Abstract
Objective
To investigate the factors associated with depression, including the serum oxytocin (OXT) levels, disease activity, activities of daily living (ADLs) and quality of life (QOL), and their effects on rheumatoid arthritis (RA).
Methods
This study included 42-RA-patients. We measured the following variables before and after 6 months of treatment with biological disease-modifying antirheumatic drugs (bDMARDs): the baseline characteristics (including age, sex, disease duration, smoking, and body mass index), the doses of prednisolone and methotrexate, the serum level of matrix metalloprotease-3, the erythrocyte sedimentation rate and the C-reactive protein level. The disease activity of RA was assessed using the Simplified Disease Activity Index (SDAI), depression was assessed using the Hamilton Depression Rating Scale (HAM-D), the ADLs were assessed using the Health Assessment Questionnaire disability index and the QOL was assessed using the Short Form (SF)-36. The serum OXT levels were determined using an enzyme-linked immunosorbent assay.
Results
The HAM-D score was significantly correlated with the SDAI, and the mental component summary score of the SF-36. However, the serum OXT levels were not correlated with the HAM-D score. The serum OXT levels before and after bDMARDs treatment did not differ to a statistically significant extent, regardless of the presence of depression. Although the differences in the serum levels of OXT were observed prior to the initiation of treatment, there was no gender difference after treatment.
Conclusion
Although RA complicated by depression may be related to the following high disease activity, a poor QOL and poor ADLs, the serum OXT levels were not directly correlated.
Keywords: rheumatoid arthritis, serum oxytocin level, depression
Introduction
Rheumatoid arthritis (RA) patients suffer from various complications. Depression, which accounts for approximately 15% of all complications, is the most common complication of RA (1). The odds ratio for depression in RA patients was 1.42 (95% confidence interval (CI): 1.3, 1.5; relative to healthy individuals) (2). The treatment of RA with biological disease-modifying antirheumatic drugs (bDMARDs) is known to be highly effective in reducing the disease activity as well as the severity of depression that occurs in association with RA (3).
The oxytocin (OXT) level-also referred to as the “happy hormone”-is reported to be decreased in various psychiatric diseases such as depression (4,5), bipolar disorder (6), schizophrenia (7,8), autism (9), eating disorders (10), developmental disorders (11) and social anxiety disorder (12). In some diseases, the OXT levels are reported to be increased or decreased, with no consistent pattern across studies. With regard to autoimmune disease, depression, anxiety and hypochondriasis have been reported in patients with Sjögren's syndrome (13) and depression has been reported in patients with fibromyalgia (14). However, there has been no previous reports on the relationship between depression and the serum OXT levels in RA patients.
Thus, our aim was to investigate the relationship between the serum OXT levels, depression and RA disease activity.
Materials and Methods
Participants
The study used a cross-sectional design. The study period was from October 1, 2005, to June 30, 2016. This multi-center study involved the Division of Rheumatology, Department of Medicine, Showa University Hospital; Showa University Koto-Toyosu Hospital; and Showa University Northern Yokohama Hospital. A total of 391 RA patients who were included in the registry (All RA patients at Showa University; ASHURA Registry) and who were reported to have undergone bDMARDs treatment at Showa University Hospital, participated in the present study. The criteria for the classification of RA complied with the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria (15). The variables were measured before the initiation of bDMARD treatment and after 6 months of treatment with bDMARDs. The variables that were investigated included the patient background, age, sex, body mass index (BMI), smoking history, history of bDMARD treatment, disease duration, dose of prednisolone, dose of methotrexate (MTX), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level and serum matrix metalloprotease-3 (MMP-3) level. The disease activity of RA was evaluated using the Simplified Disease Activity Index (SDAI) (16), depression was evaluated using the Hamilton Depression Rating Scale (HAM-D) (17), the activities of daily living (ADLs) were assessed using the Health Assessment Questionnaire Disability Index (HAQ-DI) (18), and the quality of life (QOL) was assessed using the Short Form-36 (SF-36) (19,20). The SF-36 results were analyzed according to three summary scores: the physical component summary (PCS), the mental component summary (MCS) and the role/social component summary (RCS). The HAM-D assessment was conducted under the guidance of SK. Patients were classified into two groups (depression vs. no depression), with depression defined as a HAM-D score of ≥8.
