Abstract
Staphylococcus aureus plays a major role in persistent infections and many of these species form structured biofilms on different surfaces which is accompanied by changes in gene expression profiles. Further, iron supplementation plays a critical role in the regulation of several protein(s)/enzyme function, which all aid in the development of active bacterial biofilms. It is well known that for each protein, deformylation is the most crucial step in biosynthesis and is catalyzed by peptidyl deformylase (PDF). Thus, the aim of the current study is to understand the role of iron in biofilm formation and deformylase activity of PDF. Hence, the PDF gene of S. aureus ATCC12600 was PCR amplified using specific primers and sequenced, followed by cloning and expression in Escherichia coli DH5α. The deformylase activity of the purified recombinant PDF was measured in culture supplemented with/without iron where the purified rPDF showed K m of 1.3 mM and V max of 0.035 mM/mg/min, which was close to the native PDF (K m = 1.4 mM, V max = 0.030 mM/mg/min). Interestingly, the K m decreased and PDF activity increased when the culture was supplemented with iron, corroborating with qPCR results showing 100- to 150-fold more expression compared to control in S. aureus and its drug-resistant strains. Further biofilm-forming units (BU) showed an incredible increase (0.42 ± 0.005 to 6.3 ± 0.05 BU), i.e., almost 15-fold elevation in anaerobic conditions, indicating the significance of iron in S. aureus biofilms.
Keywords: Protein maturation, PDF, Fe2+, Biofilm-forming units
Introduction
Staphylococcus aureus is a Gram-positive facultative anaerobic cocci found as a commensal pathogen associated with the skin and mucous membranes of healthy individuals. The pathogenesis of this organism includes a number of structural and secreted products like fibronectin-binding proteins (FnbA and FnbB), collagen-binding protein, clumping factor, Protein A and many other biofilm-associated proteins (Bap) facilitating the attachment of the bacterial cell to the extracellular matrix forming biofilms (Cucarella et al. 2004; Plata et al. 2009). Biofilms are structurally complex dynamic architectures of microbial communities which develop on biotic and abiotic surfaces during the growth of bacteria (Hall-Stoodley et al. 2004; Stoodley et al. 2002; Watnick and Kolter 2000). These biofilms are associated with many chronic infections such as cardiovascular diseases, arthritis, endocarditis, epilepsy, osteomyelitis, seizures, some oral health problems and indwelling device-related infections (Lembke et al. 2006).
In S. aureus and other drug-resistant strains, the differentiation of planktonic cells into sessile ones is associated with many stress factors as well as environmental factors; which may activate the complex regulatory networks in response to quorum-sensing signals (Costerton et al. 1995). Exposure of bacteria to detergents, osmolarity variation, urea, ethanol and oxidative stress induces biofilm formation. Furthermore, it is influenced by the availability of glucose and iron restriction where iron is essential for bacterial growth, energy production, nucleotide synthesis and regulation of gene expression (Rachid et al. 2000a, b; Lin et al. 2012). Numerous recent studies imply that in biofilms, multicellular organization is important to fight against unfavorable external conditions. On the other hand, anaerobic conditions enhance the transcription of the ica gene, thereby increasing the expression of PIA protein and ultimately leading to elevated biofilm formation (Boles et al. 2010; Rachid et al. 2000a, b). This was also corroborated in an earlier study from our laboratory where we showed that biofilm formation is elevated in anaerobic conditions compared to aerobic conditions (Yeswanth et al. 2013).
DNA and protein components play a major role in S. aureus biofilms through attachment of cells to extracellular surfaces and proteases (Boles and Horswill 2008). The proteolytic enzyme Rsp represses the fibronectin-binding protein (FnbA) resulting in the inhibition of biofilm formation (Lei et al. 2011). The proteolytic enzymes downregulate S. aureus biofilms; conversely, protein biosynthesis enhances the biofilm formation. Thus, protein biosynthesis and maturation are important in biofilm production in S. aureus and its drug-resistant strains (Swarupa et al. 2017). In effect, biofilm formation is influenced by several regulatory proteins. For the synthesis of these proteins, deformylation is a critical step in S. aureus which is catalyzed by peptidyl deformylase (PDF), a hydrolase enzyme with iron as the central metal ion. Its activity seems to be essential for bacterial survival, representing a crucial target for the development of antimicrobials (Leeds and Dean 2006; Swarupa et al. 2017). PDF shows a peculiar preference for iron as the central metal ion. During catalysis, it activates the water molecule enhancing deformylation. On the other hand S. aureus is known to show positive regulation on biofilm formation when the culture media is supplemented with low levels of iron (Johnson et al. 2008; Lin et al. 2012). Iron regulation on biofilm formation has been demonstrated in many organisms. Thus, iron is required for Pseudomonas aeruginosa, Escherichia coli and Vibrio cholerae biofilm formation (Banin et al. 2005; Wu and Outten 2009; Mey et al. 2005), but not required for Legionella pneumophila and Streptococcus mutans. However, iron has been shown to have positive regulations on S. aureus biofilms (Lin et al. 2012).
