Abstract
A 66-year-old man with a several year history of thrombocytopenia, pleural effusion and ascites, anasarca, and organomegaly presented with general fatigue, appetite loss, dyspnea with type II respiratory failure. The precise history of the patient and the re-evaluation of lymph node and bone marrow biopsies conducted by the previous physician indicated TAFRO syndrome. The patient's laboratory data improved for a year with tocilizumab, but then worsened to the point that the patient required artificial ventilation due to the deterioration of type II respiratory failure. The replacement of tocilizumab with rituximab yielded a steady improvement, but it was necessary to address the patient's persistent respiratory failure. Peripheral nerve disorder might have been involved with the patient's respiratory failure.
Keywords: TAFRO syndrome, tocilizumab, type II respiratory failure, rituximab, peripheral nerve disorder
Introduction
Castleman-Kojima disease (TAFRO Syndrome), a variant of multicentric Castleman's disease (MCD), is a novel systemic inflammatory disorder characterized by thrombocytopenia, anasarca, fever, myelofibrosis, renal dysfunction and organomegaly. Takai et al. were the first to report on this syndrome (1), and the disease concept was established by Kawabata et al. in 2013 (2). Previous reports have shown that patients respond to anti-interleukin (IL)-6 receptor antibodies (tocilizumab), corticosteroids, and rituximab (3-6), but there is no well-established therapy. We experienced a case of TAFRO syndrome. It took several years to reach a definite diagnosis since the initial symptoms occurred and the diagnosis was made after a re-evaluation of the pathological tissue specimens obtained at the time of biopsy which had been performed two years after the initial onset of symptoms.
Case Report
A 66-year-old man was referred to our hospital for worsening general fatigue, appetite loss, and dyspnea. He had hypertension and hypothyroidism, which were treated with candesartan and levothyroxine, respectively. He had quit smoking 30 cigarettes per day ten years previously.
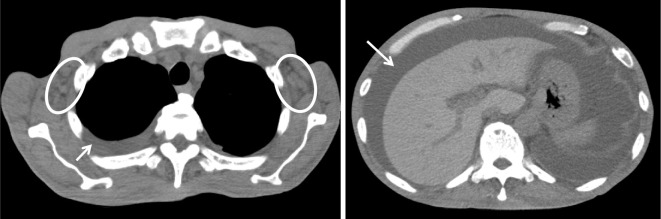
He first noticed pitting edema in both legs without any other symptoms four years previously. Two years later, he underwent several procedures to treat pleural effusion and ascites at another hospital without a definite diagnosis. One year before he was referred to our hospital, pleural effusion and ascites accumulated again. At the time, he had anemia and thrombocytopenia. Computed tomography revealed generalized lymphadenopathy (Fig. 1), and malignant lymphoma was suspected. Although a biopsy of the axillary lymph node was performed, there was no further evidence to make a definitive diagnosis. Fluorodeoxy glucose (FDG) positron emission tomography (FDG-PET) showed an abnormal uptake in the sacral region, with no other accumulation (Fig. 2). A bone marrow biopsy of the areas with a positive uptake on FDG-PET revealed no evidence of malignant lymphoma. One year after the biopsy, the patient suffered a further worsening of fatigue, appetite loss, weight loss, and respiratory discomfort. He was referred to our hospital and admitted.
Figure 1.
Mild lymphadenopathy in the axillary region (circle), pleural effusion, and ascites (arrow).
Figure 2.
