Abstract
Objective
Although schizophrenia is not a prototypic impulse-control disorder, patients report more impulsive behaviors, have higher rates of substance use, and show dysfunction in brain circuits that underlie impulsivity. We investigate impulsivity in unaffected biological relatives of schizophrenia patients to further understand the relationships between schizophrenia risk and impulse control during adolescence.
Method
Group differences in impulsivity (UPPS-P Impulsive Behavior Scale and delay discounting) were tested in 210 adolescents contrasting 39 first- and 53 second-degree biological relatives of schizophrenia patients, and 118 subjects with no schizophrenia family history (NSFH).
Results
Compared to NSFH adolescents and to second-degree relatives, first-degree relatives of schizophrenia patients had increased impulsivity-related behaviors (higher UPPS-P Perseverance, Positive Urgency and Premeditation subscale scores) and greater preference for immediate rewards (smaller AUC and larger discounting constant). Second-degree relatives did not differ significantly from NSFH adolescents on self-report impulsive behaviors or on measures of impulsive decision-making. These group differences remained even after careful consideration of potential confounding factors.
Conclusion
Impulsivity is associated with schizophrenia risk, and its severity increases with greater familial relatedness to the schizophrenia proband. Additional studies are needed to understand the role impulsivity may play in mediating schizophrenia susceptibility during adolescence.
Keywords: endophenotype, family studies, impulse control disorders, neurodevelopment, substance use disorders
Introduction
Impulsivity is a multi-dimensional construct with its core feature being impairment in the inhibition of impulses (Hofmann et al., 2009). There is no consensus on a single gold standard for the assessment of impulsivity. Various self-report questionnaires and neurocognitive-behavioral tasks are frequently used to measure impulsivity and related constructs of poor decision-making, risk taking and response inhibition. Self-report impulsivity is often weakly correlated with behavior-based measures (Caswell et al., 2015). This is consistent with the multi-dimensional nature of impulsivity, and suggests that individual dimensions may have differing yet overlapping neural substrates.
Whiteside and Lynam proposed that four distinct personality traits form discrete psychological processes that lead to impulsive behaviors (Whiteside and Lynam, 2001): 1) Urgency (or tendency to act impulsively as a result of intense emotions), 2) (lack of) Premeditation (or tendency to act without reflecting on the consequences of the act), 3) (lack of) Perseverance (inability to remain focused on a task that may be boring or difficult), and 4) Sensation Seeking (tendency to seek out new and exciting experiences). This conceptual framework, derived from personality theories and factor analysis of 8 impulsivity questionnaires, forms the basis of the UPPS Impulsive Behavior Scale, a comprehensive and widely used self-report rating scale for assessing impulsivity.
From among the different neurocognitive-behavioral tasks that have been used to measure impulsivity, the delay discounting task (de Wit et al., 2007; Mitchell, 1999) (DDT) emphasizes aspects of impulsivity that relate to the failure to consider future consequences during decision making (Ainslie, 1975). In the DDT, test subjects are presented with a series of hypothetical scenarios from which they choose between a smaller immediate reward or a larger delayed reward (e.g. Would you rather have $2 now or $10 in 1 year?). Delay discounting is the phenomenon where the current value of a future reward decreases with increased time delay to receiving the reward. More impulsive individuals tend to have a steeper rate of delay discounting, and are more likely to prefer immediate gratification over a larger delayed reward.
Impulsivity manifests in a wide range of complex behavioral phenotypes, including substance use, personality disorders, bulimia, suicidality and aggressive behaviors (Evenden, 1999). Schizophrenia (SZP) is not conventionally considered an impulse-control disorder. However, there is an accumulating literature indicating that SZP patients are more impulsive than healthy volunteers as assessed by self-report questionnaires or through experimental behavioral paradigms (Gut-Fayand et al., 2001; Hoptman et al., 2002; Ouzir, 2013). On the Barratt Impulsivity Scale (BIS), SZP patients have significantly higher ratings of impulsivity than healthy controls (Ahn et al., 2011; Amr et al., 2016; Enticott et al., 2008; Kaladjian et al., 2011; Krakowski and Czobor; Nanda et al., 2016; Reddy et al., 2014; Zhornitsky et al., 2012). Between patients with SZP, most studies (Dervaux et al., 2001; Dervaux et al., 2010; Gut-Fayand et al., 2001; Ouzir, 2013) but not all (Dervaux et al., 2004) have found that patients with concomitant drug use, or history of violence or suicidality scored higher on impulsivity than SZP patients without these behaviors. Dysfunctions in cognitive control neural circuitry postulated to mediate impulsivity have been frequently implicated in SZP patients (Aron et al., 2007; Hoptman et al., 2014).
Unaffected biological relatives of SZP patients have similar albeit less severe neurocognitive, neuroanatomic, electrophysiological and behavioral abnormalities seen in SZP patients (Boos et al., 2007; Ho, 2007; Ho and Magnotta, 2010; Keshavan et al., 2002; Lawrie et al., 1999; Thermenos et al., 2013). Such intermediate phenotypes likely result from the genetic and environmental risk factors that biological relatives shared with SZP probands (Cannon, 2005; Gottesman and Gould, 2003; Moldin, 1994). Studies using quantitative traits or endophenotypes have aided in identifying SZP susceptibility genetic loci (Freedman et al., 1997; Liu et al., 2002), and may further serve as biomarkers of SZP susceptibility useful for the early identification of SZP. To our knowledge, there has only been one family study examining impulsivity in twins of SZP patients (Fortgang et al., 2016). Impulsivity was found to be moderately heritable with 38-60% of its variance accounted by genetic factors. Twins of SZP probands were also more impulsive than healthy controls on some impulsivity measures (BIS Attentional and Nonplanning subscales) but not others (BIS Motor Impulsivity, Zuckerman Sensation-Seeking Scale (SSS) ratings or Stop Signal Task (SST) performance). Given the limited knowledge regarding impulsivity in biological relatives of SZP probands, we sought to expand on the work of Fortgang and colleagues. Therefore, we assessed both self-report impulsive behaviors as well as DDT in unaffected biological relatives of SZP patients so as to comprehensively assess facets of impulsivity that have not been previously studied.
