Abstract
A 57-year-old woman was admitted to our hospital because of a high fever, anemia, and hyperferritinemia. Since a bone marrow examination revealed hemophagocytosis, she was diagnosed with hemophagocytic syndrome (HPS). During treatment of HPS, a heliotrope rash and Gottron's sign appeared with elevated levels of serum aldolase. She also developed heart failure. She was diagnosed with dermatomyositis (DM) and associated myocarditis. Although the administration of glucocorticoids, calcineurin inhibitors, intravenous immunoglobulins, and etoposide ameliorated the clinical findings of DM and cytopenia, the fever and hyperferritinemia remained. The addition of infliximab to glucocorticoids and tacrolimus improved the fever and hyperferritinemia and enabled a reduction in the dose of prednisolone without relapse of the diseases.
Keywords: dermatomyositis, hemophagocytic syndrome, infliximab, TNF inhibitor
Introduction
Hemophagocytic syndrome (HPS), also known as hemophagocytic lymphohistiocytosis (HLH), is characterized clinically by a fever, cytopenia, and hepatosplenomegaly (1). The symptoms of HPS are attributable to excess production of inflammatory cytokines, including tumor necrosis factor (TNF)-α, and organ infiltration of activated lymphocytes and histiocytes (2).
HPS is classified into primary and secondary forms. Secondary HPS is associated with infections, malignancies, and autoimmune diseases (1). For patients with primary HPS, the HLH Study Group recommends treatment with dexamethasone, cyclosporine (Cs) A, and etoposide, followed by autologous hematopoietic stem cell transplantation (2). For patients with secondary HPS, successful treatment of the underlying diseases is essential for a good outcome (3). In autoimmune-associated HPS (AAHS), HPS occurs generally in the active phase of an underlying autoimmune disease and improves with the amelioration of the underlying disease (3). Dermatomyositis (DM) is one of the autoimmune diseases that induce AAHS.
We herein report a case of HPS complicated with DM in which HPS preceded DM. Although the administration of glucocorticoids, calcineurin inhibitors, intravenous immunoglobulins (IVIG), and etoposide ameliorated the clinical findings of DM and cytopenia, the fever and hyperferritinemia remained. The addition of infliximab to glucocorticoids and tacrolimus improved the fever and hyperferritinemia.
Case Report
The patient was a 57-year-old woman who had a medical history of idiopathic thrombocytopenic purpura (ITP) diagnosed at the age of 46. The ITP had been treated with prednisolone and remained under control after its discontinuation. In August 2012, she was admitted to a hospital with a high fever. Bone marrow aspiration was carried out because she had hepatosplenomegaly, progressive anemia (6.6 g/dL) without leukopenia or thrombocytopenia, and an increased serum ferritin level (134,439 ng/mL). The bone marrow showed increased numbers of macrophages with hemophagocytosis along with reduced numbers of erythroblasts. Tumor cell invasion was not observed. She was thus diagnosed as with HPS.
Prednisolone (40 mg/day) and CsA (200 mg/day) were initiated, but failed to improve the high fever and anemia. In October 2012, she was transferred to the Department of Hematology in our hospital. Dexamethasone (16 mg/day), CsA (200 mg/day), and etoposide (200 mg/day, twice weekly) were initiated. After four infusions of etoposide, the high fever, anemia, and serum levels of ferritin improved (10.1 g/dL and 13,465 ng/mL, respectively). When dexamethasone was subsequently reduced to 8 mg/day, she developed heliotrope rash and Gottron's sign (Fig. 1). As DM was suspected, she was transferred to our department.
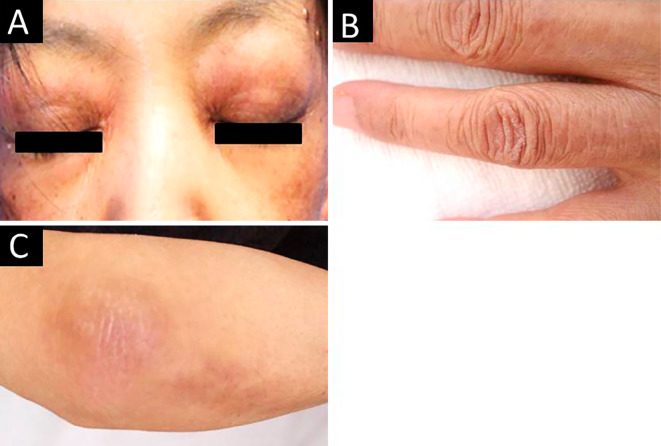
Figure 1.
