Abstract
Dendritic cells are special and powerful antigen‐presenting cells that can induce primary immune responses against tumour‐associated antigens. They can present antigens via both MHC‐I and MHC‐II, so they have the ability to stimulate both cytotoxic T lymphocytes and T helper cells. Furthermore, CD8+ cytotoxic T lymphocytes require activation by CD4+ T cells. This requires a CD4+T cell activator molecule, of which PADRE is one of the best. We chose an approach to use both of these important arms of the immune system. We prepared dendritic cells from mouse bone marrow, loaded them with our target peptides (P5 peptide alone or P5 + PADRE), and then injected these pulsed dendritic cells alone or in combination with CpG‐ODN (as adjuvant) into BALB/C mice. After the last boosting dose, mice were inoculated with TUBO cells, which overexpress HER2/neu. Two weeks after the tumour cell injection, immunological tests were performed on splenocyte suspensions, and the remaining mice were evaluated for tumour growth and survival. Our data indicate the formulation that contains PADRE plus P5 loaded onto DC in combination with CpG‐ODN was the most effective formulation at inducing immune responses. Interferon production in CD4+ and CD8+ gated cells, cytotoxicity rates of target cells and mice survival were all significantly greater in this group than in controls, and all the mice in this group were tumour‐free throughout the experiment. Based on our results and the role of HER2/neu as a candidate in human immunotherapy, this approach may be an effective cancer treatment.
Keywords: dendritic Cells, peptide vaccine, pan HLA‐DR epitope peptide, cancer
Introduction
Dendritic cells play critical roles in initiating and modulating adaptive T cell responses 1, 2, 3. They induce T cells via their ability to take up, process and present antigens and produce cytokines and chemokines 4, 5. Dendritic cells are the only antigen‐presenting cells (APCs) that are able to prime naïve T cells. Cross‐presentation of antigens via DCs is most likely the primary mechanism of CD8+ response induction 6, 7. DCs appear in two forms in their life‐times; immature DCs can take antigens, process them and present the antigen‐derived peptides on their major histocompatibility complex (MHC) molecules. Subsequently, DCs change to a phenotypically mature form, which can be distinguished by the increased expression of certain cell surface markers, including CD40, CD80 and CD86 8, 9.
One unique property of DCs is their ability to migrate from environmental sites of pathogen entrance to T cell sites in lymph nodes 10, where they prepare naïve T cells via antigen‐specific and costimulator signals. As a result, the immune system can identify invading agent molecules and their pathogenic strengths 11. In vitro DCs are derived from CD34+ precursor cells or CD14+ monocytes 12 using granulocyte–macrophage colony‐stimulating factor (GM‐CSF) 13 and other cytokines, such as IL‐4. These cytokines inhibit macrophage differentiation and induce monocyte‐derived DC production 14.
In addition to using DCs to induce specific immune responses, one well‐known strategy to increase peptide vaccine strength is to induce CD4+ T cells that have important roles in CD8+ and memory T cell responses. CD8+ T cells are especially important for responses to weakly immunogenic antigens such as tumour‐associated antigens (TAAs) 15, 16. One of the most effective molecules used to induce CD4+ responses is the pan HLA‐DR epitope peptide (PADRE) 17. PADRE is a universal, non‐specific MHC class II‐restricted epitope able to attach to more than 16 types of common HLA‐DR, I‐A b/d and I‐E b/d mouse haplotypes with high affinity. This allows it to overcome the problem of HLA polymorphism 18, 19, 20. It also has shown in clinical trials minimum toxicity 18, 21. Another group of molecules that can improve vaccine immune responses are unmethylated CpG motifs that are used as vaccine adjuvants. CpG motifs are recognized by Toll‐like receptor 9 (TLR9) and increase innate immune responses such as pro‐inflammatory cytokine release and Th1 production. Because of their stability, low cost and ease of production, CpGs are attractive to use in immune system studies 22. CpGs also increase professional APC function and generate both humoural and cellular specific immune responses 23, 24.
Based on our previous study results, the P5 peptide can induce cytotoxic T lymphocyte (CTL) responses in mice bearing HER2‐positive tumours 25. P5 peptide is derived from rat HER2/neu protein (also known as p185 or c‐erb‐B2) with 21 amino acid length (aa 5–25). The murine c‐erbB‐2 shows 93.4% homology at the nucleotide level and 94.8% homology at the amino acid level with rat c‐erbB‐2. Rat HER2/neu is 96% homologous to mouse HER2/neu and 88% homologous to human HER2/neu in overall(1).
