Abstract
A delayed diagnosis of insulinoma remains a clinical issue. Hypoglycemic symptoms can mimic neuropsychiatric disorders such as epilepsy. A 27-year-old woman with a history of epilepsy and anti-epileptic drugs (AEDs) developed repeated seizures and neuropsychiatric symptoms after a 9-year asymptomatic interval. She had received transient treatment with AEDs before the possibility of hypoglycemia was considered. Following a clinical diagnosis of insulinoma, distal pancreatectomy was performed; her seizures didn't occur again. The early diagnosis of insulinoma requires vigilance not only for hypoglycemia in patients with neuropsychiatric symptoms but also for the possible masking effects of a history of epilepsy and preceding AED usage.
Keywords: insulinoma, epilepsy, continuous glucose monitoring, hypoglycemia, misdiagnosis, anti-epileptic drug
Introduction
Insulinoma is a very rare tumor with a reported incidence of 0.5-5 per million person-years (1). A nationwide epidemiological survey in Japan showed that insulinoma comprised 65.5% of all pancreatic neuroendocrine tumors (2). The clinical clues suggesting insulinoma continue to be based on the physician's recognition of the presence of hypoglycemic symptoms, which are included in Whipple's triad (3, 4). If the relationship between patient symptoms and possible hypoglycemia are missed, blood glucose levels may not be checked in clinical settings. In addition, hypoglycemic symptoms are varied and lack specificity. They can mimic many common neuropsychiatric disorders, such as epilepsy (5). Furthermore, a previous diagnosis of epilepsy and/or a drug history of anti-epileptic drugs (AEDs) can obscure the clinical relationship between patient symptoms and possible hypoglycemia. The clinical effects of a history of epilepsy and AED prescription have not been fully examined, despite several cases of insulinoma initially misdiagnosed as epilepsy being reported (6-11).
Repeated and prolonged hypoglycemic episodes also can reduce the awareness of neurogenic and neuroglycopenic symptoms (12). In addition, normal blood glucose levels are sometimes found, especially in outpatient clinics or post-seizure situations (5). These factors can very well mask hypoglycemia and therefore delay a diagnosis of insulinoma. Regarding this issue, the utility of continuous glucose monitoring (CGM) has been reported in cases with hypoglycemia unawareness (13).
We herein report a case of insulinoma with a history of epilepsy in whom recurrent seizures developed. CGM was useful for understanding the clinical condition. Our present case highlights potentially misleading factors in the early diagnosis of insulinoma.
Case Report
A 27-year-old woman was referred to our clinic for the evaluation and treatment of hypoglycemia. At the referral, she had suffered from repeated seizures during the recent six months after a nine-year asymptomatic interval. She had no family history of hypoglycemia, neuropsychiatric disorders or multiple endocrine neoplasia type 1 (MEN1)-related abnormalities. She did not have any dietary restrictions and denied alcohol consumption. Her medical history revealed symptomatic epilepsy related to viral encephalitis at 16 years of age. She had developed generalized tonic-clonic seizures with epileptic electroencephalogram (EEG) patterns. At that time, treatment with phenytoin was started and well tolerated. As she experienced no attack recurrence for more than 2 years, phenytoin was withdrawn at 18 years of age. Hypoglycemia was not documented during the follow-up period.
However, at 27 years of age, she developed generalized chronic seizure and was transferred to the emergency department in our hospital 6 months before the above-mentioned referral. On arrival, she was semi-conscious, with a Glasgow Coma Scale (GCS) score of 14. Laboratory test results revealed normal serum glucose levels (87 mg/dL). Brain magnetic resonance imaging (MRI) and EEG showed no significant abnormalities. She was able to recall that during the seizure, she felt uncomfortable in almost the same way as she had during previous ones. Levetiracetam was administered, and she was followed-up at a neurological clinic.
Five months before the referral, her husband noticed that she had developed confusion early in the morning, but she could not recall these episodes. The frequency of such episodes was about once a week. Considering the possibility of an adverse event, levetiracetam was switched to lamotrigine and folic acid four months before the referral. However, she later experienced intermittent episodes of seizures lasting less than one minute, as well as slurred speech and hyposthenia. Their frequency gradually increased in the morning and began to occur also in the afternoon and evening. Although no apparent hypoglycemia was documented in a daytime neurological clinic, her husband found that prompt carbohydrate intake on waking in the morning seemed to improve these behavioral abnormalities. Hypoglycemia was therefore suspected as a cause of the repeated episodes, and she was referred to our clinic.
