Abstract
A 54-year-old man with polycystic liver disease received a domino liver transplantation (DLT) from a patient of hereditary ATTR amyloidosis with the transthyretin Ser50Arg mutation. Ten years after transplantation, he felt a slight numbness in his toes, and cardiac amyloidosis was simultaneously suspected upon a heart function evaluation. Biopsy specimens from the myocardium revealed transthyretin amyloidosis with the Ser50Arg mutation. Oral tafamidis therapy has inhibited the progression of neurological and cardiovascular symptoms this far. We herein report this first case of amyloid polyneuropathy and myocardial amyloidosis after DLT from hereditary ATTR amyloidosis with a transthyretin Ser50Arg mutation and discuss similar cases of other mutations.
Keywords: amyloid polyneuropathy, myocardial amyloidosis, domino liver transplantation, transthyretin, tafamidis
Introduction
Hereditary transthyretin (ATTR) amyloidosis (ATTRm) is an autosomal dominant systemic amyloidosis associated with multiorgan dysfunction involving the peripheral nerves, autonomic nervous system, cardiac muscle, and gastrointestinal tract. In systemic amyloidosis several precursor proteins, including the variant form of transthyretin (TTR), gelsolin, and apolipoprotein AI form amyloids with a fibrous structure, and amyloid deposition results in organ damage (1). ATTRm in the endemic foci of Japan often occurs in the 20s and 30s, and symptoms progress gradually, with an average survival duration of about 10 years in the absence of treatment (2). In contrast, gelsolin type amyloidosis has a good prognosis, and relatively older-onset patients are seen in the non-endemic area in Japan. The most frequent type of abnormal transthyretin (TTR) mutation in ATTRm is Val30Met (p.Val50Met), which results in sensory dominant neuropathy, autonomic dysfunction, and cardiac dysfunction (3). In addition to the TTR Val30Met mutation, Glu42Gly (p.Glu62Gly), Gly47Glu (p.Gly67Glu), Ser50Arg (p.Ser70Arg), Ser50Ile (p.Ser70Ile), Glu54Gly (p.Glu74Gly), Leu55Pro (p.Leu75Pro), Val71Ala (p.Val91Ala), Tyr114Cys (p.Tyr134Cys), and many other mutations have been reported. Although the TTR Ser50Arg mutation is a rare variant, it results in the development of various symptoms of ATTRm and has a relatively young onset age (4, 5).
Tafamidis, which stabilizes the TTR tetramer and inhibits amyloid formation, is indicated in clinical practice (6). However, liver transplantation, which inhibits variant TTR production, was conducted as radical therapy in 2,136 cases from 1990 to December 2015 (7). Domino liver transplantation (DLT) is a particularly useful choice in Japan, as liver transplantations after brain death are infrequent.
While several reports have described recipients of DLT from ATTRm donors developing amyloid polyneuropathy, most patients exhibited the TTR Val30Met mutation. Others reported mutations include one Gly47Glu mutation, one Ser50Ile, one Glu54Gly, one Leu55Pro, and two Val71Ala and Tyr114Cys mutations each (8-18).
We herein report the case of a patient who received DLT from an ATTRm patient with the TTR Ser50Arg mutation and presented with cardiac amyloidosis and polyneuropathy 10 years after DLT.
Case Report
A 66-year-old man was admitted to our hospital. His mother and elder sister had polycystic kidney disease, and his elder sister had polycystic liver disease as well. More than one maternal relative had had polycystic liver disease and had died of stroke. He presented with hematuria in his 30s and was diagnosed with polycystic kidney disease. He had started undergoing dialysis in his 40s, and polycystic liver disease had subsequently been diagnosed. At 54 years of age, his abdominal distension as a result of liver cysts rapidly worsened, and his nutritional state also deteriorated. He was added to the liver transplantation waiting list, and DLT from a 50-year-old ATTRm patient with a TTR Ser50Arg mutation was conducted. The donor had developed autonomic dysfunction, including diarrhea and orthostatic hypotension, at 46 years of age, followed by polyneuropathy and ventricular wall hypertrophy. However, the donor's cardiac and liver functions were normal.
