Abstract
Objective
The opinions about the application of pulse oximetry in diagnosis of congenital heart disease (CHD) were debatable. We performed this meta-analysis to confirm the diagnostic role of pulse oximetry screening for CHD.
Methods
Relevant articles were searched in the databases of Pubmed, Embase, Google Scholar, and Chinese National Knowledge Infrastructure (CNKI) up to April 2017. Data was processed in the MetaDiSc 1.4 software. Pooled sensitivity and specificity with 95% confidence interval (95% CI) were calculated to explain the diagnostic role of pulse oximetry screening for CHD. I2⩾50% or p < 0.05 indicated significant heterogeneity. Area under curve (AUC) of summary receiver operating characteristics (SROC) was calculated to assess its diagnostic accuracy. The robustness of overall results was evaluated by sensitivity analysis. Publication bias was evaluated by Deek's funnel plot.
Results
22 eligible articles were selected. Pooled sensitivity and specificity were 0.69 (0.67–0.72) and 0.99 (0.99-0.99), respectively. The corresponding AUC was 0.9407, suggesting high diagnostic accuracy of pulse oximetry screening for CHD. Sensitivity analysis demonstrated that the pooled results were robust. Deek's funnel plot seemed to be symmetrical.
Conclusions
Pulse oximetry screening could be used to diagnose CHD. It shows high diagnosis specificity and accuracy.
1. Introduction
Congenital heart disease (CHD) is regarded as a main cause of infant death, with an incidence of 8 in every 1000 live births [1]. It needs invasive intervention during the neonatal period and these neonates suffering this disease benefit most from early detection [2]. Prenatal diagnosis just picks up <50% of all cases [3–6]. Routine neonatal inspection fails to detect above than 50% of CHD infants. More than 55% of neonates show no murmur symptom in the nursery, and ⩽82% of them are discharged before diagnosis results are obtained [7]. Early diagnosis of CHD is crucial since the delayed diagnosis results in cardiovascular collapse, cardiac failure, and death, whereas early diagnosis during the first few days of life is difficult. Therefore, an effective screening program for CHD is necessary.
In recent years, pulse oximetry has been suggested as a diagnosis tool for CHD [8–10]. It is productive in the detection of CHD before discharge and could decrease missed cases to 4% [11, 12]. Some states of United States have made screening of pulse oximetry for CHD mandatory before discharge in hospital. Pulse oximetry can detect mild hypoxemia, which is common feature for many forms of CHD. It could recognize the cases that are not recognized by clinical examination [13]. Since the introduction of pulse oximetry to screen CHD in 1995, many studies have focused on the subject [14–17]. Despite the fact that there were differences in screening time, cut-off values, target lesions, and others among the relevant studies, the opinion is consistent that pulse oximetry screening is a useful diagnostic method of CHD. However, existing data is still insufficient to initiate a recommendation for application of pulse oximetry in routine care.
This present meta-analysis was performed to confirm the diagnostic role of pulse oximetry for CHD. The obtained results contribute to clinical application of pulse oximetry for diagnosing CHD.
2. Materials and Methods
2.1. Search Strategy
We searched the relevant articles on the databases of Pubmed, Embase, Google Scholar, and Chinese National Knowledge Infrastructure (CNKI) up to April 2017. The following keywords were used: pulse oximetry OR SpO2 AND congenital heart disease OR CHD. The references' lists of obtained articles were manually searched for eligible studies. No language restriction was applied. The studies published in abstract were not considered.
2.2. Article Selection
These obtained articles were selected according to inclusion criteria. The criteria were as follows: (a) SpO2 was assessed with pulse oximetry; (b) SpO2 was used to detect CHD subjects; (c) true positive (TP), false positive (FP), true negative (TN), and false negative (FN) or other data available for calculating them were reported. The review article, abstract article, and case reports were removed from the present analysis.
2.3. Data Extraction
Two authors were responsible for extracting data. The data included name of first author, year of publication, country, number of patients and healthy controls, screening time, screening limb, TP, FP, TN, and FN. The inconsistent opinion was solved with a discussion with the third author.
