Abstract
Purpose
To determine the impact on overall survival with different salvage therapies, including no treatment, reirradiation, systemic therapy, or radiation and systemic therapy, in participants of a phase 3 clinical trial evaluating dose-dense versus standard-dose temozolomide for patients with newly diagnosed glioblastoma.
Methods and Materials
This analysis of patients from Trial RTOG 0525 investigated the effect of reirradiation or systemic treatment after tumor progression. Survival from first progression was compared between patients receiving no therapy, systemic therapy alone, radiation alone, and both modalities. The Cox proportional hazards model was used to compare the mortality hazard, controlling for potential confounders.
Results
The analysis included 637 patients who progressed and had information on their management, excluding those who died less than half a month after progression. A total of 267 patients (42%) received neither reirradiation nor systemic treatment at progression, 24 (4%) received radiation alone, 282 (44%) received systemic treatment only, and 64 (10%) received both radiation and systemic therapy. Patients who received no treatment had a median survival of 4.8 months, lower than with radiation treatment alone (8.2 months), systemic therapy alone (10.6 months), and both radiation and systemic therapy (12.2 months). In survival models controlling for potential confounders, those who received radiation alone had modestly better survival (hazard ratio HR 0.74, 95% confidence interval [CI] 0.43–1.28), whereas those who underwent systemic therapy either without (HR 0.42, 95% CI 0.34, 0.53) or with radiation therapy (HR 0.44, 95% CI 0.30, 0.63) had better survival. There was no significant survival difference between patients who received radiation only and those who received systemic therapy (either with radiation or alone).
Conclusions
Patients who received no salvage treatment had poorer survival than those who received radiation, chemotherapy, or the combination. However, patient selection for no treatment likely reflects poorer expected prognosis. There was no significant survival difference among those receiving radiation therapy, systemic therapy, or both. Ongoing clinical trials will help define the role of reirradiation after glioblastoma progression.
Introduction
Optimal management for recurrent glioblastoma (GBM) has not been established. A plethora of monotherapy and combination therapies have been evaluated. Such approaches include surgery, reirradiation, systematic therapy either with chemotherapy and/or targeted therapeutics or antiangiogenic agents, tumor treatment fields, or some combination of these, as well as supportive care (1–6). A variety of chemotherapies have been evaluated for recurrent GBM, with modest results. Recently bevacizumab, an antivascular endothelial growth factor monoclonal antibody, was evaluated for recurrent GBM. Phase 2 studies demonstrated favorable 6-month progression-free survival and objective responses with bevacizumab for recurrent GBM, which led to its approval by the US Food and Drug Administration in 2009 for use in recurrent GBM (7–10). Currently bevacizumab is one of the most commonly used treatment options for patients with recurrent GBM in the United States. On the other hand, for patients with limited volume recurrence, reirradiation seems to provide similar overall survival (OS) in comparison with those treated with bevacizumab (11–13). Despite some evidence of improvement in progression-free survival, no significant increase in OS has been demonstrated with any particular approach (1, 14). Further investigations are needed to define the optimal choice of salvage therapy, and in particular the role of reirradiation and systemic treatment in patients with recurrent GBM.
Trial RTOG 0525 was a phase 3 clinical trial evaluating dose-dense versus standard-dose temozolomide for patients with newly diagnosed GBM (15). Patients were enrolled between January 2006 and June 2008, and primary study findings were published in 2013, where more details of the study design and results can be found (15). All patients received 60 Gy partial-brain irradiation in 2-Gy daily fractions. After progression, patients participating in this trial received variable salvage therapies (reported as nonprotocol therapy). The information on the type of nonprotocol therapy is available for analysis. The purpose of this study was to determine the impact on OS with different salvage therapies, including no treatment, reirradiation, systemic therapy, or radiation and systemic therapy, in those Trial RTOG 0525 participants. Information from this analysis may help generate new hypotheses for future clinical trials.
