Abstract
Plasma level of microbial translocation is a marker of mucosal permeability. Increased mucosal permeability ignites elevated microbial translocation and as a consequence of systemic inflammation. Pregnant women with depression have higher levels of inflammatory markers relative to pregnant women without depression, however, no studies have reported whether systemic microbial translocation will change in depressed women during pregnancy. In this study, we examined the plasma LPS level of depressed women during pregnancy. The results showed that the plasma LPS level was significantly increased in depressed mothers during their 8–12 weeks gestation compared to healthy controls. Compared to 8–12 weeks gestation, the plasma LPS levels were significantly decreased at 24–28 weeks gestation and 6–8 weeks postpartum in both depressed subjects and healthy controls. Furthermore, the plasma levels of pro-inflammatory cytokines (TNF-α and MCP/CCL2) associated with microbial translocation were significantly increased in depressed subjects during 8–12 weeks gestation compared to healthy controls. These results indicate that the level of microbial translocation is increased in depressed women during early pregnancy.
Keywords: Microbial translocation, Peripartum depression, Mucosal permeability, Inflammation
1. Introduction
Peripartum depression is a debilitating mood disorder that occurs in 8–15% of childbearing women (Byatt et al., 2016; Robakis et al., 2015). Although several psychosocial risk factors have been identified, a substantial proportion of the risk for the disorder remains unexplained and biological contributors are unclear (Babb et al., 2015). Dysregulation of the innate immune system is thought to be important in the etiology of major depression (Miller et al., 2009). Pregnant women with depression have higher levels of inflammatory markers during pregnancy, compared to non-depressed pregnant women (Osborne and Monk, 2013) but it is unclear what may be triggering this inflammatory cascade.
The passage of bacterial products from the gastrointestinal tract to extraintestinal sites is a process known as microbial translocation (Brenchley and Douek, 2012). Microbial translocation ignites a proinflammatory cytokine milieu and systemic immune activation that is thought to be important in the pathogenesis of a number of diseases (Brenchley et al., 2006; Costa et al., 2016; Jiang et al., 2009). Plasma lipopolysaccharide (LPS) is considered to be a representative biological marker of microbial translocation, stimulates immune cells through the toll like receptor 4 pathway and contributes to the pro-inflammatory cytokine milieu and systemic immune activation (Brenchley and Douek, 2012), and may contribute to several disease states including depression. Here, we tested the hypothesis that microbial translocation is associated with depression during pregnancy.
2. Methods and materials
2.1. Study subjects
These studies were approved by the Institutional Review Boards for Human Research (IRB#Pro00021511) at the Medical University of South Carolina. Participants (Table 1) were pregnant women receiving routine obstetrical care and were recruited into the study during their first prenatal visit (8–12 weeks gestation). Women were excluded if they were younger than 18 years of age, more than 12 weeks of gestation, or unable to provide informed consent. Participants provided demographic information, and were screened for depression using the Edinburgh Postnatal Depression Scale (EPDS) (Osborne and Monk, 2013). Blood samples were collected at 8–12 weeks gestation (visit 1), 24–28 weeks gestation (visit 2), and 6–8 weeks postpartum (visit 3). During these visits, participants who scored 10 or greater on the EPDS were considered depressed subjects (n = 14) and participants scored less than 9 on the EPDS were considered healthy controls (n = 14) (Table S1).
Table 1.