2.4. Statistical Analysis
Data was processed in the MetaDiSc 1.4 software. Deek's funnel plot was obtained with Stata 12.0 software. Summary sensitivity and specificity along with 95% confidence interval (95% CI) were adopted to confirm the diagnostic role of pulse oximetry screening for CHD. I2⩾50% or p < 0.05 indicated significant heterogeneity. Area under curve (AUC) of summary receiver operating characteristics (SROC) was calculated to assess the diagnostic accuracy of SpO2. The robustness of overall results was evaluated by sensitivity analysis. Publication bias was evaluated by Deek's funnel plot.
3. Results
3.1. Selection Process of Eligible Articles
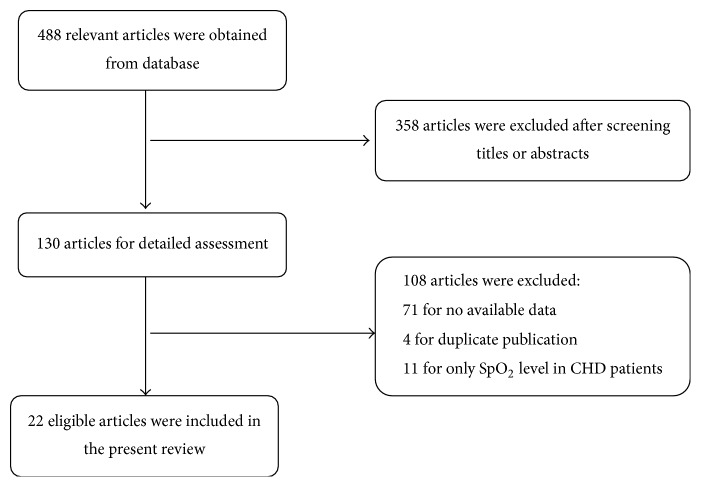
A total of 488 relevant articles were obtained after search on the databases. The titles and abstracts were screened and 358 articles were excluded. The remaining 130 articles were provided detailed assessment and 108 articles were excluded for no available data, duplicate publication, and only SpO2 level in CHD patients. Finally, 22 eligible articles were included in the present meta-analysis [8, 10–12, 14–31]. The detailed selection process was shown in Figure 1. Basic information of each study was listed in Table 1.
Figure 1.
Flow chart of articles selection. 22 articles were selected for meta-analysis.
Table 1.
Basic information of included studies.
| Author | Year | Country | Limb | Test timing, h |
|---|---|---|---|---|
| Arlettaz et al. | 2006 | Switzerland | Right or left foot | 6–12 |
| Bakr and Habib | 2005 | Egypt | Right upper and lower limbs | 31.7 (average) |
| de Wahl Granelli et al. | 2005 | Sweden | Right hand and one foot | 12–48 |
| Hoke et al. | 2002 | America | Right arm and either leg | 24 (average) |
| Koppel et al. | 2003 | America | — | >24 |
| Richmond et al. | 2002 | UK | One or other foot | >2 |
| Rosati et al. | 2005 | Italy | — | 72 h (median) |
| Zhao et al. | 2014 | China | Both on the right hand and on either foot | 6–72 |
| Riede et al. | 2010 | Germany | Foot | 24–72 |
| Mathur et al. | 2015 | India | Right upper limb and either foot | 72 (median) |
| Hu et al. | 2016 | China | Right hand and either foot | 25 (median) |
| Jones et al. | 2016 | UK | — | <24 |
| Ozalkaya et al. | 2016 | Turkey | Lower and right upper extremity | >24 |
| Jawin et al. | 2015 | Malaysia | Left foot | 20 (median) |
| Ewer et al. | 2011 | UK | Right hand and either foot | In the first few hours |
| Taksande et al. | 2013 | India | All the four limbs | Within the first 4 hours |
| van Niekerk et al. | 2016 | South Africa | — | 60 (median) |
| de-Wahl Granelli et al. | 2009 | Sweden | Right hand and either foot | 38 (median) |
| Meberg et al. | 2008 | Norway | Foot | <12 |
| Mo et al. | 2015 | China | Right hand and either foot | >24 |
| Jia et al. | 2016 | China | Right hand | 24 |
| Lu et al. | 2016 | China | Right hand and right foot | >24 |
Note. — indicates that the information was not mentioned in the article.
