Introduction
Understanding the development of chronic postsurgical pain (CPSP) following major surgery, including breast cancer surgery, has become a major concern,1–4 but there has been relatively little attention to the risk of CPSP following breast reconstruction.5 From one-fourth to one-half of women who undergo post-mastectomy breast reconstruction report persistent pain months and years after surgery.5–15 However, determining the incidence of CPSP due specifically to breast reconstruction may be confounded by methodological problems inherent in the examination of CPSP16–19 and is particularly difficult when immediate reconstructive surgery is chosen given the substantial risk of CPSP associated with mastectomy.20,21 Preliminary evidence suggests that women who receive breast reconstruction, compared with those who undergo mastectomy-alone, do not report higher rates of CPSP.7,9,22
CPSP is defined as ongoing pain that persists for at least 3 months beyond surgery, and should reflect a new onset of persistent pain that is directly attributable to the surgical procedure under study.16,17 In addition, it should be of sufficient severity to cause clinically meaningful impairment in functional ability and quality of life.17,26 Identified methodological problems with prior studies of CPSP following major surgery leave unclear whether the presence of postoperative pain reported by a particular surgical cohort is merely an estimate of the prevalence of pain in the postoperative period or actually reflects a new incidence of surgically-related pain.16, The majority of investigations of CPSP following major surgery, including breast reconstruction, are retrospective and cross-sectional in study design and fail to control for the contribution of preoperative pain when interpreting the etiology of CPSP being reported,18,19,27 and, moreover, rely on potentially inaccurate patient recall of prior pain experience.28,29 In addition, the majority of these studies fail to quantify the clinical significance of postoperative pain and thus are unable to establish whether CPSP is of sufficient clinical concern to compromise a woman’s functional ability.2,17,26 As a result, there is concern that prior investigations of CPSP may overestimate the incidence of surgery-induced and clinically relevant persistent postoperative pain.30
This study will examine the prevalence of and risk factors associated with CPSP for women seeking breast reconstruction following mastectomy. To address identified methodological limitations in previous investigations, this prospective study will examine the report of the presence and severity of persistent pain prior to surgery and at two years postoperatively. Persistent pain following surgery and site-specific (e.g., upper body and chest) physical discomfort will be compared for patients grouped by type and timing of reconstructive surgery. Additional medical factors, surgical procedure characteristics, demographic variables, and standardized measures of both anxiety and depression will also be obtained to assess their contribution to post-reconstruction reports of CPSP.
Materials and Methods
Study Setting and Participants
Patients were recruited as part of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study, a five-year prospective, multicenter cohort study of mastectomy reconstruction patients funded by the National Cancer Institute (NCI 1RO1CA152192). Appropriate Institutional Review Board (IRB) approval was obtained from all participating sites. Women 18 years or older undergoing first-time unilateral or bilateral mastectomy, and immediate or delayed breast reconstruction, were eligible for MROC participation. Figure 1 depicts the consortium enrollment process for MROC including exclusion criteria. Reasons for exclusion included early withdrawal subjects who did not complete preoperative baseline questionnaires (n = 1316), low volume procedure type (n = 213), mixed reconstruction timing (n = 37), reconstructive failure (n = 117), and less than 2-year follow-up (n = 738). Of a total of 4417 women enrolled by July 2016, a remaining sample of 1996 served as the study cohort.
Figure 1.
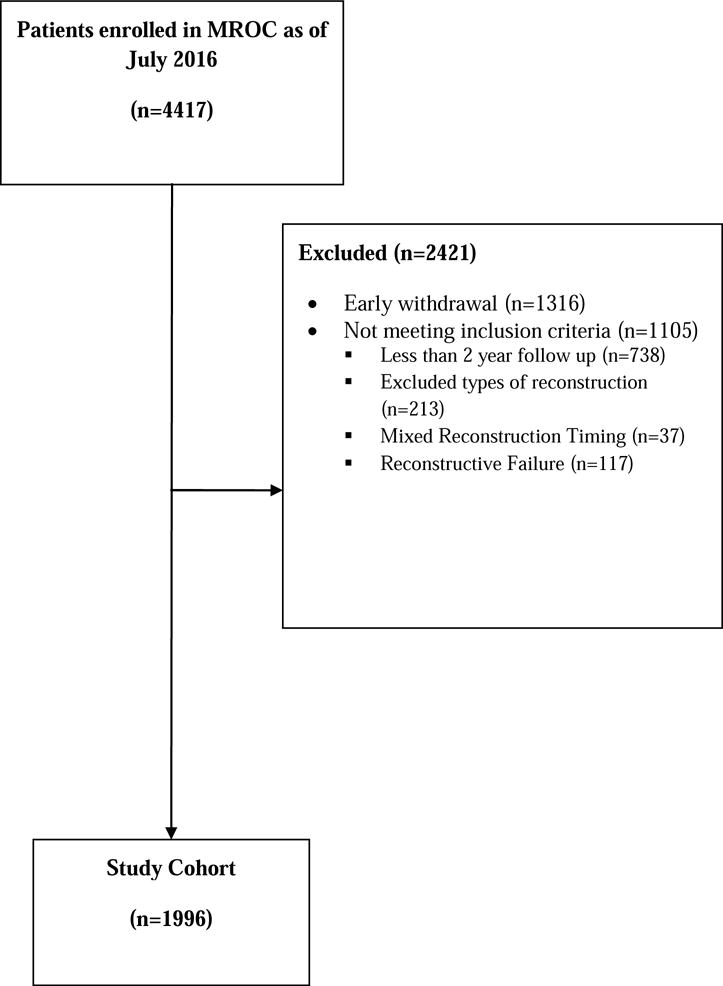
Flow chart for MROC recruitment
Study Design and Data Collection
Relevant clinical data such as medical and surgical factors were collected via medical record reviews by trained research assistants at each site. Self-administered Patient Reported-Outcome questionnaires (PROs) assessing various patient and demographic characteristics, psychological status (depression and anxiety symptom severity), pain experience, and chest and upper body discomfort were collected preoperatively and postoperatively at one week, one-year and two-years after the initiation of reconstruction. Most patients completed questionnaires electronically via the Internet in a password protected patient-portal of the study’s database system (the Velos eResearch System).