Methods and Materials
A total of 833 patients were enrolled and randomized in Trial RTOG 0525. We analyzed postprogression prognosis for patients with information regarding their nonprotocol therapy and excluded patients who died less than half a month after progression (637 analyzable patients), because there would not have been adequate time to consider/evaluate/offer a therapeutic intervention to these patients. The 637 patients were divided into 4 mutually exclusive groups according to the type of nonprotocol therapy they received: 267 patients (42%) received neither radiation treatment nor systemic treatment (chemotherapy and/or targeted therapy, such as bevacizumab); 24 patients (4%) received some form of radiation treatment (fractionated radiation therapy, radiosurgery, or brachytherapy) alone; 64 patients (10%) received some form of radiation and systemic therapy; and 282 patients (44%) received systemic treatment only. Information on the specific agent or regimen delivered was provided for only 196 (54%) of the 346 patients who received nonprotocol systemic therapy. Bevacizumab, which is indicated for recurrent GBM, was used for almost all of these patients (194; 99%); other systemic therapies that were frequently used included irinotecan (89 patients; 45%) and carboplatin (14 patients; 7%). Details on radiation therapy after recurrence were not provided in sufficient detail to meaningfully summarize.
The survival time distributions for patients in the 4 postprogression treatment groups were calculated from the date of progression using the Kaplan-Meier estimator and compared via the log-rank test. To investigate the potential influence of host prognostic factors on survival times, frequency distributions of numerous patient and disease factors were compared among the treatment groups. The χ2 test was used to compare frequencies for the original randomized treatment arm, MGMT methylation status, gender, recursive partitioning analysis (RPA) class, Karnofsky performance status (KPS), neurologic function, prior surgery, surgery upon progression, reason radiation therapy was terminated, and reason adjuvant therapy was terminated. The F test was used to compare whether distributions of time to progression, age at diagnosis, and number of adjuvant cycles differed across the groups.
Effects of the different postprogression treatments taking into account variables potentially related to both treatment choice and prognosis (confounders of treatment effects) were estimated from Cox proportional hazard models that controlled for various combinations of possible confounders. From these models, estimates and 95% confidence intervals were obtained for the relative hazards of death among the 3 treatment types compared with no postprogression treatment and to each other.
Results
Of the 833 patients who originally enrolled in Trial RTOG 0525, 664 (80%) were recorded to have progression. Salvage management details were available for 660 of 664 of these patients. At the time of this analysis, 563 of 660 participants with disease progression (85%) had died. Of these, 23 died in the first half-month after progression. To focus on patients whose outcomes were most likely affected by salvage therapy, this analysis is restricted to those 637 patients who survived at least half a month after progression (Fig. 1). Among these patients, 267 (42%) received neither radiation nor systemic treatment; 88 (14%) received some form of radiation treatment (fractionated radiation therapy, radiosurgery, or brachytherapy) with (64) or without (24) systemic therapy; and 282 (44%) received systemic treatment only. Owing to the small number of patients who received radiation treatment alone, comparisons of this group with others are of limited value, and in some analyses they were analyzed with patients who received both systemic therapy and radiation (Fig. 1).
Fig. 1.
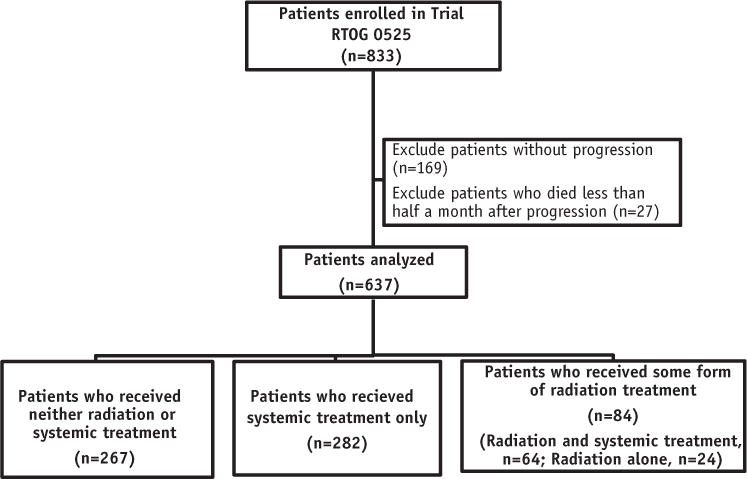
Patients included in the study.