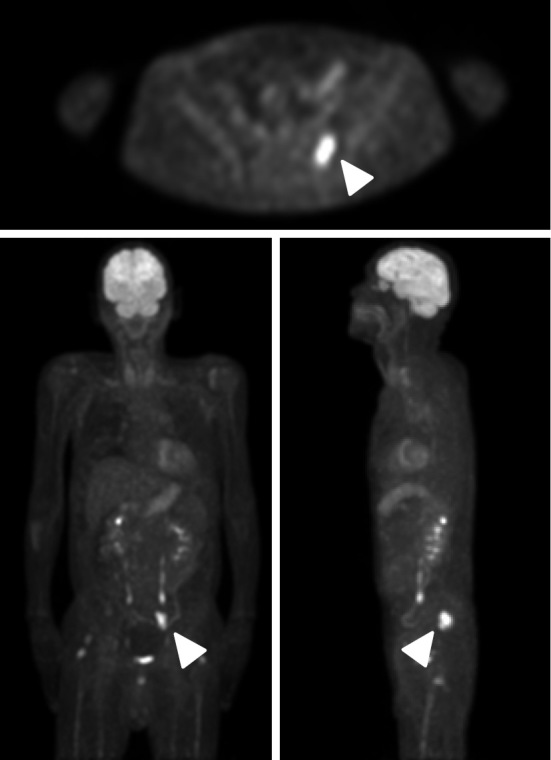
FDG positron emission tomography (FDG-PET) showing the abnormal uptake in the sacral region (arrow heads).
On admission, the patient's height and weight were 173.3 cm and 52.6 kg, respectively. He was lucid, with a blood pressure of 106/58 mmHg, a regular pulse rate of 50 beats/min, a body temperature of 37.7℃, a respiratory rate 20 breaths/min, and an oxygen saturation of 92% without the administration of oxygen. The lymph nodes in the posterior cervical region showed mild-to-moderate swelling. No other significant physical findings were apparent. The laboratory findings showed normocytic anemia, thrombocytopenia, and renal dysfunction (Table 1). Computed tomography revealed bilateral pleural effusion and moderate lymphadenopathy at the cervical, axillary, and inguinal regions. A blood gas analysis indicated type II respiratory failure, and a respiratory function test showed a restrictive defect [% vital capacity (VC), 30.1%; forced expiratogy volume (FEV) 1.0%, 93.5%].
Table 1.
Laboratory Data.
| on admission | after third tocilizumab | on exacerbation | before discharge | |
|---|---|---|---|---|
| day1 | day38 | day286 | day401 | |
| White blood cells(/μL) | 2,950 | 6,140 | 2,780 | 6,600 |
| Red blood cells(×103/μL) | 343 | 420 | 421 | 409 |
| Hemoglobin(g/dL) | 10.4 | 12.7 | 12.2 | 11.3 |
| Hematocrit(%) | 33.3 | 40.7 | 35.8 | 35.8 |
| MCV(fL) | 97.1 | 96.9 | 85.0 | 87.5 |
| Platelet counts(×103/μL) | 4.3 | 5.3 | 7.1 | 44.3 |
| Albumin(g/dL) | 3.3 | 3.3 | 3.8 | 2.9 |
| AST(IU/L) | 15 | 33 | 12 | 17 |
| ALT(IU/L) | 13 | 75 | 10 | 28 |
| LDH(IU/L) | 53 | 102 | 77 | 73 |
| ALP(IU/L) | 206 | 216 | 201 | 283 |
| Creatinine(mg/dL) | 1.55 | 0.74 | 0.91 | 0.68 |
| CRP(mg/dL) | 0.17 | 0.02 | 0.07 | 0.4 |
| IgG(mg/dL) | 2,168 | - | - | - |
| IgA(mg/dL) | 418 | - | - | - |
| IgM(mg/dL) | 39 | - | - | - |
| C3(mg/dL) | 44 | - | - | - |
| C4(mg/dL) | 17.7 | - | - | - |
| CH50(IU/L) | 33 | - | - | - |
