Abstract
We report four adult cases of ceftriaxone (CTRX)-induced pseudolithiasis and nephrolithiasis. With the exception of case 1, none of our cases showed abdominal symptoms. Our patients, who had central nervous system (CNS) infections, had been treated with CTRX (4 g/day) for 35-69 days. CTRX-induced pseudolithiasis and nephrolithiasis can appear depending on the total dose of CTRX and the duration for which it is administered. Patients with bacterial CNS infections who are treated with CTRX are typically treated with higher doses for longer periods. It should be recognized that these patients are at higher risk of developing CTRX-induced pseudolithiasis and nephrolithiasis.
Keywords: ceftriaxone (CTRX), central nervous system (CNS) infection, biliary sludge, pseudolithiasis, nephrolithiasis
Introduction
Ceftriaxone (CTRX) is used to treat central nervous system (CNS) infections such as bacterial brain abscess or meningitis. CTRX-induced pseudolithiasis and nephrolithiasis have been reported in pediatric patients (1, 2). However, this adverse event has also been reported in adult patients (3-7). We herein report the cases of four adult patients who were diagnosed of CTRX-induced pseudolithiasis and nephrolithiasis. As patients with bacterial CNS infections require higher dosages and longer periods of CTRX treatment, it is very important to recognize that these patients have a greater risk of developing CTRX-induced pseudolithiasis and nephrolithiasis.
Case Reports
Case 1
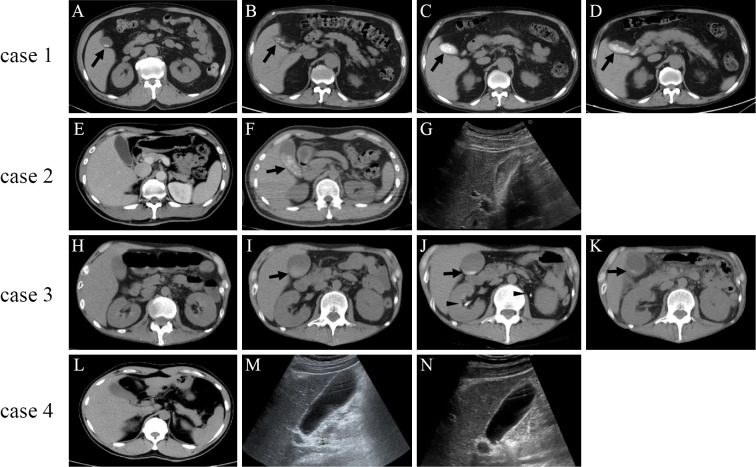
A 70-year-old man was admitted to our hospital with right-leg paralysis. He had been experiencing antineutrophilic cytoplasmic antibody (ANCA)-associated vasculitis, and was treated with oral prednisolone. Initially, he was considered to have right peroneal neuropathy due to ANCA-associated vasculitis, because his weakness was confined to the right ankle dorsiflexor. However, his weakness progressed to the right proximal leg, and subsequent cerebral magnetic resonance imaging (MRI) revealed that he had a brain abscess in the left motor cortical area. The administration of CTRX (4 g/day) and oral metronidazole was started. He already had some small gallstones before the start of CTRX therapy (Figure A). The number of gallstones increased without abdominal symptoms after 2 weeks of CTRX treatment (Figure B). After 10 weeks of CTRX treatment, he showed right hypochondrial pain. There was a further increase in the number of gallstones, and the gallbladder was filled with biliary sludge (Figure C). Acute pancreatitis due to the obstruction of the common bile duct [aspartate transaminase (AST), 109 IU/L; alanine transaminase (ALT), 45 IU/L; and amylase, 1,632 IU/L] was diagnosed. He was confined to bed because of right-leg paralysis, and did not show malnutrition with a normal body mass index (BMI) of 19.2. The patient had no other risk factors for cholelithiasis. CTRX was immediately discontinued. No gallstones were confirmed by endoscopic retrograde cholangiopancreatography (ERCP). The gallstones spontaneously resolved at 2 weeks after the discontinuation of CTRX, and the patient's pancreatitis gradually improved (Figure D).