Additionally, we contrasted first- and second-degree biological relatives to further explore how familial relatedness to the SZP proband may influence differences in impulsivity.
Materials and Methods
Sample
In this study, we evaluated 210 adolescents comprising of 92 biological relatives (39 first- and 53 second-degree relatives) of SZP patients and 118 comparison subjects with no SZP family history (NSFH). Participants and their parents/legal guardians gave written informed consent approved by the University of Iowa Human Subjects Institutional Review Board.
Study participants (aged 12-17 years) were recruited from the community via advertisements through mass emails, social media, and posting flyers at local mental healthcare providers and mental health advocacy groups. Following initial telephone screening to rule out serious medical/neurological disorders, study participants were assessed in-person to further exclude adolescents with intellectual disability (WRAT3 Reading Score (Wilikinson, 1993) <30). All subjects and their parent/legal guardian were also administered the CAPA (Child and Adolescent Psychiatric Assessment, Child Interview (Angold et al., 2008)), a semi-structured interview instrument, so as to determine lifetime history of psychiatric or substance use disorders in the adolescent study participant. Presence (or absence) of SZP family history was verified using Family History-Research Diagnostic Criteria (FH-RDC) interview administered to the study participant's parent or legal guardian. The FH-RDC has well-established reliability and validity for the assessment of SZP family history (Andreasen et al., 1977).
Sociodemographic characteristics of the sample are summarized in Table 1. The sample comprised predominantly of right-handed (82.9%) Caucasian (90.5%) adolescents (Mean age=14.8 years (SD=1.91)) with approximately equal gender distribution (51.4% males). First-degree relatives had higher rates of Major Depressive Disorder (MDD) (p=0.06) and Oppositional Defiant Disorder (ODD) (p=0.03). Otherwise, gender, mean age, ethnicity, handedness and prevalence of psychiatric disorders and drug/alcohol use did not differ significantly between first-degree relatives, second-degree relatives and NSFH (Table 1; p≥0.10). None of the study participants met DSM criteria for schizophrenia-spectrum disorders, or alcohol or drug use disorders. Thirty-four subjects (16.2%) reported current or past tobacco and/or alcohol use. There were no current or past use of other substances. Tobacco use and alcohol use did not differ significantly across the 3 comparison groups (p≥0.51).
Table 1. Sociodemographic characteristics of sample: first-degree relatives of schizophrenia patients (1°), second-degree relatives of schizophrenia patients (2°) and adolescent controls with no schizophrenia family history (NSFH).
| 1° | 2° | NSFH | Statistica (p) | |
|---|---|---|---|---|
| N | 39 | 53 | 118 | |
| Sex (Males, N (%)) | 23 (58.97) | 30 (56.60) | 55 (46.61) | 2.55 (.28) |
| Mean age (years (SD)) | 14.8 (1.49) | 14.5 (1.71) | 14.9 (2.11) | 0.97 (.38) |
| Ethnicity (White, N (%)) | 34 (87.18) | 48 (90.57) | 108 (91.53) | (.10) |
| Handednessb (L/M/R (% R)) | 1/8/30 (76.92) | 6/5/42 (79.25) | 5/11/102 (86.44) | (.12) |
| Any psychiatric disorders (N (%)) | 12 (30.77) | 9 (16.98) | 19 (16.10) | 4.28 (.12) |
| MDD (N (%)) | 9 (23.08) | 5 (9.43) | 11 (9.32) | 5.70 (.06) |
| ADHD (N (%)) | 5 (12.82) | 6 (11.32) | 10 (8.47) | 0.75 (.69) |
| ODD (N (%)) | 2 (5.13) | 0 (0) | 0 (0) | (.03) |
| Current/Past drug use (N (%)) | 6 (15.38) | 11 (20.75) | 17 (14.41) | 1.11 (.57) |
| Tobacco use (N (%)) | 2 (5.13) | 3 (5.66) | 4 (3.39) | (.72) |
| Alcohol use (N (%)) | 4 (10.26) | 10 (18.87) | 17 (14.41) | 1.35 (.51) |
sex and psychiatric diagnoses (&chi2); age (F); ethnicity and handedness (Fisher's Exact)
Annett Scale of Hand Preference (L: left; M: mixed; R: right)
MDD: Major Depressive Disorder; ADHD: Attention Deficit Hyperactivity Disorder; ODD: Oppositional Defiant Disorder
UPPS-P Impulsive Behavior Scale
We used a revised version of the UPPS that assesses the 4 original personality pathways to impulsive behaviors (Negative Urgency, Premeditation, Perseverance, and Sensation Seeking)(Whiteside and Lynam, 2001) as well as a fifth Positive Urgency subscale (Cyders et al., 2007; Lynam et al., 2006) (UPPS-P). The UPPS-P consisted of 59 statements. Subjects were instructed to indicate how much he/she agreed with each statement on a scale of 1-4 (agree strongly, agree some, disagree some or disagree strongly respectively). Since agreement with some statements while disagreement with others suggested greater impulsivity and vice versa, all responses were re-scored such that higher ratings indicated more impulsive behaviors. Each subject's subscale score is the sum of ratings of its component statements: Negative Urgency (12 statements), Premeditation (11 statements), Perseverance (10 statements), Sensation Seeking (12 statements), and Positive Urgency (14 statements). There were no missing responses from any of the subjects.
