and dietary factors. Associations between ABO blood types and the upper gastrointestinal tract diseases have been investigated in many studies. However, to the best of our knowledge, the association between ABO blood types and GDU risk had not been evaluated in a large cohort of Japanese women.
The studies by Aird et al6,14 were the first to report an association between the ABO blood group and both peptic ulcer and gastric cancer. They found that individuals with blood type O had a higher risk of peptic ulcers and that individuals with blood type A had a 20% increased risk of carcinoma of the stomach compared to those with other blood types. Recently, a large prospective population-based study performed within a cohort of Scandinavian blood donors included in the Scandinavian Donations and Transfusions (SCANDAT) study confirmed that blood type O is associated with a higher risk of peptic ulcers and that blood group A is associated with a higher risk of gastric cancer.15 Despite the evidence supporting the link between the ABO blood group and gastric cancer, a case-control analysis of data of the European Institute of Oncology did not confirm the aforementioned relationship.16
Regarding GDU, our results are aligned with the previous abovementioned studies. While gastric cancer did not show any association with blood type A, as reported previously, the associations tended to be similar for GDU. However, the association between blood type O and increased risk of gastric cancer did not reach statistical significance. It is possible that the lack of significance was a result of the limited numbers of gastric cancer cases (n = 45) in this study. Therefore, our results need to be confirmed in further studies analyzing a greater number of incident cases of gastric cancer. However, our results were supported by the significant association observed between GDU and gastric cancer. Women with GDU were at higher risk of developing gastric cancer in our study. This finding is in agreement with previous reports that found an association between benign gastric ulcers and gastric cancers and probably reflects common risk factors (ie, mainly H. pylori infection).17,18 Taken together, these results suggest that women with blood type O might have an increased susceptibility to gastroduodenal ulceration and may be at higher risk of developing gastric cancer.
The mechanisms underlying the associations between the ABO blood group and the upper gastrointestinal tract diseases remain uncertain. However, several lines of biological evidence might explain the associations observed. Alteration in the antigenic and genetic structures and expression of blood groups may influence malignant progression by changing cell motility, sensitivity to apoptosis, and immune surveillance.2 A genome-wide association analysis suggests that SNPs at the ABO gene locus are associated with circulating levels of tumor necrosis factor alpha, soluble intercellular adhesion molecule-1, soluble E-selectin, and soluble P-selectin.19–22 Thus, ABO blood group alleles may influence the systemic inflammatory state and immune response of certain malignances and diseases.22–24
Previously, it was speculated that individuals with blood type O have excessive gastric production of hydrochloric acid and consequently are susceptible to duodenal ulceration.25 Thus, the experimental results indicate that the gastric secretory cell mass is probably larger in individuals with blood type O than those with blood type A.26 Additionally, glycosyltransferase activity, encoded by the ABO gene, has been associated with circulating levels of von Willebrand factor (VWF) and the risk of venous thromboembolism.23,24 It has been reported that plasma VWF levels are 25–35% lower in subjects with blood type O than in non-O individuals.27 Horwich et al28 investigated subjects with duodenal ulcers and healthy controls and reported a significant increase of bleeding duodenal ulcers in subjects with blood type O compared with the controls.
H. pylori infection is considered a major risk factor of both gastric cancer and peptic ulcer.29 Because of the high prevalence of H. pylori infection in the Japanese population, the incidence of peptic ulcer and gastric cancer is much higher in Japanese individuals than in individuals of European descent.30 H. pylori infection rates vary with age, with higher rates among individuals born before 1950 and lower for those born thereafter, owing to the massive improvement in hygiene and the economic environment in Japan in the post-war decades.29 Our results showed that women born before 1956 were at significantly higher risk of GDU than were those born after 1955, which is consistent with the previously established association between H. pylori infection, birth year, and the incidence of GDU in the Japanese population. Furthermore, we found that women with blood type O born pre-1956 had a higher cumulative incidence of GDU than those with blood type O born post-1955. These findings seem to explain why the older generation of Japanese women, especially those with blood type O, had a higher incidence of GDU.
The mechanism of the association between H. pylori infection and blood groups has been reported in several studies.31–34 It was reported that the increased susceptibility of subjects with blood type O to develop peptic ulcer might be attributable to the higher density of H. pylori colonization and higher inflammatory responses to H. pylori compared with individuals with other blood types.35 It has also been shown that epithelial cells of individuals with blood type O bound significantly more to H. pylori than epithelial cells of individuals with other blood types.32 Interestingly, it has been reported that the blood group antigen-binding adhesion has been shown to mediate adherence of H. pylori to Lewis b receptors in the gastric epithelium.33,34 These fucosylated blood group antigens are highly expressed in the gastrointestinal epithelium, as well as on the surface of red blood cells, endothelium, kidney, and genitourinary epithelium.36,37 Collectively, these findings suggest that the increased risk of women with blood type O to develop GDU observed in our study might be attributable to the higher H. pylori colonization and to the mediated adhesion of H. pylori to the gastroduodenal epithelium observed in individuals with blood type O.