| IL-6(pg/mL) | 4.97 | 62.55 | 64.15 | 18.76* |
| VEGF(pg/mL) | 151 | 1,590* | ||
| antinuclear antibody | negative | |||
| thyroglobulin antibody(IU/mL) | 495.7 | |||
| thyroid peroxidase antibody(IU/mL) | 148.1 | |||
| PAIgG(ng/107 cells) | 20.9 | |||
| acetylcholie receptor antibody | negative | |||
| HHV-8 DNA PCR | negative | |||
| analysis of blood gases | ||||
| pH | 7.310 | 7.336 | 7.327 | 7.397 |
| pCO2 (mmHg) | 62.0 | 64.2 | 64.1 | 48.2 |
| pO2 (mmHg) | 58.5 | 77.1 | 56.7 | 71.6 |
| HCO3 (mmol/L) | 30.5 | 33.5 | 32.8 | 29.0 |
AST: asparate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, VFGF: vascular endothelial growth factor, PAIgG: platelet associated IgG, HHV-8: human herpes virus 8
* these data were obtained after discharge (day 434)
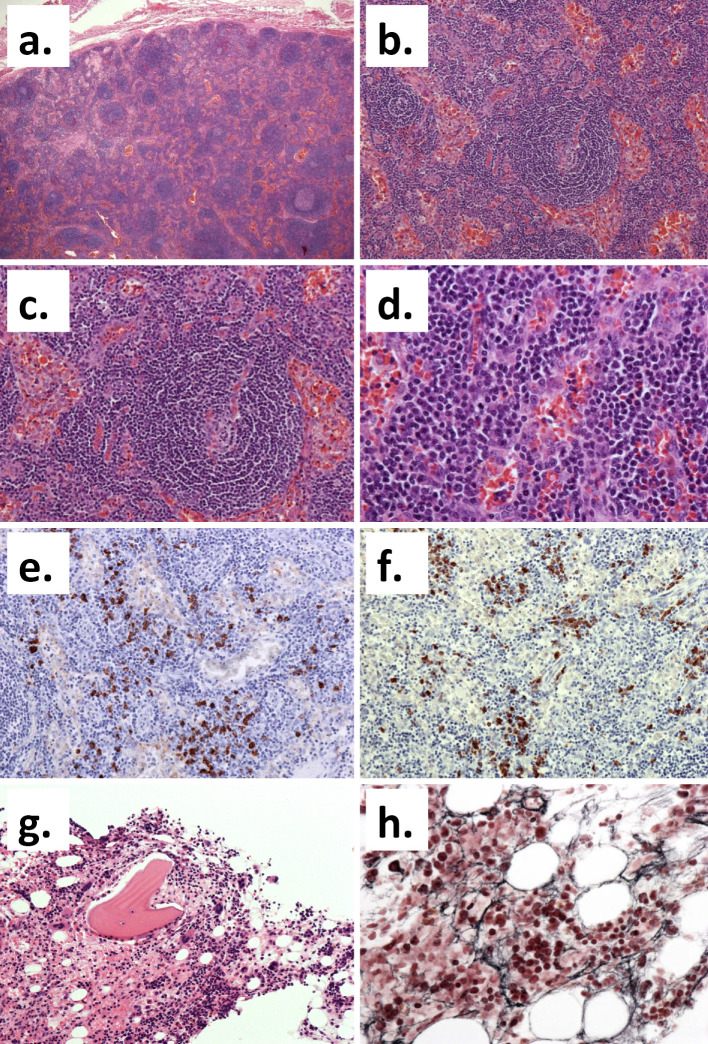
TAFRO syndrome was considered as one of the differential diagnoses based on the presence of thrombocytopenia, recurrent pleural effusion and ascites, renal dysfunction, and moderate systemic lymphadenopathy. We therefore conducted further evaluations. An anti-nuclear antibody test was negative, while anti-thyroid antibody and platelet-associated IgG tests were positive. The patient's serum IL-6 levels were within the normal range, but his serum vascular endothelial growth factor (VEGF) level was elevated (151.0 pg/mL). We then re-evaluated the pathological tissue specimens obtained by the previous medical institution. The left axillary lymph node biopsy specimen revealed atrophic germinal centers, an expansion of the interfollicular zone, and arborized blood vessels. We noted the infiltration of small lymphocytes and plasma cells (Fig. 3a-d); the infiltrating plasma cells showed no monoclonality (Fig. 3e and f). A bone marrow biopsy specimen showed increased numbers of bone marrow megakaryocytes and reticular fibers (Fig. 3g and h). We considered that his clinical course, imaging findings and histological findings were highly consistent with TAFRO syndrome.
Figure 3.