Skin rashes of the patient. Heliotrope rash (A) and Gottron’s sign on the hand (B) and elbow (C) were noted.
When transferred, she presented with skin rashes, conjunctival pallor, and hepatosplenomegaly. Her muscle strength could not be estimated because of her poor physical status. The blood data were as follows: white blood cells 14,200/μL (neutrophils 91.0%, lymphocytes 4.0%, monocytes 5.0%), hemoglobin 8.7 g/dL, platelets 24.9×104/μL, fibrinogen 349 mg/dL, fibrin/fibrinogen degradation products 7.0 μg/mL, aspartate aminotransferase 71 U/L, alanine aminotransferase 102 U/L, lactate dehydrogenase 799 U/L, creatine kinase (CK) 16 U/L, aldolase 16.4 U/L (normal range 2.1-6.1 U/L), myoglobin 12 ng/mL (<106 ng/mL), C-reactive protein 1.84 mg/dL, ferritin 53,966 ng/mL, and soluble interleukin (IL)-2 receptor 1,140 IU/L (145-519 U/mL). Anti-nuclear, anti-Jo-1, and anti-MDA-5 antibodies were negative. Herpes simplex virus (HSV) 1, HSV2, human herpes virus (HHV) 6, HHV7, HHV8, Epstein-Barr virus, cytomegalovirus, varicella zoster virus, and parvovirus B19 nucleic acids in the serum were undetectable with polymerase chain reaction. Blood cultures were negative for aerobic and anaerobic bacteria. Repeated bone marrow aspiration revealed hemophagocytosis without atypical cells. Fluorine-18 (18F) fluorodeoxyglucose positron emission tomography-CT revealed a normal 18F-fluorodeoxyglucose uptake. A random skin biopsy revealed no atypical cells. A skin biopsy from the elbow revealed liquefactive degeneration of the basal epidermal layer and dermal mucinosis. Magnetic resonance imaging of the muscles or electromyogram was not performed because of her poor physical status. Since her dermatological and dermatopathological features were compatible with DM, she was diagnosed with DM.
Under treatment with dexamethasone and CsA, she developed a high fever again and progressive thrombocytopenia (2.6×104/μL) without leukopenia or anemia. We diagnosed the thrombocytopenia as associated with the exacerbation of HPS, since the serum levels of ferritin increased to 196,204 ng/mL with the fever, while the elevation of platelet-associated IgG levels was marginal (48 ng/107 cells; normal range: <46 ng/107 cells). We added methylprednisolone pulse therapy followed by prednisolone (60 mg/day) and etoposide (200 mg/day, twice weekly). However, the high fever, thrombocytopenia, and hyperferritinemia persisted. A week after the emergence of the skin rashes, she developed acute heart failure. Electrocardiogram depicted low voltage without ST-segment elevation or T-wave inversion. Echocardiography depicted diffuse hypokinesia with a reduced ejection fraction (10%), without any findings compatible with Takotsubo-shaped cardiomyopathy, which has been reported as a cardiac involvement of HPS (4). Serum levels of troponin I, brain natriuretic peptide, and aldolase were elevated (0.97 ng/mL, 4,103 pg/mL, and 27 U/L, respectively). She was diagnosed with DM-associated myocarditis since the cardiac abnormalities developed after the emergence of the skin rashes and along with the elevation of serum levels of aldolase, which indicated exacerbation of DM. IVIG (20 g/day for consecutive 5 days) administration led to improvements of the heart failure, the skin rashes, and the levels of troponin I and aldolase, but not of the high fever and thrombocytopenia. The serum levels of ferritin decreased but not normalized (8,874 ng/mL). We re-administered etoposide and changed CsA to tacrolimus. These treatments eradicated the skin rashes, improved the thrombocytopenia, and normalized the serum levels of aldolase. Since the fever persisted and the serum levels of ferritin increased to 10,888 ng/mL, IVIG administration was repeated but only reduced the ferritin levels moderately (6,739 ng/mL).