Therefore, the present study is aimed to understand the iron-responsive regulation of biofilms as well as PDF activity in S. aureus ATCC12600 and its drug-resistant strains.
Materials and methods
Culture identification and characterization
The study was performed on 12 S. aureus strains isolated from raw milk. Among these, eight strains were isolated from raw milk collected from local milk vendors and the rest of the four from dairy herds (Swarupa et al. 2014). Further, these strains were grown on Baird Parker agar plate at 37 °C (Baird-Parker 1962). A single black, shiny colored colony from the plate was picked and grown in brain heart infusion (BHI) broth for isolation of genomic DNA and preparation of the cytosolic fraction (Yeswanth et al. 2013; Prasad et al. 2013).
Cloning, expression and purification of PDF
Forward (TATGTTAACAATGAAAGAC) and reverse primers (TACAGCATCTGTATGTGG) for PCR amplification of PDF gene were commercially synthesized from Eurofins Private Ltd, Bangaluru, India. The gene amplification was carried out under the following conditions: denaturation at 94 °C for 10 min, renaturation at 94 °C for 45 s, annealing at 45 °C for 45 s, amplification at 72 °C for 2 min and extension at 72 °C. The PCR product was purified and sequenced and the obtained sequence was deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/submit). This obtained sequence of S. aureus was compared with PDF sequences of other bacteria using ClustalX2 (Jeanmougin et al. 1998).
Further, the amplified product was cloned into the Sma I site of the pQE30 vector and transformed into E. coli DH5α. The thus formed recombinant clone was propagated in LB broth containing 50 µg/ml ampicillin and on reaching to mid log phase (OD540 = 0.4–0.5), 0.75 mM IPTG was added to induce PDF gene expression in the recombinant clone and allowed to grow for a further 5 h at 37 °C. The bacteria were collected by centrifugation and lysed by sonication at 50 Hz for 40 cycles. The lysate was centrifuged, the debris removed and the supernatant centrifuged at 30,000 rpm at 4 °C for 90 min. From the supernatant, the recombinant PDF was purified by passing through nickel metal chelate agarose column containing His-tagged proteins, since affinity chromatography represents one of the most effective protein purification methods (Prasad et al. 2013).
Kinetic characterization of PDF
Peptidyl deformylase in the cytosolic fraction of S. aureus was identified by performing formate dehydrogenase-coupled assay (Lazennec and Meinnel 1997). The reaction mixture contained 12 mM NAD+, 1 mM ZnCl2, 4.5 U/ml FDH and 10 μl of either crude (cytosolic fraction of S. aureus) or pure recombinant PDF. The reaction assay was done at 37 °C using For-Met-Ala as substrate. The absorbance was recorded at 340 nm using Cyberlab spectrophotometer, USA. The deformylase activity was measured by calculating the amount of NAD reduced to NADH, and the kinetic parameters V max and K M were calculated using the Hanes–Woolf plot ([S] vs [S]/V).
Effect of iron on PDF activity
To examine the effect of iron on bacterial growth, the culture was supplemented with increasing concentrations of iron ranging from 20 to 120 μM and the absorbance was recorded at 540 nm. Additionally, a PDF assay was also repeated as described above by supplementing increasing concentrations of iron at a constant substrate concentration.
Reverse transcription PCR
Using MEDOX-Easy™ Spin column total RNA Minipreps Kit, total RNA was isolated from the bacteria and the purity was determined using a UV–visible spectrophotometer (Cyberlab spectrophotometer, USA) (Venkatesh et al. 2015). The samples near 2.0 only were used in the expression studies. Further, this RNA was converted into cDNA using high-capacity cDNA reverse transcription kit (Applied biosystems, Foster City, CA) in a thermocycler calibrated at 25 °C for 10 min, 37 °C for 120 min and 85 °C for 5 min (Srikanth et al. 2015).
Real-time PCR (qPCR)
Using multiscribe reverse transcriptase and random hexamers, cDNA was synthesized for quantitative polymerase chain reaction with SYBR select master mix in ABI 7300 for 40 cycles (Gibco, Invitrogen). Subsequently, PDF expression was quantified for S. aureus ATCC12600 and its drug-resistant cultures (LMV, MRSA) (Swarupa et al. 2014) supplemented with iron using the formula ΔΔC T = ΔC Tr (iron supplemented culture) − ΔC T (culture without iron supplementation) and relative expression was calculated using (Livak and Schmittgen 2001; Swarupa et al. 2017; Venkatesh et al. 2015).