Table 1 shows patient baseline (Trial RTOG 0525 initial study entry) and specific postentry characteristics by postprogression therapy groups. There was some evidence that patients may have been selected for their postprogression treatment on the basis of baseline characteristics, with those receiving no additional therapy or radiation only tending to be older and of poorer RPA class. With respect to postentry characteristics, the number of adjuvant chemotherapy cycles received was greater among patients who received radiation (either with or without systemic therapy after progression). All other patient characteristics were distributed similarly among the postprogression treatment groups.
Table 1.
Characteristics of patients by postprogression treatment group
| Characteristic | Neither radiation nor systemic therapy | Radiation therapy only | Systemic therapy only | Both radiation therapy and systemic therapy | P for difference |
|---|---|---|---|---|---|
| Original treatment group | .2968 | ||||
| Arm 1 | 125 (47) | 10 (42) | 151 (54) | 35 (55) | |
| Arm 2 | 142 (53) | 14 (58) | 131 (46) | 29 (45) | |
| MGMT methylation | .8961 | ||||
| Methylated | 65 (24) | 3 (13) | 74 (26) | 15 (23) | |
| Unmethylated | 179 (67) | 18 (75) | 185 (66) | 43 (67) | |
| Indeterminate | 14 (5) | 2 (8) | 18 (6) | 4 (6) | |
| Invalid | 9 (3) | 1 (4) | 5 (2) | 2 (3) | |
| Gender | .5747 | ||||
| Male | 150 (56) | 13 (54) | 167 (59) | 32 (50) | |
| Female | 117 (44) | 11 (46) | 115 (41) | 32 (50) | |
| RPA class | .0005 | ||||
| 3 | 35 (13) | 5 (21) | 65 (23) | 23 (36) | |
| 4 | 174 (65) | 13 (54) | 175 (62) | 35 (55) | |
| 5 | 58 (22) | 6 (25) | 42 (15) | 6 (9) | |
| KPS at randomization | .0199 | ||||
| ≤70 | 47 (18) | 7 (29) | 34 (12) | 5 (8) | |
| >70 | 220 (82) | 17 (71) | 248 (88) | 59 (92) | |
| Neurologic function at randomization | .1989 | ||||
| 0–no symptoms | 78 (29) | 6 (25) | 108 (38) | 27 (42) | |
| 1 | 129 (48) | 10 (42) | 127 (45) | 28 (44) | |
| 2 | 38 (14) | 4 (17) | 28 (10) | 5 (8) | |
| 3/4–severe symptoms | 22 (8) | 4 (17) | 19 (7) | 4 (6) | |
| Surgery at trial entry | .3615 | ||||
| Biopsy | 12 (4) | 0 (0) | 9 (3) | 1 (2) | |
| Partial resection | 114 (43) | 13 (54) | 109 (39) | 32 (50) | |
| Total resection | 141 (53) | 11 (46) | 164 (58) | 31 (48) | |
| Surgery at progression | <.0001 | ||||
| None | 225 (84) | 10 (42) | 176 (62) | 27 (42) | |
| Stereotactic biopsy | 3 (1) | 1 (4) | 4 (2) | 4 (6) | |
| Partial resection | 22 (8) | 4 (17) | 33 (12) | 13 (20) | |
| Total resection | 14 (5) | 4 (4) | 58 (21) | 16 (25) | |
| Other | 3 (1) | 5 (21) | 11 (4) | 4 (6) | |
| Reason radiation therapy terminated | .1522 | ||||
| Treatment completed | 261 (99) | 23 (100) | 279 (100) | 62 (97) | |
| Other | 2 (1) | 0 (0) | 1 (0) | 2 (3) | |
| Reason adjuvant therapy terminated | .4719 | ||||
| Treatment completed | 28 (14) | 5 (26) | 35 (16) | 8 (17) | |
| Disease progression | 126 (63) | 9 (47) | 143 (66) | 33 (69) | |
| Other | 46 (23) | 5 (26) | 38 (18) | 7 (15) | |
| Time to progression (wk), mean (SD) | 7.5 (7.7) | 9.7 (7.5) | 7.9 (7.7) | 7.5 (6.2) | .5706 |
| Age at diagnosis (y), mean (SD) | 58.0 (11.0) | 55.6 (13.1) | 54.6 (10.7) | 50.3 (11.0) | <.0001 |
| Cycles of adjuvant therapy, mean (SD) | 5.2 (3.6) | 6.7 (3.5) | 5.2 (3.5) | 6.5 (3.6) | .0185 |
Abbreviations: KPS = Karnofsky performance status; RPA = recursive partitioning analysis.