Clinical characteristics.
| Healthy controls | Depressed patients | P value a | |
|---|---|---|---|
| Numbers of subjects | 14 | 14 | |
| Age (years) median b | 30 (25–32) | 29 (23–33) | 0.61 |
| Maternal BMI at visit 1 (kg/m2) b | 33.0 (26.3–38.3) | 32.5 (25.5–37.3) | 0.92 |
| Gravidity b | 2.0 (2.0–3.3) | 2.5 (1.0–4.0) | 0.97 |
| Parity b | 1.0 (0.8–1.0) | 0.5 (0.0–1.3) | 0.33 |
| Total gestation weeks b | 39.0 (37.8–40.0) | 39.5 (38.0–40.3) | 0.39 |
| Baby birth weight (oz) b | 115.5 (108.8–130.3) | 122.5 (107.5–136.3) | 0.46 |
| Therapies of depression | No | No | |
| Previous psychiatric disorders | No | No | |
| Alcohol drinking (past 12 h) | No | No | |
| Systemic antibiotic treatment (past 6 months) | No | No | |
| Fetal sex (%) | > 0.99 | ||
| Male | 35.7 | 42.9 | |
| Female | 64.3 | 57.1 | |
| Delivery type (%) | 0.33 | ||
| Vaginal | 85.7 | 70.0 | |
| Cesarean | 14.3 | 30.0 | |
| Ethnicity (%) | > 0.99 | ||
| Caucasian | 50.0 | 50.0 | |
| Africa American | 42.9 | 42.9 | |
| Asian | 7.1 | 7.1 | |
| Education (%) | 0.97 | ||
| Less than high school+ | 7.1 | 21.4 | |
| High school | 28.6 | 42.9 | |
| Some college | 35.7 | 28.6 | |
| College or higher | 28.6 | 7.1 | |
| Family income (%) | > 0.99 | ||
| Less than $20,000+ | 14.3 | 28.6 | |
| $20,000–$49,999 | 35.7 | 35.7 | |
| $50,000–$74,999 | 35.7 | 35.7 | |
| $75,000 or more | 14.3 | 0.0 | |
| Smokers (%) | 0.80 | ||
| Never | 92.9 | 71.4 | |
| Former | 7.1 | 21.4 | |
| Current | 0.0 | 7.1 |
P values compared between the two study groups were analyzed by Mann Whitney test (non-paired).
Data are median (interquartile range) values.
2.2. Plasma levels of LPS
Plasma were stored in a 1.5 mL microtube at 80 °C until they were thawed for study. LPS level in Plasma was then quantified using limulus amebocyte lysate QCL-1000 kit (Lonza, Walkersville, USA) as descripted in our previous studies (Jiang et al., 2009). Data were shown in median (interquartile range [IQR]).
2.3. Plasma levels of TNF-α, IL-6, IL-1β and MCP/CCL2
Plasma cytokines levels (TNF-α, IL-6, IL-1β and MCP/CCL2) were measured using the Bio-Plex Pro™ Human Chemokine Panel, 40-plex (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's instruction.
2.4. Statistical analysis
In the pre-specified hypothesis, we were interested in the comparisons of results from depression versus from healthy controls; therefore, p-values from comparing depression to each of control groups were not adjusted for multiple comparisons (Rothman, 1990). Therefore statistical significance was assessed using Mann Whitney U tests. A multi-variable linear regression model was used to analyze the differences in plasma LPS after adjusting age, race and BMI using SAS (Version 9.3, Cary, NC, USA). P ≤ 0.05 was considered statistical significance.