Delay Discounting Task (DDT)
The computerized DDT we used was based on the experimental paradigm previously developed by Richards and colleagues (Richards et al., 1999). Details of the DDT have been previously described (Ho et al., 2016) (see Supplemental Materials). In brief, the DDT comprised of a series of two hypothetical monetary rewards from which study participants chose either a smaller ($0.50 to $10) immediately available reward or a $10 standard reward in the future (2, 30, 180 or 365 days later). Based on the study participant's responses, a random adjustment algorithm then derived an indifference point for each of the 5 different delays (i.e. 0, 2, 30, 180 and 365 days). Next, two DDT measures were generated from these indifference points: area under the curve (AUC; calculated by summing the resulting trapezoids and expressed as a proportion AUC (Myerson et al., 2001)) and discounting constant (k; computed using nonlinear regression least-squares fit, and natural log-transformed (ln k) to normalize its skewed distribution). Smaller AUC or larger k indicate preference for immediate rewards or greater impulsivity (Myerson et al., 2001).
Thirteen participants (2 first-degree relatives, 1 second-degree relative and 10 NSFH adolescents) did not show consistent discounting behavior (see Supplemental Materials). These subjects were excluded from statistical analyses leaving 197 subjects with valid discounting data. Subjects with versus without valid delay discounting did not differ significantly on age, gender distribution, ethnicity, handedness, or presence of psychiatric disorders and drug use (p≥0.22).
Statistical Analysis
Differences in UPPS-P subscale scores and delay discounting (proportion AUC and ln k) between the 3 comparison groups were assessed using general linear models (GLM). In each GLM, the dependent measure was the UPPS-P score or delay discounting measure while the independent measure was grouping (first-degree relatives, second-degree relatives or NSFH). A significant main effect of group would suggest that the dependent measure (UPPS-P score or delay discounting measure) differed significantly between groups. Age, gender and presence of any psychiatric disorders/substance use were entered as covariates. Even though age and gender did not differ significantly between the 3 groups, both variables have been previously shown to contribute to variance in impulsivity (Steinberg et al., 2008; Weafer and de Wit, 2014). Presence of psychiatric disorders/substance use was included as a covariate so as to statistically control for any effects MDD, ADHD, ODD and/or current or past drug use may have on impulsivity. When the ANCOVA yielded significant main effects of group membership (p≤.05, 2-sided test), post hoc pair-wise group comparisons used the Tukey's method (Q statistic) to adjust for multiple comparisons.
Results
On the UPPS-P, there were statistically significant main effects of group on Premeditation, Perseverance and Positive Urgency subscale scores (Table 2; F≥3.14, p≤.04). Post hoc pair-wise comparisons found that first-degree relatives of SZP patients were more impulsive; having significantly higher Perseverance and Positive Urgency subscale scores when compared to NSFH and to second-degree relatives (Table 2 and Figure 1; Q≥2.45, p≤.04). First-degree relatives also had significantly higher Premeditation subscale scores than second-degree relatives (Q=2.47, p=.04), but Premeditation ratings did not differ significantly between first-degree relatives and NSFH (p=.13). There were no significant group differences between second-degree relatives and NSFH on Premeditation, Perseverance and Positive Urgency subscale scores (Q≤0.97, p≥.60). Main effects of group on Negative Urgency or on Sensation Seeking subscale scores were also not statistically significant (Table 2; F≤1.73, p≥.18).
Table 2. Measures of impulsivity (UPPS-P Impulsive Behavior Scale and delay discounting) and pair-wise independent group comparisons (Tukey's test Q (p)) between first-degree relatives of schizophrenia patients (1°), second-degree relatives of schizophrenia patients (2°) and adolescent controls with no schizophrenia family history (NSFH).
| Impulsivity Measures (Mean (SD)) | Groupa F (p) | Pair-wise Comparison (Q (p)) | |||||
| 1° | 2° | NSFH | 1° vs. NSFH | 1° vs. 2° | 2° vs. NSFH | ||
| UPPS-P | |||||||
| N | 39 | 53 | 118 | ||||
| Negative Urgency | 29.08 (6.99) | 27.22 (6.21) | 26.92 (6.78) | 1.73 (.18) | - | - | - |
| Premeditation | 23.89 (4.85) | 21.53 (4.05) | 22.26 (4.75) | 3.14 (.04) | 1.95 (.13) | 2.47 (.04) | 0.97 (.60) |
| Perseverance | 21.67 (4.19) | 19.23 (4.10) | 19.75 (4.52) | 4.11 (.02) | 2.45 (.04) | 2.74 (.02) | 0.75 (.73) |
| Sensation Seeking | 34.84 (6.48) | 35.12 (7.25) | 35.64 (6.31) | 0.27 (.76) | - | - | - |
| Positive Urgency | 30.33 (9.72) | 25.90 (6.84) | 26.57 (8.12) | 4.31 (.01) | 2.63 (.03) | 2.72 (.02) | 0.52 (.86) |
| Delay Discounting | |||||||
| N | 37 | 52 | 108 | ||||
| AUC | 0.36 (0.25) | 0.52 (0.32) | 0.54 (0.31) | 5.00 (.008) | 3.13 (.006) | 2.40 (.04) | 0.49 (.88) |
| ln k | -3.55 (2.63) | -5.02 (2.73) | -5.01 (2.43) | 4.98 (.008) | 3.05 (.007) | 2.64 (.02) | 0.08 (.99) |
AUC: Proportion under the curve
ln k: natural log of discounting constant
Covariates: age, gender and presence of any psychiatric disorders/substance use
Figure 1.