Description of Surgical Procedures and Sample Composition
Based on patient preference, reconstructive surgery for this study included one of seven available techniques. Most commonly, patients underwent implant-based reconstructions, either one-staged, direct-to-implant techniques (DTI; n = 93, 4.7%) or two-staged procedures (TE/I; n = 1263, 63.3%) in which implant placement was preceded by a temporary tissue expander. The study cohort also included women receiving latissimus dorsi procedures (LD; n = 64, 3.2%), or four types of abdominally-based tissue flap procedures: pedicle transverse rectus abdominis musculocutaneous flaps (PTRAM; n = 77, 3.9%), free TRAM (FTRAM; n = 87, 4.4%), deep inferior epigastric artery perforator (DIEP; n = 350, 17.5%), and superficial inferior epigastric artery perforator (SIEA; n = 62, 3.1%) flaps.
To determine if CPSP is related to selected medical and surgical factors associated with reconstructive surgery, patients were assessed for their body mass index (BMI), and lymph node management (none, sentinel lymph node biopsy [SLNB] or axillary lymph node dissection [ALND]) at the time of reconstruction. In addition to surgical procedure type (TE/I, DTI, LD, PTRAM, FTRAM, DIEP, SIEA), patients were further categorized by laterality (unilateral vs. bilateral), and timing of reconstruction (immediate vs. delayed). To assess the influence of adjuvant cancer therapies, patients were also stratified for a history and timing of both radiation and chemotherapy (none, before reconstruction, during or after reconstruction).
Measures
Pain Assessment
For the three pain metrics, participants were asked to provide a single global rating of their current level of pain experience per each metric’s standard instructions. The same questionnaire format was repeated for all preoperative and postoperative pain assessments. Overall pain intensity was assessed by the Numerical Pain Rating Scale (NPRS),31 which provides a single measure of overall pain intensity drawn from an ordinal numerical scale reflecting increasing pain severity, ranging from 0 to 10. A broader measure of pain experience was obtained by the McGill Pain Questionnaire-Short Form (MPQ-SF).32 The MPQ-SF contains 15 descriptors of pain experience (11 sensory, 4 affective) and provides separate measures of the sensory and affective components of pain experience. The sensory pain rating (MPQ-Sensory Rating) quantifies the sensory dimensions of pain experience including mechanical, spatial and temporal characteristics (range 0 – 33) while the affective pain rating (MPQ-Affective Rating) provides a measure of the subjective unpleasantness or suffering associated with pain (range 0 – 12). For both the NPRS and MPQ-Sensory Rating and MPQ-Affective Rating, higher scores indicate more severe pain experience.
Chest and Upper Body Discomfort
Symptoms of chest and upper body discomfort associated with reconstructive surgery were assessed by The BREAST-Q.33,34 The BREAST-Q includes a Physical Well-being: Chest and Upper Body Scale utilized in this study, which solicits problems over the prior two weeks with shoulder, neck, back, arm, chest muscle and rib pain, complaints of discomfort such as tightness, pulling, or throbbing, and reports of related activity limitations. Higher scores on the BREAST-Q indicate better physical well-being and less pain.
Psychological Distress
To assess the severity of anxiety and depressive symptoms, each participant completed the Generalized Anxiety Disorders Scale (GAD-7)35 and Patient Health Questionnaire (PHQ-9).36 The GAD-7 and PHQ-9 scales share a common format whereby subjects report the frequency of various symptoms that may have occurred during the previous two weeks and individual items are summed to derive a composite score, with a higher score indicating more severe symptom complaints. The GAD-7 has a range from 0–21, and a cut point score ≥10 demonstrates a sensitivity of 89% and specificity of 82% for a diagnosis of generalized anxiety disorder. The PHQ-9 score can range from 0–27 and a score ≥10 has a sensitivity of 88% and a specificity of 88% for major depression.