Figure.
Plain abdominal computed tomography (CT) and ultrasonography images of the cases involving patients with central nervous system infections who were treated with intravenous ceftriaxone (CTRX). Case 1: Some tiny gallstones were present before the initiation of CTRX therapy (A, arrow). The number of gallstones increased after 2 weeks of CTRX treatment (B, arrow). The number of gallstones further increased, and the gallbladder was filled with biliary sludge after 10 weeks of CTRX treatment (C, arrow). The gallstones and biliary sludge resolved at 2 weeks after the discontinuation of CTRX (D, arrow). Case 2: Neither gallstones nor biliary sludge was present before the initiation of CTRX treatment (E). Numerous gallstones with biliary sludge appeared after 7 weeks of CTRX treatment (F, arrow). Abdominal ultrasonography performed at 2 weeks after the discontinuation of treatment showed a reduction in the number of gallstones and the amount of sludge (G). Case 3: Neither gallstones nor biliary sludge was present before the initiation of CTRX therapy (H). The faint appearance of biliary sludge occurred after 2 weeks of CTRX treatment (I, arrowhead). In addition to the presence of condensed biliary sludge at the bottom of the gallbladder (J, arrow), bilateral nephrolithiasis (J, arrowhead) appeared after 5 weeks of CTRX treatment. The biliary sludge and nephrolithiasis resolved at 5 weeks after the discontinuation of CTRX (K, arrow). Case 4: Neither gallstones nor biliary sludge was present before the initiation of CTRX treatment (L). Abdominal ultrasonography performed after 10 days of CTRX treatment (M) and after discontinuation of CTRX treatment (N) did not show gallstones or biliary sludge.
Case 2
A 39-year-old man was admitted to our hospital with severe headache and fever. He showed Kernig's sign, stiff neck, and cerebrospinal fluid abnormality (initial pressure, 28 cmH2O; cell count, 2,009 /μL; polymorphonucleocytes, 97%; and glucose, 2 mg/dL). Abdominal computed tomography (CT) before treatment did not show any gallbladder abnormalities (Figure E). The patient was diagnosed with bacterial meningitis, and intravenous CTRX (4 g/day) was initiated. After 7 weeks of CTRX treatment, abdominal CT clearly demonstrated multiple gallstones with biliary sludge (Figure F). His BMI was 22.5, and the patient had no risk factors for developing cholelithiasis. CTRX therapy was discontinued. The patient never showed abdominal symptoms or elevated serum liver enzyme levels. Abdominal ultrasonography at 2 weeks after the discontinuation of CTRX showed a reduction in the amounts of gallstones and sludge (Figure G).
Case 3
A 35-year-old man was admitted to our hospital with a long history of myasthenia gravis. Cerebral MRI revealed multiple brain abscesses. Intravenous CTRX (4 g/day), clindamycin, and oral metronidazole were started. There was no sludge when CTRX was started (Figure H), however, after 2 weeks of CTRX treatment, abdominal CT showed a faint appearance of biliary sludge (Figure I). The patient did not have abdominal symptoms, and his serum liver enzyme levels were not elevated. After 5 weeks of CTRX treatment, the biliary sludge appeared to have condensed at the bottom of the gallbladder (Figure J, arrow), and bilateral nephrolithiasises appeared (Figure J, arrowhead). The serum levels of direct bilirubin and γ-glutamyl transpeptidase were elevated at this point. He had been bedridden for a long time, and showed aphagia due to the progression of general myathenic symptoms. These conditions and remarkable weight loss (BMI: 15.6) might be the risk factors for developing cholelithiasis. The biliary sludge and nephrolithiasis resolved at 5 weeks after the discontinuation of CTRX treatment (Figure K).