Biofilm producing assay
Staphylococcus aureus ATCC12600 was grown in both LB and BHI broth at 37 °C. From this grown culture, 200 μl was transferred into each well of 96-well plates and incubated at 37 °C for 24 h. After incubation, bound biofilms in each well were washed with phosphate buffer saline, pH 7.4, followed by staining with 0.4% crystal violet. After washing with distilled water, the absorbance was recorded at 570 nm. From the absorbance values, the biofilm units were calculated (Amaral et al. 2005; Yeswanth et al. 2013) as:
Further, the biofilms were stained with calcofluor white for 10 min and visualized under fluorescence microscope at 400×.
Results
Characterization of PDF
The PDF gene was PCR amplified from the genomic DNA of S. aureus ATCC12600, The 0.5 kb amplicon observed on 1% agarose gel electrophoresis was subjected to sequencing and the obtained sequence was deposited in GenBank (GenBank accession number: JX311310) (Fig. 1). This sequence showed complete similarity with the PDF gene sequence present in all the strains of S. aureus genome sequence present in the database and in the strains used in this study. However, conspicuous variations were observed with PDFs of other organisms (Fig. 2). After ascertaining the sequence of PDF, the gene was cloned in the Sma I of pQE30 vector in −1 frame and transformed into E. coli DH5α (Fig. 1) and the generated clone was named PVSR-1. The insert in the clone was again sequenced and the sequence showed 100% identity with the deposited sequence in the GenBank. The stable expression of PDF gene in PVSR-1 clone could be induced at 0.75 mM IPTG concentration. From the cytosolic fraction of the induced PVSR-1 clone, pure recombinant PDF was obtained by passing through nickel metal chelate agarose column, which exhibited a molecular weight of 20 kDa in 10% SDS-PAGE corresponding to the size of the insert cloned (Fig. 1). Enzyme kinetic results of recombinant PDF showed K M of 1.3 mM, while native PDF present in the cytosolic fraction of S. aureus showed a K M of 1.4 mM (Table 1).
Fig. 1.
Cloning strategy of PDF. a Genomic DNA isolated from S. aureus. M. supermix DNA ladder ranging from 33.5 to 0.5 kb. L1–L7 genomic DNA. b PCR amplification of PDF. Lane M. supermix DNA ladder. Lanes L4–L7 PCR products showing 0.5 kb PCR product. c Chromatogram showing the PDF gene sequence. d NCBI-generated GenBank flat file showing the gene sequence. e pQE30 vector showing the SmaI recognition sequence with multiple cloning sites. f Electrophoretogram showing the expression of 20 kDa recombinant protein of PDF in 10% SDS-PAGE. Lane M: medium-size protein marker ranging from 97 kDa to 3.5 kDa (Merck Pvt Ltd). Lane L1: crude cell lysate of S. aureus ATCC12600. Lane L2: uninduced cell lysate of PVSR-1 clone. Lane L3: induced cell lysate of PVSR-1 clone. Lane L4: pure recombinant PDF eluted from nickel metal chelate column chromatography
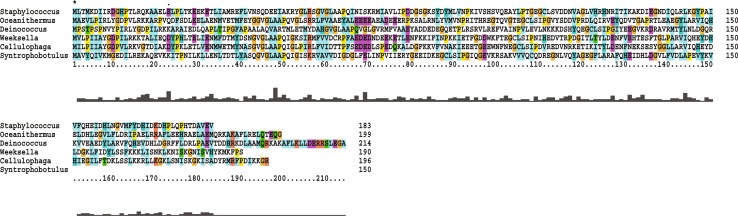
Fig. 2.
Multiple sequence alignment of PDF of S. aureus ATCC12600 with PDFs of other bacteria. “Asterisk” indicates the conserved regions
Table 1.