3.2. Diagnostic Role of Pulse Oximetry Screening for CHD
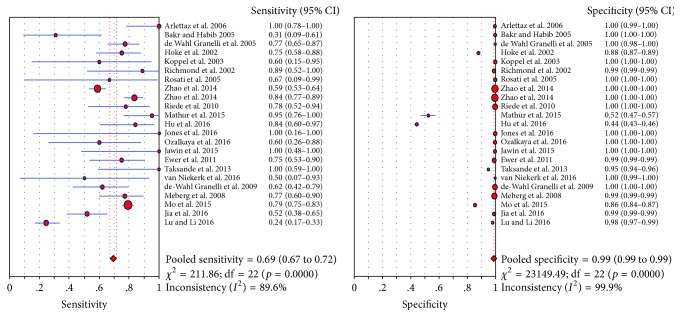
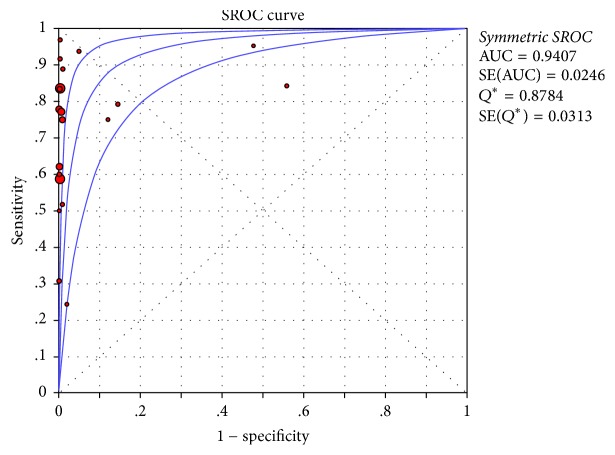
Pulse oximetry screening showed high specificity in detecting CHD (specificity: 0.99), while having relatively low sensitivity (0.69) (Figure 2). In the analyses of sensitivity and specificity, we observed significant heterogeneity (sensitivity: p = 0.0000, I2 = 89.6%; specificity: p = 0.0000, I2 = 99.9%). The corresponding AUC was 0.9407, suggesting high diagnostic accuracy of pulse oximetry screening for CHD (Figure 3).
Figure 2.
Diagnostic sensitivity and specificity of pulse oximetry screening for congenital heart disease (CHD). The sensitivity and specificity were 0.69 (0.67–0.72) and 0.99 (0.99-0.99), respectively.
Figure 3.
Area under curve (AUC) of SROC curve. AUC was 0.9407.
3.3. Sensitivity Analysis and Publication Bias Detection
Robustness of pooled results was assessed by sensitivity analysis by deleting one study each time. The analysis indicated that the pooled results were robust. Deek's funnel plot was used to assess publication bias. The funnel plot seemed to be symmetrical (Figure 4).
Figure 4.
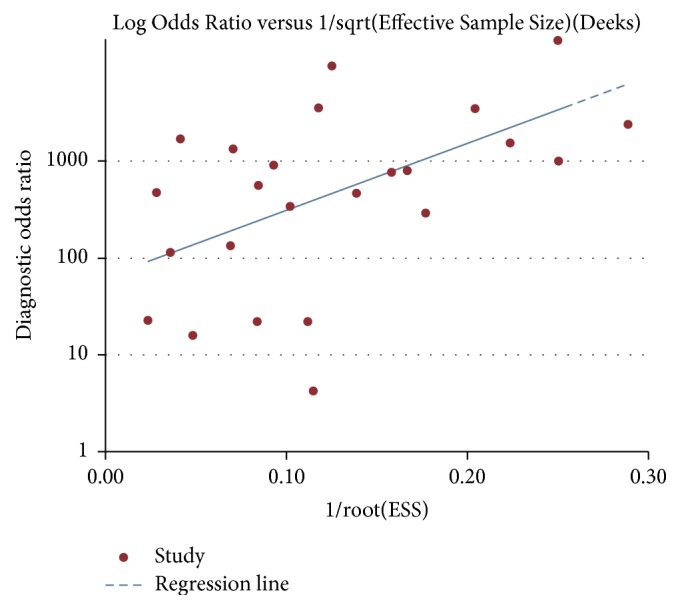
Deek's funnel plot. We found no significant publication bias in the present meta-analysis.