The exclusion criteria were as follows: antidepressant use, pregnancy, lactation and comorbidities aside from depression affecting the serum OXT level (bipolar disorder, schizophrenia, autism, eating disorders, developmental disorders and social anxiety disorder). There were no restrictions on the use of other antirheumatic drugs or nonsteroidal anti-inflammatory drugs. There were no limits on age or disease duration. Patients who requested that the examination be stopped and patients who were judged by a doctor to be inappropriate for the study were excluded.
The measurement of serum OXT
The serum samples obtained at the baseline assessment were stored at normal temperature for 30 minutes, centrifuged at 1,500×g for 10 minutes and then stored at -80℃ until the analysis. The serum OXT levels were measured using an enzyme-linked immunosorbent assay (ELISA) (Catalog No. ADI-900-153A; ENZO Life Sciences, Farmingdale, USA). Blood samples were collected in the morning because the serum OXT levels exhibit diurnal variation. The solid-phase extraction of the serum samples was performed to eliminate the effects of potentially interacting molecules, as described previously (21). Briefly, an equal volume (125 μL) of 0.1% trifluoracetic acid in water (TFA-H2O) was added to the serum sample (125 μL) and centrifuged at 17,000×g for 15 minutes at 4℃, after which the supernatant was collected. A C18 Sep-Pak column (200 mg; Bachem, San Carlos, USA) was equilibrated with 1 mL of acetonitrile and then 4 times with 3 mL of 0.1% TFA-H2O. The supernatant was applied to the C18 Sep-Pak column and washed 4 times with 3 mL of 0.1% TFA-H2O; the flow-through fraction was discarded. The sample was eluted slowly by applying a 3-mL solution of 60% acetonitrile and 40% 0.1% TFA-H2O followed by collection in a plastic tube. Next, the solvent was evaporated at 4℃ using a vacuum centrifugal concentrator and was stored at -20℃ until analysis.
Statistical analyses
We analyzed (1) the differences in background characteristics due to the presence or absence of depression (2), the correlation coefficients between HAM-D and the other factors (3), the correlation coefficients between the serum OXT levels and other factors, and (4) the serum OXT levels before and after bDMARD treatment. The following statistical analyses were performed: a Mann-Whitney U test, chi-squared test, Pearson's moment correlation coefficient and a repeated-measure analysis of variance (ANOVA). The JMP Pro 13.0 software program (SAS Institute, Cary, USA) was used to perform all of the statistical analyses. We obtained written informed consent from all of the patients who enrolled in the study. The study received approval from the Bio-Ethics Committee of the Department of Medicine, Showa University School of Medicine (No. 1950).
Results
Of the 391 patients in the ASHURA Registry considered for the study, 349 patients were excluded due to primary failure, secondary failure, complications, lack of data, lack of serum samples, transfer, withdrawal from the study, or other circumstances.
At the baseline, 42 patients were included; 12 were complicated by depression and 30 were not complicated by depression. The median SDAI among patients with depression was 24 (interquartile range, 20-31), and differed significantly from that in patients without depression (p=0.035). However, the serum OXT levels and the other parameters of the two groups did not differ to a statistically significant extent (Table 1, Figure).
Table 1.
Summary of Demographics and Baseline Characteristics of 42 RA Patients.