3. Results
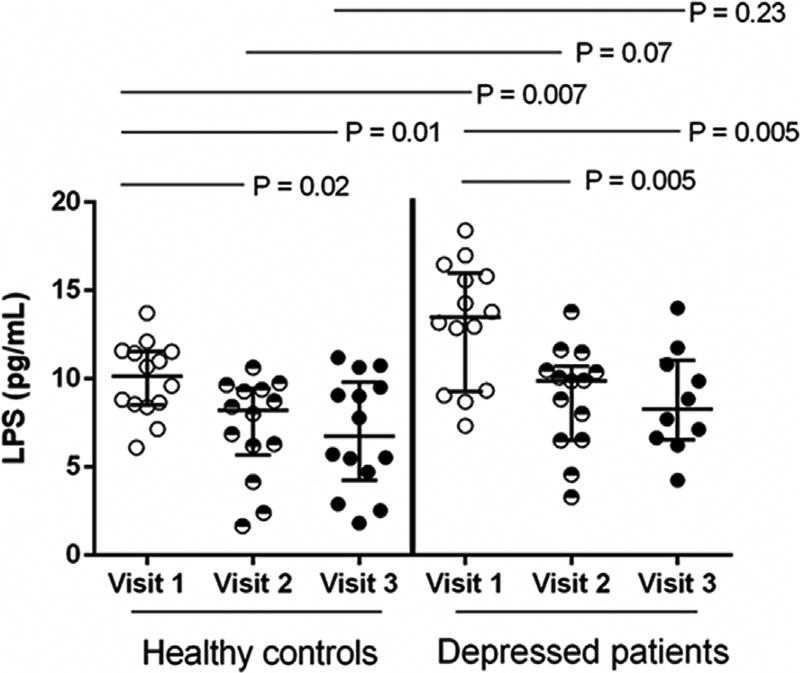
In order to assess systemic microbial translocation, we examined the plasma LPS level. Compared to visit 1, the plasma LPS level was significantly decreased at visit 2 and visit 3 in both healthy controls (P = 0.02 and P = 0.01) and depressed subjects (P = 0.005 and P = 0.005) (Fig. 1). Notably, the plasma LPS level was significantly increased in depressed subjects at visit 1 compared to healthy controls (P = 0.007), even after controlling age, race, and BMI (P = 0.01). The plasma LPS levels at visit 2 and visit 3 in depressed subjects were marginally increased compared to health controls (P = 0.07 and P = 0.23) but did not achieve statistically significant differences (Fig. 1).
Fig. 1.
Increased plasma levels of LPS in depressed women during early pregnancy compared to healthy controls. Plasma LPS levels in healthy control women and depressed women during pregnancy (median ± IQR). Mann Whitney U test.
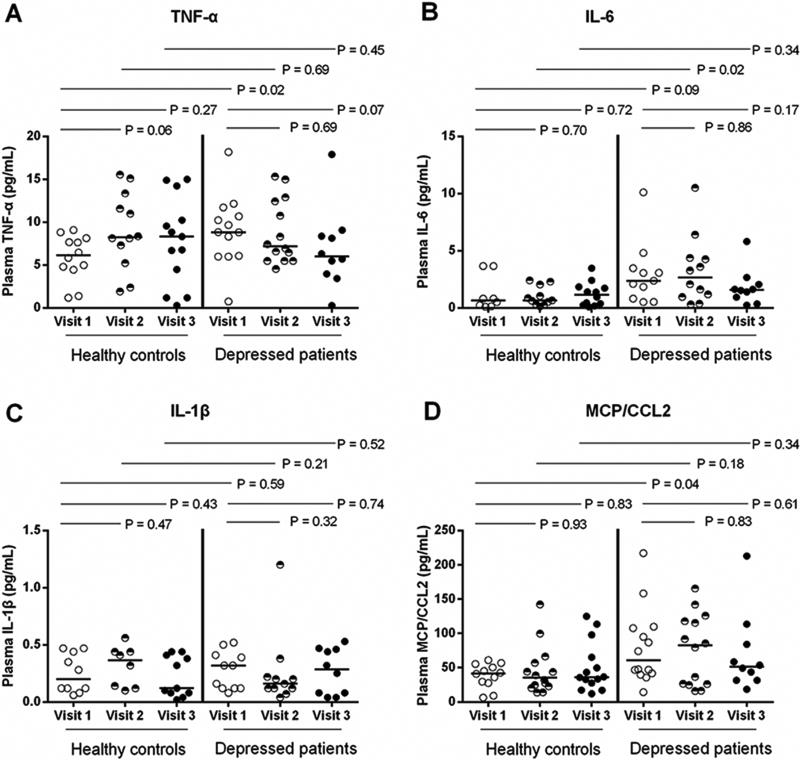
To further verify our finding, we compare the levels of pro-inflammatory cytokines in early pregnancy (Fig. 2). The results showed that the plasma TNF-α and MCP/CCL2 levels were significantly increased in depressed subjects at visit 1 compared to healthy controls (P = 0.02, and P = 0.04). Interestingly, plasma IL-6 level was increased in depressed subjects at visit 2 but not at the other visits compared to healthy controls (P = 0.02). However, plasma levels of IL-6 and IL-1β at visit 1 were similar among depressed and healthy women.