Statistical Analysis
Patients at or beyond two-years following the initiation of breast reconstruction surgery were included for the analysis. For each of the four primary outcome measures (NPRS, MPQ-Sensory Rating, MPQ-Affective Rating, BREAST-Q), unadjusted within-patient changes from preoperative to one- and two-year postoperative assessment points were examined to determine whether pain persisted beyond the pain level at the onset of initial reconstruction surgery. Mixed-effects regression modeling was used to assess the relationship between preoperative patient-specific factors and acute postoperative pain (independent variables) and two-year postoperative pain (dependent variable) for each pain and bodily discomfort outcome measure, while accounting for between-center variability using random intercepts for centers (hospitals). For MPQ-Sensory Rating, MPQ-Affective Rating, and BREAST-Q Physical Well-being, a linear mixed-effects regression model was employed to model each pain outcome. For the NPRS, we dichotomized the pain score into moderate to severe pain (NPRS > 3) versus not, and modeled moderate-to-severe using a generalized linear mixed-effects model with logit link. For all models, the independent variables included age, BMI, preoperative anxiety score (GAD-7), depression score (PHQ-9), acute postoperative pain, and a number of surgical procedure factors including laterality (unilateral or bilateral), timing (immediate or delayed), procedure type (TE/Implant, DTI, LD, PTRAM, FTRAM, DIEP or SIEA), lymph node management (SLNB, ALND or none), adjuvant radiation therapy (before reconstruction, during/after reconstruction vs. none) and adjuvant chemotherapy (during/after reconstruction, both before and during/after reconstruction or none). The primary regression model included the preoperative values of the dependent variable to adjust for baseline differences in pain level. All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC), and statistical significance was set at 0.05.
RESULTS
Study Participants
Table 1 lists the clinical and demographic characteristics of the analytic cohort (N = 1996). The mean age was 49.5 (SD = 10.2) years old. Mean BMI was 26.5 (SD = 5.6). The majority of patients received immediate (92.7%) and bilateral (53.8%) reconstruction. For lymph node management, 47.6% of women underwent SLNB and 25.9% ALND. The majority of women did not receive radiation therapy (70.3%) or chemotherapy before, during or after reconstruction (52.7%). In general, participants were reporting mild levels of depressive (PHQ-9; mean 5.1 (SD = 4.8) and anxiety (GAD-7; mean 5.2 SD = 5.0) symptoms.
Table 1.
Clinical and Demographic Characteristics of Patients (n=1996)
| Variable | |
|---|---|
| Age, Mean (SD) | 49.5 (10.2) |
| BMI, Mean (SD) | 26.5 (5.6) |
| Extent of disease, No. (%) | |
| Local | 1285 (64.5) |
| Regional | 527 (26.5) |
| Metastatic | 13 (0.7) |
| None | 167 (8.4) |
| Laterality, No.(%) | |
| Unilateral | 922 (46.2) |
| Bilateral | 1074 (53.8) |
| Lymph Node Status, No.(%) | |
| None | 529 (26.5) |
| SLNB | 950 (47.6) |
| ALND | 517 (25.9) |
| Timing, No.(%) | |
| Immediate | 1851 (92.7) |
| Delayed | 145 (7.3) |
| Procedure type, No.(%) | |
| TE | 1263 (63.3) |
| DTI | 93 (4.7) |
| FTRAM | 87 (4.4) |
| PTRAM | 77 (3.9) |
| DIEP | 350 (17.5) |
| LD | 64 (3.2) |
| SIEA | 62 (3.1) |
| Radiation, No.(%) | |
| Before reconstruction | 246 (12.3) |
| During/after reconstruction | 347 (17.4) |
| None | 1403 (70.3) |
| Chemotherapy, No.(%) | |
| Before reconstruction | 353 (17.7) |
| During/after reconstruction | 549 (27.5) |
| Both before and during/after reconstruction | 42 (2.1) |
| None | 1052 (52.7) |
| Preoperative depression (PHQ-9), Mean (SD) | 5.1 (4.8) |
| Preoperative anxiety (GAD-7), Mean (SD) | 5.2 (5.0) |
Prevalence and Intensity of Mean Preoperative and Postoperative Pain and Body Discomfort
Table 2 lists the means, standard deviations and severity category (for the NPRS) for the measures of pain and upper chest/body discomfort preoperatively and at one week, one-year and two-year post-reconstruction for the study sample. There was a significant increase in pain intensity as measured by the NPRS comparing preoperative pain and pain severity at 2–years (P = 0.046) but the absolute change in mean pain rating and narrow standard deviation suggest that this was not a clinically meaningful change. Similarly, there were more complaints of bodily discomfort on the BREAST-Q (P < 0.001) at two-years, but the statistical parameters again indicate little clinically meaningful differences from preoperative status. Conversely, there was a significant decrease from baseline in pain report on the MPQ-Affective Pain Rating (P < 0.001) at two-years postoperatively. Examination of the pain ratings from preoperative to postoperative follow-up intervals revealed little change in average pain for the MPQ-Sensory Rating (3.2, 3.3, 3.1) and NPRS (1.1, 1.3, 1.2), with somewhat decreasing pain ratings for the MPQ-Affective Rating (1.6, 0.9, 0.8) In general, these pain ratings seen at baseline, one-year and two-years are associated with a mild level of clinical pain intensity.37 Categorizing pain intensity ratings on the NPRS demonstrated no meaningful variation across the time intervals. Comparing pain intensity preoperatively and at the postoperative follow-ups, about one-half of women reported no pain, approximately 35–40% reported mild pain (NPRS 1–3), 8–9% indicated moderately severe pain (NPRS 4–6) and only 2–3% reported severe pain (NPRS 7–10). There was no discernible change in the frequency of moderately severe and severe pain when comparing preoperative and two-year pain ratings (McNemar’s test; P = 0.083). For the BREAST-Q: Physical Well-being: Chest/Upper Body Scale, similar to the other pain measures, there was substantial worsening of bodily discomfort at the acute postoperative assessment at one week, but recovery to near preoperative levels of comfort at both one-year and two-year follow-up (78.8, 57.9, 75.9, 76.6).