At her first visit, she appeared alert and asymptomatic but her laboratory test results showed a low plasma glucose level (29 mg/dL) and relatively high serum levels of insulin (8.13 μU/mL) and C-peptide (2.61 ng/mL). She reported no increased appetite and no bodyweight gain during the past six months. Although early hospitalization for the evaluation and treatment of hyperinsulinemic hypoglycemia was planned, she soon afterward lost consciousness and was immediately admitted to our department a few days before the scheduled date. On admission, the point-of-care glucose level was 39 mg/dL. Her mental status immediately improved with intravenous injection of glucose. Her GCS score was 15; height, 160 cm; weight, 48.6 kg; axillary temperature, 37.4℃; pulse, 75 bpm; saturation of peripheral blood oxygen, 100% (room air); and blood pressure, 103/66 mmHg. There were no remarkable findings on a physical examination except for bruises to her head.
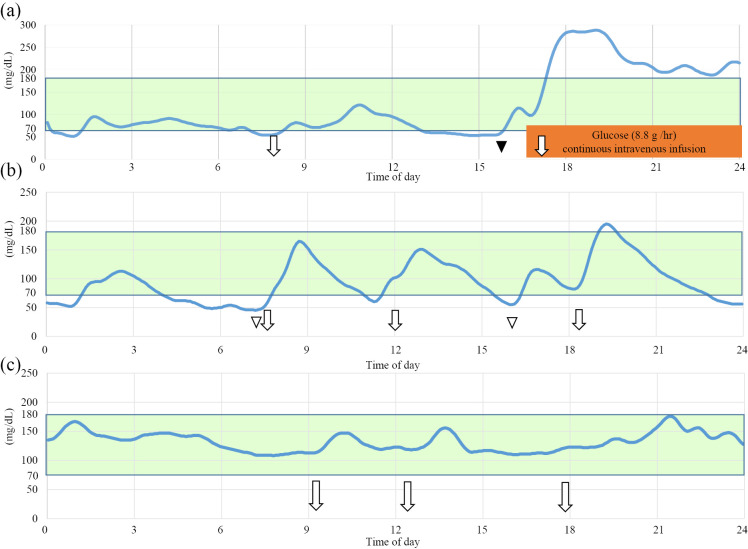
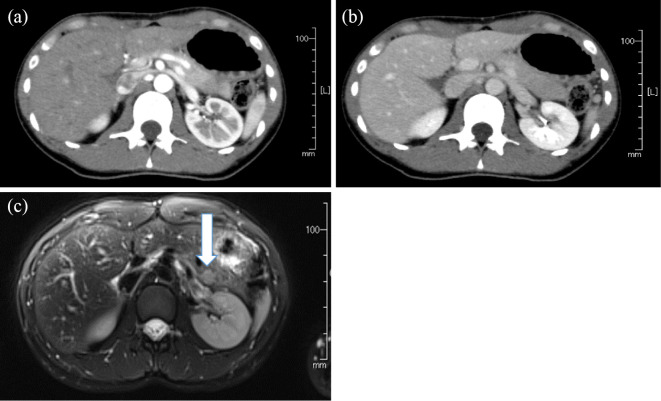
The results of fasting blood samples analyzed the day after admission are shown in Table 1. The concentrations of β-hydroxybutyric acid, adrenalin, noradrenalin, glucagon, and cortisol were 112 μMol/L, 77 μg/dL, 215 μg/dL, 138 pg/mL, and 8.9 μg/dL, respectively. Since she had no history of anti-diabetic agents and none of the laboratory data suggested other endocrine diseases (Table 1), she was examined for insulinoma. Other MEN1-associated abnormalities were not suggested (Table 1). She was subjected to a 72-hour fasting test, which was stopped approximately 7 hours after beginning the test because her plasma glucose level reached ≤45 mg/dL (Table 2, Fig. 1a). Although she remained asymptomatic, we stopped the test for her safety due to the possibility of hypoglycemia unawareness. Despite the low glucose level (45 mg/dL), serum levels of insulin (10.9 μU/mL) and C-peptide (3.09 ng/mL) were not suppressed (Fajans index 0.24, Grunt index 4.13, Turner index 72.7 and Taminato index 434.5). While abdominal computed tomography (CT) scans showed no obvious abnormalities in the pancreas (Fig. 2a and b), abdominal MRI revealed a 10-mm nodule in the tail of the pancreas that showed a moderate signal intensity on T2-weighted images (Fig. 2c) and a high signal intensity on diffusion-weighted ones. Endoscopic ultrasonography also showed a hyperechoic homogenous tumor in the tail of the pancreas.