Values are number (percentage) unless otherwise noted. Information on KPS was missing in 446 participants; information on termination of radiation therapy was not available in 7 participants; information on termination of adjuvant therapy was not available for 154 participants; information on the cycles of adjuvant therapy was not available for 82 participants.
Survival time distributions were found to differ between patients according to the postprogression management categories (Fig. 2, Table 2). Table 2 provides estimates of the median survival and average deaths per week by treatment group. The patients who received neither radiation treatment nor systemic treatment had the poorest outcome (with median survival of 4.8 months), significantly lower than those who received radiation (for all patients either with or without systemic therapy, 11.3 months, P<.05) or systemic therapy alone (10.5 months, P<.05). The small group of patients who underwent radiation only had somewhat better survival (8.2 months) than those receiving no additional treatment.
Fig. 2.
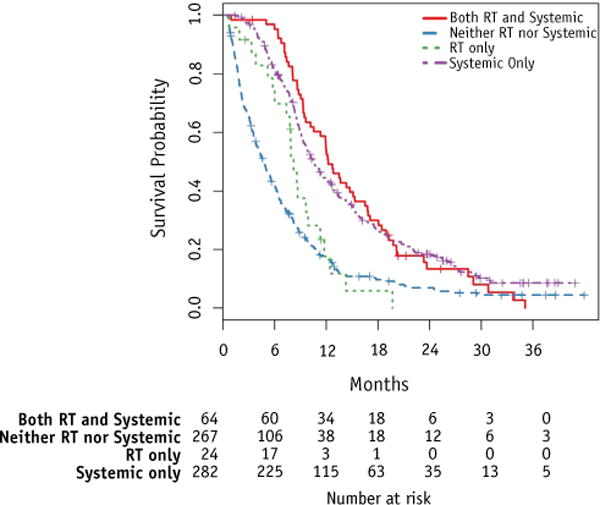
Kaplan-Meier survival estimates by treatment group. Abbreviation: RT = radiation therapy.
Table 2.
Average monthly death rate and Kaplan-Meier estimates of median survival by treatment group
| Treatment group | No. of patients | No. of deaths | Average deaths per mo | Estimated median (range) survival (mo) |
|---|---|---|---|---|
| Neither radiation nor systemic therapy | 267 | 237 | 0.133 | 4.80 (3.81–5.58) |
| Radiation therapy only | 24 | 21 | 0.108 | 8.21 (6.01–9.89) |
| Systemic therapy only | 282 | 229 | 0.064 | 10.55 (9.53–12.06) |
| Both radiation and systemic therapy | 64 | 58 | 0.064 | 12.22 (10.18–15.12) |
Figure 2 graphically illustrates survival by group. Although patients in the 4 management groups were statistically different from each other (P<.0001), pairwise comparisons found that survival time distributions were specifically different between patients who received no treatment and patients who received radiation therapy (with or without systemic therapy, P<.0001), and also between patients who received no treatment and those who received systemic therapy only (P<.0001). However, patients who received systemic therapy only and patients who received some radiation therapy did not significantly differ in their survival (P=.38).