Enzyme kinetics of PDF
| S. no | Crude PDF activity | rPDF activity | PDF activity with iron |
|---|---|---|---|
| K M | 1.4 mM | 1.3 mM | 0.1 mM |
| V max | 0.030 mM/mg/min | 0.035 mM/mg/min | 0.053 mM/mg/min |
| Specific activity | 0.007 mM of NADH/mg/min | 0.015 mM of NADH/mg/min | 0.069 mM of NADH/mg/min |
The PDF activity remains the same in the crude and recombinant fractions; however, it has been increased with iron supplementation
Effect of iron on PDF activity
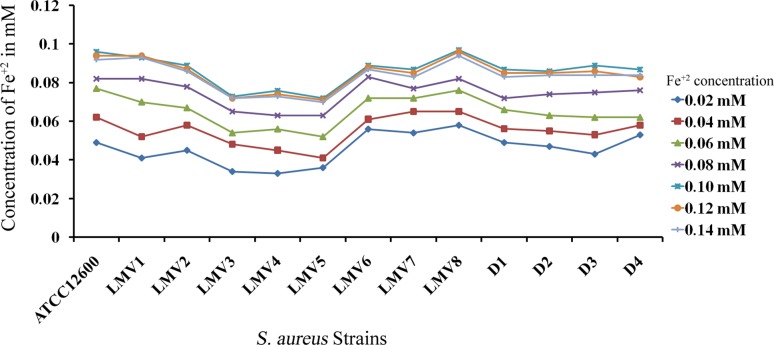
To examine the effect of iron on S. aureus growth, the culture was supplemented with increasing concentrations of iron and absorbance was recorded (Fig. 3). The PDF activity was assessed in the cytosolic fractions of these iron-supplemented cultures. The kinetic results revealed that PDF activity increased steadily with iron supplementation up to 0.1 mM, and beyond this concentration the PDF activity remained constant. Similar results were recorded for PDF in all the S. aureus strains used in the study (Swarupa et al. 2017) (Table 2).
Fig. 3.
Growth curves of S. aureus and other drug-resistant strains supplemented with different concentrations of iron. LMV1 to LMV8 are the S. aureus isolates from raw milk samples of local milk vendors. D1–D4 are S. aureus isolates from dairy herds. All strains showed maximum absorbance at 0.1 mM of iron concentration
Table 2.
Biofilm-forming units of S. aureus and other drug-resistant strains
| S. no | Strain type | Biofilm-forming units in LB (BU) | Biofilm-forming units in BHI (BU) | Biofilm-forming units in culture supplemented with iron (BU) |
|---|---|---|---|---|
| 1 | S. aureus ATCC12600 | 0.023 ± 0.005 | 0.42 ± 0.005 | 6.3 ± 0.05 |
| 2 | LMV1 | 0.021 ± 0.0052 | 0.423 ± 0.0045 | 6.31 ± 0.06 |
| 3 | LMV2 | 0.021 ± 0.0044 | 0.427 ± 0.0051 | 6.34 ± 0.052 |
| 4 | LMV3 | 0.018 ± 0.0045 | 0.392 ± 0.0052 | 5.78 ± 0.051 |
| 5 | LMV4 | 0.017 ± 0.0053 | 0.375 ± 0.0049 | 5.63 ± 0.049 |
| 6 | LMV5 | 0.018 ± 0.0051 | 0.386 ± 0.0055 | 5.24 ± 0.045 |
| 7 | LMV6 | 0.021 ± 0.0053 | 0.412 ± 0.0052 | 6.18 ± 0.055 |
| 8 | LMV7 | 0.023 ± 0.0046 | 0.423 ± 0.0051 | 6.23 ± 0.051 |
| 9 | LMV8 | 0.022 ± 0.0048 | 0.428 ± 0.0052 | 6.28 ± 0.064 |
| 10 | D1 | 0.021 ± 0.0049 | 0.431 ± 0.0059 | 6.34 ± 0.052 |
| 11 | D2 | 0.021 ± 0.0051 | 0.437 ± 0.0052 | 6.41 ± 0.054 |
| 12 | D3 | 0.024 ± 0.0047 | 0.424 ± 0.0060 | 6.39 ± 0.049 |
| 13 | D4 | 0.022 ± 0.0052 | 0.429 ± 0.0054 | 6.25 ± 0.053 |
S. aureus and other drug-resistant strains showed increased biofilm-forming units in anaerobic conditions compared to the strains grown under aerobic conditions. Also, strains supplemented with iron showed 15-fold higher expression of BU
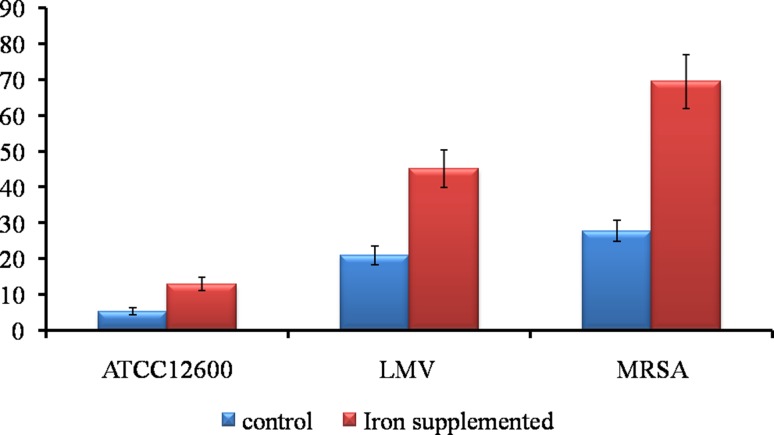
The real-time PCR expression analysis revealed iron supplementation increased the PDF expression by 142.6-fold in S. aureus ATCC12600, while in drug-resistant strains of S. aureus LMV (local milk vendors) 116-fold of elevated expression was observed. Interesting results were seen in MRSA strains (LMV3-5) where 150.4-fold of increased PDF expression was observed compared to the control (cultures without iron supplement) (Fig. 4).