Table 1.
Laboratory Data of the Patient at Fasting.
| Complete blood count | Cl | 103 | mEq/L | Selected hormones | ||||
| WBC | 9,600 | /μL | Ca | 8.6 | mg/dL | TSH | 2.99 | μIU/mL |
| RBC | 426×104 | /μL | IP | 4.1 | mg/dL | free T4 | 1.1 | ng/dL |
| Hb | 13.3 | g/dL | T-Chol | 108 | mg/dL | ACTH | 25.3 | pg/mL |
| Plt | 24.9×104 | /μL | CK | 177 | mg/dL | Cortisol | 18.5 | μg/dL |
| CRP | <0.1 | mg/dL | GH | 4.62 | ng/mL | |||
| Biochemistry | IGF-1 | 338 | ng/mL | |||||
| AST | 20 | IU/L | Plasma glucose | 63 | mg/dL | PRL | 25.2 | ng/mL |
| ALT | 11 | IU/L | HbA1c | 4.9 | % | LH | 8.0 | mIU/mL |
| ALP | 149 | IU/L | Insulin | 10.1 | μU/mL | FSH | 2.9 | mIU/mL |
| LDH | 176 | IU/L | C-peptide | 3.31 | ng/mL | Estradiol | 53.3 | pg/mL |
| T-Bil | 0.8 | mg/dL | Fajans index | 0.16 | (normal range<0.3) | Glucagon | 110 | pg/mL |
| TP | 6.5 | g/dL | Turner index | 30.6 | (normal range<50) | Gastrin | 87 | pg/mL |
| ALB | 4.2 | g/dL | Lactate | 6.3 | mg/dL | PTH-intact | 54 | pg/mL |
| Amy | 235 | IU/L | Pyruvic acid | 0.53 | mg/dL | Adrenalin | 36 | pg/dL |
| BUN | 6 | mg/dL | Acetoacetic acid | 30.1 | μmol/L | Noradrenalin | 200 | pg/mL |
| Cr | 0.74 | mg/dL | 3-β- hydroxybutyric acid | 48.5 | μmol/L | Dopamine | <20 | pg/dL |
| Na | 139 | mEq/L | Anti-insulin antibody | <125 | nU/mL | |||
| K | 3.6 | mEq/L | ||||||
Fasting blood samples were analyzed the next day after admission to our hospital for investigation of hypoglycemia. WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Plt: platelet, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, T-Bil: total bilirubin, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, Cr: creatinine, T-Chol: total cholesterol, CK: creatine kinase, CRP: C-reactive protein, HbA1c: hemoglobin A1c, IGF-1: insulin like growth factor-1
Table 2.
Results of 72-hour Fasting Test.
| 7 hr | 30 min after glucagon | |
|---|---|---|
| Plasma glucose (mg/dL) | 45 | 123 |
| Insulin (μU/mL) | 10.9 | 15.4 |
| C-peptide (ng/mL) | 3.09 | 3.31 |
The 72-hour fasting test was performed the next day after admission to our hospital for investigation of spontaneous hypoglycemia. Glucagon (1 mg) was administered after confirming plasma glucose ≤45 mg/dL.
Figure 1.
Representative daily summaries of continuous glucose monitoring (CGM) findings. (a) On the day of the fasting test, (b) before the operation while taking diazoxides, (c) three months after the operation. Meals are shown with arrows, oral glucose intake for hypoglycemia with a white arrowhead, 1 mg glucagon administration with a black arrowhead.