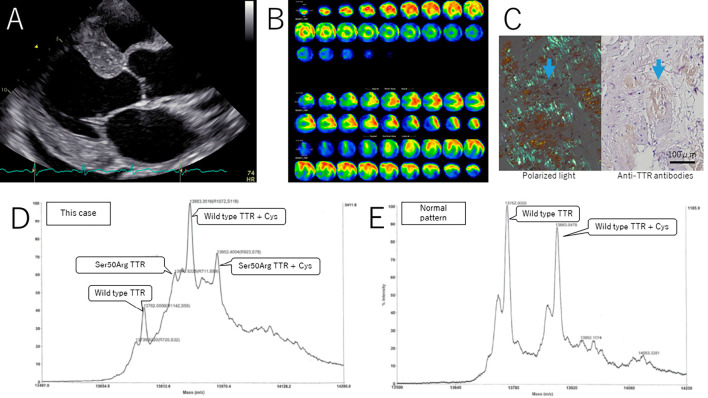
Six and nine years after DLT, our patient visited the neurology department for peripheral neuropathy tests, but a neurological examinations and nerve conduction studies (NCS) demonstrated no signs of neuropathy (Table 1). Ten years after DLT (at 64 years of age), he noticed a slight numbness in his toes. He also had unruptured cerebral aneurysms and decided to undergo a clipping operation. He was referred to the cardiology department for a heart function evaluation before the operation. Echocardiography demonstrated left ventricular hypertrophy, left atrial wall thickness, leaflet thickness, and pericardial effusion (Figure A). 99mTc-PYP scintigraphy showed diffuse accumulation enhancement in the left ventricular myocardium (Figure B). Cardiac amyloidosis was suspected, at which time he visited the neurology department for a reevaluation of neuropathy.
Table 1.
Nerve Conduction Study Findings of Left Extremities in This Patient.
| Nerve | Years from LT | DL (ms) | MCV (m/s) | CMAP(D/P) (mV) | F latency (ms) | SCV (ms) | SNAP (µV) |
|---|---|---|---|---|---|---|---|
| Median | 9 | 3.7 | 49.0 | 5.7/4.0 | 30.6 | 54.5 | 17.8 |
| 12 | 4.4 | 45.5 | 4.6/4.2 | 31.9 | 53.7 | 11.8 | |
| Ulnar | 9 | 3.2 | 51.3 | 12.8/10.8 | 29.8 | 56.3 | 21.8 |
| 12 | 3.2 | 45.6 | 10.6/8.7 | 31.5 | 50.6 | 17.0 | |
| Tibial | 9 | 4.3 | 36.3 | 4.3/4.2 | 61.1 | ||
| 12 | 5.4 | 34.0 | 1.8/1.3 | 58.0 | |||
| Peroneal | 9 | 5.2 | 36.9 | 2.9/2.3 | 58.7 | ||
| 12 | 5.4 | 34.8 | 0.8/0.9 | - | |||
| Sural | 9 | 46.1 | 3.8 | ||||
| 12 | 42.6 | 3.3 |
LT: liver transplantation, DL: distal latency, MCV: motor conduction velocity, CMAP (D/P): compound motor action potential (distal/ proximal), SCV: sensory conduction velocity, SNAP: sensory action potential
Figure.
Laboratory findings of echocardiography, 99mTc-PYP scintigraphy, myocardial biopsy and mass spectroscopy of TTR protein. A: Echocardiography demonstrated left ventricular hypertrophy, left atrial wall thickness, leaflet thickness, and pericardial effusion. B: 99mTc-PYP scintigraphy showed diffuse accumulation enhancement in the left ventricular myocardium. C: Myocardial biopsy findings. The positive image of this specimen in polarized light examination (left) matched that of anti-TTR antibodies (right). D: Ser50Arg TTR was found in mass spectroscopy of TTR protein.
