Abstract
Extramedullary relapse (EMR) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is relatively rare. The most commonly reported sites in acute lymphoblastic leukemia (ALL) patients after allo-HSCT are soft tissue and the central nervous system, and the gastrointestinal system is an uncommon site. We herein report a unique case with massive hematemesis resulting from gastrointestinal relapse of ALL after allo-HSCT. Upper gastrointestinal endoscopy showed bleeding from a 1.5-cm submucosal tumorous lesion with central ulceration on the anterior wall of the stomach. At the same time, computed tomography revealed extramedullary relapse at the breast and bilateral adrenal glands.
Keywords: extramedullary gastric relapse, acute lymphoblastic leukemia, allogeneic hematopoietic stem cell transplantation
Introduction
Extramedullary relapse (EMR) after allogeneic hematopoietic stem cell transplantation (allo-HSCT) is relatively rare (1, 2). The incidence of EMR after allo-HSCT has been reported to range from 6-20% (1, 3, 4). In general, the incidence of EMR after allo-HSCT is higher in patients with acute lymphoblastic leukemia (ALL) than in those with acute myeloid leukemia (AML) (1, 5). A recent report showed that the estimated 10-year cumulative incidence of EMR after allo-HSCT was 12.9% in patients with ALL (1). The most commonly reported sites in ALL patients after allo-HSCT are soft tissue and the central nervous system (1, 6), and the gastrointestinal system is an uncommon site (2, 6-8). There have been only a few case reports of gastrointestinal relapse after allo-HSCT (2, 7, 8). We herein report a unique case with massive hematemesis resulting from gastrointestinal relapse of ALL after allogeneic bone marrow transplantation (BMT).
Case Report
A 55-year-old woman with t(1;19)(q23;p13.3), E2A-PBX1-positive acute B lymphoblastic leukemia (B-ALL) underwent bone marrow transplantation (BMT) from a human leukocyte antigen (HLA)-matched unrelated male donor after myeloablative conditioning with a regimen including cyclophosphamide and total body irradiation. The patient's disease status was hematological complete remission at the time of BMT. Tacrolimus (FK) and short-term methotrexate were used for the prophylaxis of graft-versus-host disease (GVHD). On day 42 post-BMT, the patient developed acute (grade II) GVHD of the skin and gut, which improved following treatment with FK (2.4 mg/day) and enteric-coated capsules of beclomethasone dipropionate (BDP) (8 mg/day). This result permitted FK tapering and the discontinuation of BDP capsules 8 months after BMT. However, the patient developed mild chronic GVHD (cGVHD) of the mouth and skin 12 months after BMT. At that time, the dose of FK was 1.0 mg/day. cGVHD stabilized with increased doses of immunosuppressive agents, including FK (1.4 mg/day). Treatment with FK (1.0 mg/day) was necessary for controlling the chronic mouth GVHD.
Twenty-one months after BMT, the patient experienced massive hematemesis and was admitted to our hospital. The laboratory findings demonstrated a white blood cell count of 8.0×103/μL (52% neutrophils, 43.5% lymphocytes, and 0.5% blasts), a hemoglobin level of 9.6 g/dL, a platelet count of 12.6×104 /μL, and a lactate dehydrogenase (LDH) level of 1,154 IU/L. Computed tomography (CT) revealed a 2.0-cm right subcutaneous breast mass and 2.5-cm bilateral adrenal masses. Upper gastrointestinal endoscopy showed bleeding from a 1.5-cm submucosal tumorous lesion with central ulceration on the anterior wall of the gastric angle (Fig. 1a and b). A pathological examination revealed infiltration of lymphoblasts that were positive for CD10, CD19, CD20, and terminal deoxynucleotidyl transferase (TdT) (Fig. 2). A Y-chromosome was not detected in these lymphoblasts by fluorescence in situ hybridization (FISH) analysis for sex chromosomes. The bone marrow aspirate showed a hypercellular marrow with 80.2% lymphoblasts of the same phenotype as those found in the pathological examination of the stomach. A real-time quantitative polymerase chain reaction (RQ-PCR) analysis of the BM sample showed positivity for E2A-PBX1. We therefore diagnosed the patient with relapse of B-ALL.
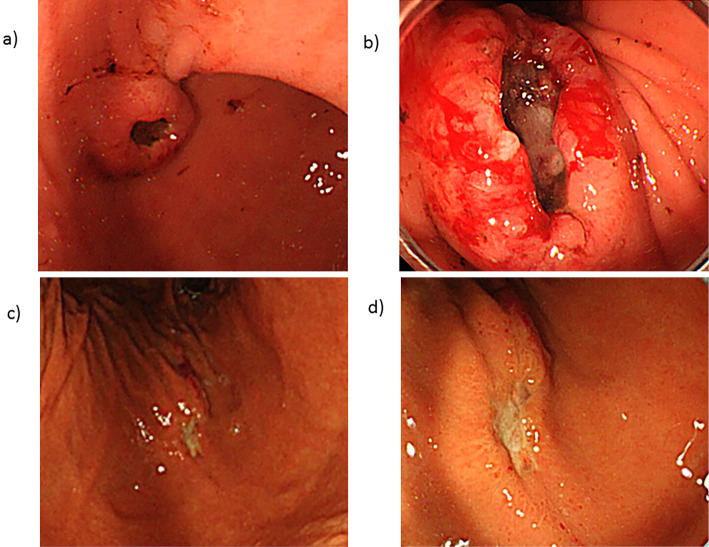
Figure 1.
(a, b) Upper gastrointestinal endoscopy on admission revealed a 1.5-cm submucosal tumorous lesion with central ulceration on the anterior wall of the gastric angle. (c, d) Upper gastrointestinal endoscopy on day 51 after re-induction chemotherapy showed marked improvement in the submucosal tumorous lesion compared to admission.