| with depression | without depression | p | ||
|---|---|---|---|---|
| n | 12 | 30 | ||
| Age (years) | 62 (50-72) | 51 (40-63) | 0.079* | |
| Sex (female: male) | 10:2 | 23:7 | 0.491** | |
| Body mass index | 21 (20-23) | 21 (20-23) | 0.606* | |
| Smoking history (yes : no) | 6:6 | 22:8 | 0.277** | |
| bDMARD-naïve or switch | 7:5 | 23:7 | 0.938** | |
| Disease duration (years) | 8.5 (3.5-24.3) | 2 (0.90-6.3) | 0.210* | |
| Prednisolone dosage (mg/d) | 1 (0-5) | 0 (0-5) | 0.581* | |
| MTX dosage (mg/w) | 9 (5.5-10) | 9 (6.5-11.5) | 0.541* | |
| ESR (mm/H) | 30 (11-54) | 17 (8-43) | 0.411* | |
| CRP (mg/dL) | 1.3 (0.31-3.7) | 0.56 (0.2-1.5) | 0.427* | |
| Serum MMP-3 level (ng/mL) | 151 (84-378) | 114 (65-191) | 0.316* | |
| Serum OXT level (pg/mL) | 76 (59-81) | 74 (58-93) | 0.796* | |
| SDAI | 24 (20-31) | 20 (13-27) | 0.035* | |
| TJC | 6 (4.75-12.75) | 4 (3-6.75) | 0.079* | |
| SJC | 2 (2-4) | 3 (2-4) | 0.362* | |
| PtGA (VAS, mm) | 64 (58.75-73.5) | 54.5 (24.25-69.5) | 0.094* | |
| PGA (VAS, mm) | 71 (41.25-76) | 38 (22.5-74.25) | 0.315* | |
| HAQ-DI | 0.8125 (0.125-1.625) | 0.375 (0-0.75) | 0.055* | |
| HAM-D | 10 (9-12.25) | 3 (1-5) | 0.000* | |
| SF-36 | PCS | 31 (22-36) n=11 | 34 (23-42) n=29 | 0.671* |
| MCS | 48 (36-52) n=11 | 53 (48-55) n=29 | 0.101* | |
| RCS | 37 (34-46) n=11 | 57 (35-62) n=29 | 0.214* | |
median (interquartile range). bDMARDs: biological disease-modifying antirheumatic drugs, MTX: methotrexate, ESR: erythrocyte sedimentation rate, MMP-3: matrix metalloproteinase-3, OXT: oxytocin, SDAI: simplified disease activity index, TJC: tender joint count, SJC: swollen joint count, PtGA: patient’s global assessment, PGA: physician’s global assessment, HAQ-DI: Health Assessment Questionnaire Disability Index, HAM-D: Hamilton Depression Rating Scale, SF-36: Short Form-36, PCS: physical component summary, MCS: mental component summary, RCS: role/social component summary, *analysis using a Mann-Whitney U test, **analysis using a chi-squared test for independence
Figure.
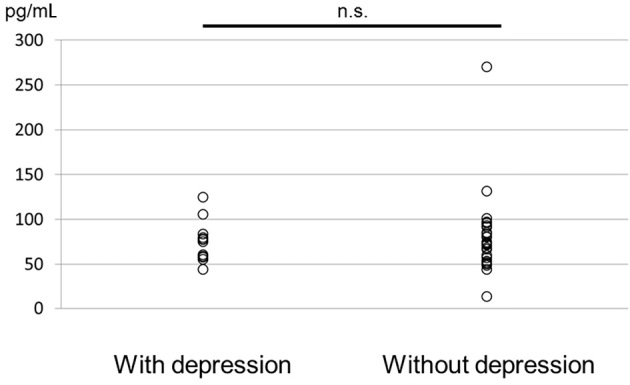
The serum OXT levels before bDMARD treatment.
The SDAI (r=0.422, p=0.008) and the MCS score (r=-0.555, p=0.000) on the SF-36 were significantly correlated with the HAM-D score. The tender joint count (TJC) (r=0.390, p=0.014), the patient's global assessment (PtGA) (r=0.380, p=0.017), the physician's global assessment (PGA) (r=0.391, p=0.014), and the HAQ-DI (r=0.347, p=0.031) were weakly correlated with the HAM-D score. However, the serum OXT levels were not significantly correlated with the HAM-D score (r=0.083, p=0.617) and no other factors were significantly correlated with the serum OXT levels (Table 2).
Table 2.