On a physical examination, a subcutaneously fixed superficial artery on the right arm was observed, but no findings of cardiac tamponade, including paradoxical pulse, heart sound attenuation, or jugular venous distention, were observed. A neurological examination revealed a decreased deep tendon reflex in the upper and lower extremities and abnormal sensation in the left lower extremity, but no muscle weakness, thermal hypoalgesia, hypopallesthesia, or orthostatic hypotension were observed. Autonomic dysfunction was screened by the Japanese version of scales for outcomes in Parkinson's disease - autonomic questionnaire; there were no subjective signs of problems.
A blood examination detected mild hepatic dysfunction and renal dysfunction equivalent to dialysis. Blood glucose and vitamin B12 levels were within normal limits, and major autoantibodies were absent. A TTR gene mutation was not detected in a whole-genome sequence of the patient's peripheral blood. NCS revealed decreased motor conduction velocity, compound motor action potential, and sensory nerve action potential in the left lower limb, and F wave latencies in the upper and lower extremities were delayed, but these findings did not meet the criteria of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (Table 1). The coefficient of variation of the R-R intervals in the electrocardiogram was 1.87% (normal range >2.0%). A metaiodobenzylguanidine scan was not conducted.
A myocardial biopsy revealed TTR-type amyloidosis. The positive image of this specimen in polarized light examination matched that of anti-TTR antibodies, but not those of anti-AA, anti-IGLCκ, or anti-IGLCγ antibodies (Figure C). These findings corresponded to TTR-type amyloidosis. Expectedly, Ser50Arg TTR was found in the patient's serum (Figure D).
As the symptoms of neuropathy were relatively mild, a nerve biopsy was not conducted. Based on the clinical course and examination results, including the myocardial biopsy, causes of neuropathy other than ATTRm were unlikely. It was suggested that this patient had developed iatrogenic ATTRm a long time after DLT. In addition, as there was no accompanying osteolesion, dialysis amyloidosis was unlikely in this patient.
Based on the diagnosis and with the patient's consent, we started tafamidis to inhibit symptom progression. While only a relatively short time has passed since starting treatment, there has been no deterioration of the neurological or cardiovascular symptoms in this patient over the past two years.
Discussion
Previous cases in which patients developed ATTRm after DLT showed TTR Val30Met, Gly47Glu, Ser50Ile, Glu54Gly, Leu55Pro, Val71Ala, or Tyr114Cys mutations; this is the first report of a case with a Ser50Arg mutation (Table 2). The ATTRm with TTR Ser50Arg mutation was characterized by neuropathic pain, walking disabilities, weight loss, diarrhea, orthostatic hypotension, and cardiomyopathy (5, 19).
Table 2.
Previous Cases of Hereditary ATTR Amyloidosis after Domino Liver Transplantation.
| Reference | Gender | Age at DLT | Age at neuropathy onset | Cause of DLT | Histology | TTR variant | Symptom | ||
|---|---|---|---|---|---|---|---|---|---|
| motor | sensory | Autonomic | |||||||
| 8 | M | 47 | 55 | HCV cirrhosis, HCC | Nerve, GI wall | Val30Met | - | + | - |
| 9 | F | 50 | 57 | PBC | GI wall | Val30Met | - | + | - |
| 10 | F | 65 | 72 | HCC, HCV cirrhosis | GI wall | Gly47Glu | - | + | - |
| 11 | M | 54 | 63 | Hemangioendothelioma HCV cirrhosis | Nerve | Val30Met | + | + | + |
| 12 | M | 54 | 59 | HCC, HBV, HCV cirrhosis | Nerve, LSG | Val30Met | - | + | - |