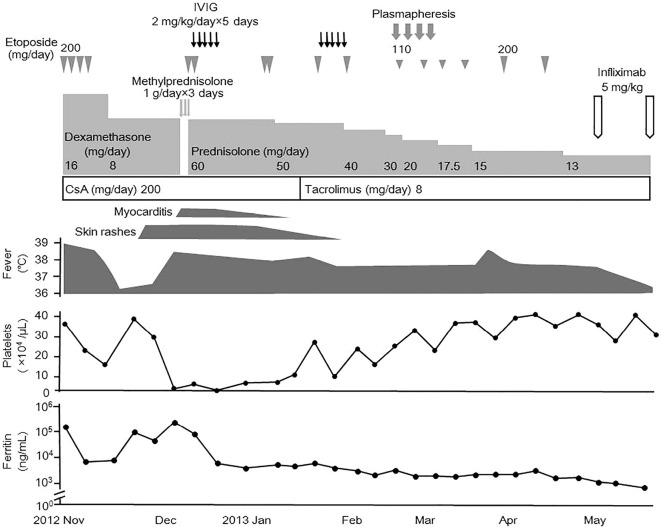
Therefore, we started plasmapheresis in addition to prednisolone, tacrolimus, and etoposide, but noted no improvement. We performed bone marrow aspiration, which revealed marked hemophagocytosis. Instead of continuing etoposide and plasmapheresis, we treated her with infliximab (5 mg/kg) two weeks after the last administration of etoposide. One week after the administration of infliximab, the high fever improved. Although the serum levels of ferritin were not normalized (1,578 ng/mL), the doses of prednisolone were tapered successfully without a fever or cytopenia. She has since been well-controlled with prednisolone (5 mg/day), tacrolimus (3 mg/day), and infliximab (6 mg/kg, every 6 weeks). The abnormal findings and their responsiveness to the treatments are depicted in Fig. 2.
Figure 2.
Clinical course of the patient. CsA: cyclosporine A, IVIG: intravenous immunoglobulin
Discussion
The patient suffered from HPS complicated with DM. HPS preceded DM and persisted even after the amelioration of DM. Ten other cases of DM-associated HPS have been reported (5-11). DM developed earlier than or simultaneously with HPS. In 7 out of the 10 cases, HPS improved along with the amelioration of DM (5-8). DM and HPS did not achieve remission in two cases (9, 10). In one case, HPS persisted after the amelioration of DM, as in our case (11). A juvenile case in which HPS preceded DM was also reported (12). These findings indicate that HPS does not always emerge as a complication of DM, suggesting that HPS shares underlying pathologies with DM.
In the present case, the addition of infliximab to conventional therapies improved HPS. Although the therapeutic effect of TNF inhibitors on primary HPS has not been reported, TNF inhibitors have been used successfully in the treatment of autoimmune-associated HPS, such as systemic lupus erythematosus (SLE)- and adult onset Still's disease (AOSD)-associated HPS (13-15). TNF inhibitors may be a therapeutic option in autoimmune-associated HPS.
In the present case, the serum levels of TNF-α (3.7 pg/mL) and IL-6 (6.2 pg/mL) were not elevated prior to the administration of infliximab. Similarly, TNF-α was not elevated in a case of SLE-associated HPS treated successfully with infliximab (13). The efficacy of infliximab in these cases indicates that TNF-α plays a key role in HPS, even though the serum levels of TNF-α were not elevated.
The effect of TNF inhibitors on DM remains unclear. In some cases, infliximab was effective against refractory DM (16-18). However, a clinical trial revealed that infliximab had limited efficacy on polymyositis (PM)/DM (18). The administration of TNF inhibitors had been reported to exacerbate PM/DM in some cases (19). Therefore, when using infliximab for HPS complicated with DM, DM should be controlled with conventional therapies.