Figure 2.
Abdominal early-phase (a) and delayed-phase contrast-enhanced (b) computed tomography images showing no obviously abnormal findings. (c) T2-weighted magnetic resonance imaging showing a moderate-signal-intensity pancreatic tumor (shown with arrow).
Selective arterial calcium injection (SACI) testing also was performed. A significant selective increase in insulin from a baseline level of 24.6 μU/mL to a peak level of 172.1 μU/mL at 40 seconds after stimulation was observed in the splenic artery but not in the gastroduodenal, dorsal pancreatic, or inferior pancreaticoduodenal arteries. These findings support the localization of the insulinoma in the tail of the pancreas.
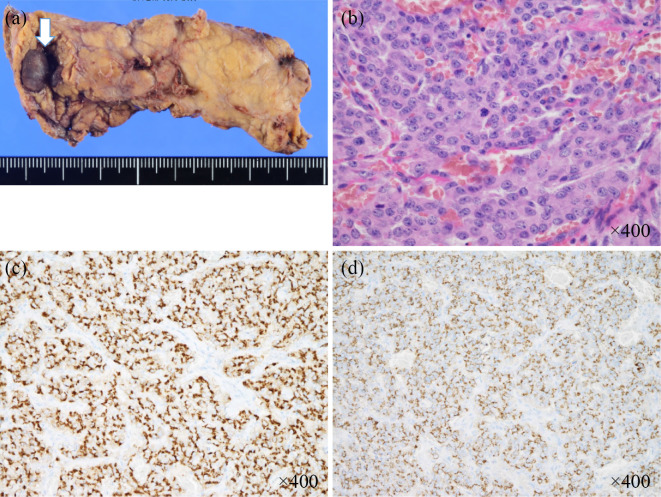
Although diazoxide (300 mg per day in three divided doses each 8 hours) and frequent snacks seemed to eliminate her apparent symptoms, CGM revealed hypoglycemia in the early morning, a time at which she had often shown various neuropsychiatric symptoms before admission (Fig. 1b). Seven weeks after admission, distal pancreatectomy was performed because intraoperative ultrasonography showed that the tumor was close to the main pancreatic duct. A pathological examination revealed a tumor 12 mm diameter in the proximal part of the pancreatic tail (Fig. 3a and b). Immunochemical studies demonstrated positive staining for chromogranin A (Fig. 3c) and synaptophysin as well as insulin (Fig. 3d). The tumor was found to be negative for gastrin and glucagon on an immunohistochemical analysis. The tumor was therefore pathologically confirmed to be an insulinoma. The Ki-67 proliferative indices of these tumors were 4.2%. The patient's pathological staging was determined to be T1bN0M0 (stage IA) and T1N0M0 (stage I), according to the American Joint Committee on Cancer/Unio Internationalis Contra Cancrum (AJCC/UICC) and European neuroendocrine tumor society tumor-node-metastasis (TNM) staging system, respectively. Postoperatively, the patient experienced no further hypoglycemic symptoms, including various neuropsychiatric ones. CGM also showed improved glycemic fluctuations (Fig. 1c). Seventeen weeks after the surgery, lamotrigine was withdrawn; there have been no recurrent seizures since then.
Figure 3.
Pathological examinations confirmed the tumor in the pancreatic distal region to be insulinoma. (a) Macroscopic image (arrow), (b) Hematoxylin and Eosin staining, (c) diffusely positive chromogranin A staining on an immunostaining analysis, (d) diffusely positive insulin staining on an immunostaining analysis.
Discussion
Insulinoma is a rare neuroendocrine tumor that produces excess endogenous insulin, resulting in hypoglycemia (4). A misdiagnosis or delayed diagnosis of insulinoma can readily progress to coma or death (6, 14). The clinical diagnosis of insulinoma requires confirmation of the presence of hypoglycemia with inappropriate insulin secretion and identification of a tumor mass. In terms of the latter, novel and promising technologies for localization have been developed recently (15). However, a necessary clinical first step is a physician's high suspicion of hypoglycemia. Although previous studies have shown that the median time to a diagnosis is 24 months, with a range from 1 month to 30 years (16), a delayed diagnosis remains a clinical issue (17). Thus, any factors that might wrongly allay a physician's suspicion of hypoglycemia and its associated symptoms should be highlighted.