| M | 72 | 75 | HCC, HBV cirrhosis | Nerve, LSG | Val30Met | - | + | + | |
| M | 60 | 65 | HBV cirrhosis | Nerve | Val30Met | - | + | + | |
| M | 45 | 53 | HCV, alcoholic cirrhosis | Nerve, LSG | Val30Met | - | + | - | |
| 13 | M | 59 | 68 | HBV & HCV cirrhosis | AFT | Val30Met | + | + | + |
| 14 | M | 35 | 46 | PSC | AFT | Val30Met | - | + | - |
| 15 | F | 43 | 52 | HBV, HCC | GI wall, AFT | Glu54Gly | - | + | + |
| 16 | M | 66 | ND | HCC, HCV cirrhosis | Nerve, GI wall | Unclear* | + | + | + |
| M | 62 | ND | alcoholic cirrhosis | Nerve, GI wall | Unclear* | - | + | + | |
| M | 60 | ND | HBV cirrhosis | Nerve | Unclear* | - | + | + | |
| M | 60 | ND | HCV cirrhosis | Nerve | Unclear* | - | - | - | |
| M | 64 | ND | HCC, HCV cirrhosis | ND | Unclear* | - | - | - | |
| F | 62 | ND | HCV cirrhosis | ND | Unclear* | - | - | - | |
| M | 64 | ND | HCC, HCV cirrhosis | Nerve | Unclear* | - | + | + | |
| F | 61 | ND | alcoholic cirrhosis | ND | Unclear* | - | - | - | |
| M | 58 | ND | HCC, HCV cirrhosis | ND | Unclear* | - | - | + | |
| M | 61 | ND | HCC, HBV cirrhosis | GI wall | Unclear* | - | - | - | |
| M | 66 | ND | alcoholic cirrhosis | ND | Unclear* | - | - | - | |
| M | 59 | ND | HCC, HCV cirrhosis | Nerve, GI wall | Unclear* | - | - | - | |
| F | 61 | ND | HCV cirrhosis | GI wall | Unclear* | - | - | - | |
| M | 66 | ND | HCV cirrhosis | ND | Unclear* | - | - | + | |
| M | 62 | ND | HCV cirrhosis | ND | Unclear* | - | - | - | |
| M | 64 | ND | HCC, HCV cirrhosis | GI wall | Unclear* | - | - | + | |
| F | 62 | ND | HCC, HCV cirrhosis | ND | Unclear* | - | - | - | |
| 17 | M | 62 | 67 | Cirrhosis, HCC | AFT | Val71Ala | ND | ND | ND |
| 18 | F | 50 | ND | PBC | + | Val30Met | - | + | - |
| M | 35 | ND | cirrhosis | + | Val30Met | - | + | + | |
| M | 41 | ND | PSC | - | Val30Met | - | - | - | |
| M | 30 | ND | cirrhosis | + | Tyr114Cys | - | - | - | |
| M | 60 | ND | HBV cirrhosis | + | Ser50Ile | - | + | - | |
| F | 52 | ND | PBC | - | Val30Met | - | - | - | |
| F | 53 | ND | HBV cirrhosis | + | Val30Met | - | - | - | |
| F | 35 | ND | BA | + | Val30Met | - | + | - | |
| M | 40 | ND | HCV cirrhosis, HCC | - | Val30Met | - | - | - | |
| F | 23 | ND | BA | - | Val30Met | - | - | - | |
| M | 57 | ND | HCV cirrhosis, HCC | - | Val30Met | - | - | - | |
| M | 18 | ND | BA | + | Val30Met | - | - | - | |
| M | 58 | ND | cirrhosis | + | Val30Met | - | + | + | |
| M | 43 | ND | HCV cirrhosis | - | Val30Met | - | - | - | |
| M | 56 | ND | cirrhosis, HCC | - | Val30Met | - | - | - | |
| M | 58 | ND | HCV cirrhosis, HCC | - | Val30Met | - | - | - | |
| M | 54 | ND | HBV cirrhosis | - | Val30Met | - | - | - | |
| M | 35 | ND | PBC | - | Val30Met | - | - | - | |
| F | 45 | ND | cirrhosis | - | Val30Met | - | - | - | |
| M | 53 | ND | HCV cirrhosis, HCC | - | Leu55Pro | - | - | - | |
| M | 62 | ND | HCV cirrhosis | - | Val30Met | - | - | - | |
| M | 59 | ND | HBC cirrhosis, HCC | - | Val30Met | - | - | - | |
| This case | M | 54 | 64 | Polycystic liver | Myocardium | Ser50Arg | - | + | - |
M: male, F: female, DLT: domino liver transplantation, HCV: hepatitis C virus, PBC: primary biliary cirrhosis, HCC: hepatocellular carcinoma, HBV: hepatitis B virus, PSC: primary sclerosing cholangitis, BA: biliary atresia, GI: gastrointestinal tract, LSG: labial salivary gland, AFT: abdominal fat tissue, TTR: transthyretin, ND: not described
* Unclear means that except for one case of Val71Ala mutation, all other patients were Val30Met mutation, but it was unspecified which is Val 71 Ala mutation.