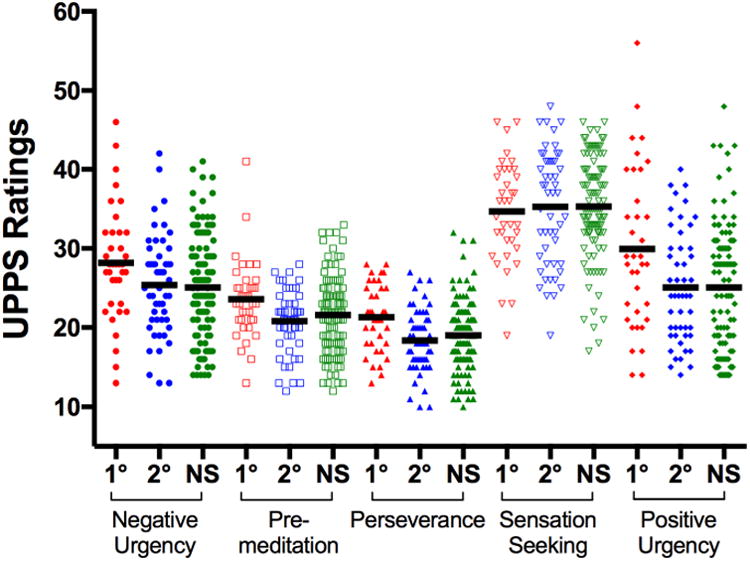
Scatterplots and Mean (horizontal bar) UPPS-P Impulsive Behavior Scale subscale scores of first-degree biological relatives of schizophrenia patients (1°), second-degree biological relatives of schizophrenia patients (2°) and adolescent controls with no schizophrenia family history (NS).
For delay discounting, main effects of group were significant on both AUC and discounting constant (Table 2; F≥4.98, p≤.008). Again, first-degree relatives showed greater impulsivity with significantly smaller AUC and larger k than both NSFH and second-degree relatives (Table 2 and Figure 2; Q≥2.40, p≤.04). Second-degree relatives and NSFH did not differ significantly on AUC or discounting constant (Q≤0.49, p≥.88).
Figure 2.
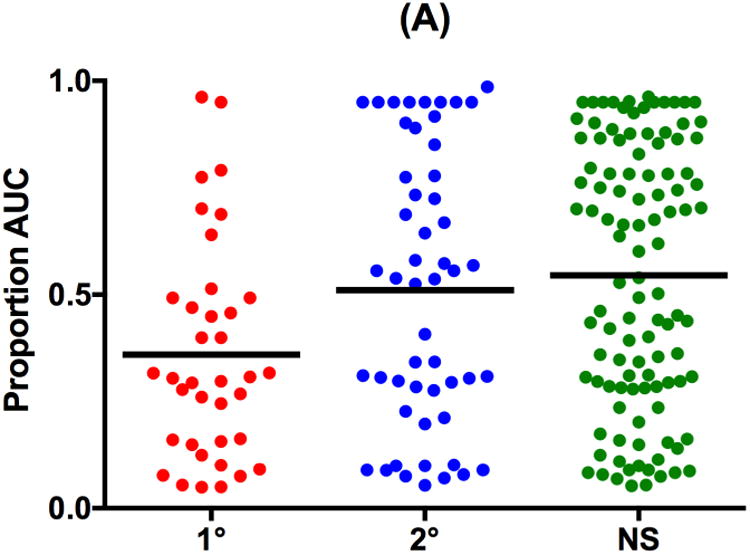
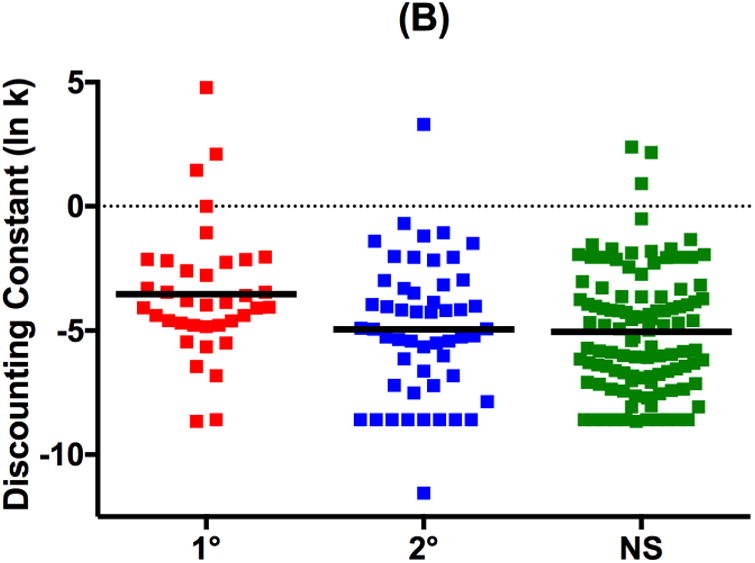
Scatterplots and Mean (horizontal bar) delay discounting measures of (A) Proportion Under-the-Curve (AUC) and (B) natural log-transformed discounting constant k (ln k) in first-degree biological relatives of schizophrenia patients (1°), second-degree biological relatives of schizophrenia patients (2°) and adolescent controls with no schizophrenia family history (NS).
Secondary Analyses
We conducted additional analyses to further verify that the group differences in impulsivity had not been confounded by psychiatric disorders or by substance use. First, we restricted the ANCOVA to only subjects without psychiatric disorders or tobacco/alcohol use (Supplemental Table S1). Main effects of group were similar with results based on the entire study sample (Table 2). Second, we repeated the ANCOVA on the entire study sample adding a group-by-presence of psychiatric disorders interaction term (Supplemental Table S2). Again, main effects of group remained statistically significant for those 3 UPPS-P subscale scores and for delay discounting (p≤.04). More importantly, the interaction term was not statistically significant (p≥.06). Therefore, these additional analyses suggest that group differences in impulsivity were independent of psychiatric diagnoses or substance use.
Positive Urgency and Negative Urgency scores in our sample were highly correlated (Pearson r=0.72, p=<.0001 for the whole sample; r=0.72, 0.56 and 0.75 for first-degree relatives, second-degree relatives and NSFH respectively). Previous research has recommended combining Positive Urgency and Negative Urgency scores to assess the overall urgency trait when both measures are not expected to differentially predict impulsive behaviors (Smith and Cyders, 2016). On summing Negative Urgency and Positive Urgency scores in our study participants, we found a significant group effect on total urgency scores (F=3.46, p=0.03); such that first-degree relatives had significantly higher total urgency when compared to NSFH (Mean (SD)=59.41 (15.53) and 53.49 (13.95) respectively; Q=2.47, p=0.04) and to second-degree relatives (Mean (SD)=53.12 (11.53); Q=2.31, p=0.05).