Correlation Coefficient between Serum OXT Levels, HAM-D Score and Other Factors.
| OXT | HAM-D | |||||
| r | p | r | p | |||
| Age (years) | -0.026 | 0.874 | 0.253 | 0.121 | ||
| Sex (female) | 0.206 | 0.208 | -0.021 | 0.898 | ||
| Body mass index | 0.046 | 0.782 | -0.090 | 0.586 | ||
| Smoking history (yes) | 0.143 | 0.385 | -0.248 | 0.129 | ||
| bDMARD-naïve | -0.091 | 0.821 | 0.027 | 0.872 | ||
| Disease duration (years) | -0.055 | 0.741 | 0.153 | 0.353 | ||
| Prednisolone dosage (mg/d) | 0.029 | 0.860 | 0.286 | 0.077 | ||
| MTX dosage (mg/w) | 0.255 | 0.117 | 0.113 | 0.492 | ||
| ESR (mm/H) | -0.254 | 0.119 | 0.109 | 0.509 | ||
| CRP (mg/dL) | -0.235 | 0.151 | 0.171 | 0.299 | ||
| Serum MMP-3 level (ng/mL) | -0.099 | 0.549 | 0.159 | 0.334 | ||
| Serum OXT level | Ref | 0.083 | 0.617 | |||
| SDAI | -0.055 | 0.741 | 0.422 | 0.008 | ||
| TJC | 0.037 | 0.823 | 0.390 | 0.014 | ||
| SJC | -0.252 | 0.122 | -0.093 | 0.575 | ||
| PtGA (VAS, mm) | 0.197 | 0.228 | 0.380 | 0.017 | ||
| PGA (VAS, mm) | 0.013 | 0.937 | 0.391 | 0.014 | ||
| HAQ-DI | 0.101 | 0.540 | 0.347 | 0.031 | ||
| HAM-D | 0.083 | 0.617 | Ref | |||
| SF-36 | PCS | -0.099 | 0.551 | -0.165 | 0.316 | |
| MCS | -0.261 | 0.109 | -0.555 | 0.000 | ||
| RCS | 0.015 | 0.926 | -0.165 | 0.315 | ||
Analyses by Pearson’s moment correlation coefficient. MTX: methotrexate, ESR: erythrocyte sedimentation rate, MMP-3: matrix metalloproteinase-3, OXT: oxytocin, SDAI: simplified disease activity index, TJC: tender joint count, SJC: swollen joint count, PtGA: patient’s global assessment, PGA: physician’s global assessment, HAQ-DI: Health Assessment Questionnaire Disability Index, HAM-D: Hamilton Depression Rating Scale, SF-36: Short Form-36, PCS: physical component summary, MCS: mental component summary, RCS: role/social component summary
The serum OXT levels before and after bDMARD treatment, did not differ to a statistically significant extent, regardless of the presence of depression. Thus, no treatments were observed to improve the serum OXT levels (Table 3, 4). Although differences in the serum levels of OXT prior to the initiation of treatment were observed between men [median, 65 (25-75% quartile, 53-70)] and women [83 (60-94); p=0.045], there was no gender difference in OXT levels after treatment (data not shown). No gender difference was observed in the serum OXT levels of patients with or without depression.
Table 3.
Serum OXT Levels and HAM-D Score before and after bDMARD Treatment.
| OXT | HAM-D | ||||
|---|---|---|---|---|---|
| Before | After 6 months | Before | After 6 months | ||
| With depression | 76 (59-81) | 68 (56-74) | 10 (9-12.25) | 2 (2-7.5) | |
| Without depression | 74 (58-93) | 77 (66-88) | 3 (1-5) | 1 (0-2) | |
Median (interquartile range). OXT: oxytocin, HAM-D: Hamilton Depression Rating Scale, bDMARD: biological disease-modifying antirheumatic drug
Table 4.
Serum OXT Levels before and after bDMARDs Treatment.
| f value | p value | f value (0.95) | |
|---|---|---|---|
| Interaction | 0.102 | 0.752 | 4.085 |
| Interindividual variation | 0.659 | 0.422 | 4.085 |
| Individual variation | 0.729 | 0.398 | 4.085 |
Analysis by repeated measure ANOVA. OXT: oxytocin, bDMARD: biological disease-modifying antirheumatic drug, ANOVA: analysis of variance
Discussion
In this report, we first measured the serum OXT levels in RA patients. Although disease activity, the MCS in SF-36, TJC, PtGA, PGA and HAQ-DI were found to be correlated with depression in RA, the serum OXT levels were not.