To assess postprogression treatment taking into account patient factors (Table 1) that may influence survival, hazard regression models were used. Table 3 shows hazard ratios for models (1) with no covariates, equivalent to comparisons in Figure 2; (2) controlling for time to progression, which although statistically similar among the groups could be responsible for residual confounding; (3) controlling for the 3 variables (age at diagnosis, RPA, and cycles of adjuvant treatment) that showed statistical differences in distribution between the groups; (4) controlling for those 3 variables, surgery at progression, and time to progression; and (5) controlling for all available covariates. Table 3 displays the hazard ratios for death for the 3 groups relative to no further therapy. Relative to no postprogression treatment, patients who received radiation therapy had a decreased risk of death, with an approximate 20% reduction not accounting for potential confounders and a 26% reduction in the fully adjusted model; this reduction in risk, however, did not reach statistical significance. From Table 3 it can also be seen that (1) death hazard reductions for systemic therapy alone and systemic therapy with radiation were nearly the same and associated with significantly better survival than no treatment; and (2) radiation therapy alone, although demonstrating a smaller effect, does not differ significantly from the other 2 intervention types (because confidence intervals on hazard ratios overlap). The observed benefit associated with receipt of systemic therapy was large, with a >50% reduction in risk of death relative to no treatment in the adjusted models (P<.0001; Table 3).
Table 3.
Hazard ratios for death by treatment group (unadjusted and multivariable models)
| Treatment group | Model 1 (n=637) |
Model 2 (n=637) |
Model 3 (n=555) |
Model 4 (n=555) |
Model 5 (n=478) |
|---|---|---|---|---|---|
| Neither radiation nor systemic therapy | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Radiation therapy only | 0.78 (0.50–1.22) | 0.79 (0.51–1.24) | 0.74 (0.45–1.23) | 0.84 (0.50–1.39) | 0.74 (0.43–1.28) |
| Systemic therapy only | 0.43 (0.36–0.52) | 0.43 (0.36–0.52) | 0.42 (0.34–0.51) | 0.44 (0.36–0.54) | 0.42 (0.34–0.53) |
| Both radiation and systemic therapy | 0.42 (0.32–0.56) | 0.42 (0.32–0.56) | 0.45 (0.32–0.62) | 0.49 (0.35–0.69) | 0.44 (0.30–0.63) |
Abbreviations as in Table 1.
Values in parentheses are 95% confidence intervals. Model 1: No covariates. Model 2: Controlling for time to progression. Model 3: Controlling for age at diagnosis, RPA, and cycles of adjuvant treatment. Model 4: Controlling for age at diagnosis, RPA, cycles of adjuvant treatment, surgery at progression, and time to progression. Model 5: Controlling for all measure confounders (assigned treatment group, MGMT methylation status, gender, RPA, KPS at randomization, neurologic function at randomization, prior surgery, surgery at progression, reason for radiation therapy termination, reason for adjuvant termination, time to progression, age at diagnosis, and cycles of adjuvant therapy).
Discussion
Optimal treatment for patients with recurrent GBM remains a challenge. In the present study we focused on the efficacy of reirradiation and/or systemic treatment as salvage options. Our analysis demonstrated trends toward better survival for patients who received any salvage treatment, either radiation, systemic therapy, or the combination, as compared with those who did not. The median survival of patients who did not receive treatment was only 4.8 months. Patients who received systemic therapy, either with or without radiation, had a >50% reduction in mortality risk relative to those receiving no further treatment. With respect to whether reirradiation might yield equal benefit to systemic therapy, there were too few patients in the radiation-alone group to make any reliable determination, although survival seemed modestly better.
Systemic therapy has been widely used as second-line therapy for recurrent GBM. The majority of patients with recurrent GBM are offered systemic therapy at the time of progression. Among patients who received systemic treatment only in the present study, although the type of systemic therapy was reported in only approximately 50% of cases, among those for whom this information was available, bevacizumab was nearly always used. The median OS after progression of 10.6 months for these patients is similar to other reported bevacizumab trials in recurrent disease (7–9, 16).