Fig. 2.
Plasma levels of TNF-α, IL-6, IL-1β and MCP/CCL2 in healthy and depressed pregnant women. Plasma levels of cytokines, including TNF-α (A), IL-6 (B), IL-1β (C) and MCP/CCL2 (D) were analyzed by using ELISA kits according to the manufacturer's instruction. The data were shown as median. Mann Whitney U test.
4. Discussion
Elevated plasma LPS level, which is translocated from impaired mucosal barriers, will lead to the production of pro-inflammatory cytokines and a state of aberrant immune activation (Brenchley and Douek, 2012; Brenchley et al., 2006). Research has demonstrated that women with depression may have higher levels of inflammatory markers (IL-6 and TNF-α) related to microbial toll-like receptor signaling pathway in early pregnancy (Haeri et al., 2013). Recently, study showed that plasma IgA and IgM against commensal gram-negative bacteria were associated with depression during pregnancy (Roomruangwong et al., 2017). However, IgA and IgM against bacteria represent host humoral immune responses rather than the direct measurement of bacterial products. In the current study, we found that plasma LPS level was significantly increased in depressed women during early pregnancy. Consistently, the plasma TNF-α and MCP/CCL2 levels were significantly increased in depressed subjects at visit 1. These findings suggest that the mucosal permeability may be disrupted in early pregnant women with depression, which induces higher levels of microbial translocation and inflammation. Furthermore, Pazos et al. had reported that there is a strengthened immune barrier function at late pregnancy (Pazos et al., 2012), which is consistent with our findings that showed there is a decreased plasma LPS level at late pregnancy compared to early pregnancy in both healthy and depressed women.
In addition, previous reports have shown that massive hormonal changes during the peripartum period may trigger perinatal depression (Agrati and Lonstein, 2016; Klier et al., 2007). For example, during pregnancy, certain plasma estrogens increase up to 1000-fold, with a return to near prepregnancy levels generally within the first few hours postpartum (Russell et al., 2001). Importantly, studies from others and ours have also suggested that estradiol and human choionic gonadotophin (hCG) may have the ability to increase the mucosal permeability (Herr et al., 2013; Kuruca et al., 2017; Zhou et al., 2017). Consequently, the alteration of sex hormones in pregnant women with depression may result in a disruption of mucosal barriers, and subsequently ignite an elevated microbial translocation and systemic Inflammation.
In conclusion, based on our finding, early diagnosis and treatment interventions of depressed mothers may be imperative. Several confounding variables (e.g. fetal sex, type II diabetes) may need to be controlled in order to establish a causal inference link between bacterial translocation and postnatal depression. However, the limitation of the small size in our study prevents us to draw further conclusions. Future research should aim at investigating the interplay among sex hormones, microbial translocation and pro-inflammatory cytokines, and aim at reconstitution of disrupted mucosa during pregnancy.
Supplementary Material
Acknowledgments
We are indebted to all study participants.
Role of the funding sources
This work was supported by National Institutes of Health Grants AI091526 and AI128864 (to WJ), the Medical Research Service at the Ralph H. Johnson VA Medical Center Merit Grant VA CSRD MERIT CX001211 (to WJ), Building Interdisciplinary Research Careers in Women's Health Career Development Award K12HD055885 (to CG), South Carolina Clinical and Translational Research Institute Pilot Grant Program Grant UL1 TR000062 (to CG), and Career Development Award from the National Institute on Drug Abuse Grant 1K23DA039318-01 (to CG).