Case 4
A 36-year-old man with frontal sinus empyema was admitted to our hospital due to tonic-clonic seizure. Cerebral MRI revealed a brain abscess in the right frontal lobe. The patient was diagnosed with secondary epilepsy due to brain abscess and treatment with intravenous CTRX (4 g/day), vancomycin (2 g/day), and oral metronidazole (1.5 g/day) were administered. Abdominal CT and ultrasonography were performed before and after the administration of CTRX; however, neither pseudolithiasis nor biliary sludge appeared during 5 weeks of CTRX treatment (Figure L, M, N). The patient's BMI was 22.0, and he had no risk factors for cholelithiasis.
Discussion
CTRX is a third-generation broad-spectrum cephalosporin that has a beneficial accessibility across the blood-brain barrier, and which is used in the treatment of CNS infections such as bacterial brain abscess or meningitis. After CTRX is intravenously administered and distributed throughout the body, it is excreted by the biliary and urinary systems (8), and condenses in the gallbladder. Additionally, CTRX can easily combine with serum calcium and produce biliary sludge. CTRX-induced nephrolithiasis or urolithiasis have also reported (6, 9, 10). CTRX-induced pseudolithiasis, biliary sludge, and nephrolithiasis have been repeatedly reported in pediatric patients with CNS infections. However, this adverse event has also been reported in adult patients. CTRX-induced pseudolithiasis has been reported to appear in 17-43% of children of 1 month to 18 years of age who are treated with CTRX (50-100 mg/kg/day) for 4-22 days (1, 11). In contrast, among adult patients pseudolithiasis was reported to appear in 5% of patients who were treated with CTRX (2 g/day) for 10-14 days (12), 21% of patients who were treated with CTRX (2 g/day) for 14 days (4), and 25% of patients who were treated with CTRX (3 g/day) for 4-17 days (3). Abdominal pain was only reported in 2 of these previous reports, and the biliary sludge resolved at 7-26 days after the discontinuation of CTRX. In contrast, CTRX-induced nephrolithiasis is was only reported in 1 of 86 pediatric patients (1.2%) who were treated with CTRX (13). To date, there have been no prospective studies on CTRX-induced nephrolithiasis in adults.
CTRX is frequently used for the treatment of bacterial CNS infections, and is typically administered at relatively high doses (e.g., 4 g/body/day) for longer periods. The present four patients, who all showed CNS infections, were treated with CTRX (4 g/day) for 35-69 days, with the total dose of 140-276 g for treatment (Table). Three out of the four patients developed pseudolithiasis or biliary sludge; however, there was a substantial decrease in the amount of pseudolithiases and sludge at 2-5 weeks after the discontinuation of CTRX.
Table.
Clinical Summary of Cases of Progressive Cholelithiasis Associated with Intravenous Ceftriaxone (CTRX) Administration.
| Case | Age (years) | Sex | Diagnosis | Amount of CTRX | Duration of CTRX | Clinical symptoms |
|---|---|---|---|---|---|---|
| 1 | 70 | Male | Brain abscess | 4 g/day | 69 days | Abdominal pain |
| 2 | 39 | Male | Bacterial meningitis | 4 g/day | 47 days | None |
| 3 | 35 | Male | Brain abscess | 4 g/day | 39 days | None |
| 4 | 36 | Male | Brain abscess | 4 g/day | 35 days | None |
CTRX-induced pseudolithiasis or biliary sludge can appear depending on the total dose or the duration of CTRX treatment. The occurrence of pseudolithiasis or biliary sludge increases when CTRX was administered at doses of >2 g/day for 9 days (14). The other factors that can promote the production of pseudolithiasis include rapid intravenous infusion, depression of gallbladder contractility, fasting, and dehydration (11). Although cases 3 and 4 were of similar age and received a similar total amount of CTRX, case 3 developed biliary sludge and nephrolithiasis while case 4 did not. The patient in case 3 had multiple risk factors, including immobilization and aphagia due to myasthenia gravis. In contrast, the patient in case 4 had no risk factors. The difference in risk factors might have been responsible for this discrepancy,