UPPS-P subscale scores were weakly correlated with both measures of delay discounting (Pearson absolute r (|r|): Median |r|=0.04 and 0.06 for AUC and ln k respectively; Range=0.01 to 0.12). Lastly, there were no statistically significant main effects of familial clustering on UPPS-P subscale scores or on delay discounting (see Supplemental Table S3). Inclusion of familial clustering into the statistical models did not substantially alter the main effects of group.
Discussion
In this study, we assessed impulsivity in adolescent biological relatives of SZP patients who are without SZP diagnoses. Compared to matched adolescents without SZP family history or to second-degree relatives of SZP patients, first-degree relatives of SZP patients were more impulsive; reporting higher levels of impulsivity-related behaviors (i.e. UPPS-P ratings for lack of perseverance, positive urgency and lack of premeditation (in comparison to second-degree relatives only)) and greater preference for immediate rewards during delay discounting. Second-degree relatives, on the other hand, were no different from adolescents without SZP family history on both self-report and behavioral measures of impulsivity. These results were unchanged with secondary statistical analyses that made further adjustments for the effects of psychiatric diagnoses, substance use and familial clustering. Hence, when viewed in conjunction with prior studies finding increased impulsivity in SZP patients and in their twins, our results suggest that impulsivity is associated with SZP risk, and its severity increases with greater familial relatedness to the SZP proband.
Our findings are consistent with most of the results from the only other published SZP family study examining impulsivity (Fortgang et al., 2016). Together, both studies support the notion that impulsivity serves as an endophenotype for SZP even though SZP may not be typically considered an impulse control disorder. Despite differing with respect to the sampling of biological relatives and in the choice of impulsivity measures studied, these two studies provide convergent evidence indicating that biological relatives of SZP patients are more impulsive. Whether in adult twins (73.8% of whom were dizygotic in the Fortgang et al study) or among adolescent siblings or children (current study), first-degree biological relatives of SZP patients scored higher than healthy controls without SZP family history on specific facets of self-report impulsivity (BIS Attentional and Nonplanning Impulsivity, and UPPS-P Perseverance and Positive Urgency subscales respectively). However, other self-report impulsivity measures (including sensation-seeking) were no different among biological relatives in either studies. On the other hand, laboratory measures of impulsivity were significantly higher in biological relatives from the current study (delay discounting assessing impulsivity relating to failure to consider future consequences during decision making) but were not significantly different in the Fortgang et al study (Stop-Signal Task which evaluates response inhibition). Another reason that may have contributed to differences in findings between these 2 family studies is that the SZP co-twins in the Fortgang et al study were older (Mean age=51.5 years), and therefore unlikely to develop SZP. In contrast, approximately 9% (based on 15% of first-degree and 5% of second-degree relatives have SZP diagnosis) of our adolescent biological relatives may develop SZP later in their lives. Such “pre-schizophrenics” may have inadvertently raised the group's mean impulsivity.
In a recent meta-analysis, Berg and colleagues reviewed the large and accumulating literature regarding the psychopathological correlates of the UPPS (Berg et al., 2015). They found that Negative Urgency had the largest effects on a wide range of impulsive behaviors manifesting as alcohol and substance use, borderline personality disorder, bulimia, suicidal ideation and suicide attempts, aggression and obsessive-compulsive symptoms. Among these diverse impulsive behaviors, alcohol and substance use had the strongest and most consistent associations with elevated UPPS ratings. However, because only one study to-date has used the UPPS for assessing impulsivity in SZP (Hoptman et al., 2014), this meta-analysis did not include studies of SZP patients.
Nonetheless, Hoptman and colleagues showed that SZP patients had significantly elevated Negative Urgency and Positive Urgency scores than healthy controls (Hoptman et al., 2014). Premeditation, Perseverance or Sensation Seeking subscale scores in SZP patients did not differ significantly from control subjects. Our study, on the other hand, found that Positive Urgency but not Negative Urgency subscale scores were significantly higher among first-degree biological relatives of SZP patients when compared to controls without SZP family history.
Analogous to Hoptman et al study of SZP patients (Hoptman et al., 2014), Premeditation and Sensation Seeking scores in biological relatives of SZP patients did not differ significantly from controls without SZP family history. Our first-degree relatives, however, had significantly higher (lack of) Perseverance scores than control subjects. Since Hoptman et al and our study are the only publications to-date that have examined UPPS-P scores in SZP patients and in their biological relatives respectively, these similarities and differences in self-report impulsivity-related behaviors associated with SZP will require replication in future studies.
Consistent with previous research (e.g.(Cyders and Coskunpinar, 2010; Settles et al., 2014)), Positive Urgency and Negative Urgency subscale scores in our sample were also highly and positively correlated (Pearson r ranged between 0.56 to 0.75). Even though both subscales are frequently associated with each other, and most impulsive behaviors (including alcohol and drug use, risky sexual behaviors and gambling) are related to both Negative Urgency and Positive Urgency (Baker et al., 2004), there is increasing interest in the field to distinguish between these two constructs and understand their underlying neurobiological basis in impulsivity (Smith and Cyders, 2016). Further research into the effects of intense emotions on impulsivity may be especially relevant for impulsive behaviors that are associated with only one extreme mood state but not with the other (e.g. Negative Urgency but not Positive Urgency is correlated with bulimia (Cyders et al., 2007)). Smith and Cyders (Smith and Cyders, 2016) suggested combining Positive Urgency and Negative Urgency subscale scores to assess the overall urgency trait when both measures do not predict psychopathology differently; as would have been expected based on Hoptman et al's finding in SZP patients (Hoptman et al., 2014).