Table 2.
Preoperative and Postoperative Pain Scores
| Pain measure | Pre-op
|
One week post-op
|
One year post-op
|
Two years post-op
|
||||
|---|---|---|---|---|---|---|---|---|
| n1 | Mean (SD) or % | n1 | Mean (SD) or % | n1 | Mean (SD) or % | n1 | Mean (SD) or % | |
| MPQ sensory | 1773 | 3.2 (4.5) | 1584 | 10.5 (6.3) | 1248 | 3.3 (4.3) | 1121 | 3.1 (4.2) |
| MPQ affective | 1750 | 1.6 (2.1) | 1529 | 2.9 (2.3) | 1242 | 0.9 (1.6) | 1112 | 0.8 (1.5) |
| NPRS | 1800 | 1.1 (1.7) | 1566 | 3.9 (2.1) | 1337 | 1.3 (1.8) | 1166 | 1.2 (1.7) |
| NPRS pain severity category | ||||||||
| None (0) | 980 | 54.4 | 44 | 2.8 | 635 | 47.5 | 593 | 50.9 |
| Mild (1–3) | 634 | 35.2 | 659 | 42.1 | 543 | 40.6 | 445 | 38.2 |
| Moderate (4–6) | 149 | 8.3 | 674 | 43.0 | 121 | 9.1 | 104 | 8.9 |
| Severe (7–10) | 37 | 2.1 | 189 | 12.1 | 38 | 2.8 | 24 | 2.1 |
| BREAST-Q physical well-being chest and upper body | 1989 | 78.8 (14.8) | 1727 | 57.9 (12.7) | 1425 | 75.9 (14.7) | 1261 | 76.6 (14.6) |
Denotes number of cases with complete PROs.
Factors Associated with Chronic Postsurgical Pain and Chest/Upper Body Discomfort at 2-Year Follow-up
The regression analyses for predicting CPSP (MPQ-Sensory and MPQ-Affective Ratings) and ratings of bodily discomfort at two-years are listed in Table 3. The regression analysis for predicting CPSP based on moderate-to-severe pain intensity on the NPRS is listed in Table 4.
Table 3.
Factors Associated with Postoperative Two Years MPQ-sensory, MPQ-affective, and BREAST-Q Physical Well-being based on Linear Mixed-Effects Regression Model with Sites as Random Intercepts and Weighted by Non-response
| Variable | MPQ-sensory
|
MPQ-affective
|
BREAST-Q Physical well-being chest and upper body
|
|||
|---|---|---|---|---|---|---|
| Beta | p | Beta | p | Beta | p | |
| Age | 0.02 | 0.105 | 0.00 | 0.742 | −0.06 | 0.182 |
| BMI | 0.06 | 0.020 | 0.01 | 0.115 | −0.31 | <.001 |
| Bilateral Reconstruction | 0.60 | 0.037 | 0.09 | 0.363 | 0.38 | 0.659 |
| Delayed Reconstruction | −0.58 | 0.365 | −0.22 | 0.343 | 2.86 | 0.129 |
| Procedure type | ||||||
| TE | -Reference- | -Reference- | -Reference- | |||
| DTI | 0.00 | 0.997 | 0.02 | 0.919 | 0.92 | 0.629 |
| FTRAM | 2.48 | 0.000 | 0.84 | 0.000 | −0.62 | 0.774 |
| PTRAM | 1.19 | 0.080 | 0.04 | 0.877 | −3.93 | 0.068 |
| DIEP | 1.11 | 0.004 | 0.33 | 0.013 | 1.44 | 0.289 |
| LD | 0.42 | 0.606 | −0.13 | 0.630 | 0.22 | 0.930 |
| SIEA | 2.37 | 0.003 | 1.24 | <.0001 | 2.83 | 0.273 |
| Lymph Node Status | ||||||
| None | -Reference- | -Reference- | -Reference- | |||
| SLNB | −0.20 | 0.550 | −0.08 | 0.519 | 0.50 | 0.651 |
| ALND | −0.60 | 0.156 | 0.04 | 0.788 | 0.59 | 0.659 |
| Radiation | ||||||
| None | -Reference- | -Reference- | -Reference- | |||
| Before reconstruction | −0.32 | 0.528 | −0.12 | 0.507 | 0.37 | 0.812 |
| During or after reconstruction | 1.19 | 0.004 | 0.08 | 0.595 | −6.07 | <.001 |
| Chemotherapy | ||||||
| None | -Reference- | -Reference- | -Reference- | |||
| Before reconstruction | 0.30 | 0.466 | 0.12 | 0.436 | −2.58 | 0.044 |
| During or after reconstruction | 0.11 | 0.741 | 0.31 | 0.011 | 0.46 | 0.655 |
| Both before and during/after reconstruction | 0.91 | 0.343 | 1.10 | 0.001 | −3.05 | 0.265 |
| Preoperative pain score1 | 0.21 | <.001 | 0.14 | <.001 | 0.33 | <.001 |
| Acute postoperative pain score1 | 0.11 | <.001 | 0.05 | 0.034 | 0.24 | <.001 |
| Preoperative depression score, PHQ-9 | 0.08 | 0.081 | 0.03 | 0.055 | −0.26 | 0.055 |
| Preoperative anxiety score, GAD-7 | −0.01 | 0.740 | −0.01 | 0.671 | 0.10 | 0.428 |
Refers to the pain score of the corresponding outcome.