According to previous reports, only 0-19% of patients with CTRX-induced pseudolithiasis and biliary sludge showed abdominal symptoms 0-19% (11, 15, 16). In contrast, CTRX-induced nephrolithiasis can cause anuria and colicky abdominal pain (6). Among the present four cases only case 1 (in which the highest total amount of CTRX was administered) showed abdominal symptoms. As patients with bacterial CNS infections require higher doses and longer durations of CTRX treatment, it is very important to recognize that these patients have a higher risk of developing CTRX-induced pseudolithiasis and nephrolithiasis. If the long-term administration of CTRX is necessary, it may cause cholangitis and pancreatitis. Thus, it is necessary to perform follow-up ultrasonography or CT. Even if pseudolithiasis or nephrolithiasis occurs, it can be expected to disappear or decrease after the discontinuation of CTRX.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet 17: 1411-1413, 1988. [DOI] [PubMed] [Google Scholar]
- 2. Araz N, Okan V, Demirci M, Araz M. Pseudolithiasis due to ceftriaxone treatment for meningitis in children: report of 8 cases. Tohoku J Exp Med 211: 285-290, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet 15: 165, 1989. [DOI] [PubMed] [Google Scholar]
- 4. Heim-Duthoy KL, Caperton EM, Pollock R, Matzke GR, Enthoven D, Peterson PK. Apparent biliary pseudolithiasis during ceftriaxone therapy. Antimicrob Agents Chemother 34: 1146-1149, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bickford CL, Spencer AP. Biliary sludge and hyperbilirubinemia associated with ceftriaxone in an adult: case report and review of the literature. Pharmacotherapy 25: 1389-1395, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Li ZL, Li HL, Chen HW, et al. Anuria and abdominal pain induced by ceftriaxone-associated ureterolithiasis in adults. Int Urol Nephrol 45: 73-76, 2013. [DOI] [PubMed] [Google Scholar]
- 7. Shima A, Suehiro T, Takii M, Soeda H, Hirakawa M. Reversible ceftriaxone-induced pseudolithiasis in an adult patient with maintenance hemodialysis. Case Rep Nephrol Dial 5: 187-191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richards DM, Heel RC, Brogden RN, Speight TM, Avey GS. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 27: 469-527, 1984. [DOI] [PubMed] [Google Scholar]
- 9. de Moor RA, Egberts AC, Schröder CH. Ceftriaxone-associated nephrolithiasis and biliary pseudolithiasis. Eur J Pediatr 158: 975-977, 1999. [DOI] [PubMed] [Google Scholar]
- 10. Lozanovski VJ, Gucev Z, Avramoski VJ, Kiroyski I, Makreski P, Tasic V. Ceftriaxone associated urolithiasis in a child with hypercalciuria. Hippokratia 15: 181-183, 2011. [PMC free article] [PubMed] [Google Scholar]
- 11. Biner B, Oner N, Celtik C, et al. Ceftriaxone-associated biliary pseudolithiasis in children. J Clin Ultrasound 34: 217-222, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Cometta A, Gallot-Lavallee-Villars S, Iten A, et al. Incidence of gallbladder lithiasis after ceftriaxone treatment. J Antimicrob Chemother 25: 689-695, 2006. [DOI] [PubMed] [Google Scholar]
- 13. Ustyol L, Bulut MD, Agengin K, et al. Comparative evaluation of ceftriaxone- and cefotaxime-induced biliary pseudolithiasis or nephrolithiasis: a prospective study in 154 children. Hum Exp Toxicol 36: 547-553, 2017. [DOI] [PubMed] [Google Scholar]
- 14. Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology 99: 1772-1778, 1990. [DOI] [PubMed] [Google Scholar]
- 15. Papadopoulou F, Efremidis S, Karyda S, et al. Incidence of ceftriaxone-associated gallbladder pseudolithiasis. Acta Paediatr 88: 1352-1355, 1999. [DOI] [PubMed] [Google Scholar]
- 16. Ozturk A, Kaya M, Zeyrek D, Ozturk E, Kat N, Ziylan SZ. Ultrasonographic findings in ceftriaxone: associated biliary sludge and pseudolithiasis in children. Acta Radiol 46: 112-116, 2005. [DOI] [PubMed] [Google Scholar]