ELAAWCRWGFLLALLPPGIAG. Amino acids in boldface type are those in rat HER2/neu, which are different from those in HER2/neu murine sequence 25. The goal of the current study was to overcome the peptide vaccine limitations, such as its weak immunogenicity 26, binding to non‐professional APCs, and rapid degradation by tissue and serum peptidases 27. Here, DCs were prepared in vitro from mouse bone marrow stem cells. Target peptides were loaded onto the prepared DCs, and pulsed DCs with or without CpG‐ODN were injected into mice. Immunological in vitro tests were performed on mice splenocyte suspensions to assess the immune responses. Mice inoculated with TUBO overexpressing HER2/neu cells were analysed for tumour growth and survival.
Materials and methods
Mice
BALB/C, 4‐ to 6‐week‐old female mice were bought from the Pasteur Institute (Tehran, Iran). The mice were kept in the animal house of the Pharmaceutical Research Center (Mashhad, Iran) under controlled conditions of 23°C room temperature, relative humidity of 65% in 12/12‐hr light/dark cycles with free access to water and animal food. All animal experiments were carried out under the approval of the Institutional Ethical Committee and Research Advisory Committee of Mashhad University of Medical Sciences.
Cell lines
TUBO cells, a cloned cell line that overexpresses HER2/neu, kindly provided by Dr. Pier‐Luigi Lollini (Department of Clinical and Biological Sciences, University of Turin, Orbassano, Italy), were cultured in Dulbecco's modified Eagle's medium (DMEM) and supplemented with 20% foetal bovine serum (FBS). A murine colon carcinoma cell line, CT26, was purchased from the Pasteur Institute and cultured in RPMI‐1640 medium supplemented with 10% FBS.
Peptides, protein and oligonucleotide
The P5 (ELAAWCRWGFLLALLPPGIAG) and PADRE (AKFVAAWTLKAAA) peptides were synthesized by Peptron Inc. (Daejeon, South Korea). OVA‐FITC protein was purchased from Invitrogen (Carlsbad, CA, USA). CpG‐ODN1826 (5‐TCCATGACGTTCCTGACGTT‐3) was purchased from Mycrosynth (Balgach, Switzerland) 28.
Generation of DCs
Dendritic cells were generated via a 7‐day protocol. Briefly, on day 0 the mouse bone marrow cells were collected and cultured in Iscove's modified Dulbecco's media (IMDM) (Invitrogen) supplemented with 10% FCS (Gibco, Gaithersburg, MD, USA) containing 25 ng/ml of GM‐CSF and 5 ng/ml of IL‐4. On days 3 and 5, cells were seeded in new plates. On day 7, 1 μg/ml of lipopolysaccharide (LPS) was added to the cells and the cultures were incubated at 37°C for 6 hrs. After 6 hrs, the DCs were mature.
Peptide pulsing of DCs
2.5 μg/ml of each peptide was added to the mature DCs and incubated for 1 hr at 37°C.
Immunization
After the incubations with peptides, the DCs were washed and counted. 5 × 105 of these DCs containing 0.5 μg of peptide were injected into each mouse. Vaccinations with peptide alone were performed with 100 μg/mouse.
BALB/C mice (Eight per group) were subcutaneously injected three times at 2‐week intervals with the following formulas: buffer (as a control group), CpG, P5, P5 plus CpG, PADRE, PADRE plus CpG, DCs pulsed with P5, DCs pulsed with P5 plus CpG, DCs pulsed with P5 plus PADRE or DCs pulsed with P5 plus PADRE plus CpG.
ELISpot
ELISpot assays were performed to assess IFN‐ production from splenocytes in response to the different formulas as previously described with mouse ELISpot kits from U‐CyTech (Utrecht, the Netherlands) 29. Briefly, ELISpot plates were coated with anti‐IFN‐ antibody and incubated overnight at 4°C. The following day, splenocyte suspensions of 3 × 105, 2 × 105 and 1 x 105 cells were added to the wells and incubated at 37°C for 24 hrs. Spots were counted with the Kodak 1D software package (version 3.5, Eastman Kodak, Rochester, New York) in triplicate wells and the mean ± S.E.M. was expressed as spot‐forming units (SFUs)/106 splenocytes.