The level of OXT, also known as the “happy hormone”, is reported to be decreased in various psychiatric diseases, including depression (4,5). It has been reported that the intranasal administration of OXT improves refractory major depression (22). The methods of assessing the serum OXT level included an ELISA and a radioimmunoassay (RIA). In recent years, most reports evaluated the serum OXT levels via an ELISA using a kit from ENZO; thus, an ELISA was used in the present study.
In previous reports, the serum OXT levels in women with post-traumatic stress disorder (PTSD) associated with a traffic accident, PTSD associated with other events, and women with schizopherenia were reported to be 69.5±32.7 pg/mL (23), 101.59±55.89 pg/mL (24), and 100±35 pg/mL (25), respectively. Attention deficit hyperactivity disorder (ADHD) (26,27) and autism spectrum disorder (ASD) (28,29) in children as well as treatment-resistant depression in adolescents (30) are reported to be associated with OXT levels. Depression was only reported to be associated with OXT levels based on the RIA method (5,31,32). The serum OXT levels show no fixed value and vary with disease, age, and gender. Fibromyalgia is the only connective tissue disease for which the OXT levels have been reported (14); the OXT levels have not been previously reported in patients with RA. In a study of healthy subjects, the OXT levels were in elderly women and men were 140±133 pg/mL and 50±38 pg/mL, respectively (33) (Table 5). Although the serum OXT levels in RA patients were lower than those of healthy elderly subjects, the difference was small, even in comparison to other psychiatric diseases.
Table 5.
Serum Oxytocin Levels across Clinical Conditions.
| Reference | Disease | Age | Sex | Serum OXT Level | Unit | Method |
|---|---|---|---|---|---|---|
| 21 | PTSD | 44.9 ± 15.6 | female | 69.5 ±32.7 | pg/mL | ELISA |
| 34.8 ± 14.4 | male | 65.5 ± 23.3 | pg/mL | ELISA | ||
| 24 | PTSD | no data | male | 101.6 ± 55.9 | pg/mL | ELISA |
| 25 | Schizophrenia | 44.7 ± 2.4 | both | 100 ± 35 | pg/mL | ELISA |
| 26 | ADHD | 6-15 | both | 60.7 ± 37.1 | pg/mL | ELISA |
| 27 | ADHD | 7-18 | both | 37.62 ± 9 | μIU/mL | ELISA |
| 28 | ASD | 2-9 | both | 124.1 ± 90.6 | pg/mL | ELISA |
| 29 | ASD | 21.8 ± 2.0 | both | 21.8 ± 2.0 | pg/mL | ELISA |
| 30 | TRDIA | 14.40 ± 1.71 | both | 394.3 ± 371.23 | pg/mL | ELISA |
| non-TRDIA | 12.89 ± 1.99 | both | 94.34 ± 31.24 | pg/mL | ELISA | |
| 5 | Depression | 42.5 ± 12.8 | female | 8.98 ± 7.28 | ng/mL | RIA |
| male | 5.70 ± 4.54 | ng/mL | RIA | |||
| 31 | Depression | 40.6 ± 14.7 | both | 1.2 ± 0.2 | ng/mL | RIA |
| 32 | Depression | 19-59 | both | 3.67 ± 1.34 | ng/mL | RIA |
| 14 | Fibromyalgia | 27-61 | both | 15 ± 5 | pmol/L | RIA |
| 33 | Healthy elderly | 65- | male | 50 ± 38 | pg/mL | EIA |
| female | 140 ± 133 | pg/mL | EIA |
Mean ± standard deviation values or range are used. OXT: Oxytocin, PTSD: post traumatic stress disorder, ADHD: attention deficit hyperactivity disorder, TRDIA: treatment resistant depression in adolescents, ASD: autism spectrum disorder, ELISA: enzyme-linked immunosorbent assay, RIA: radioimmunoassay, EIA: enzyme immunoassay
In this study, we hypothesized that after treatment with bDMARDs, the RA disease activity would decrease, the depression status would improve, and that the serum OXT levels would increase. Previous reports have suggested that OXT has an anti-inflammatory function (34). However, the results showed that there was no difference in the serum OXT levels before and after bDMARDs treatment.