When we summed Negative Urgency and Positive Urgency subscale scores in our study participants, first-degree relatives had significantly higher total urgency when compared to the other two comparison groups. Notwithstanding this and despite the highly correlated Negative Urgency and Positive Urgency subscale scores, only Positive Urgency (but not Negative Urgency) was significantly higher in first-degree relatives when these two subscales were analyzed separately. Thus, our study suggests that positive and negative extreme mood states may differentially influence impulsivity in SZP. Additional studies are still needed to further clarify the roles of Positive Urgency and Negative Urgency on impulsivity in SZP patients and their biological relatives.
Unlike UPPS, there have been more studies investigating delay discounting in SZP. However, findings regarding delay discounting in SZP have also been somewhat mixed. Some studies found that SZP patients were more impulsive showing steeper discounting than healthy controls (Ahn et al., 2011; Heerey et al., 2007; Weller et al., 2014). Other investigators have reported no significant differences in delay discounting in SZP patients (Avsar et al., 2013; MacKillop and Tidey, 2011; Wing et al., 2012). Although earlier studies suggested that greater discounting seen in SZP patients may have been confounded by cigarette smoking (MacKillop and Tidey, 2011; Wing et al., 2012), more recent reports indicate that after controlling for the effects of tobacco, alcohol and drug use on delay discounting, SZP diagnosis was still associated with steeper discounting (Ahn et al., 2011; Weller et al., 2014). To the best of our knowledge, no previous studies have investigated delay discounting in biological relatives of SZP patients.
Second-degree relatives were less impulsive than first-degree relatives, and were more like adolescents without SZP family history on self-report impulsive behaviors and on delay discounting. A similar pattern has been reported for magnetic resonance (MR) brain imaging measures and cognitive performance among biological relatives of SZP patients. Compared to first-degree relatives, second-degree relatives have less severe abnormalities in MR spectroscopy metabolite levels within limbic brain regions (Capizzano et al., 2011) and in relational memory performance (Onwuameze et al., 2016).
Self-reports and behavioral measures of impulsivity have consistently been shown to be only weakly correlated (Caswell et al., 2015; Enticott et al., 2008; Nolan et al., 2011). Even though correlation coefficients (r) between UPPS-P scores and delay discounting in the current study were also small (absolute r≤0.12), both categories of impulsivity measures were significantly elevated in first-degree biological relatives of SZP patients. Nonetheless, our study would have been strengthened by including other behavioral paradigms that not only assess the multiple facets of impulsivity more comprehensively but that are also abnormal in SZP patients (e.g. Stop-signal and continuous performance tasks for evaluating response inhibition; Stroop and flanker tasks on resistance to distractor interference; cued recall test on resistance to proactive interference, etc.). Although our overall study sample size has adequate statistical power to detect group differences of “small” effect sizes (Cohen's d≥0.21 at α=.05 and β=0.2), the current study is limited by the relatively modest number of first-degree relatives. For post hoc 2-group comparisons, we have statistical power to detect only “medium” effect size group differences (d≥0.60, 0.52 and 0.47 for 1° versus 2° relatives, 1° relatives versus NSFH and 2° relatives versus NSFH respectively; α=.05 and β=0.2). Consequently, first-degree relatives did not differ significantly from NSFH on UPPS-P Premeditation even though d=0.34. This is likely due to Type II error related to reduced statistical power. Another weakness in the current study is that we did not have a comparison group of SZP patients on whom impulsivity was assessed. Although the FH-RDC is a valid and reliable instrument to assess SZP family history, our study would have been further strengthened if we had administered diagnostic interviews to the SZP probands. Despite these limitations, the UPPS-P and delay discounting task we examined here assess different aspects of impulsivity; which, in turn, are likely mediated by distinct yet connected brain circuits.
Lesions in ventral striatum or within the orbitofrontal cortex have been shown to increase impulsivity in animals (Cardinal et al., 2001; Mar et al., 2011; Mobini et al., 2002). These findings support the role of prefrontal brain regions in exerting top-down cognitive control over limbic brain structures in the evaluation of rewards and in mediating impulsivity (Bush et al., 2000; Ernst et al., 2005; Kuhnen and Knutson, 2005; Milham and Banich, 2005; Miller, 2000; O'Doherty et al., 2003; Peters and Buchel, 2010; Soloff et al., 2017). It has been suggested that predominant prefrontal influence over limbic activation leads to “safe” behaviors (Cox et al., 2010; Miller and Cohen, 2001; Smith and Jonides, 1999). Conversely, imbalances in frontal-striatal neural connections that favor the striatum may bias toward the drive for immediate rewards, maladaptive decision-making and impulsivity. Aberrant cognitive control may underlie increased impulsivity among SZP patients (Aron et al., 2007; Hoptman et al., 2014). Elevated UPPS-P Positive Urgency and Negative Urgency ratings in SZP patients were associated with reduced prefrontal cortical thickness, and with poor functioning within competing prefrontal neural networks that sub-serve cognitive control (Hoptman et al., 2014). Similar abnormalities in brain volume deficits and in functional connectivity may also underlie increased impulsivity in our sample of first-degree biological relatives as well. However, this will require confirmation in future studies.