Table 4.
Factors Associated with Postoperative Two Years Moderate to Severe NPRS based on Generalized Linear Mixed-Effects Regression Model with Sites as Random Intercepts and Weighted by Non-response
| Variable | OR | p |
|---|---|---|
| Age | 1.03 | 0.007 |
| BMI | 1.05 | 0.006 |
| Bilateral Reconstruction | 1.02 | 0.908 |
| Delayed Reconstruction | 0.60 | 0.269 |
| Procedure type | ||
| TE | -Reference- | |
| DTI | 0.54 | 0.292 |
| FTRAM | 1.73 | 0.205 |
| PTRAM | 1.64 | 0.266 |
| DIEP | 1.22 | 0.454 |
| LD | 0.94 | 0.915 |
| SIEA | 1.43 | 0.445 |
| Lymph Node Status | ||
| None | -Reference- | |
| SLNB | 0.81 | 0.414 |
| ALND | 0.76 | 0.402 |
| Radiation | ||
| None | -Reference- | |
| Before reconstruction | 1.12 | 0.743 |
| During or after reconstruction | 1.54 | 0.129 |
| Chemotherapy | ||
| None | -Reference- | |
| Before reconstruction | 1.04 | 0.897 |
| During or after reconstruction | 1.37 | 0.196 |
| Both before and during/after reconstruction | 3.54 | 0.015 |
| Preoperative pain score1 | 1.41 | <.001 |
| Acute post-operative pain score1 | 1.21 | <.001 |
| Preoperative depression score, PHQ-9 | 1.08 | 0.012 |
| Preoperative anxiety score, GAD-7 | 1.02 | 0.546 |
Refers to the pain score of the corresponding outcome.
Demographic and Medical/Surgical Factors and CPSP
Older age was associated with more severe pain on the NPRS (P = 0.007). Higher BMI was associated with CPSP for both the MPQ-Sensory Rating (P = 0.020), NPRS (P = 0.006) and body discomfort scores (P < 0.001). Bilateral reconstruction was associated with CPSP on the MPQ-Sensory Rating (P = 0.037). Radiation therapy during or after reconstruction was associated with more severe pain on the MPQ-Sensory Rating (P = 0.001) and physical discomfort on the BREAST-Q Physical Well-being scale (P < 0.001). Having chemotherapy prior to reconstruction was associated with more upper body and chest discomfort (P = 0.044) while chemotherapy during or after breast reconstruction (P = 0.011), and chemotherapy both before and during/after reconstruction (P = 0.001), was associated with more severe pain on the MPQ-Affective Rating. Chemotherapy both before, and during/after reconstruction was also related to higher NPRS scores (P = 0.015). With regard to type of reconstructive surgery, women undergoing FTRAM (P < 0.001), DIEP (P = 0.011), and SIEA (P = 0.043) each reported increased pain compared to women receiving TE/I reconstruction on the MP-Sensory Rating. Similarly, on the MPQ-Affective Rating, FTRAM (P = 0.001), DIEP (P = 0.002), and SIEA (P = 0.001) procedures were associated with higher pain severity than TE/I reconstruction. Lymph node status and timing of reconstruction were not associated with any of the outcome measures.
Pain and Mood Factors
Higher preoperative pain and acute postoperative pain were both strongly associated with more severe pain and bodily discomfort symptoms at two-year assessment for all outcome measures (MPQ-Sensory and Affective Ratings, NPRS, BREAST-Q, all P < .001 except acute postoperative pain and MPQ-Affective Rating, P = 0.034). Preoperative depression scores correlated with more severe pain for the NPRS (P = 0.012) and marginally with both the MPQ-Affective Rating (P = 0.055) and Chest/Upper body discomfort scale (P = 0.055). Anxiety ratings did not demonstrate an association with measures of CPSP or chest/upper body discomfort.