4. Discussion
CHD is a series of heterogeneous disorders that catheter intervention or surgery is mandatory to achieve patients' survival. Clinical examination shows limitation in detecting all forms of CHD [32, 33]. Heart murmurs, one of the hallmarks of CHD, may be misleading or absent due to the reduced ventricular function, prolonged decline of pulmonary vascular resistance, and underlying anatomy. Although prenatal diagnosis is widely applied, a large proportion of CHD neonates are still not diagnosed before being discharged and after birth [34, 35], which may be strengthened by earlier discharge and certain postnatal care [36]. It has been thought that the application of prenatal ultrasound, clinical observation, and physical examination may be sufficient for early diagnosis of CHD [37]. This opinion may be true under specific circumstances; however, the prerequisites possibly do not exist in the majority of hospitals. Thus, a broad consensus that efficient diagnostic tool for CHD is urgently needed has been achieved.
Pulse oximetry is an accurate and noninvasive test for quantification of hypoxaemia. The application of this method for diagnosing CHD is based on the theory that undetectable hypoxaemia in clinic exists in most life-threatening cases. Pulse oximetry screening for CHD has gained more attention over the last decade. It has been demonstrated to be cost-effective and acceptable to the mothers [38, 39]. The existing protocol of pulse oximetry to detect CHD is restricted to 24 to 48 hours of age for neonates in well infant nursery [40].
Our meta-analysis included as many eligible articles as possible via systematic search. These obtained articles were selected carefully according to inclusion criteria. Moreover, the quality of included articles was high. Besides, the results were based on 22 eligible studies involving both Western and Asian countries. Therefore, our results were reliable. The pooled results suggested that combined sensitivity, specificity, and AUC were 0.69 (0.67–0.72), 0.99 (0.99-0.99), and 0.9407, respectively, which is similar to the previous meta-analysis [41].
Among the included articles, the results showed great differences. In the study by Mathur et al., pulse oximetry readings were taken at admission from 950 neonates and the diagnostic sensitivity, specificity, positive predictive value, and negative predictive value were 95.2%, 52.4%, 9.5%, and 99.5%. The diagnostic specificity was poor. Similarly, Hu et al. reported that diagnostic specificity of pulse oximetry screening for CHD was just 44.22%. Meanwhile, Niekerk et al. reported that the diagnostic sensitivity of pulse oximetry was merely 50%, while the specificity was 99.9%. On the contrary, Arlettaz et al. investigated the contribution of pulse oximetry to the early detection of CHD in newborns and found that the sensitivity and specificity were 100% and 99.7%, respectively. In the study of Jones et al., the estimated sensitivity and specificity were 100% and 99.8% of pulse oximetry screening for diagnosing CHD. Thus, the conclusion of our analysis is significant to confirm the diagnostic role of pulse oximetry screening.
However, we must acknowledge that there were limitations in the present meta-analysis. First, cut-off value, diagnosis criteria, target location, and test timing of pulse oximetry were inconsistent among the included studies, which might affect the diagnostic accuracy of pulse oximetry screening. And the significant heterogeneity in the present analysis might result from these variances. Besides, the difference in the severity of CHD also might influence the accuracy of pulse oximetry screening.
5. Conclusion
In conclusion, pulse oximetry screening may serve as a valuable diagnostic tool with high accuracy for CHD. The diagnostic sensitivity and specificity are 0.69 (0.67–0.72) and 0.99 (0.99-0.99), respectively.