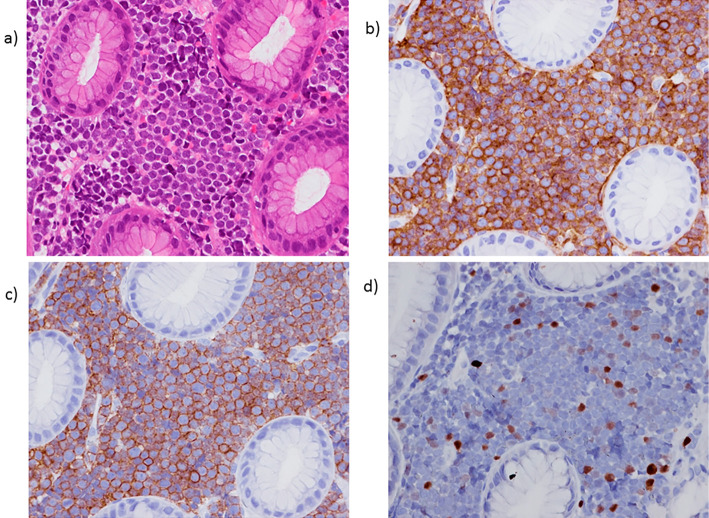
Figure 2.
(a) A gastric biopsy revealed the infiltration of monomorphic lymphoid cells with fine nuclear chromatin and prominent nucleoli in the lamina propria. Hematoxylin and Eosin staining. The tumor cells showed diffuse immunoreactivity for CD10 (b) and CD19 (c) but focal immunoreactivity for TdT (d).
Hematological complete remission was achieved in the bone marrow aspirate 36 days after the patient received re-induction therapy, including vincristine, L-asparaginase, daunorubicin, cyclophosphamide, and prednisolone. A CT scan at the same time showed marked improvement of the subcutaneous breast masses and bilateral adrenal masses. Furthermore, upper gastrointestinal endoscopy also showed marked improvement in the submucosal tumorous lesion compared to admission (Fig. 1: c, d vs. a, b) .
Discussion
Regarding the relapse sites of leukemia after allo-HSCT, it has been reported that 63% of relapses occur in the bone marrow (BM) only, 14% occur in the BM and extramedullary (EM) sites, and 23% occur in EM sites only (4). This frequency was similar between AML and ALL (4). Furthermore, the gastrointestinal tract has been reported as a rare site of relapse after allo-HSCT in both AML and ALL (6).
Regarding lymphoid neoplasms, more-differentiated B-cell neoplasms, such as lymphomas and chronic lymphocytic leukemia, may involve the gastrointestinal tract because of their origin, or traffic to gut-associated lymphoid tissue (GALT) (7). However, ALL cells derived from precursor B-cells may lack recognition capacity and therefore display tropism for GALT (7). Under these mechanisms, ALL infiltration of the gastrointestinal tract would be uncommon. Furthermore, the manifestations of gastrointestinal leukemia are nonspecific, and many of the symptoms after allo-HSCT are similar to those associated with GVHD (7, 8). Afflicted patients have complained of abdominal pain, nausea, and diarrhea in the past. However, hematemesis as in the present case has not been reported as the first symptom of leukemia relapse after allo-HSCT. In addition, our patient did not have any abdominal pain, nausea, or diarrhea. When patients with ALL present with gastrointestinal symptoms following allo-HSCT, EMR should be distinguished from GVHD.
In summary, the gastrointestinal system is a very rare site of EMR of ALL after allo-HSCT. Such a relapse would lead to the development of severe and acute bleeding, like that observed in this case. Clinicians should be alert to the possibility of gastric relapse when patients with ALL present with gastrointestinal symptoms following allo-HSCT.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Ge L, Ye F, Mao X, et al. Extramedullary relapse of acute leukemia after allogeneic hematopoietic stem cell transplantation: different characteristics between acute myelogenous leukemia and acute lymphoblastic leukemia. Biol Blood Marrow Transplant 20: 1040-1047, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Ciarallo A, Makis W, Novales-Diaz JA, Michel RP. Extramedullary gastric relapse of acute lymphoblastic leukemia following allogeneic stem cell transplant: staging with F-18 FDG PET/CT. Clin Nucl Med 36: e90-e92, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Shi JM, Meng XJ, Luo Y, et al. Clinical characteristics and outcome of isolated extramedullary relapse in acute leukemia after allogeneic stem cell transplantation: a single-center analysis. Leuk Res 37: 372-377, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Lee JH, Choi SJ, Lee JH, et al. Anti-leukemic effect of graft-versus-host disease on bone marrow and extramedullary relapses in acute leukemia. Haematologica 90: 1380-1388, 2005. [PubMed] [Google Scholar]
- 5. Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant 32: 835-842, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Cunningham I. Extramedullary sites of leukemia relapse after transplant. Leuk Lymphoma 47: 1754-1767, 2006. [DOI] [PubMed] [Google Scholar]
- 7. Weisdorf D, Arthur D, Rank J, Blazar B, Gajl-Peczalska K, Snover D. Gastric recurrence of acute lymphoblastic leukaemia mimicking graft-versus-host disease. Br J Haematol 71: 559-561, 1989. [DOI] [PubMed] [Google Scholar]
- 8. Kletzel M, Meitar D, El-Youssef M, Cohn SL. Gastrointestinal relapse of leukemia, mimicking acute graft vs. host disease, following a stem cell transplant. Med Pediatr Oncol 34: 287-289, 2000. [DOI] [PubMed] [Google Scholar]