Discussion
Among the major findings of this study, we replicated in a breast reconstruction cohort known associations of preoperative pain,38,39 acute postoperative pain,38–40 and preoperative depression level22,38 with severity of long-term postoperative pain following breast cancer surgery. More noteworthy, however, careful examination of our data suggests that CPSP following breast reconstruction may be of less clinical concern as a direct consequence of breast reconstruction than suggested by previous investigations of major surgery, including mastectomy and breast reconstruction. A methodological limitation of our study concerns our measures of pain, which represented a single global measure and thus could not ascertain a specific relationship between surgery site and postoperative pain. However, nearly half of our subjects reported some level of persistent pain prior to surgery, comparable to investigations of CPSP associated with breast cancer surgery.38 This substantial rate of preoperative pain suggests that, at least for some patients, the report of postoperative pain may simply reflect a presurgical pain condition that persists unperturbed through the course of reconstructive surgery and postoperative care. This may explain the consistent evidence for preoperative pain as a predictor of CPSP.19,38
For the entire sample, while women reported increased pain intensity comparing preoperative scores and at two-years, the pain metrics indicated this increase to be of minimal clinical significance. Of clinical relevance, examination of pain ratings for preoperative, one-year and two-year assessments revealed little significant change in absolute mean pain ratings. Also encouraging is the observation that the postoperative prevalence of pain in our sample is relatively modest, with only 9 % and 2 % reporting moderate or severe pain, respectively, and these prevalence data were not significantly different from preoperative pain severity scores. As over 90 % of our sample underwent mastectomy at the time of reconstructive surgery, these findings also challenge the results of previous studies reporting a substantial incidence of new onset persistent pain following mastectomy, described in a range from 20–60% across studies.20–22
We found that a number of medical and surgical variables held association with CPSP in our prospective analysis. Both radiation therapy and chemotherapy, each established as cancer treatments with potential risk for the development of persistent pain following breast cancer surgery,44–46 were selectively associated with CPSP measures for our sample. Of some surprise, we found evidence for a positive association between our pain measures and BMI. To our knowledge this is the first empirical evidence demonstrating an association between BMI and post-reconstruction chronic pain and is line with previous reports describing greater clinical morbidity associated with higher BMI following reconstruction surgery.48–51 Among surgical procedure variables, we found that bilateral reconstruction held a relationship with reports of pain at follow-up. This is likely due to the more extensive nature of bilateral mastectomy and reconstructive surgeries for the majority of our sample, and replicates prior evidence for the contribution of more pervasive surgery and CPSP.33,57,58
Comparing reconstruction procedure types, women undergoing FTRAM, DIEP and SIEA reconstruction all reported more severe pain than the TE/I group. Comparative studies on the risk of CPSP following TE/I and AFR reconstructive surgeries have produced mixed findings demonstrating greater postoperative pain with TE/I compared to breast reconstruction without implants,14 equivocal evidence for increased pain with TE/I reconstruction,15 more pain following AFR surgery compared to TE/I reconstruction,12 or no differences in the severity of persistent postoperative pain between the two procedure types.7,10,11 One explanation for this lack of agreement among previous studies may be that relative differences in postoperative pain among procedure types may evolve over time. This has been the observation in our own study population. An earlier analysis of post-reconstruction pain from our center observed that TE/I reconstruction was associated with higher levels of severe acute (1 week) postoperative pain43 and subacute (3 month) chest/upper body dysfunction and discomfort15 when compared to AFR surgeries. However, in the current two-year analysis, AFR patients appear to fare somewhat worse compared to those undergoing TE/I procedures. These data collectively suggest an interaction of time by procedure type in determining levels of persistent pain following reconstructive surgery.
Our study has a number of methodological limitations. Given its clinical nature, patients by necessity selected their choice of reconstruction procedure type and thus there may be some degree of selection bias in considering our findings. Secondly, surgical patients with low volume procedure type, mixed reconstruction timing, and reconstructive failure were excluded from the analysis which may reduce the generalizability of the results, although it is unclear whether any of these groups would be at greater or lesser risk for reconstruction-related CPSP. Moreover, the substantial reduction in the sample size at follow-up due to the absence of completed PROs may have spuriously influenced our with-in subject statistical comparisons. Fourth, as most of our patients (93%) received immediate reconstruction at the time of mastectomy, we could not determine which (if either) of the combined surgical procedures may have actually resulted in a new onset of persistent postoperative pain. In addition, our measures of pain represented a single global measure, and thus we could not ascertain a specific relationship between surgery site and postoperative pain that would further implicate a causal link between reconstructive surgery and obtained measures of CPSP. Finally, the 11 surgical centers that participated in this study undoubtedly shared differences in surgical techniques and acute postoperative pain management, which likely contributed to variability in the level of postoperative pain experienced by patients.
Conclusion
Our prospective study of breast reconstruction outcome found limited evidence for increased rate and severity of CPSP as suggested by previous investigations. Future delineation of CPSP associated with reconstructive surgery will require greater methodological rigor to better determine its potential risk and etiology, and thereby allow for the proper counsel of patients seeking breast reconstructive surgery.
Highlights.