The strengths of the present study include a large study population, a high proportion of followed-up participants, and the availability of detailed lifestyle data, which allowed us to examine confounding and effect modification by several exposures of interest. Although the use of self-reported blood type may have introduced some exposure misclassification, the high concordance between self-reported blood type and serologic/genotypic testing in a subset of participants suggests that these health professionals report their blood type with a high degree of accuracy. The limitations of the present study relate to the effect of H. pylori infection. This issue was not investigated in our study because we lacked information on such infection for all subjects. Our cohort questionnaire did not ask the women to specify whether they had gastric or duodenal ulcers. Moreover, no significantly increased risk of GDU was evident in women with blood type O in the prospective analysis. This finding might have resulted from the small number of incident GDU cases in the relatively short 4-year follow-up period and/or the possible effect of more common administration of bacterial elimination therapy for H. pylori infection in the past decade.
In summary, the results from this ambispective analysis of a cohort of Japanese women (the JNHS cohort) showed that women, particularly those born before 1956, with blood type O (independent of other important risk factors in the Japanese population) have an increased susceptibility to gastroduodenal ulceration.
ACKNOWLEDGMENTS
We appreciate the cooperation of the Japanese nurses that participated in the present study. Additionally, we wish to thank Dr. Kazue Nagai, Ms. Masayo Kamio, and Ms. Satomi Shimizu for their help with data collection and management.
Funding: This study was supported partly by a Grant-in-Aid for Scientific Research (B: 22390128 to KH) from the Japan Society for the Promotion of Science.
Conflicts of interest: The authors declare they have no conflict of interest with respect to this research and paper.
Author Contributions: All authors contributed to this work. L.A. wrote the draft of the paper, analyzed the data, and performed the genotyping validation of ABO blood types. Y.I. and Y.S. provided advice on statistical analysis and double-checked the analyses. JS.L. and S.S. initiated and designed the study as the JNHS steering committee members. J.NS. and H.O. performed the validation of ABO blood types. K.H. was responsible for developing the study concept, design, and acquisition and analysis of data as the principal investigator of the JNHS. All authors were involved in the interpretation of the data and revision of the manuscript, and all read and approved the final manuscript.
REFERENCES
- 1.Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev. 2005;19:200–209. 10.1016/j.tmrv.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim Biophys Acta. 1999;1473:247–266. 10.1016/S0304-4165(99)00183-X [DOI] [PubMed] [Google Scholar]
- 3.Szulman AE. The histological distribution of the blood group substances in man as disclosed by immunofluorescence: II. The H antigen and its relationto A and B antigens. J Exp Med. 1962;115:977–996. 10.1084/jem.115.5.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang BL, He N, Huang YB, Song FJ, Chen KX. ABO blood groups and risk of cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15:4643–4650. 10.7314/APJCP.2014.15.11.4643 [DOI] [PubMed] [Google Scholar]
- 5.Liumbruno GM, Franchini M. Hemostasis, cancer, and ABO blood group: the most recent evidence of association. J Thromb Thrombolysis. 2014;38:160–166. 10.1007/s11239-013-1027-4 [DOI] [PubMed] [Google Scholar]
- 6.Aird I, Bentall HH, Mehigan JA, Roberts JA. The blood groups in relation to peptic ulceration and carcinoma of colon, rectum, breast, and bronchus; an association between the ABO groups and peptic ulceration. BMJ. 1954;2:315–321. 10.1136/bmj.2.4883.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najeeb ST, Mushtaq A. Relationship between blood groups and ulcers: revisiting the pioneer work. J Pak Med Assoc. 2012;62:1367. [PubMed] [Google Scholar]
- 8.Hayashi K, Mizunuma H, Fujita T, et al. Design of the Japan Nurses’ Health Study: a prospective occupational cohort study of women’s health in Japan. Ind Health. 2007;45:679–686. 10.2486/indhealth.45.679 [DOI] [PubMed] [Google Scholar]
- 9.Kato C, Shimada J, Hayashi K. Sleepiness during shift work in Japanese nurses: A comparison study using JESS, SSS, and actigraphy. Sleep Biol Rhythms. 2012;10:109–117. 10.1111/j.1479-8425.2011.00528.x [DOI] [Google Scholar]
- 10.Maeno T, Ohta A, Hayashi K, et al. Impact of reproductive experience on women’s smoking behaviour in Japanese nurses. Public Health. 2005;119:816–824. 10.1016/j.puhe.2004.10.017 [DOI] [PubMed] [Google Scholar]
- 11.Nakao M, Matsuo K, Hosono S, et al. ABO blood group alleles and the risk of pancreatic cancer in a Japanese population. Cancer Sci. 2011;102:1076–1080. 10.1111/j.1349-7006.2011.01907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanikawa C, Urabe Y, Matsuo K, et al. A genome-wide association study identifies two susceptibility loci for duodenal ulcer in the Japanese population. Nat Genet. 2012;44:430–434, s431–432. 10.1038/ng.1109 [DOI] [PubMed] [Google Scholar]