Acknowledgments
This work was supported by the Science and Technology Development Plan Project in Medicine and Health Care of Shandong Province under Grant no. 2015WS0044.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Caiju Du and Dianmei Liu were involved in the conception and design, or analysis and interpretation of the data; Guojing Liu was involved in the interpretation of the data; Huaixin Wang revised the manuscript critically for intellectual content. All the authors approve the final version to be published and agree to be accountable for all aspects of the work.
References
- 1.Payne R. M., Johnson M. C., Grant J. W., Strauss A. W. Toward a molecular understanding of congenital heart disease. Circulation. 1995;91(2):494–504. doi: 10.1161/01.CIR.91.2.494. [DOI] [PubMed] [Google Scholar]
- 2.Chang R.-K. R., Gurvitz M., Rodriguez S. Missed diagnosis of critical congenital heart disease. JAMA Pediatrics. 2008;162(10):969–974. doi: 10.1001/archpedi.162.10.969. [DOI] [PubMed] [Google Scholar]
- 3.Abu-Harb M., Wyllie J., Hey E., Richmond S., Wren C. Presentation of obstructive left heart malformations in infancy. Archives of Disease in Childhood - Fetal and Neonatal Edition. 1994;71(3):F179–F183. doi: 10.1136/fn.71.3.F179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bull C. Current and potential impact of fetal diagnosis on prevalence and spectrum of serious congenital heart disease at term in the UK. The Lancet. 1999;354(9186):1242–1247. doi: 10.1016/S0140-6736(99)01167-8. [DOI] [PubMed] [Google Scholar]
- 5.Du Z.-D., Roguin N., Barak M. Clinical and echocardiographic evaluation of neonates with heart murmurs. Acta Paediatrica. 1997;86(7):752–756. doi: 10.1111/j.1651-2227.1997.tb08580.x. [DOI] [PubMed] [Google Scholar]
- 6.Gregory J., Emslie A., Wyllie J., Wren C. Examination for cardiac malformations at six weeks of age. ADC - Fetal and Neonatal Edition. 1999;80(1):F46–F48. doi: 10.1136/fn.80.1.F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varan B., Tokel K., Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Archives of Disease in Childhood. 1999;81(1):49–52. doi: 10.1136/adc.81.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koppel R. I., Druschel C. M., Carter T., et al. Effectiveness of pulse oximetry screening for congenital heart disease in asymptomatic newborns. Pediatrics. 2003;111(3):451–455. doi: 10.1542/peds.111.3.451. [DOI] [PubMed] [Google Scholar]
- 9.Reich J., Miller S., Brogdon B. The use of pulse oximetry to detect congenital heart disease. ACC Current Journal Review. 2003;12(4):p. 92. doi: 10.1016/S1062-1458(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 10.Richmond S., Reay G., Abu Harb M. Routine pulse oximetry in the asymptomatic newborn. ADC - Fetal and Neonatal Edition. 2002;87(2):F83–F88. doi: 10.1136/fn.87.2.F83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de-Wahl Granelli A., Wennergren M., Sandberg K., et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: a Swedish prospective screening study in 39,821 newborns. BMJ (Clinical research ed.) 2009;338:p. a3037. doi: 10.1136/bmj.a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riede F. T., Wörner C., Dähnert I., Möckel A., Kostelka M., Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine-results from a prospective multicenter study. European Journal of Pediatrics. 2010;169(8):975–981. doi: 10.1007/s00431-010-1160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donnell C. P. F., Omar C., Kamlin F., Davis P. G., Carlin J. B., Morley C. J. Clinical assessment of infant colour at delivery. ADC - Fetal and Neonatal Edition. 2007;92(6):F465–F467. doi: 10.1136/adc.2007.120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arlettaz R., Bauschatz A. S., Mönkhoff M., Essers B., Bauersfeld U. The contribution of pulse oximetry to the early detection of congenital heart disease in newborns. European Journal of Pediatrics. 2006;165(2):94–98. doi: 10.1007/s00431-005-0006-y. [DOI] [PubMed] [Google Scholar]