A previous report described a patient with cadaveric DLT in Japan; the authors of that patient's report had observed no de novo ATTRm cases among recipients of DLT but cautioned that careful attention to the development of ATTRm in the future was needed (20). Confirming this prediction, a number of cases of ATTRm after DLT were reported, starting in 2005 (Table 2) (8-18).
Of the 1,112 DLT cases (7), more than 10 have developed ATTRm. The average duration from DLT to the onset of polyneuropathy was 7.4 years (to the extent that it was confirmed, 3-11 years). The present case showed a relatively long duration to development compared with other cases. However, the period to develop ATTRm was shorter than previously considered. ATTRm was previously suspected to develop 20 to 30 years after DLT (21), but this was not entirely true. Liver transplantation, including DLT, is an effective choice for radical therapy for ATTRm, but the recipient's risk of developing iatrogenic ATTRm in the future should be considered. Tafamidis, a TTR tetramer stabilizer, or diflunisal (22), immunotherapy (23), gene conversion therapy (24), and RNAi therapy (25) may become mainstream alternatives to DLT in the future.
The NCS findings of this case suggested relatively demyelinating changes, which did not meet the criteria of CIDP, unlike axonal or small fiber neuropathy observed in typical ATTRm. The neurological symptoms of this case were not similar to those of the donor patient; indeed, autonomic dysfunction was inconspicuous in this case. Dissociative sensory disturbance was not obvious, and symptoms might not have been remarkable because the neuropathy was in an early stage. There were some cases in non-endemic area of Japan that had disturbances with myelinated fibers from an early stage of ATTRm (26). The present case exhibited different clinical findings from typical cases in the endemic area of Japan, but the finding of PYP scintigraphy was coincident with the findings from previous ATTRm cases (27).
Autonomic dysfunction is a significant prognostic element of ATTRm; the prognosis improves if the onset and development of autonomic dysfunction are inhibited, as in the present case. In addition, this case confirmed that regular follow-up after DLT from ATTRm is very important for the early detection of neurological symptoms. We will follow this case closely, particularly with respect to the inhibitory effects of tafamidis on not only peripheral neuropathy but also systemic organs, such as myocardial dysfunction.
Conclusions
We herein reported the first case of amyloid polyneuropathy and myocardial amyloidosis at 10 years after DLT from a donor with ATTRm and a TTR Ser50Arg mutation and reviewed similar cases of other TTR mutations. Regular follow-up after DLT from an ATTRm donor and the early detection of neurological symptoms were very important.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Dr. Yoshiki Sekijima (Shinshu University) for his cooperation in the measurement of serum mutant TTR and Dr. Taro Yamashita (Kumamoto University) for his cooperation in the myocardial pathology investigation.
References
- 1. Plante-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol 10: 1086-1097, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Ando Y, Nakamura M, Araki S. Transthyretin-related familial amyloidotic polyneuropathy. Arch Neurol 62: 1057-1062, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Koike H, Tanaka F, Hashimoto R, et al. . Natural history of transthyretin Val30Met familial amyloid polyneuropathy: analysis of late-onset cases from non-endemic areas. J Neurol Neurosurg Psychiatry 83: 152-158, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Shirota Y, Iwata A, Ishiura H, et al. . A case of atypical amyloid polyneuropathy with predominant upper-limb involvement with the diagnosis unexpectedly found at lung operation. Intern Med 49: 1627-1631, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez-Duarte A, Soto KC, Martinez-Banos D, et al. . Familial amyloidosis with polyneuropathy associated with TTR Ser50Arg mutation. Amyloid 19: 171-176, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Coelho T, Maia LF, da Silva AM, et al. . Long-term effects of tafamidis for the treatment of transthyretin familial amyloid polyneuropathy. J Neurol 260: 2802-2814, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Familial Amyloidotic Polyneuropathy World Transplant Registry and Domino Liver Transplant Registry [Internet]. [cited 2016 Oct. 25]. Available from : http://www.fapwtr.org/ .