- 13.Fujita Y, Tanimura M, Tanaka K. The distribution of the ABO blood groups in Japan. Jinrui Idengaku Zasshi. 1978;23:63–109. 10.1007/BF02001790 [DOI] [PubMed] [Google Scholar]
- 14.Aird I, Bentall HH, Roberts JA. A relationship between cancer of stomach and the ABO blood groups. Br Med J. 1953;1:799–801. 10.1136/bmj.1.4814.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgren G, Hjalgrim H, Rostgaard K, et al. Risk of gastric cancer and peptic ulcers in relation to ABO blood type: a cohort study. Am J Epidemiol. 2010;172:1280–1285. 10.1093/aje/kwq299 [DOI] [PubMed] [Google Scholar]
- 16.Iodice S, Maisonneuve P, Botteri E, Sandri MT, Lowenfels AB. ABO blood group and cancer. Eur J Cancer. 2010;46:3345–3350. 10.1016/j.ejca.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 17.Hansson LE, Nyrén O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. N Engl J Med. 1996;335:242–249. 10.1056/NEJM199607253350404 [DOI] [PubMed] [Google Scholar]
- 18.Take S, Mizuno M, Ishiki K, et al. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–1042. 10.1111/j.1572-0241.2005.41384.x [DOI] [PubMed] [Google Scholar]
- 19.Barbalic M, Dupuis J, Dehghan A, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19:1863–1872. 10.1093/hmg/ddq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paré G, Chasman DI, Kellogg M, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4:e1000118. 10.1371/journal.pgen.1000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paterson AD, Lopes-Virella MF, Waggott D, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29:1958–1967. 10.1161/ATVBAHA.109.192971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi L, Cornelis MC, Kraft P, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19:1856–1862. 10.1093/hmg/ddq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiggins KL, Smith NL, Glazer NL, et al. ABO genotype and risk of thrombotic events and hemorrhagic stroke. J Thromb Haemost. 2009;7:263–269. 10.1111/j.1538-7836.2008.03243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolpin BM, Kraft P, Xu M, et al. Variant ABO blood group alleles, secretor status, and risk of pancreatic cancer: results from the pancreatic cancer cohort consortium. Cancer Epidemiol Biomarkers Prev. 2010;19:3140–3149. 10.1158/1055-9965.EPI-10-0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke CA. Correlations of ABO Blood Groups with Peptic Ulcer, Cancer, and Other Diseases. Am J Hum Genet. 1959;11:400–404. [PMC free article] [PubMed] [Google Scholar]
- 26.Hanley WB. Hereditary Aspects of Duodenal Ulceration: Serum-pepsinogen Level in Relation to ABO Blood Groups and Salivary ABH Secretor Status. Br Med J. 1964;1:936–940. 10.1136/bmj.1.5388.936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ Jr, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69:1691–1695. [PubMed] [Google Scholar]
- 28.Horwich L, Evans DA, McConnell RB, Donohoe WT. ABO blood groups in gastric bleeding. Gut. 1966;7:680–685. 10.1136/gut.7.6.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiota S, Murakawi K, Suzuki R, Fujioka T, Yamaoka Y. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol. 2013;7:35–40. 10.1586/egh.12.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419–424. 10.1136/pgmj.2004.029330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Pendu J, Marionneau S, Cailleau-Thomas A, Rocher J, Le Moullac-Vaidye B, Clément M. ABH and Lewis histo-blood group antigens in cancer. APMIS. 2001;109:9–31. 10.1111/j.1600-0463.2001.tb00011.x [DOI] [PubMed] [Google Scholar]
- 32.Alkout AM, Blackwell CC, Weir DM. Increased inflammatory responses of persons of blood group O to Helicobacter pylori. J Infect Dis. 2000;181:1364–1369. 10.1086/315375 [DOI] [PubMed] [Google Scholar]
- 33.Ishijima N, Suzuki M, Ashida H, et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256–25264. 10.1074/jbc.M111.233601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Styer CM, Hansen LM, Cooke CL, et al. Expression of the BabA adhesin during experimental infection with Helicobacter pylori. Infect Immun. 2010;78:1593–1600. 10.1128/IAI.01297-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heneghan MA, Moran AP, Feeley KM, et al. Effect of host Lewis and ABO blood group antigen expression on Helicobacter pylori colonisation density and the consequent inflammatory response. FEMS Immunol Med Microbiol. 1998;20:257–266. 10.1111/j.1574-695X.1998.tb01135.x [DOI] [PubMed] [Google Scholar]
- 36.Ilver D, Arnqvist A, Ogren J, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. 10.1126/science.279.5349.373 [DOI] [PubMed] [Google Scholar]
- 37.Marionneau S, Cailleau-Thomas A, Rocher J, et al. ABH and Lewis histo-blood group antigens, a model for the meaning of oligosaccharide diversity in the face of a changing world. Biochimie. 2001;83:565–573. 10.1016/S0300-9084(01)01321-9 [DOI] [PubMed] [Google Scholar]