- 15.Bakr A. F., Habib H. S. Combining pulse oximetry and clinical examination in screening for congenital heart disease. Pediatric Cardiology. 2005;26(6):832–835. doi: 10.1007/s00246-005-0981-9. [DOI] [PubMed] [Google Scholar]
- 16.Hoke T. R., Donohue P. K., Bawa P. K., et al. Oxygen saturation as a screening test for critical congenital heart disease: A preliminary study. Pediatric Cardiology. 2002;23(4):403–409. doi: 10.1007/s00246-002-1482-8. [DOI] [PubMed] [Google Scholar]
- 17.Rosati E., Chitano G., Dipaola L., De Felice C., Latini G. Indications and limitations for a neonatal pulse oximetry screening of critical congenital heart disease. Journal of Perinatal Medicine. 2005;33(5):455–457. doi: 10.1515/JPM.2005.080. [DOI] [PubMed] [Google Scholar]
- 18.de Wahl Granelli A., Mellander M., Sunnegårdh J., Sandberg K., Ostman-Smith I. Screening for duct-dependant congenital heart disease with pulse oximetry: a critical evaluation of strategies to maximize sensitivity. Acta paediatrica (Oslo, Norway : 1992) 2005;94(11):1590–1596. doi: 10.1111/j.1651-2227.2005.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Q. M., Ma X. J., Ge X. L., et al. Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: a prospective study. Lancet. 2014;384(9945):747–754. doi: 10.1016/S0140-6736(14)60198-7. [DOI] [PubMed] [Google Scholar]
- 20.Mathur N. B., Gupta A., Kurien S. Pulse oximetry screening to detect cyanotic congenital heart disease in sick neonates in a neonatal intensive care unit. Indian Pediatrics. 2015;52(9):769–772. doi: 10.1007/s13312-015-0714-y. [DOI] [PubMed] [Google Scholar]
- 21.Hu X.-J., Zhao Q.-M., Ma X.-J., et al. Pulse oximetry could significantly enhance the early detection of critical congenital heart disease in neonatal intensive care units. Acta Paediatrica. 2016;105(11):e499–e505. doi: 10.1111/apa.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones A. J., Howarth C., Nicholl R., Mat-Ali E., Knowles R. The impact and efficacy of routine pulse oximetry screening for CHD in a local hospital. Cardiology in the Young. 2016;26(7):1397–1405. doi: 10.1017/S1047951115002784. [DOI] [PubMed] [Google Scholar]
- 23.Özalkaya E., Akdağ A., Şen I., Cömert E., Melek Yaren H. Early screening for critical congenital heart defects in asymptomatic newborns in Bursa province. The Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(7):1105–1107. doi: 10.3109/14767058.2015.1035642. [DOI] [PubMed] [Google Scholar]
- 24.Jawin V., Ang H.-L., Omar A., Thong M.-K. Beyond critical congenital heart disease: Newborn screening using pulse oximetry for neonatal sepsis and respiratory diseases in a Middle-Income Country. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0137580.e0137580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewer A. K., Middleton L. J., Furmston A. T., et al. Pulse oximetry screening for congenital heart defects in newborn infants (PulseOx): A test accuracy study. The Lancet. 2011;378(9793):785–794. doi: 10.1016/S0140-6736(11)60753-8. [DOI] [PubMed] [Google Scholar]
- 26.Taksande A. M., Lakhkar B., Gadekar A. Accuracy of pulse oximetry screening for detecting critical congenital heart disease in the newborns in rural hospital of Central India. Images Paediatr Cardiol. 2013;15(4):5–10. [PMC free article] [PubMed] [Google Scholar]