In vitro CTL activity assay
Using ex vivo‐expanded splenocytes, in vitro CTL assays were performed as described previously 25.
Briefly, TUBO or CT26 (as negative control) cells were resuspended in DMEM with 20% FBS. Calcein AM (Invitrogen) at a final concentration of 12.5 μM was added to the cells and the cells were incubated for 1 hr in the dark at 37°C. Target cells loaded with Calcein AM (1.2 × 105 cells/well) were cocultured with the three different concentrations of splenocytes for 4 hrs at 37°C. The fluorescence intensity was measured at 485 nm of excitation and 538 nm of emission via a fluorescent plate reader (FLX 800, Bio‐Tek Instruments Inc. Beverly, MA, USA). The mean percentage of the triplicate wells was calculated by the following formula: [(release by CTL − release by targets alone)/(release by 2% Triton X‐100 − release by targets alone)] ×100.
Intracellular cytokine assay via flow cytometry
Splenocytes (106 cells/ml) in a medium containing 1 μl/ml Golgi Plug™ with 2 μg/ml PMA/ionomycin cocktail were stimulated at 37°C for 4 hrs. Cell surface markers were stained with anti‐phytoerythrin (PE)‐cy5 CD4 and anti‐PE‐cy5 CD8 antibodies and incubated. The cells were then stained with anti‐PE‐IL4 and FITC anti‐IFN‐ antibodies (Cytofix/Cytoperm™; BD Biosciences, San Jose, CA, USA) and analysed by FACSCalibur (BD Biosciences). All antibodies were from BD Biosciences.
Uptake assay
To assess the protein uptake by prepared DCs, mature DCs were incubated with 2.5 μg/ml of OVA‐FITC at 37 and 4°C (as a control) for 1 hr. Antigen uptake by DCs was analysed by FACS and the mean fluorescence intensity (MFI) measured.
In vivo challenge of immunized mice with TUBO cells
To study tumour growth, 2 weeks after the last boost, vaccinated mice were inoculated subcutaneously with 5 × 105 TUBO cells in their right flanks. The tumours were measured with a calliper and volumes were calculated using the formula (a × b × c) × 0.5 mm3 25.
Mice were monitored for 100 days after injection.
Statistical analysis
The results were analysed using one‐way anova with GraphPad Prism version 6 (GraphPad software, San Diego, CA, USA). In the case of a significant P value, the multiple comparison Tukey test was used to compare the means of different formulas.
The log‐rank test (GraphPad Prism, version 6) was used to analyse mice survival. When P < 0.05, results were considered significant.
Results
Phenotype of mature and immature DCs
On day 5 and 7, DCs were harvested and characterized using DC surface marker antibodies (anti‐CD11c, anti‐CD40, anti‐CD80, anti‐CD86 and anti‐IA/IE). As shown in Figure 1, MHC and costimulatory molecule expression are greater in mature than in immature DCs and most of the DCs were mature after LPS stimulation.
Figure 1.
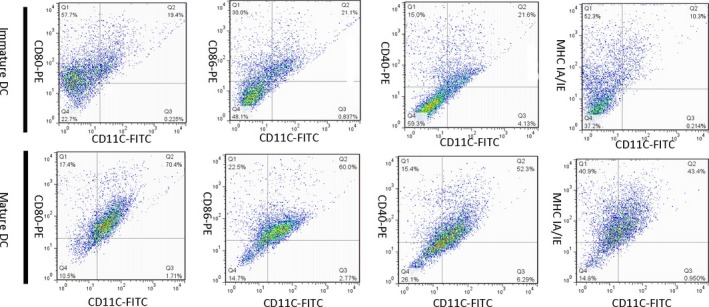
CD11c, CD40, CD80 (B7‐1), CD86 (B7‐2) and MHC IA/IE expression on immature and mature DCs. Expression of these markers is greater in mature than in immature DCs. Dendritic cells were generated from mouse bone marrow and matured by the addition of LPS. The dot plot represents cell percentages in the mature and immature states.
Uptake assay
The endocytic capability of prepared DCs at 37°C was greater than at 4°C and the geometric means at 37 and 4°C were 382 and 87.7, respectively (Fig. 2). Overall, the generated DCs took up peptides with high efficacy.