A delay in a diagnosis can be caused by several factors (5). First, insulinoma can exhibit various neurogenic and neuroglycopenic symptoms. These can mimic neuropsychiatric symptoms, including unconsciousness, confusion, seizure, personality change, and bizarre behavior in most patients (16, 18). In addition, over half of patients with these symptoms are initially misdiagnosed with neuropsychiatric disorders, such as epilepsy (5, 16). However, a correct diagnosis of epilepsy is also challenging in clinical settings and can lead to inappropriate treatment with AEDs (5, 7, 16). Furthermore, although EEG is one of the most common diagnostic tools for epilepsy, it is well known that severe hypoglycemia also can cause epileptic discharge in vivo and abnormal results on EEG (19). While several previous reports have described cases of insulinoma initially misdiagnosed as epilepsy (6-11), our case developed repeated seizures after a nine-year asymptomatic interval. EEG and brain MRI in addition to the history of epilepsy did not exclude the possibility of recurrent epilepsy and thereby inhibited further exploration of her hypoglycemia. Her symptoms were found to be resistant to AEDs at the time, although she had been highly responsive to AEDs nine years earlier. This suggested that the pathogenesis of her recent seizures differed from that of nine years earlier, in addition to her epileptic EEG patterns with viral encephalitis and long asymptomatic interval. We should therefore note that in current clinical settings, simply a high suspicion for hypoglycemia induced by insulinoma in patients with neuropsychiatric symptoms is insufficient, and the early and accurate detection of an insulinoma additionally requires attention to the possible masking effect of a history of epilepsy and AED use.
Second, repeated and prolonged hypoglycemic episodes can induce unawareness of neurogenic and neuroglycopenic symptoms (12). As previously reported (20), our case showed no neurogenic symptoms, which might have obscured the clinical relationships between the symptoms and possible hypoglycemia due to the lack of specificity of neuroglycopenic symptoms. In addition, normal serum glucose levels may be found at an outpatient clinic even in patients with insulinoma, due to dietary intake effects and counterregulatory hormones (5). Indeed, in our case, hypoglycemia was not revealed in routine laboratory examinations performed at a daytime neurological clinic. Our case showed symptoms especially in the early morning, so a fasting blood sampling in the morning should be considered during follow-up once the possibility of a relationship between symptoms and hypoglycemia is recognized. However, in actual clinical settings, some patients have difficulty traveling to the hospital for a fasted blood sample early in the morning. On this point, CGM shows potential utility in revealing the presence and tendency of hypoglycemia in our case, as previously reported (13).
In addition, our case did not show any increased appetite or body weight gain, although these are also characteristic symptoms of insulinoma (16, 21). AEDs can also cause a masking effect on hunger and weight gain, possibly through a reduced appetite (10). While our case was taking levetiracetam and lamotrigine, new-generation AEDs with superior efficacy and tolerability (22), these AEDs may have masked the increased appetite and body weight gain associated with insulinoma.
Finally, the negative results of CT scans in our case are also suggestive. As previously shown, CT scans have an accuracy rate of only 55% for diagnosing insulinoma (23), and multiple modalities are required for the accurate detection of this entity. Given that CT is one of most frequently used tools for abdominal screening, especially in emergency departments, a physician's vigilance for insulinoma remains essential.
In summary, we reported a case of insulinoma with various potentially misleading factors that might have impeded an early diagnosis of the disease, including a history of epilepsy and AEDs, unawareness of hypoglycemic symptoms, blood sampling at an outpatient clinic, usage of AEDs and negative results on CT.
The authors state that they have no Conflict of Interest (COI).
Takaaki Murakami and Takafumi Yamashita contributed equally to this work.