During the last decade there has been increased interest in reirradiation as a salvage measure for patients with recurrent GBM. Reirradiation is frequently administered in the form of stereotactic radiosurgery or as hypofractionated radiation therapy (12, 17–22). Fogh et al (12) reported on a cohort of patients receiving a median dose of 35 Gy delivered in 10 fractions. These results were promising, with median survival time from reirradiation of 11.2 months. Moreover, it seems that the combination of bevacizumab with stereotactic radiosurgery or fractionated stereotactic radiation therapy (FSRT) may provide superior outcomes when compared with either treatment alone. A prospective trial showed median OS of 12.5 months for patients treated with FSRT and bevacizumab (21). In the present study we have a heterogeneous group of patients. Patients included received a variety of radiation regimens (stereotactic radiosurgery, FSRT, or brachytherapy). Different dose and fractionation schedules were used, and these details were not available for analysis. Of those patients who received reirradiation, approximately 25% received radiation as the only salvage therapy, and the remainder received some type of systemic treatment in addition to radiation. The overall median survival for the entire reirradiation cohort is 11.3 months. Although in the present study the OS of patients who received radiation treatment is similar to that of those who received systemic therapy alone, it is hard to draw definitive conclusions about the value of reirradiation, owing to selection bias. The role of reirradiation with bevacizumab for recurrent GBM is being evaluated in the randomized Trial RTOG 0525, which recently completed accrual.
Limitations of this analysis must be acknowledged, with the key issue being selection bias with respect to treatment received after progression. Specifically, patients who did not receive radiation or systemic treatment most likely represent those with less favorable conditions as perceived by the treating physician, whereas those selected for additional therapy, and in particular combination therapy, may have had better prognostic factors at the time of retreatment decision making. As a result, we may observe improved survival mainly due to patient factors (eg, KPS, neuro-cognition, age, quality of life). Although identifying the optimum salvage therapy may improve survival, it is equally important to identify those patients who would benefit from salvage therapy versus those for whom supportive care alone is most appropriate. We did examine potential confounding factors (Table 1) and incorporated these factors into models (Table 3), but residual confounding by unmeasured factors may persist. In particular, important factors that may mediate survival or act as surrogates for expected prognosis, such as surgery type at progression, were included, but control of confounding may still not be adequate. Methods such as propensity score analysis were considered, but the covariates available did not prove strong predictors of treatment class on which to stratify or match, and thus results of such analyses would resemble those of the adjusted estimates presented here. More informative factors, such as KPS, neurocognitive measures, and other patient factors at the time of progression, were not reliably collected. Nonetheless, the adjusted results are provocative and suggest benefits of salvage radiation and systemic therapy in the recurrent setting. The number of patients who only received radiation treatment as salvage treatment is very low, which makes the accurate assessment of the benefit of radiation alone difficult.
In conclusion, salvage treatments for patients with GBM after progression were highly variable. Patients who received no salvage treatment had significantly lower survival than those who received radiation treatment, chemotherapy, or a combination of both. However, these results may reflect poorer functional status or other prognostic determinants among the untreated patients. There was no significant survival difference between patients who received systemic therapy only and patients who received radiation therapy. Our data suggest a benefit to salvage treatment in the setting of progressive/recurrent GBM. Despite these provocative findings, owing to limitations of this retrospective analysis, the role of reirradiation in the management of recurrent GBM patients, particularly in the setting of bevacizumab, is yet to be defined and points to the need for prospective trials.
Summary.
Optimal treatment for glioblastoma patients who progress after standard Treatment chemoradiotherapy remains unknown. We analyzed data from Trial RTOG 0525 to investigate the effect of reirradiation or systemic treatment on survival after progression. Salvage treatment options were found to be highly variable. Patients who received no salvage treatment had significantly shorter survival than those treated after progression. There was no significant survival difference among patients receiving systemic therapy (alone or with radiation) or radiation alone.