Fig. 4.
Real-time PCR analysis showing the quantitative expression of PDF in S. aureus ATCC12600 and other drug-resistant strains LMV and MRSA. Control represents the cultures untreated with iron
Biofilm-forming assay
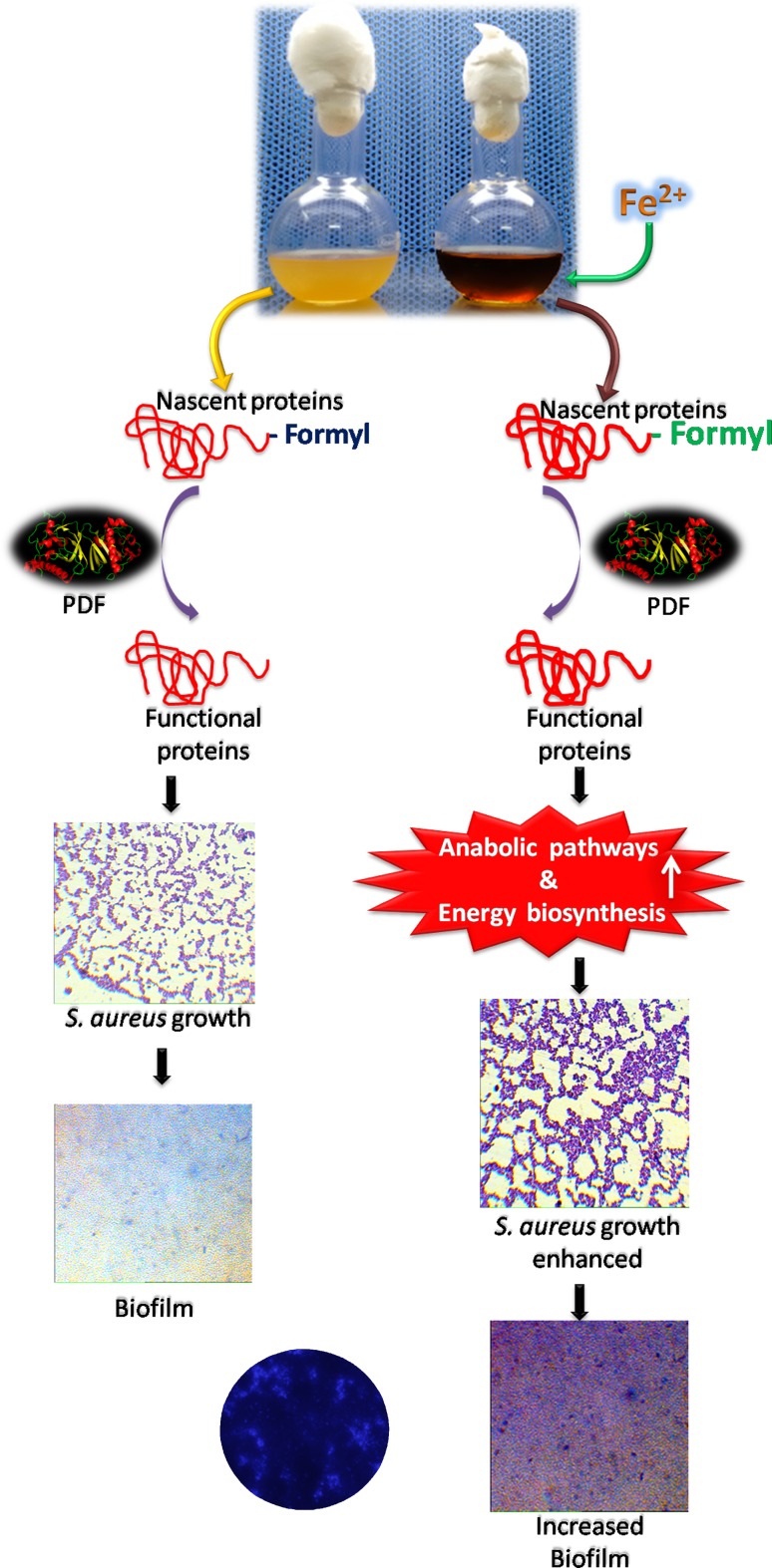
Biofilm-forming assay was carried out in a microtiter plate as described by the Coffey and Anderson method (Coffey and Anderson 2014; Yeswanth et al. 2013; Swarupa et al. 2017). 0.2 ml of fully grown cultures of S. aureus ATCC12600 and other drug-resistant strains of S. aureus were taken in 96-well microtiter plates and incubated for 1 day. The unbound bacteria were rinsed off, whereas the bound bacteria were stained with crystal violet to visualize the biofilm. Further, these biofilms were solubilized in broth and quantified spectrophotometrically. Interesting results were obtained showing increased biofilm units in all the S. aureus strains grown in the BHI broth compared with LB. Also when the culture media was supplemented with iron, it showed 15-fold enhanced elevation in BU correlating with elevated PDF activity (Table 2). The calcofluor white staining also showed that the biofilm units had increased with iron supplementation (Fig. 5).