In the treatment of HPS, the diagnosis of the underlying diseases is essential. The present patient developed periorbital edematous violaceous eruption and erythematous patches on the extensor surfaces of the elbows, which were concordant with heliotrope rash and Gottron's papules in DM. In addition, a skin biopsy revealed dermal mucinosis, which is a characteristic finding of DM (20). HPS manifests via various skin rashes, including an erythematous rash. However, dermal mucinosis has not been reported in HPS-associated skin rashes (21). Therefore, we diagnosed the skin rashes as being associated with DM.
In the present case, the serum levels of CK were low. In the spectrum of inflammatory myopathy, the degree of muscle injury varies (22). Similar to the present case, some cases of DM without elevation of serum CK levels have been reported (23). In addition, the systemic inflammation in HPS might have contributed to the reduction in the CK levels in the present case. The CK activity is maintained with glutathione in the circulation (24). Under systemic inflammatory conditions, increased oxidative stress decreases the serum levels of glutathione, which eventually decreases the CK activity (24-26).
The improvement of the fever after the administration of infliximab in our case might have come from the discontinuation of etoposide, since a fever is one of its adverse effects (27). Generally, a drug fever improves rapidly after the discontinuation of the causal drug (28). In our case, the fever persisted for three weeks after the last administration of etoposide. In addition, the fever improved one week after the administration of infliximab. Therefore, we concluded that infliximab improved the fever.
In conclusion, we herein described a case of HPS that preceded DM and persisted after the amelioration of DM. Additional therapies, such as TNF inhibitors, for HPS might be necessary when therapies for DM are insufficient to improve HPS.
Author's disclosure of potential Conflicts of Interest (COI).
Hitoshi Kohsaka: Honoraria, Ono Pharmaceutical; Research funding, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Ono Pharmaceutical, Astellas Pharma. and Eisai.
References
- 1. Janka GE, Lehmberg K. Hemophagocytic syndromes - an update. Blood Rev 28: 135-142, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 48: 124-131, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndromes in adults. Arthritis Rheum 66: 2297-2307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takeoka Y, Nakamae M, Nakamae H, et al. Two cases of ampulla (takotsubo-shaped) cardiomyopathy associated with hemophagocytic lymphohistiocytosis. Acta Haematol 117: 205-210, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Thomas A, Appiah J, Langsam J, Parker S, Christian C. Hemophagocytic lymphohistiocytosis associated with dermatomyositis: a case report. Conn Med 77: 481-485, 2013. [PubMed] [Google Scholar]
- 6. Kaieda S, Yoshida N, Yamashita F, et al. Successful treatment of macrophage activation syndrome in a patient with dermatomyositis by combination with immunosuppressive therapy and plasmapheresis. Mod Rheumatol 25: 962-966, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Yajima N, Wakabayashi K, Odai T, et al. Clinical features of hemophagocytic syndrome in patients with dermatomyositis. J Rheumatol 35: 1838-1841, 2008. [PubMed] [Google Scholar]
- 8. Mizoguchi F, Takada K, Ishikawa K, Mizusawa H, Kohsaka H, Miyasaka N. A case of dermatomyositis with rhabdomyolysis, rescued by intravenous immunoglobulin. Mod Rheumatol 25: 646-648, 2015. [DOI] [PubMed] [Google Scholar]
- 9. Madaule S, Porte L, Couret B, Arlet-Suau E. Fatal haemophagocytic syndrome in the course of dermatomyositis with anti-Mi2 antibodies. Rheumatology (Oxford) 39: 1157-1158, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Bazan-Socha S, Zolcinski M, Szostek M, et al. A fatal case of acquired hemophagocytic lymphohistiocytosis (macrophage activation syndrome) in the initial course of dermatomyositis with anti-Jo-1 antibody. Int J Rheum Dis in press. [DOI] [PubMed] [Google Scholar]