Acknowledgments
This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology Statistics and Data Management Center [SDMC]), and UG1CA189867 (NCI community Oncology Research Program [NCORP]) from the National Cancer Institute, National Cancer Institute of National Institutes of Health under award number R25CA057699 and Merck & Co.
M.P.M. reports consulting fees from BMS, Celldex, Roche, Novartis, Cavion, Varian, Agenus, Insys, and Remedy, consulting fees and grant funding from Novocure, research support from Cellectar, fees from Pharmacyclics (Board of Directors), and Monteris (Data Safety Monitor Board [DSMB]), outside the submitted work. D.T.B. reports honoraria for medical advisory board consultation from Vascular Biogenics, Ltd, outside the submitted work. L.S. reports travel expenses from Varian Medical Systems, outside the submitted work.
References
- 1.Kirkpatrick JP, Sampson JH. Recurrent malignant gliomas. Semin Radiat Oncol. 2014;24:289–298. doi: 10.1016/j.semradonc.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyle T, Baptista J, Winfield J, et al. Mechlorethamine, vincristine, and procarbazine chemotherapy for recurrent high-grade glioma in adults: A phase II study. J Clin Oncol. 1990;8:2014–2018. doi: 10.1200/JCO.1990.8.12.2014. [DOI] [PubMed] [Google Scholar]
- 3.Brandes AA, Scelzi E, Zampieri P, et al. Phase II trial with BCNU plus alpha-interferon in patients with recurrent high-grade gliomas. Am J Clin Oncol. 1997;20:364–367. doi: 10.1097/00000421-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Postma TJ, Heimans JJ, Luykx SA, et al. A phase II study of paclitaxel in chemonaïve patients with recurrent high-grade glioma. Ann Oncol. 2000;11:409–413. doi: 10.1023/a:1008376123066. [DOI] [PubMed] [Google Scholar]
- 5.Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–1388. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]
- 6.Prados MD, Yung WK, Fine HA, et al. Phase 2 study of BCNU and temozolomide for recurrent glioblastoma multiforme: North American Brain Tumor Consortium study. Neuro Oncol. 2004;6:33–37. doi: 10.1215/S1152851703000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 8.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 10.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereo-tactic reirradiation in recurrent gliomas: Long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–8869. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 12.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieder C, Astner ST, Mehta MP, et al. Improvement, clinical course, and quality of life after palliative radiotherapy for recurrent glioblastoma. Am J Clin Oncol. 2008;31:300–305. doi: 10.1097/COC.0b013e31815e3fdc. [DOI] [PubMed] [Google Scholar]
- 14.Weller M, Cloughesy T, Perry JR, et al. Standards of care for treatment of recurrent glioblastoma–are we there yet? Neuro Oncol. 2013;15:4–27. doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desjardins A, Reardon DA, Coan A, et al. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118:1302–1312. doi: 10.1002/cncr.26381. [DOI] [PubMed] [Google Scholar]
- 17.Combs SE, Widmer V, Thilmann C, et al. Stereotactic radiosurgery (SRS): Treatment option for recurrent glioblastoma multiforme (GBM) Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JD, Siglin J, Yamoah K, et al. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: More is not always better. J Neurooncol. 2015;124:215–221. doi: 10.1007/s11060-015-1825-y. [DOI] [PubMed] [Google Scholar]
- 19.Cabrera AR, Cuneo KC, Vredenburgh JJ, et al. Stereotactic radiosurgery and bevacizumab for recurrent glioblastoma multiforme. J Natl Compr Canc Netw. 2012;10:695–699. doi: 10.6004/jnccn.2012.0072. [DOI] [PubMed] [Google Scholar]
- 20.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82:2018–2024. doi: 10.1016/j.ijrobp.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park KJ, Kano H, Iyer A, et al. Salvage gamma knife stereotactic radiosurgery followed by bevacizumab for recurrent glioblastoma multiforme: A case-control study. J Neurooncol. 2012;107:323–333. doi: 10.1007/s11060-011-0744-9. [DOI] [PubMed] [Google Scholar]