Fig. 5.
Growth regulation and biofilm establishing property of S. aureus with/without iron supplementation. PDF catalyzes the functional protein synthesis that aids in biofilm formation. The culture, supplemented with iron, enhances the PDF activity which further increases growth and biofilm formation
Discussion
The emergence of multidrug-resistant strains has complicated the management and treatment of S. aureus infections. Moreover, many of these chronic infections are associated with bacterial growth in the form of adherent colonies encased in a large matrix formed mainly of proteins that constitutes a biofilm (Amaral et al. 2005). S. aureus biofilm-forming ability is one of the most important virulence mechanisms that not only allows the bacteria to adhere to biotic and abiotic surfaces; but also reduces the phagocytic ability thereby providing immunoprotective function (Costerton et al. 1995; Begun et al. 2007; Götz 2002; Vuong et al. 2004). Among others, FnbA, FnbB, (fibronectin-binding proteins) SasC, SasG (surface proteins), SpaA (IgG-binding protein), and Bap (biofilm-associated protein) are important in promoting biofilm formation. In addition, several regulatory proteins have been shown to affect biofilm formation (O’gara 2007). Although several external factors influence the attachment and accumulation of bacteria, polysaccharide intracellular adhesion protein (PIA) plays a crucial role in forming the biofilm matrix; however, PIA-deleted mutants still possessed similar abilities to form biofilms (Otto 2008).
Staphylococcus aureus expresses several different MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) that have the ability to bind to human matrix proteins such as fibrinogen and fibronectin (Merino et al. 2009). In addition, the extracellular proteases have been shown to affect biofilm formation. The AraC and XylS family regulator Rsp represses biofilm formation by suppressing the cell wall-anchored proteins. Here, Rsp represses biofilm formation by downregulating the FnbA and Spa proteins. It is noteworthy to mention here that Spa-deleted S. aureus did not form biofilms and also S. aureus grown in the presence of IgG did not show biofilm formation. However on providing Protein A to the media, the biofilm formation was restored (Merino et al. 2009). In addition, Rsp upregulates a number of genes encoding proteases, which could have an impact on S. aureus biofilms by cleaving the cell surface proteins and secreted proteins, both at the post-translational stage and at the mature functional stage (Lei et al. 2011). Thus, proteins involved in post-translational modifications forming mature proteins are important in the formation of S. aureus biofilms (Islam et al. 2014). Several studies have emphasized that proteins play a pivotal role in the formation of the biofilm matrix and polypeptide synthesis follows its maturation to form a functional protein molecule. For each protein, deformylation is the most critical step which is catalyzed by the action of PDF. It removes the formyl group from formylated methionine, forming mature proteins (Swarupa et al. 2017). PDF, being an iron-dependent enzyme, shows positive regulation in its presence. Thus, the current study aims at understanding the iron-responsive regulation on PDF activity and biofilm formation. Hence, the gene encoding PDF was amplified from S. aureus genomic DNA as the template strand (Swarupa et al. 2017). The results showed a 0.5 kb gene product, confirming the presence of PDF (Fig. 1). Further kinetic characterization results showed that the deformylase activity of native PDF remained very close to rPDF; however, the activity is increased with the addition of iron (Table 1). Iron acts as an electron-withdrawing group by interacting with four water molecules favoring the formation of the hydroxide ligand. Further, it allows the interconversion between tetra and penta co-ordination, resulting in enhanced activity of the substrate. These results were further corroborated with qPCR results (Table 2, Fig. 4). Iron also downregulates the enzymes of TCA cycle such as isocitrate dehydrogenase and aconitase, thus creating high reductive conditions leading to more anabolic biosynthesis (Ledala et al. 2014; Prasad et al. 2013; Somerville et al. 2003), which affects the biofilm mode of growth in S. aureus. It appears that, in addition to bacterial growth, iron is essential for energy generation in the system by signaling the nucleotide synthesis and changing the gene expression profiles. Thus, iron is important for primary attachment and PIA synthesis (Lin et al. 2012). Numerous recent studies imply that in biofilms, multicellular organization is important to fight against unfavorable external conditions. On the other hand, anaerobic conditions enhance the transcription of the ica gene, thereby increasing the expression of PIA protein and ultimately leading to elevated biofilm formation (Boles et al. 2010; Rachid et al. 2000a, b) in S. aureus and other drug-resistant strains tested (Table 2, Fig. 5).