The histological findings. (a-d) The histological appearance of the left axillary lymph node (Hematoxylin and Eosin (H&E) staining). (a) The basic structure of the lymph node is obscure (original magnification: ×40). (b) Many lymphoid follicles with unclear atrophic germinal centers and the expansion of the interfollicular zone are apparent (original magnification: ×100). (c) The peripheral mantle layer was developed with a concentric cellular distribution (original magnification: ×200). (d) Arborized blood vessels were present and the infiltration of small lymphocytes and plasma cells in the interfollicular zone was noted (original magnification: ×400). (e, f) Kappa (e) and Lambda (f) immunostaining showed no clear monoclonality (original magnification: ×200). (g, h) The histological appearance of the bone marrow. (g) An increase in the number of megakaryocytes was evident on H&E staining (original magnification: ×200). (h) Silver impregnation staining showed an increase in reticular fibers (original magnification: ×400).
We initiated treatment with tocilizumab (8.0 mg/kg) on day 3 of hospitalization; this was continued bi-weekly along with prednisolone (20 mg/day). A gradual improvement was noted in the patient's oral intake and activities of daily living (ADL), and the laboratory findings (such as thrombocytopenia and renal dysfunction) improved. However, his type II respiratory failure did not show sufficient improvement. He discharged on day 35 and the bi-weekly tocilizumab therapy was continued. We considered the efficacy of the therapy to be sufficient. From day 148, the therapy was administered every 3 weeks; then, once every 4 weeks from day 211.
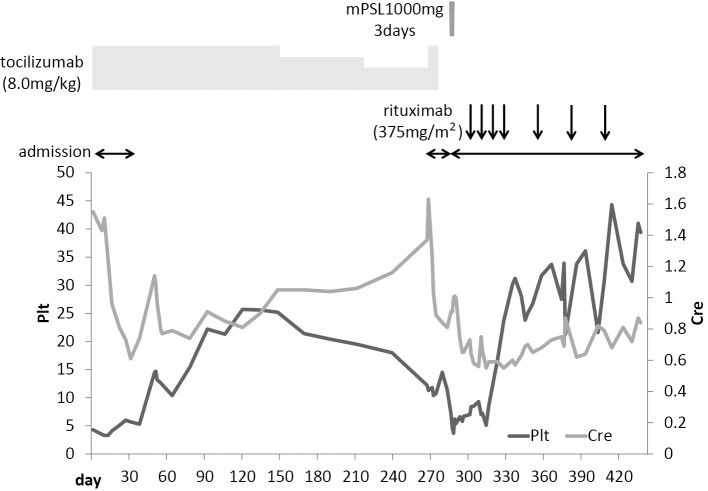
The patient was readmitted on day 267 due to thrombocytopenia and renal dysfunction. Sputum, urine and blood cultures were all negative. Based on these findings, we were of the opinion that the patient's TAFRO syndrome had worsened, and the tocilizumab frequency was increased to bi-weekly. The patient's overall condition seemed to improve and he was discharged on day 283. However, immediately after discharge, the patient experienced further general malaise, and in addition to thrombocytopenia and renal dysfunction, an exacerbation of type II respiratory failure was confirmed. The patient was readmitted on day 286. CT revealed mild fluid accumulation in the left chest, but no other significant changes were apparent. Methylprednisolone pulse therapy (1,000 mg/day for 3 days) was started on day 288, followed by prednisolone (PSL; 60 mg/day). However, the effects were insufficient. The patient's respiratory condition continued to deteriorate, and he became dependent on an artificial ventilator. We initiated treatment rituximab [500 mg, weekly (375 mg/m2)]. The laboratory findings showed some improvements (Table 1). His respiratory showed only limited improvement. Thus, on day 314, tracheotomy was performed, after which an artificial ventilator was used intermittently. After the 4th round of rituximab, rituximab was administered once every 4 weeks. The patient was discharged on day 442 (Fig. 4). However, the type II respiratory failure persisted. Although some patients with Castleman's disease have been reported to be complicated by myasthenia gravis with respiratory failure (7, 8), the patient was negative for anti-acetylcholine receptor antibodies. A nerve conduction study showed axonal involvement demyelination changes (Table 2), and peripheral nerve involvement was suspected. Since then, the patient has continued to receive outpatient treatment with rituximab (500 mg, once every 4 weeks), and no worsening has been noted in his overall condition.