Nearly half of women undergoing breast reconstruction report pain prior to surgery
There is no meaningful change in pain prevalence following reconstructive surgery
Preoperative and acute postoperative pain predict chronic pain after reconstruction
Greater study design rigor necessary to determine risk of post-reconstruction pain
Acknowledgments
This study was supported by a grant from the National Institutes of Health/National Cancer Institute NIH Grant #1R01CA152192 (E.G.W., A.L.P.) and, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748 (A.L.P.). The sponsoring agency had no contribution to the design, data collection, analysis or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 2.Lavand’homme P. Transition from acute to chronic pain after surgery. Pain. 2017;158:S50–S54. doi: 10.1097/j.pain.0000000000000809. [DOI] [PubMed] [Google Scholar]
- 3.Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12:725–746. doi: 10.1016/j.jpain.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Vadivelu N, Schreck M, Lopez J, Kodumudi G, Narayan D. Pain after mastectomy and breast reconstruction. Am Surg. 2008;74:285–296. [PubMed] [Google Scholar]
- 5.Tsoi B, Ziolkowski NI, Thoma A, Campbell K, O’Reilly D, Goeree BA. Systematic review on the patient-reported outcomes of tissue-expander/implant vs autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients. J Am Coll Surg. 2014;218:1038–1048. doi: 10.1016/j.jamcollsurg.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 2003;80:39–48. doi: 10.1023/A:1024435101619. [DOI] [PubMed] [Google Scholar]
- 7.De Oliveira GS, Jr, Bialek JM, Nicosia L, McCarthy RJ, Chang R, Fitzgerald P, Kim JY. Lack of association between breast reconstructive surgery and the development of chronic pain after mastectomy: a propensity matched retrospective cohort analysis. The Breast. 2014;23:329–333. doi: 10.1016/j.breast.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Hickey OT, Nugent NF, Burke SM, Hafeez P, Mudrakouski AL, Shorten GD. Persistent pain after mastectomy with reconstruction. J Clin Anesth. 2011;23:482–488. doi: 10.1016/j.jclinane.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Klit A, Mejdahl MK, Gärtner R, Elberg JJ, Kroman N, Anderson KG. Breast reconstruction with an expander prosthesis following mastectomy does not cause additional persistent pain: a nationwide cross-sectional study. J Plast Reconstr Aesthet Surg. 2013;66:1652–1658. doi: 10.1016/j.bjps.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Mullan MH, Wilkins EG, Goldfarb S, Lowery JC, Smith DM, Wickman M, Sandelin K. Prospective analysis of psychosocial outcomes after breast reconstruction: Cross-cultural comparisons of 1-year postoperative results. J Plast Reconstr Aesthet Surg. 2007;60:503–508. doi: 10.1016/j.bjps.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Nelson JA, Fischer JP, Pasick C, Nelson P, Chen AJ, Fosnot J, Selber JC, Wu LC, Serletti J. Chronic pain following abdominal free flap breast reconstruction: a prospective pilot analysis. Ann Plast Surg. 2013;71:278–282. doi: 10.1097/SAP.0b013e31828637ec. [DOI] [PubMed] [Google Scholar]
- 12.Roth RS, Lowery JC, Davis J, Wilkins EG. Persistent pain following postmastectomy breast reconstruction: long-term effects of type and timing of surgery. Ann Plast Surg. 2007;58:371–376. doi: 10.1097/01.sap.0000239810.38137.84. [DOI] [PubMed] [Google Scholar]
- 13.Roth RS, Lowery JC, Wilkins EG. Preoperative affective distress and somatic complaints predict persistent pain after postmastectomy breast reconstruction. Eur J Plast Surg. 2007;29:277–233. [Google Scholar]
- 14.Wallace MS, Wallace AM, Lee J, Dobke MK. Pain with breast surgery: a survey of 282 women. Pain. 1996;66:195–205. doi: 10.1016/0304-3959(96)03064-3. [DOI] [PubMed] [Google Scholar]
- 15.Weichman KE, Hamill JB, Kim HM, Chen X, Wilkins EG, Pusic AL. Understanding the recovery phase of breast reconstruction: patient-reported outcomes correlated to the type and timing of reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:1370–1378. doi: 10.1016/j.bjps.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth. 2008;101:77–86. doi: 10.1093/bja/aen099. [DOI] [PubMed] [Google Scholar]
- 17.Werner MU, Kongsgaard UE. Defining persistent post-surgical pain: is an update required? Br J Anaesth. 2014;113:1–4. doi: 10.1093/bja/aeu012. [DOI] [PubMed] [Google Scholar]
- 18.Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Persistent post-surgical pain in a general population: prevalence and predictors in the Tromsø Study. Pain. 2012;153:1390–1396. doi: 10.1016/j.pain.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Kalso E. Persistent post-surgery pain: research agenda for mechanisms, prevention, and treatment. Br J Anaesth. 2013;111:9–12. doi: 10.1093/bja/aet211. [DOI] [PubMed] [Google Scholar]
- 20.Stevens PE, Dibble SL, Miaskowski C. Prevalence, characteristics, and impact of postmastectomy pain syndrome: an investigation of women’s experiences. Pain. 1995;61:61–68. doi: 10.1016/0304-3959(94)00162-8. [DOI] [PubMed] [Google Scholar]
- 21.Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99:604–610. doi: 10.1038/sj.bjc.6604534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreiber KL, Martel MO, Shnol H, Shaffer JR, Greco C, Viray N, et al. Persistent pain in postmastectomy patients: comparison of psychophysical, medical, surgical, and psychosocial characteristics between patients with and without pain. Pain. 2013;154:660–668. doi: 10.1016/j.pain.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisotzky E, Hanrahan N, Lione T, Maltser S. Deconstructing postmastectomy syndrome: implications for physiatric management. Phys Med Rehabil Clin N Am. 2017;28:153–169. doi: 10.1016/j.pmr.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Ducic I, Seiboth LA, Iorio ML. Chronic postoperative breast pain: danger zones for nerve injuries. Plast Reconstr Surg. 2011;127:41–46. doi: 10.1097/PRS.0b013e3181f9587f. [DOI] [PubMed] [Google Scholar]