Conclusion
Staphylococcus aureus forms biofilms on various surfaces through attachment and accumulation of bacteria which is regulated by the expression of several proteins. The protein formation in S. aureus starts with deformylation, which is the essential step catalyzed by PDF. Iron is the key metal required for the proper functioning of PDF, which in turn influences bacterial growth, energy production and nucleotide biosynthesis, all of which have an influence on the genesis of the biofilm. Increased PDF activity is correlated with active synthesis of proteins in the organism, paving the way to the formation of biofilms.
Acknowledgements
We sincerely acknowledge Sri Venkateswara Institute of Medical Sciences (SVIMS University) for providing facilities to carry out the Ph.D. work. This paper forms a part of the Ph.D. thesis work submitted to the SVIMS University, Tirupati, Andhra Pradesh, India.
Funding
We are highly thankful to the Indian Council of Medical Research (ICMR), for providing research fund to carry out the work (File no: 80/832-2013-ECD-I), New Delhi. This work was also supported by Sri Balaji Arogya Vara Prasadini scheme (SBAVP) (Grant no: SBAVP-RG/Ph.D/02).
Compliance with ethical standards
Conflict of interest
No conflict of interest declared.
References
- Amaral MM, Coelho LR, Flores RP, Souza RR, Silva-Carvalho MC, et al. The predominant variant of the Brazilian epidemic clonal complex of methicillin-resistant Staphylococcus aureus has an enhanced ability to produce biofilm and to adhere to and invade airway epithelial cells. J Infect Dis. 2005;192(5):801–910. doi: 10.1086/432515. [DOI] [PubMed] [Google Scholar]
- Baird-Parker AC. An improved diagnostic and selective medium for isolating coagulase positive Staphylococci. J Appl Bacteriol. 1962;25:12–19. doi: 10.1111/j.1365-2672.1962.tb01113.x. [DOI] [Google Scholar]
- Banin E, Vasil ML, Greenberg EP. Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA. 2005;102:11076–11081. doi: 10.1073/pnas.0504266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begun J, Gaiani JM, Rohde H, Mack D, Calderwood SB, et al. Staphylococcal biofilm exopolysaccharide protects against Caenorhabditis elegans immune defenses. Plos Pathog. 2007;3(4):e57. doi: 10.1371/journal.ppat.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4(4):e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Thoendel M, Roth AJ, Horswill AR. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5(4):e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey BM, Anderson GG. Biofilm formation in the 96-well microtiter plate. Methods Mol Biol. 2014;1149:631–641. doi: 10.1007/978-1-4939-0473-0_48. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Cucarella C, Tormo MA, Ubeda C, Trotonda MP, Monzón M, et al. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun. 2004;72(4):2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Islam N, Kim Y, Ross JM, Marten MR. Proteomic analysis of Staphylococcus aureus biofilm cells grown under physiologically relevant fluid shear stress conditions. Proteome Sci. 2014;12:21. doi: 10.1186/1477-5956-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23(10):403–405. doi: 10.1016/S0968-0004(98)01285-7. [DOI] [PubMed] [Google Scholar]
- Johnson M, Cockayne A, Morrissey JA. Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect Immun. 2008;76(4):1756–1765. doi: 10.1128/IAI.01635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec C, Meinnel T. Formate dehydrogenase-coupled spectrophotometric assay of peptide deformylase. Anal Biochem. 1997;244(1):180–182. doi: 10.1006/abio.1996.9910. [DOI] [PubMed] [Google Scholar]