Figure 2.
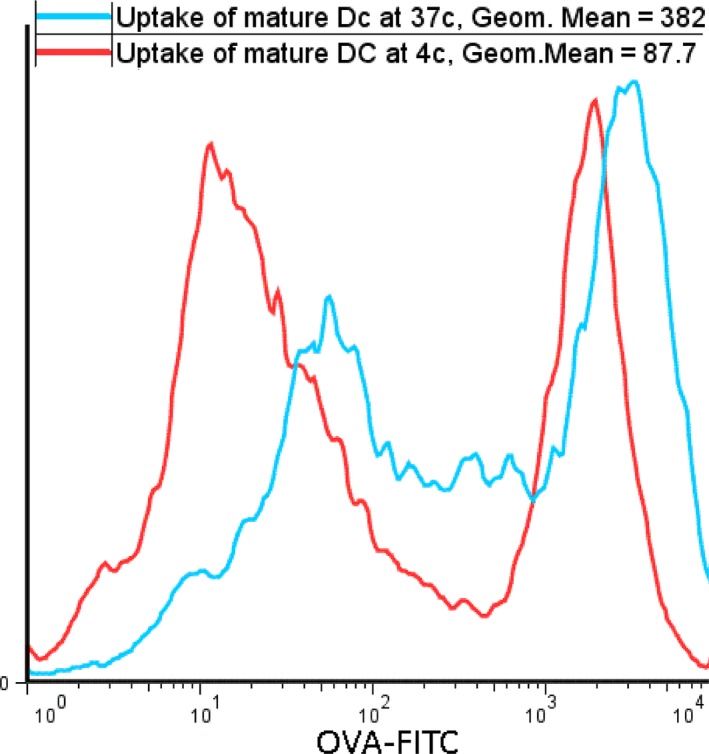
Uptake of OVA‐FITC by DCs. The uptake assay was performed to analyse the endocytic ability of prepared DCs. Dendritic cells took up more OVA‐FITC 37°C than at 4°C. The fluorescent intensity corresponds to the uptake.
Intracellular cytokine assay via flow cytometric analysis
To analyse the effect of the different formulas on induction of CD4+ and CD8+ T cells, their cytokine profiles were assessed. Induction of IFN‐γ by CD8+ cells by P5 and PADRE peptides alone or with CpG was not significantly different from control. However, IFN‐γ release was significantly increased when the peptides were loaded onto DCs. Immunization with P5 plus PADRE‐loaded DCs along with CpG stimulated IFN‐γ secretion by CTLs, and significantly more IFN‐γ was detected in gated CD8+ cells than in the control or other treatment groups (P < 0.001) (Fig. 3C). A similar significant effect was observed in the IFN‐γ‐producing CD4+ cells (Fig. 3A) (P = 0.0005). In contrast, IL‐4 production was not affected in CD4+ cells, indicating that humoural immunity was not significantly induced in the vaccinated groups (Fig. 3B).
Figure 3.
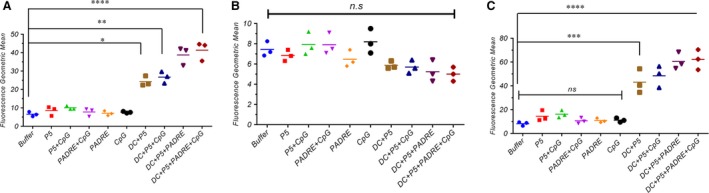
Geometric mean fluorescence intensity (MFI) of IFN‐γ in gated CD4+ cells (A), IL‐4 in gated CD4+ cells (B) and IFN‐γ in gated CD8+ cells (C). Cytokine profile frequency was analysed by FACS analysis to determine the immune response rate. Splenocytes were stained by anti‐CD4 and anti‐CD8 antibodies. After stimulation with PMA and ionomycin, cells were stained with anti‐IFN‐γ and anti‐IL‐4 antibodies. Data are expressed as the mean ± S.E.M. (n = 3). Results were analysed by one‐way anova, and statistically significant differences are designated as follows: ns: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
In vitro IFN‐γ assay by ELISpot
P5 alone on DCs caused no statistically significant increase in IFN‐γ; however, DCs loaded with both P5 and PADRE peptides and co‐administered with CpG resulted in significantly greater IFN‐γ production from T cells than controls, which supports the flow cytometry result (P < 0.001) (Fig. 4). However, the level of IFN‐γ in pulsed DCs with peptides without CpG was slightly less than with this formulation with CpG. Our data indicate that the immune responses in mice vaccinated with DCs loaded with both of two targeted peptides are significantly greater than peptides alone or DCs loaded with P5 alone.