Footnotes
Contributions
C.G. and W.J. conceived and designed the experiments; C.G., A.P., and W.J. collected the sample information, contributed to reagents and materials; Z.Z., E.O., R.L., and Z.L. performed the experiments and analyzed the data; and Z.Z., C.G., and W.J. wrote the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2017.11.009.
References
- Agrati D, Lonstein JS. Affective changes during the postpartum period: influences of genetic and experiential factors. Horm. Behav. 2016;77:141–152. doi: 10.1016/j.yhbeh.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Babb JA, Deligiannidis KM, Murgatroyd CA, Nephew BC. Peripartum depression and anxiety as an integrative cross domain target for psychiatric preventative measures. Behav. Brain Res. 2015;276:32–44. doi: 10.1016/j.bbr.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu. Rev. Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Byatt N, Xiao RS, Dinh KH, Waring ME. Mental health care use in relation to depressive symptoms among pregnant women in the USA. Arch. Womens Ment. Health. 2016;19:187–191. doi: 10.1007/s00737-015-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa FR, Françozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS, Ramos SG, Câmara NO, de Zoete MR, Palm NW. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J. Exp. Med. 2016;213:1223–1239. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeri S, Baker AM, Ruano R. Do pregnant women with depression have a proinflammatory profile? J. Obstet. Gynaecol. Res. 2013;39:948–952. doi: 10.1111/jog.12017. [DOI] [PubMed] [Google Scholar]
- Herr D, Fraser HM, Konrad R, Holzheu I, Kreienberg R, Wulff C. Human chorionic gonadotropin controls luteal vascular permeability via vascular endothelial growth factor by down-regulation of a cascade of adhesion proteins. Fertil. Steril. 2013;99:1749–1758. e1746. doi: 10.1016/j.fertnstert.2013.01.120. [DOI] [PubMed] [Google Scholar]
- Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J. Infect. Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier CM, Muzik M, Dervic K, Mossaheb N, Benesch T, Ulm B, Zeller M. The role of estrogen and progesterone in depression after birth. J. Psychiatr. Res. 2007;41:273–279. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kuruca SE, Karadenizli S, Akgun-Dar K, Kapucu A, Kaptan Z, Uzum G. The effects of 17β-estradiol on blood brain barrier integrity in the absence of the estrogen receptor alpha; an in-vitro model. Acta. histochem. 2017;119:638–647. doi: 10.1016/j.acthis.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C. Perinatal depression - the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology. 2013;38:1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Sperling RS, Moran TM, Kraus TA. The influence of pregnancy on systemic immunity. Immunol. Res. 2012;54:254–261. doi: 10.1007/s12026-012-8303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robakis TK, Williams KE, Crowe S, Kenna H, Gannon J, Rasgon NL. Optimistic outlook regarding maternity protects against depressive symptoms post-partum. Arch. Womens Ment. Health. 2015;18:197–208. doi: 10.1007/s00737-014-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roomruangwong C, Kanchanatawan B, Sirivichayakul S, Anderson G, F Carvalho A, Duleu S, Geffard M, Maes M. IgA/IgM responses to gram-negative bacteria are not associated with perinatal depression, but with physio-somatic symptoms and activation of the tryptophan catabolite pathway at the end of term and postnatal anxiety. Cns Neurol. Disord-Dr. 2017;16:472–483. doi: 10.2174/1871527316666170407145533. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Russell JA, Douglas AJ, Ingram CD. Brain preparations for maternity-adaptive changes in behavioral and neuroendocrine systems during pregnancy and lactation. An overview. Prog. Brain Res. 2001;133:1–38. doi: 10.1016/s0079-6123(01)33002-9. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhang L, Ding M, Luo Z, Yuan S, Bansal MB, Gilkeson G, Lang R, Jiang W. Estrogen decreases tight junction protein ZO-1 expression in human primary gut tissues. Clin. Immunol. 2017;183:174–180. doi: 10.1016/j.clim.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.