- Ledala N, Zhang B, Seravalli J, Powers R, Somerville GA. Influence of iron and aeration on Staphylococcus aureus growth, metabolism, and transcription. J Bacteriol. 2014;196(12):2178–2189. doi: 10.1128/JB.01475-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds JA, Dean CR. Peptide deformylase as an antibacterial target: a critical assessment. Curr Opin Pharmacol. 2006;6(5):445–452. doi: 10.1016/j.coph.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Lei MG, Cue D, Roux CM, Dunman PM, Lee CY. Rsp inhibits attachment and biofilm formation by repressing fnbA in Staphylococcus aureus MW2. J Bacteriol. 2011;193(19):5231–5241. doi: 10.1128/JB.05454-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembke C, Podbielski A, Hidalgo-grass C, Jonas L, Hanski E, Kreikemeyer B. Characterization of biofilm formation by clinically relevant serotypes of group A streptococci. Appl Environ Microbiol. 2006;72(4):2864–2875. doi: 10.1128/AEM.72.4.2864-2875.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MH, Shu JC, Huang HY, Cheng YC. Involvement of iron in biofilm formation by Staphylococcus aureus. PLoS One. 2012;7(3):e34388. doi: 10.1371/journal.pone.0034388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, et al. Protein A-mediated multicellular behaviour in Staphylococcus aureus. J Bacteriol. 2009;191(3):832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey AR, Craig SA, Payne SM. Characterization of Vibrio cholerae RyhB: the RyhB regulon and role of ryhB in biofilm formation. Infect Immun. 2005;73:5706–5719. doi: 10.1128/IAI.73.9.5706-5719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270(2):179–188. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata K, Rosato AE, Wegrzyn G. Staphylococcus aureus as an infectious agent: overview of biochemistry and molecular genetics of its pathogenicity. Acta Biochim Pol. 2009;56(4):597–612. [PubMed] [Google Scholar]
- Prasad UV, Vasu D, Kumar YN, Kumar PS, Yeswanth S, et al. Cloning, expression and characterization of NADP-dependent isocitrate dehydrogenase from Staphylococcus aureus. Appl Biochem Biotechnol. 2013;169(3):862–869. doi: 10.1007/s12010-012-0027-8. [DOI] [PubMed] [Google Scholar]
- Rachid S, Cho S, Ohlsen K, Hacker J, Ziebuhr W (2000) Induction of Staphylococcus epidermidis biofilm formation by environmental factors: the possible involvement of the alternative transcription factor SigB. In Emödy L, Blum-Oehler, Hacker J, Pal T (eds) Genes and proteins underlying microbial urinary tract virulence. Plenum Press, New York, pp159–166 [DOI] [PubMed]
- Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesion expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44(12):3357–3363. doi: 10.1128/AAC.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville GA, Cockayne A, Dürr M, Peschel A, Otto M, et al. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J Bacteriol. 2003;185(22):6686–6694. doi: 10.1128/JB.185.22.6686-6694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth L, Sunitha MM, Venkatesh K, Santhosh Kumar P, Chandrasekhar C, Vengamma B, Sarma PVGK. Anaerobic glycolysis and HIF1α expression in haematopoietic stem cells explains its quiescence nature. J Stem Cells. 2015;10(2):97–106. [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Swarupa V, Rupa Sundari A, Prasad UV, Yeswanth S, Hari Prasad O, et al. Identification of methicillin resistant Staphylococcus aureus in raw cow milk through amplification of mecA. J Pure Appl Microbiol. 2014;8:4909–4915. [Google Scholar]
- Swarupa V, Chaudhary A, Sarma PV. Effect of 4methoxy 1methyl 2oxopyridine 3carbamide on S. aureus by inhibiting UDPMurNAc-pentapeptide, peptidyl deformylase and uridine monophosphate kinase. J Appl Microbiol. 2017;122(3):663–675. doi: 10.1111/jam.13378. [DOI] [PubMed] [Google Scholar]
- Venkatesh K, Srikanth L, Vengamma B, Chandrasekhar C, Prasad BC, Sarma PV. In vitro transdifferentiation of human cultured CD34+ stem cells into oligodendrocyte precursors using thyroid hormones. Neurosci Lett. 2015;588:36–41. doi: 10.1016/j.neulet.2014.12.050. [DOI] [PubMed] [Google Scholar]
- Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182:2675–2679. doi: 10.1128/JB.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Outten FW. IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J Bacteriol. 2009;191:1248–1257. doi: 10.1128/JB.01086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeswanth S, Nanda kumar Y, Venkateswara Prasad U, Swarupa V, Koteswara rao V, et al. Cloning and characterization of l-lactate dehydrogenase gene of Staphylococcus aureus. Anaerobe. 2013;24:43–48. doi: 10.1016/j.anaerobe.2013.09.003. [DOI] [PubMed] [Google Scholar]