- 27.van Niekerk A. M., Cullis R. M., Linley L. L., Zühlke L. Feasibility of pulse oximetry pre-discharge screening implementation for detecting critical congenital heart lesions in newborns in a secondary-level maternity hospital in the Western Cape, South Africa: The ‘POPSICLe’ study. South African Medical Journal. 2016;106(8):817–821. doi: 10.7196/SAMJ.2016.v106i8.10071. [DOI] [PubMed] [Google Scholar]
- 28.Meberg A., Brügmann-Pieper S., Due R., Jr., et al. First Day of Life Pulse Oximetry Screening to Detect Congenital Heart Defects. Journal of Pediatrics. 2008;152(6):761–765. doi: 10.1016/j.jpeds.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 29.Mo Z. D., Guo X. L., Wang W. J. Effectiveness of pulse oximetry for the screening of congenital heart disease in newborn infants. Chinese Journal of Neonatology. 2015;30(5):343–346. [Google Scholar]
- 30.Jia A. Q., Wu J. H., Guo A. Y. Analysis of intervals of pulse oximetry in congenital heart disease screening. J Clin Pediatr. 2016;34(5):357–359. [Google Scholar]
- 31.Lu X. Y., Li Y. Comparison between pulse oximetry and traditional method in screening congenital heart disease. Chinese Journal of Clinical Rational Drug Use. 2016;9(10A):152–153. [Google Scholar]
- 32.Ainsworth S. B., Wyllie J. P., Wren C. Prevalence and clinical significance of cardiac murmurs in neonates. ADC - Fetal and Neonatal Edition. 1999;80(1):F43–F45. doi: 10.1136/fn.80.1.F43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wren C., Richmond S., Donaldson L. Presentation of congenital heart disease in infancy: Implications for routine examination. ADC - Fetal and Neonatal Edition. 1999;80(1):F49–F53. doi: 10.1136/fn.80.1.F49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang R.-K. R., Rodriguez S., Klitzner T. S. Screening newborns for congenital heart disease with pulse oximetry: Survey of pediatric cardiologists. Pediatric Cardiology. 2009;30(1):20–25. doi: 10.1007/s00246-008-9270-8. [DOI] [PubMed] [Google Scholar]
- 35.Wren C., Reinhardt Z., Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. ADC - Fetal and Neonatal Edition. 2008;93(1):F33–F35. doi: 10.1136/adc.2007.119032. [DOI] [PubMed] [Google Scholar]
- 36.Mellander M., Sunnegårdh J. Failure to diagnose critical heart malformations in newborns before discharge - An increasing problem? Acta Paediatrica. 2006;95(4):407–413. doi: 10.1080/08035250500541910. [DOI] [PubMed] [Google Scholar]
- 37.Sendelbach D. M., Jackson G. L., Lai S. S., Fixler D. E., Stehel E. K., Engle W. D. Pulse oximetry screening at 4 hours of age to detect critical congenital heart defects. Pediatrics. 2008;122(4):e815–e820. doi: 10.1542/peds.2008-0781. [DOI] [PubMed] [Google Scholar]
- 38.Ewer A., Furmston A., Middleton L., et al. Pulse oximetry as a screening test for congenital heart defects in newborn infants: a test accuracy study with evaluation of acceptability and cost-effectiveness. Health Technology Assessment. 2012;16(2) doi: 10.3310/hta16020. [DOI] [PubMed] [Google Scholar]
- 39.Powell R., Pattison H. M., Bhoyar A., et al. Pulse oximetry screening for congenital heart defects in newborn infants: An evaluation of acceptability to mothers. ADC - Fetal and Neonatal Edition. 2013;98(1):F59–F63. doi: 10.1136/fetalneonatal-2011-301225. [DOI] [PubMed] [Google Scholar]
- 40.Kemper A. R., Mahle W. T., Martin G. R., et al. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011;128(5):e1259–e1267. doi: 10.1542/peds.2011-1317. [DOI] [PubMed] [Google Scholar]
- 41.Thangaratinam S., Brown K., Zamora J., Khan K. S., Ewer A. K. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. The Lancet. 2012;379(9835):2459–2464. doi: 10.1016/S0140-6736(12)60107-X. [DOI] [PubMed] [Google Scholar]