The complex inter-relationships between impulsivity, prefrontal cognitive control, substance use and SZP susceptibility may be best understood within a neurodevelopmental perspective. Adolescence is a period of accelerated brain remodeling. Axons undergo pruning leading to thinning of prefrontal and parietal cortical gray matter (Giedd et al., 1999; Sowell et al., 1999). There is also increased neuronal myelination associated with white matter (WM) brain volume enlargements (Mathalon et al., 2001; Sowell et al., 2003). Such maturational changes are thought to bring about cognitive, emotional and social maturation as adolescents grow into adulthood. Compared to adults, adolescents are more impulsive (Arnett, 1992). Increased adolescent impulsivity has been shown to be related to a “maturational gap”, i.e. earlier maturing fronto-striatal reward circuits are under inadequate regulation by later maturing cognitive control centers in the prefrontal cortex (Casey et al., 2008; Nelson et al., 2002). An emerging body of literature suggests that adolescent WM brain maturation enhances prefrontal inhibition on the ventral striatum, and decreases impulsivity as adolescents matures into adults (Berns et al., 2009; van den Bos et al., 2015). In studies on delay discounting, preference for immediate rewards has been associated with reduced brain WM maturity (Bjork et al., 2009; Ho et al., 2016; Olson et al., 2009; Yu, 2012). Adolescence is also the most likely period for substance use initiation, and impulsive individuals are more likely to use drugs (Perry and Carroll, 2008). In SZP, heavy adolescent marijuana use has been associated with a two-fold increased risk for the disorder in later life (Henquet et al., 2005). Animals studies support the plausibility that heavy adolescent marijuana use may be a causal factor for SZP (Schneider and Koch, 2003; Verrico et al., 2014). However, a recent study reported that besides marijuana, alcohol and other drug use during adolescence also increases SZP risk (Nielsen et al., 2017). Although Nielsen and colleagues did not specifically assess impulsivity, increased SZP risk associated with adolescent drug use may be mediated through impulsivity - either via the increased likelihood for substance use initiation that comes from greater impulsivity, or through disrupted cognitive control neural circuits that are common to impulsivity and SZP. More studies will be needed to understand such complex inter-relationships.
In conclusion, impulsivity is associated with schizophrenia susceptibility. Impulsivity-related measures may serve as endophenotypes useful in advancing knowledge regarding the neurobiological underpinnings and risk factors in schizophrenia, including understanding the nature of the link between adolescent marijuana use and increased vulnerability for the disorder.
Supplementary Material
Acknowledgments
None
Funding: This work was supported by the National Institute of Health (MH097751), NARSAD Distinguished Investigator Award, Nellie Ball Research Trust, and the Herbert and Nancy Townsend Endowed Schizophrenia Research Fund.
Footnotes
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O'Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120(4):911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82(4):463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Amr M, Elsayed H, Ibrahim IMA. Impulsive behavior and its correlates among patients with schizophrenia in a tertiary care psychiatry setting in Mansoura. Asian J Psychiatr. 2016;22:111–115. doi: 10.1016/j.ajp.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Angold A, Cox A, Prendergast M, Rutter M, Simonoff E. Child and Adolescent Psychiatric Assessment, Child Interview, Version 5.0. Duke University; Durham, NC: 2008. [DOI] [PubMed] [Google Scholar]
- Arnett J. Reckless Behavior in Adolescence - a Developmental Perspective. Dev Rev. 1992;12(4):339–373. [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avsar KB, Weller RE, Cox JE, Reid MA, White DM, Lahti AC. An fMRI investigation of delay discounting in patients with schizophrenia. Brain Behav. 2013;3(4):384–401. doi: 10.1002/brb3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the UPPS for psychopathology. Psychol Assess. 2015;27(4):1129–1146. doi: 10.1037/pas0000111. [DOI] [PubMed] [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4(8):e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry. 2009;65(8):710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulated cortex. Trends in cognitive sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Curr Opin Psychiatry. 2005;18(2):135–140. doi: 10.1097/00001504-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Capizzano AA, Toscano JL, Ho BC. Magnetic resonance spectroscopy of limbic structures displays metabolite differences in young unaffected relatives of schizophrenia probands. Schizophr Res. 2011;131(1-3):4–10. doi: 10.1016/j.schres.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292(5526):2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell AJ, Bond R, Duka T, Morgan MJ. Further evidence of the heterogeneous nature of impulsivity. Personality and Individual Differences. 2015;76:68–74. doi: 10.1016/j.paid.2014.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Gotimer K, Roy AK, Castellanos FX, Milham MP, Kelly C. Your resting brain CAREs about your risky behavior. PLoS One. 2010;5(8):e12296. doi: 10.1371/journal.pone.0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Is urgency emotionality? Separating urgent behaviors from effects of emotional experiences. Pers Individ Dif. 2010;48(7):839–844. doi: 10.1016/j.paid.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol Assess. 2007;19(1):107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- de Wit H, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42(1):111–121. [Google Scholar]
- Dervaux A, Baylé FJ, Laqueille X, Bourdel MC, Le Borgne MH, Olié JP, Krebs MO. Nicotine use in schizophrenia and disinhibition. Psychiatry Res. 2004;128(3):229–234. doi: 10.1016/j.psychres.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Dervaux A, Bayle FJ, Laqueille X, Bourdel MC, Le Borgne MH, Olie JP, Krebs MO. Is substance abuse in schizophrenia related to impulsivity, sensation seeking, or anhedonia? The American journal of psychiatry. 2001;158(3):492–494. doi: 10.1176/appi.ajp.158.3.492. [DOI] [PubMed] [Google Scholar]
- Dervaux A, Laqueille X, Bourdel MC, Olié JP, Krebs MO. Impulsivity and Sensation Seeking in Alcohol Abusing Patients with Schizophrenia. Front Psychiatry. 2010;1:135. doi: 10.3389/fpsyt.2010.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott PG, Ogloff JR, Bradshaw JL. Response inhibition and impulsivity in schizophrenia. Psychiatry Res. 2008;157(1-3):251–254. doi: 10.1016/j.psychres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Fortgang RG, Hultman CM, van Erp TG, Cannon TD. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: testing for shared endophenotypes. Psychological medicine. 2016;46(7):1497–1507. doi: 10.1017/S0033291716000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. The American journal of psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gut-Fayand A, Dervaux A, Olie JP, Loo H, Poirier MF, Krebs MO. Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry Res. 2001;102(1):65–72. doi: 10.1016/s0165-1781(01)00250-5. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12(3):213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31(3):608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Ho BC. MRI brain volume abnormalities in young, nonpsychotic relatives of schizophrenia probands are associated with subsequent prodromal symptoms. Schizophr Res. 2007;96(1-3):1–13. doi: 10.1016/j.schres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Koeppel JA, Barry AB. Cerebral white matter correlates of delay discounting in adolescents. Behav Brain Res. 2016;305:108–114. doi: 10.1016/j.bbr.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Magnotta V. Hippocampal volume deficits and shape deformities in young biological relatives of schizophrenia probands. NeuroImage. 2010;49(4):3385–3393. doi: 10.1016/j.neuroimage.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and Self-Control From a Dual-Systems Perspective. Perspect Psychol Sci. 2009;4(2):162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. The American journal of psychiatry. 2014;171(9):939–948. doi: 10.1176/appi.ajp.2014.13111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Volavka J, Johnson G, Weiss E, Bilder RM, Lim KO. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52(1):9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychological medicine. 2011;41(2):291–299. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58(2-3):173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Krakowski MI, Czobor P. Proneness to aggression and its inhibition in schizophrenia: Interconnections between personality traits, cognitive function and emotional processing. Schizophr Res. doi: 10.1016/j.schres.2016.11.038. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353(9146):30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- Liu H, Heath SC, Sobin C, Roos JL, Galke BL, Blundell ML, Lenane M, Robertson B, Wijsman EM, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(6):3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynam DR, Smith GT, Whiteside SP, Cyders MA. The UPPS-P: Assessing five personality pathways to impulsive behavior (Technical Report) Purdue University; West Lafayette: 2006. [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology (Berl) 2011;216(1):91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31(17):6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(2):148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Human brain mapping. 2005;25(3):328–335. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature reviews. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160(3):290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- Moldin SO. Indicators of liability to schizophrenia: perspectives from genetic epidemiology. Schizophr Bull. 1994;20(1):169–184. doi: 10.1093/schbul/20.1.169. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda P, Tandon N, Mathew IT, Padmanabhan JL, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. Impulsivity across the psychosis spectrum: Correlates of cortical volume, suicidal history, and social and global function. Schizophr Res. 2016;170(1):80–86. doi: 10.1016/j.schres.2015.11.030. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Dev Psychopathol. 2002;14(3):499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Nielsen SM, Toftdahl NG, Nordentoft M, Hjorthoj C. Association between alcohol, cannabis, and other illicit substance abuse and risk of developing schizophrenia: a nationwide population based register study. Psychological medicine. 2017:1–10. doi: 10.1017/S0033291717000162. [DOI] [PubMed] [Google Scholar]
- Nolan KA, D'Angelo D, Hoptman MJ. Self-report and laboratory measures of impulsivity in patients with schizophrenia or schizoaffective disorder and healthy controls. Psychiatry Res. 2011;187(1-2):301–303. doi: 10.1016/j.psychres.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onwuameze OE, Titone D, Ho BC. Transitive inference deficits in unaffected biological relatives of schizophrenia patients. Schizophr Res. 2016;175(1-3):64–71. doi: 10.1016/j.schres.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzir M. Impulsivity in schizophrenia: A comprehensive update. Aggr Violent Behav. 2013;18(2):247–254. [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200(1):1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213(2):135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Reddy LF, Lee J, Davis MC, Altshuler L, Glahn DC, Miklowitz DJ, Green MF. Impulsivity and Risk Taking in Bipolar Disorder and Schizophrenia. Neuropsychopharmacology. 2014;39(2):456–463. doi: 10.1038/npp.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71(2):121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28(10):1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Settles RE, Zapolski TC, Smith GT. Longitudinal test of a developmental model of the transition to early drinking. J Abnorm Psychol. 2014;123(1):141–151. doi: 10.1037/a0035670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Smith GT, Cyders MA. Integrating affect and impulsivity: The role of positive and negative urgency in substance use risk. Drug Alcohol Depend. 2016;163(1):S3–S12. doi: 10.1016/j.drugalcdep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff PH, Abraham K, Burgess A, Ramaseshan K, Chowdury A, Diwadkar VA. Impulsivity and aggression mediate regional brain responses in Borderline Personality Disorder: An fMRI study. Psychiatry Research: Neuroimaging. 2017;260:76–85. doi: 10.1016/j.pscychresns.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature neuroscience. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Dev Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Keshavan MS, Juelich RJ, Molokotos E, Whitfield-Gabrieli S, Brent BK, Makris N, Seidman LJ. A review of neuroimaging studies of young relatives of individuals with schizophrenia: A developmental perspective from schizotaxia to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2013;162(7):604–635. doi: 10.1002/ajmg.b.32170. [DOI] [PubMed] [Google Scholar]
- van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Adolescent impatience decreases with increased frontostriatal connectivity. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1423095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated Delta9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. The American journal of psychiatry. 2014;171(4):416–425. doi: 10.1176/appi.ajp.2013.13030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, de Wit H. Sex differences in impulsive action and impulsive choice. Addict Behav. 2014;39(11):1573–1579. doi: 10.1016/j.addbeh.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RE, Avsar KB, Cox JE, Reid MA, White DM, Lahti AC. Delay discounting and task performance consistency in patients with schizophrenia. Psychiatry Res. 2014;215(2):286–293. doi: 10.1016/j.psychres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. [Google Scholar]
- Wilikinson GS. The Wide Range Achievement Test (WRAT-3) 3rd. Wide Range, Inc.; Wilmington: 1993. [Google Scholar]
- Wing VC, Moss TG, Rabin RA, George TP. Effects of cigarette smoking status on delay discounting in schizophrenia and healthy controls. Addict Behav. 2012;37(1):67–72. doi: 10.1016/j.addbeh.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Yu R. Regional white matter volumes correlate with delay discounting. PLoS One. 2012;7(2):e32595. doi: 10.1371/journal.pone.0032595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Rizkallah É, Pampoulova T, Chiasson JP, Lipp O, Stip E, Potvin S. Sensation-seeking, social anhedonia, and impulsivity in substance use disorder patients with and without schizophrenia and in non-abusing schizophrenia patients. Psychiatry Res. 2012;200(2–3):237–241. doi: 10.1016/j.psychres.2012.07.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