References
- 1. Service FJ, McMahon MM, O'Brien PC, Ballard DJ. Functioning insulinoma-incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 66: 711-719, 1991. [DOI] [PubMed] [Google Scholar]
- 2. Ito T, Igarashi H, Nakamura K, et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol 50: 58-64, 2015. [DOI] [PubMed] [Google Scholar]
- 3. Whipple AO, Frantz VK. Adenoma of islet cells with hypeinsulinism: a review. Ann Surg 101: 1299-1335, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol 16: 4519-4525, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding Y, Wang S, Liu J, et al. Neuropsychiatric profiles of patients with insulinomas. Eur Neurol 63: 48-51, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Graves TD, Gandhi S, Smith SJ, Sisodiya SM, Conway GS. Misdiagnosis of seizures: insulinoma presenting as adult-onset seizure disorder. J Neurol Neurosurg Psychiatry 75: 1091-1092, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aupy J, Benoilid A, Sarhan M, Dalvit C, Valenti MP, Hirsch E. Misleading features of neuroimaging and electroencephalography: insulinoma misdiagnosed as temporal lobe epilepsy. Epileptic Disord 15: 93-97, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Dion MH, Cossette P, St-Hilaire JM, Rasio E, Nguyen DK. Insulinoma misdiagnosed as intractable epilepsy. Neurology 62: 1443-1445, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Deleo F, Matricardi S, Didato G, et al. An unusual behavioural and motor paroxysmal disorder caused by insulinoma-related hypoglycemia: a possible cause of epilepsy misdiagnosis. Seizure 23: 909-911, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki K, Miyamoto M, Miyamoto T, Hirata K. Insulinoma with early-morning abnormal behavior. Intern Med 46: 405-408, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Park SH, Kim DW. Insulinoma presenting as medically intractable temporal lobe epilepsy. J Epilepsy Res 4: 21-23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitrakou A, Ryan C, Veneman T, et al. Hierarchy of glycemic thresholds for counterregulatory hormone secretion, symptoms, and cerebral dysfunction. Am J Physiol 260: E67-E74, 1991. [DOI] [PubMed] [Google Scholar]
- 13. Munir A, Choudhary P, Harrison B, Heller S, Newell-Price J. Continuous glucose monitoring in patients with insulinoma. Clin Endocrinol 68: 912-918, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Tesfaye N, Seaquist ER. Neuroendocrine response to hypoglycemia. Ann N Y Acad Sci 1212: 12-28, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamoto Y, Ishimori T, Sano K, et al. Clinical efficacy of dual-phase scanning using 68Ga-DOTATOC-PET/CT in the detection of neuroendocrine tumours. Clin Radiol 71: 1069.e1-1069.e5, 2016. [DOI] [PubMed] [Google Scholar]
- 16. Dizon AM, Kowalyk S, Hoogwerf BJ. Neuroglycopenic and other symptoms in patients with insulinomas. Am J Med 106: 307-310, 1999. [DOI] [PubMed] [Google Scholar]
- 17. Imamura M, Nakamoto Y, Uose S, Komoto I, Awane M, Taki Y. Diagnosis of functioning pancreaticoduodenal neuroendocrine tumors. J Hepatobiliary Pancreat Sci 22: 602-609, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Stefanini P, Carboni M, Patrassi N, Basoli A. Beta-islet cell tumors of the pancreas: results of a study on 1,067 cases. Surgery 75: 597-609, 1974. [PubMed] [Google Scholar]
- 19. Del Campo M, Abdelmalik PA, Wu CP, Carlen PL, Zhang L. Seizure-like activity in the hypoglycemic rat: lack of correlation with the electroencephalogram of free-moving animals. Epilepsy Res 83: 243-248, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Mitrakou A, Fanelli C, Veneman T, et al. Reversibility of unawareness of hypoglycemia in patients with insulinomas. N Engl J Med 329: 834-839, 1993. [DOI] [PubMed] [Google Scholar]
- 21. Rokutan M, Yabe D, Komoto I, et al. A case of insulinoma with non-alcoholic fatty liver disease: roles of hyperphagia and hyperinsulinemia in pathogenesis of the disease. Endocr J 62: 1025-1030, 2015. [DOI] [PubMed] [Google Scholar]
- 22. French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 62: 1261-1273, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Placzkowski KA, Vella A, Thompson GB, et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab 94: 1069-1073, 2009. [DOI] [PubMed] [Google Scholar]