- 11. Yamashita H, Matsuki Y, Shimizu A, et al. Hemophagocytic lymphohistiocytosis complicated by central nervous system lesions in a patient with dermatomyositis: a case presentation and literature review. Mod Rheumatol 23: 386-392, 2013. [DOI] [PubMed] [Google Scholar]
- 12. Poddighe D, Cavagna L, Brazzelli V, Bruni P, Marseglia GL. A hyper-ferritinemia syndrome evolving in recurrent macrophage activation syndrome, as an onset of amyopathic juvenile dermatomyositis: a challenging clinical case in light of the current diagnostic criteria. Autoimmun Rev 13: 1142-1148, 2014. [DOI] [PubMed] [Google Scholar]
- 13. Ideguchi H, Ohno S, Takase K, et al. Successful treatment of refractory lupus-associated haemophagocytic lymphohistiocytosis with infliximab. Rheumatology (Oxford) 46: 1621-1622, 2007. [DOI] [PubMed] [Google Scholar]
- 14. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndromes in adults. Arthritis Rheum 66: 2297-2307, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henzan T, Nagafuji K, Tsukamoto H, et al. Success with infliximab in treating refractory hemophagocytic lymphohistiocytosis. Am J Hematol 81: 59-61, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Efthimiou P, Schwartzman S, Kagen LJ. Possible role for tumour necrosis factor inhibitors in the treatment of resistant dermatomyositis and polymyositis: a retrospective study of eight patients. Ann Rheum Dis 65: 1233-1236, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dold S, Justiniano ME, Marquez J, Espinoza LR. Treatment of early and refractory dermatomyositis with infliximab: a report of two cases. Clin Rheumatol 26: 1186-1188, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Coyle K, Pokrovnichka A, French K, et al. A Randomized, double-blind, placebo-controlled trial of infliximab in patients with polymyositis and dermatomyositis. Arthritis Res 58: s293, 2008. [Google Scholar]
- 19. Dastmalchi M, Grundtman C, Alexanderson H, et al. A high incidence of disease flares in an open pilot study of infliximab in patients with refractory inflammatory myopathies. Ann Rheum Dis 67: 1670-1677, 2008. [DOI] [PubMed] [Google Scholar]
- 20. Callen JP. Cutaneous manifestations of dermatomyositis and their management. Curr Rheumatol Rep 12: 192-197, 2010. [DOI] [PubMed] [Google Scholar]
- 21. Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology 230: 234-243, 2015. [DOI] [PubMed] [Google Scholar]
- 22. Sontheimer RD. Skin manifestations of systemic autoimmune connective tissue disease: diagnostics and therapeutics. Best Pract Res Clin Rheumatol 18: 429-462, 2004. [DOI] [PubMed] [Google Scholar]
- 23. Carter JD, Kanik KS, Vasey FB, Valeriano-Marcet J. Dermatomyositis with normal creatine kinase and elevated aldolase levels. J Rheumatol 28: 2366-2367, 2001. [PubMed] [Google Scholar]
- 24. Gunst JJ, Langlois MR, Delanghe JR, De Buyzere ML, Leroux-Roels GG. Serum creatine kinase activity is not a reliable marker for muscle damage in conditions associated with low extracellular glutathione concentration. Clin Chem 44: 939-943, 1998. [PubMed] [Google Scholar]
- 25. Kwon WY, Suh GJ, et al. Plasma glutathione reductase activity and prognosis of septic shock. J Surg Res 200: 298-307, 2016. [DOI] [PubMed] [Google Scholar]
- 26. Bavunoglu I, Genc H, Konukoglu D, et al. Oxidative stress parameters and inflammatory and immune mediators as markers of the severity of sepsis. J Infect Dev Ctries 10: 1045-1052, 2016. [DOI] [PubMed] [Google Scholar]
- 27. Pajtler KW, Tippelt S, Siegler N, et al. Intraventricular etoposide safety and toxicity profile in children and young adults with refractory or recurrent malignant brain tumors. J Neurooncol 128: 463-471, 2016. [DOI] [PubMed] [Google Scholar]
- 28. Patel RA, Gallagher JC. Drug fever. Pharmacotherapy 30: 57-69, 2010. [DOI] [PubMed] [Google Scholar]