There are several possible reasons for our unexpected results. First, it is possible that no significant difference was detected due to the low number of cases of RA with depression. The bDMARDs that were used to treat RA might have affected the OXT levels. The anti-inflammatory effect of oxytocin may not be involved in RA. The old age of the patients in comparison to other diseases for which reports are available makes comparisons difficult. Although a slight gender difference was observed in OXT levels before the start of bDMARD treatment, there was no gender difference after treatment. The serum OXT levels were higher in men with RA than in healthy elderly men but they were lower in women. It is important to note that there is no defined normal OXT level; it is therefore difficult to judge whether the levels in this patient population were high or low. The serum OXT level also varies according to disease, age, and sex; thus, it is necessary to study the serum OXT levels in a disease other than those that have been previously reported and to consider a large number of cases.
Our study is associated with 4 key limitations. First, the number of cases that were included in the analysis was small (42 cases); thus, the small sample size might have contributed to the lack of statistical significance of the results. Second, we did not perform a radiographic evaluation of the joints, although we were aware that a radiographic evaluation would be expected to influence depression. Third, no socioeconomic factors were included in our analysis. Fourth, we did not investigate the history of major depression.
Conclusion
RA complicated with depression may be related to high disease activity, a poor QOL and poor ADLs. However, the serum OXT levels may not be directly related to RA complicated with depression.
Author's disclosure of potential Conflicts of Interest (COI).
Yusuke Miwa: Research funding, Astellas Pharma, Mitsubishi Tanabe Pharma Corporation, Pfizer Japan and Chugai Pharmaceutical. Tsuyoshi Kasama: Research funding, Mitsubishi Tanabe Pharma Corporation and AbbVie CK.
Acknowledgement
Cooperation on data collection: all members of the Rheumatoid Arthritis Group of Showa University (ASHURA groups): Nobuyuki Yajima, Takeo Isozaki, Kuninobu Wakabayashi, Sakiko Isojima, Mayu Saito, Nao Oguro, Yuzo Ikari, Takahiro Tokunaga, Yoko Miura, Sho Ishii, Shinichiro Nishimi, Airi Nishimi, Mika Hatano, Kosuke Sakurai, Yoichi Toyoshima and Katsunori Inagaki.
Measurement of serum OXT levels: Hidetoshi Arakawa, Yoshihiro Sano, Koji Karasawa in the Department of Analytical Biochemistry, School of Pharmacy, Showa University and Hiroko Takeuchi in Division of Rheumatology, Showa University.
Data entry and management: Hiroka Mitsuhashi and Yuko Mitamura.
References
- 1. Dougados M, Soubrier M, Antunez A, et al. . Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 73: 62-68, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Margaretten ME, Katz P, Schmajuk G, Yelin E. Missed opportunities for depression screening in patients with arthritis in the United States. J Gen Intern Med 28: 1637-1642, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miwa Y, Isojima S, Saito M, et al. . Comparative study of infliximab therapy and methotrexate monotherapy to improve the clinical effect in rheumatoid arthritis patients. Intern Med 55: 2581-2585, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Cochran DM, Fallon D, Hill M, Frazier JA. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry 21: 219-247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ozsoy S, Esel E, Kula M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res 169: 249-252, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31: 736-756, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Strauss GP, Keller WR, Koenig JI, Gold JM, Frost KH, Buchanan RW. Plasma oxytocin levels predict social cue recognition in individuals with schizophrenia. Schizophr Res 162: 47-51, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin NY, Park HY, Jung WH, et al. . Effects of oxytocin on neural response to facial expressions in patients with schizophrenia. Neuropsychopharmacology 40: 1919-1927, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdulamir HA, Abdul-Rasheed OF, Abdulghani EA. Low oxytocin and melatonin levels and their possible role in the diagnosis and prognosis in Iraqi autistic children. Saudi Med J 37: 29-36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawson EA, Holsen LM, Santin M, et al. . Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J Clin Psychiatry 74: e451-e457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu XJ, Zhang HF, Shou XJ, et al. . Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring. Physiol Behav 138: 13-20, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Hoge EA, Pollack MH, Kaufman RE, Zak PJ, Simon NM. Oxytocin levels in social anxiety disorder. CNS Neurosci Ther 14: 165-170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karaiskos D, Mavragani CP, Sinno MH, et al. . Psychopathological and personality features in primary Sjögren's syndrome-associations with autoantibodies to neuropeptides. Rheumatology (Oxford) 49: 1762-1769, 2010. [DOI] [PubMed] [Google Scholar]
- 14. Anderberg UM, Uvnas-Moberg K. Plasma oxytocin levels in female fibromyalgia syndrome patients. Z Rheumatol 59: 373-379, 2000. [DOI] [PubMed] [Google Scholar]
- 15. Aletaha D, Neogi T, Silman AJ, et al. . 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69: 1580-1588, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum 52: 2625-2636, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56-62, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ziebland S, Fitzpatrick R, Jenkinson C, Mowat A. Comparison of two approaches to measuring change in health status in rheumatoid arthritis: the Health Assessment Questionnaire (HAQ) and modified HAQ. Ann Rheum Dis 51: 1202-1205, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol 51: 1037-1044, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Fukuhara S, Ware JE Jr, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol 51: 1045-1053, 1998. [DOI] [PubMed] [Google Scholar]
- 21. Nishi D, Hashimoto K, Noguchi H, Matsuoka Y. Serum neuropeptide Y in accident survivors with depression or posttraumatic stress disorder. Neurosci Res 83: 8-12, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Scantamburlo G, Hansenne M, Geenen V, Legros JJ, Ansseau M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: an open trial. Eur Psychiatry 30: 65-68, 2015. [DOI] [PubMed] [Google Scholar]
- 23. Nishi D, Hashimoto K, Noguchi H, Kim Y, Matsuoka Y. Serum oxytocin, posttraumatic coping and C-reactive protein in motor vehicle accident survivors by gender. Neuropsychobiology 71: 196-201, 2015. [DOI] [PubMed] [Google Scholar]
- 24. Cao C, Wang L, Wang R, Qing Y, Zhang J. Oxytocin is associated with PTSD's anxious arousal symptoms in Chinese male earthquake survivors. Eur J Psychotraumatol 5: 26530, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldman M, Marlow-O'Connor M, Torres I, Carter CS. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr Res 98: 247-255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sasaki T, Hashimoto K, Oda Y, et al. . Decreased levels of serum oxytocin in pediatric patients with Attention Deficit/Hyperactivity Disorder. Psychiatry Res 228: 746-751, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Demirci E, Ozmen S, Kilic E, Oztop DB. The relationship between aggression, empathy skills and serum oxytocin levels in male children and adolescents with attention deficit and hyperactivity disorder. Behav Pharmacol 27: 681-688, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Husarova VM, Lakatosova S, Pivovarciova A, et al. . Plasma oxytocin in children with autism and its correlations with behavioral parameters in children and parents. Psychiatry Investig 13: 174-183, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jansen LM, Gispen-de Wied CC, Wiegant VM, Westenberg HG, Lahuis BE, van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord 36: 891-899, 2006. [DOI] [PubMed] [Google Scholar]
- 30. Sasaki T, Hashimoto K, Oda Y, et al. . Increased serum levels of oxytocin in 'Treatment Resistant Depression in Adolescents (TRDIA)' Group. PLoS One 11: e0160767, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parker KJ, Kenna HA, Zeitzer JM, et al. . Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res 178: 359-362, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scantamburlo G, Hansenne M, Fuchs S, et al. . Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 32: 407-410, 2007. [DOI] [PubMed] [Google Scholar]
- 33. Kutake Y, Imamura Y, Mizogushi Y, et al. . In female mild cognitive impairment and Alzheimer type dementia, peripheral oxytocin concentration is lower than healthy elderly. Jpn J Psychiatr Neurol (supple): S528, 2016(in Japanese). [Google Scholar]
- 34. Gutkowska J, Jankowski M. Oxytocin: old hormone, new drug. Pharmaceuticals (Basel) 2: 168-183, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