Figure 4.
The clinical course. Plt: platelet, Cre: creatinine, mPSL: methylprednisolone
Table 2.
Nerve Conduction Study.
| motor | ||||||
|---|---|---|---|---|---|---|
| median | Ulnar | Tibial | ||||
| right | left | right | left | right | left | |
| Latency(ms) | 6.16 | 6.75 | 3.67 | 3.15 | - | - |
| Amplitude(mV) | 2.49 | 0.50 | 6.05 | 3.89 | 0.73 | 1.54 |
| Nerve conduction velocity (m/s) | 28.0 | 17.1 | 39.1 | 40.2 | 30.1 | 28.9 |
| sensory | ||||||
| median | Ulnar | Sural | ||||
| right | left | right | left | right | left | |
| Amplitude(μV) | Not evoked | Not evoked | 14.6 | 5.10 | 4.40 | 4.80 |
| Nerve conduction velocity (m/s) | 49.4 | 41.9 | 49.3 | 37.9 | ||
Discussion
In the present case, it took several years after the onset of symptoms to reach a definite diagnosis of TAFRO syndrome. The re-evaluation of lymph node and bone marrow specimens obtained by the previous hospital was important for making the diagnosis.
We examined the present case based on the diagnostic criteria for TAFRO syndrome, which were proposed by Masaki et al. in 2016 (9). Our patient fulfilled all of the major and minor categories. Although the patient had a slight fever, which became elevated a few times (to ≥37.5℃) before the therapy was initiated, his C-reactive protein (CRP) level did not increase throughout the clinical course, as was noted in previous reports on TAFRO syndrome (10). Based on his clinical course, the laboratory data and the pathological findings, we considered that malignancies such as lymphoma, infectious disease, IgG4-related disease, hepatic cirrhosis, and thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS) could be excluded. With regard to polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome, the present case exhibited peripheral nerve disorder, but showed no monoclonal gammopathy or skin symptoms; thus, the likelihood of POEMS syndrome was low. Furthermore, the clinical course and laboratory findings were not indicative of an autoimmune disease such as systemic lupus erythematosus. However, in addition to platelet-associated (PA)IgG antibody positivity, which is an immunological abnormality found in some patients with TAFRO syndrome (3, 11, 12), the patient was positive for anti-thyroid peroxidase and anti-thyroglobulin antibodies. Thus, the possible involvement of some sort of autoimmune response cannot be ruled out. The patient's disease severity was classified as slightly severe (grade 3: anasarca, 3 points; thrombocytopenia, 2 points; fever, 1 point; and renal insufficiency, 1 point). Taking these factors into consideration, we are of the opinion that the diagnosis of TAFRO syndrome was appropriate.
In this case, although tocilizumab and rituximab yielded some improvement, the patient's type II respiratory failure did not show a sufficient improvement at any point in the clinical course, and the patient became fully dependent on artificial ventilation. A case of myocardial damage as an organ disorder accompanying TAFRO syndrome has been reported (13), but we were unable to find any cases in the literature that were further complicated by type II respiratory failure. Although we identified several reports of patients with Castleman's disease complicated by lung damage (14-16), each of the cases involved abnormalities of the lung parenchyma, indicating that the mechanism was different from that of our patient. Cases of Castleman's disease complicated by myasthenia gravis have been reported (7, 17, 18); however, the fact that our patient was negative for anti-AChR antibodies as well as other symptoms was inconsistent with myasthenia gravis. We confirmed a peripheral nerve disorder in our patient, which could have been involved in the type II respiratory failure. There have been no other reports of TAFRO syndrome accompanied by peripheral nerve disorder. TAFRO syndrome and POEMS syndrome are similar to Castleman's disease. Peripheral neuropathy usually dominates the clinical picture of POEMS syndrome. We hypothesize that under certain conditions, TAFRO syndrome could affect peripheral nerve disorder. On the other hand, the patient's peripheral nerve disorder showed very little response to treatment; thus, the involvement of another disease cannot be ruled out.