- 25.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104:1–13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- 26.VanDenKerkhof EG, Peters ML, Bruce J. Chronic pain after surgery: time for standardization? a framework to establish core risk factor and outcome domains for epidemiological studies. Clin J Pain. 2013;29:2–8. doi: 10.1097/AJP.0b013e31824730c2. [DOI] [PubMed] [Google Scholar]
- 27.Gewandter JS, Dworkin RH, Turk DC, Farrar JT, Fillingim RB, Gilron I, et al. Research design considerations for chronic pain prevention clinical trials: IMMPACT recommendations. Pain. 2015;156:1184–1197. doi: 10.1097/j.pain.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasmuth T, Estlanderb AM, Kalso E. Effect of present pain and mood on the memory of past postoperative pain in women treated surgically for breast cancer. Pain. 1996;68:343–347. doi: 10.1016/s0304-3959(96)03219-8. [DOI] [PubMed] [Google Scholar]
- 29.Nikolajsen L, Sorensen HC, Jensen TS, Kehlet H. Chronic pain after Caesarean section. Acta Anaesthesiol Scand. 2004;48:111–116. doi: 10.1111/j.1399-6576.2004.00271.x. [DOI] [PubMed] [Google Scholar]
- 30.Kehlet H, Rathmell JP. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 2010;112:514–515. doi: 10.1097/ALN.0b013e3181cf423d. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 32.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 33.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: The BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. doi: 10.1097/PRS.0b013e3181aee807. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serlin RC, Mendoza TR, Nakamura Y. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 37.Miaskowski C, Cooper B, Paul SM, West C, Langford D, Levine JD, et al. Identification of patient subgroups and risk factors for persistent breast pain following breast cancer surgery. J Pain. 2012;13:1172–1187. doi: 10.1016/j.jpain.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413–2422. doi: 10.1097/j.pain.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 39.Bruce J, Thornton AJ, Powell R, Marfizo S, Powell R, Johnston M, et al. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: A population-based cohort study. Pain. 2014;155:232–243. doi: 10.1016/j.pain.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 40.Peters ML, Sommer M, de Rijke JM, Kessels F, Heineman E, Patijn J, et al. Somatic and psychologic predictors of long-term unfavorable outcome after surgical intervention. Ann Surg. 2007;245:487–494. doi: 10.1097/01.sla.0000245495.79781.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poleshuck EL, Katz J, Andrus CH, Hogan LA, Jung BF, Kulick DI, et al. Risk factors for chronic pain following breast cancer surgery: a prospective study. J Pain. 2006;7:626–634. doi: 10.1016/j.jpain.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni AR, Pusic AL, Hamill JB, Kim HM, Qi J, Wilkins EG, et al. Factors associated with acute postoperative pain following breast reconstruction. JPRAS Open. 2017;11:1–13. doi: 10.1016/j.jpra.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giglio P, Gilbert MR. Neurologic complications of cancer treatment. Curr Oncol Rep. 2010;12:50–59. doi: 10.1007/s11912-009-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung BF, Herrmann D, Griggs J, Oaklander AL, Dworkin RH. Neuropathic pain associated with nonsurgical treatment of breast cancer. Pain. 2005;118:10–14. doi: 10.1016/j.pain.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Pereira S, Fontes F, Sonin T, Dias T, Fragoso M, Castro-Lopes JM, et al. Neurological complications of breast cancer: A prospective cohort study. The Breast. 2015;24:582–587. doi: 10.1016/j.breast.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Stubblefield MD, Levine A, Custodio CM, Fitzpatrick T. The role of botulinum toxin type A in the radiation fibrosis syndrome: a preliminary report. Arch Phys Med Rehabil. 2008;89:417–421. doi: 10.1016/j.apmr.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 47.Huo J, Smith BD, Giodano SH, Reece GP, Shih Y-CT. Post-mastectomy breast reconstruction and its subsequent complications: a comparison between obese and non-obese women with breast cancer. Breast Cancer Res Treat. 2016;157:373–383. doi: 10.1007/s10549-016-3832-x. [DOI] [PubMed] [Google Scholar]
- 48.Selber JC, Kurichi JE, Vega SJ, Sonnad SS, Serletti JM. Risk factors and complications in free TRAM flap breast reconstruction. Ann Plast Surg. 2006;56:492–497. doi: 10.1097/01.sap.0000210180.72721.4a. [DOI] [PubMed] [Google Scholar]
- 49.Boquiren VM, Hack TF, Roanne L, Thomas RL, Tower A, Kwan WB, et al. A longitudinal analysis of chronic arm morbidity following breast cancer surgery. Breast Cancer Res Treat. 2016;157:413–425. doi: 10.1007/s10549-016-3834-8. [DOI] [PubMed] [Google Scholar]
- 50.Dean LT, DeMichele A, LeBlanc M, Stephens-Shields A, Li SQ, Colameco C, et al. Black breast cancer survivors experience greater upper extremity disability. Breast Cancer Res Treat. 2015;154:117–125. doi: 10.1007/s10549-015-3580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