- 8. Stangou AJ, Heaton ND, Hawkins PN. Transmission of systemic transthyretin amyloidosis by means of domino liver transplantation. N Engl J Med 352: 2356, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Goto T, Yamashita T, Ueda M, et al. . Iatrogenic amyloid neuropathy in a Japanese patient after sequential liver transplantation. Am J Transplant 6: 2512-2515, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Barreiros AP, Geber C, Birklein F, Galle PR, Otto G. Clinical symptomatic de novo systemic transthyretin amyloidosis 9 years after domino liver transplantation. Liver Transpl 16: 109, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Conceicao I, Evangelista T, Castro J, et al. . Acquired amyloid neuropathy in a Portuguese patient after domino liver transplantation. Muscle Nerve 42: 836-839, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Adams D, Lacroix C, Antonini T, et al. . Symptomatic and proven de novo amyloid polyneuropathy in familial amyloid polyneuropathy domino liver recipients. Amyloid 18 (Suppl 1): 174-177, 2011. [DOI] [PubMed] [Google Scholar]
- 13. Pradotto L, Franchello A, Milesi A, et al. . Amyloid polyneuropathy following domino liver transplantation. Muscle Nerve 45: 918-919, 2012. [DOI] [PubMed] [Google Scholar]
- 14. Obayashi K, Yamashita T, Tasaki M, et al. . Amyloid neuropathy in a younger domino liver transplanted recipient. Muscle Nerve 43: 449-450, 2011. [DOI] [PubMed] [Google Scholar]
- 15. Abdelfatah MM, Hayman SR, Gertz MA. Domino liver transplantation as a cause of acquired familial amyloid polyneuropathy. Amyloid 21: 136-137, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Lladó L, Baliellas C, Casasnovas C, et al. . Risk of transmission of systemic transthyretin amyloidosis after domino liver transplantation. Liver Transpl 16: 1386-1392, 2010. [DOI] [PubMed] [Google Scholar]
- 17. van den Berg MP, Slart RH, Blokzijl H, et al. . Transthyretin-derived (ATTR) amyloidotic cardiomyopathy after receiving a domino liver allograft. Circulation 132: e216-e217, 2015. [DOI] [PubMed] [Google Scholar]
- 18. Misumi Y, Narita Y, Oshima T, et al. . Recipient aging accelerates acquired transthyretin amyloidosis after domino liver transplantation. Liver Transpl 22: 656-664, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Furukawa K, Ikeda S, Okamura N, et al. . Cardiac positron-emission tomography images with an amyloid-specific tracer in familial transthyretin-related systemic amyloidosis. Circulation 125: 556-557, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Wakayama K, Jin MB, Furukawa H, et al. . Cadaveric domino liver transplantation: the first case in Japan. J Hepatobiliary Pancreat Surg 11: 445-448, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Furtado A, Tome L, Oliveira FJ, Furtado E, Viana J, Perdigoto R. Sequential liver transplantation. Transplant Proc 29: 467-468, 1997. [DOI] [PubMed] [Google Scholar]
- 22. Berk JL, Suhr OB, Obici L, et al. . Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA 310: 2658-2667, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terazaki H, Ando Y, Fernandes R, Yamamura K, Maeda S, Saraiva MJ. Immunization in familial amyloidotic polyneuropathy: counteracting deposition by immunization with a Y78F TTR mutant. Lab Invest 86: 23-31, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Nakamura M, Ando Y, Nagahara S, et al. . Targeted conversion of the transthyretin gene in vitro and in vivo. Gene Ther 11: 838-846, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Coelho T, Adams D, Silva A, et al. . Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369: 819-829, 2013. [DOI] [PubMed] [Google Scholar]
- 26. Koike H, Misu K, Sugiura M, et al. . Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 63: 129-138, 2004. [DOI] [PubMed] [Google Scholar]
- 27. Bokhari S, Castaño A, Pozniakoff T, et al. . (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 6: 195-201, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]