We reported our experiences in the treatment of a patient with TAFRO syndrome that was resistant to tocilizumab but which responded favorably to rituximab. Much about the mechanism underlying the onset of TAFRO syndrome remains unknown and the treatment options for this condition have yet to be established. The further accumulation of case studies is required to determine the association between TAFRO syndrome and peripheral nerve disorder. We anticipate that the present case will provide valuable insight to this end.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsho Ketsueki (Jpn J Clin Hematol) 51: 320-325, 2010(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 2. Kawabata H, Takai K, Kojima M, et al. Castleman-Kojima disease (TAFRO syndrome) : a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly : a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop 53: 57-61, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Inoue M, Ankou M, Hua J, Iwaki Y, Hagihara M, Ota Y. Complete resolution of TAFRO syndrome (thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly) after immunosuppressive therapies using corticosteroids and cyclosporin A : a case report. J Clin Exp Hematop 53: 95-99, 2013. [DOI] [PubMed] [Google Scholar]
- 4. Yamaga Y, Tokuyama K, Kato T, et al. Successful treatment with cyclosporin A in tocilizumab-resistant TAFRO syndrome. Intern Med 55: 185-190, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: a case report and systematic review. Mod Rheumatol 1-6, 2016(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara S, Mochinaga H, Nakata H, et al. Successful treatment of TAFRO syndrome, a variant type of multicentric Castleman disease with thrombotic microangiopathy, with anti-IL-6 receptor antibody and steroids. Int J Hematol 103: 718-723, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Ishikawa K, Kato T, Aragaki M, et al. A case of Castleman's disease with myasthenia gravis. Ann Thorac Cardiovasc Surg 20 (Suppl): 585-588, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Wang S, Chen SW, Cai SL, Jin BY. A case report of retroperitoneal pararenal Castleman's disease associated with myasthenia gravis. World J Surg Oncol 12: 331, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol 103: 686-692, 2016. [DOI] [PubMed] [Google Scholar]
- 10. Ozawa T, Kosugi S, Kito M, et al. Efficacy of rituximab for TAFRO syndrome, a variant type of multicentric Castleman's disease. Rinsho Ketsueki (Jpn J Clin Hematol) 55: 350-355, 2014(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 11. Tedesco S, Postacchini L, Manfredi L, et al. Successful treatment of a Caucasian case of multifocal Castleman's disease with TAFRO syndrome with a pathophysiology targeted therapy - a case report. Exp Hematol Oncol 4: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takai K, Nikkuni K, Momoi A, Nagai K, Igarashi N, Saeki T. Thrombocytopenia with reticulin fibrosis accompanied by fever, anasarca and hepatosplenomegaly : a clinical report of five cases. J Clin Exp Hematop 53: 63-68, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Hiramatsu S, Ohmura K, Tsuji H, et al. Successful treatment by rituximab in a patient with TAFRO syndrome with cardiomyopathy. Jpn J Clin Immunol 39: 64-71, 2016. [DOI] [PubMed] [Google Scholar]
- 14. Morise M, Shimomoto H, Honda T, Mori Y. A case of multicentric Castleman's disease with pulmonary involvement. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 41: 59-65, 2003(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 15. Nei T, Oiwa T, Saitoh Y, et al. A case of multicentric Castleman disease showing diffuse cystic change in the lung. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 44: 468-473, 2006(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 16. Ohno N, Fujiyama S, Ooi M, et al. Effectiveness of humanized anti-interleukin-6 receptor antibody (tocilizumab) for Castleman's disease mainly with pulmonary involvement. Nihon Naika Gakkai Zasshi (J Jpn Soc Inter Med) 96: 988-990, 2007(in Japanese). [DOI] [PubMed] [Google Scholar]
- 17. Westphal FL, Lima LC, Santana LC, Netto JC, Amaral VC, Silva Mdos S. Castleman's disease associated with follicular dendritic cell sarcoma and myasthenia gravis. J Bras Pneumol 36: 819-823, 2010. [DOI] [PubMed] [Google Scholar]
- 18. Lee SK, Kim DH, Son BS. Castleman's disease with myasthenia gravis. Korean J Thorac Cardiovasc Surg 45: 199-201, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]