Figure 4.
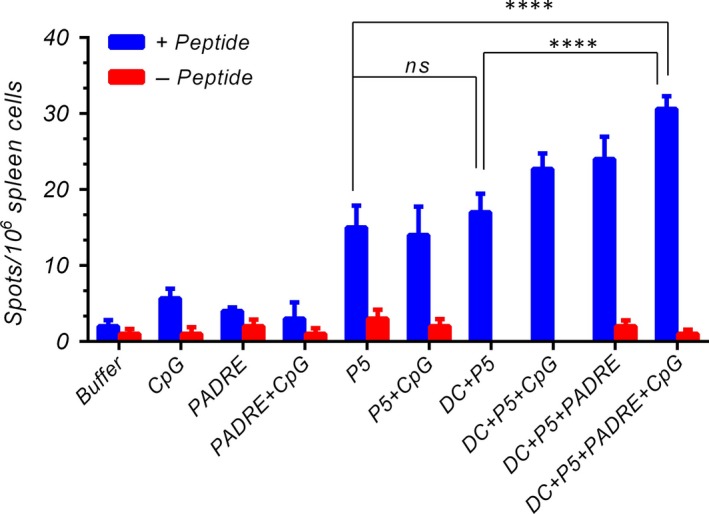
In vitro IFN‐γ production was analysed in vaccinated mice 2 weeks after the last booster by ELISpot assay. Splenocytes were harvested and treated with (+peptide) or without peptides (‐peptide) and the IFN‐γ secretion was determined. The data indicate the mean ± S.E.M. (n = 3). Statistical significances were designated as follows: ns: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Cytotoxicity assay
The specific responses of CTL cells against cancer cells in groups immunized with pP5 or P5 + CpG or PADRE and PADRE + CpG were no greater than those of the control group (Fig. 5). As expected, vaccination with DCs + P5 + PADRE + CpG was best able to induce CTLs to recognize and kill the TUBO cells (P < 0.001). At all E/T ratios, the per cent of TUBO cell killing by CD8+ cells was greatest with this formula. CTLs did not kill HER2/neu‐negative CT26 cells, indicating that the cytotoxic response was antigen‐specific.
Figure 5.
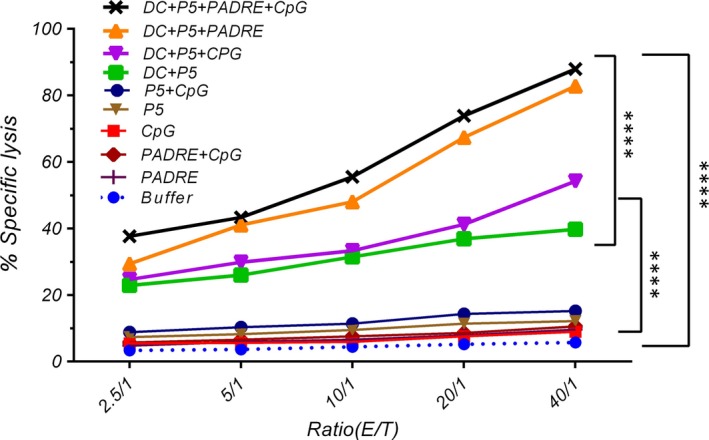
Induction of specific CTL potency for tumour cell killing by the different formulations was assessed by in vitro CTL activity assay. Five different effector to target cell ratios were tested. HER2/neu‐expressing TUBO cells and CT26 cells (as a negative control) were labelled by Calcein AM and co‐incubated with different ratios of splenocytes. The data are expressed as means ± S.E.M.s (n = 3). E: effector cells and T: target cells. One‐way anova was employed and statistically significant differences are shown as follows: ns: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Tumour size and survival
Pulsed DCs showed significantly greater antitumour activity than the control group (P < 0.001). P5 or PADRE alone as a peptide vaccine with or without CpG inhibited tumour growth significantly less than the same peptide loaded on DCs (Figs 6 and 7A).
Figure 6.
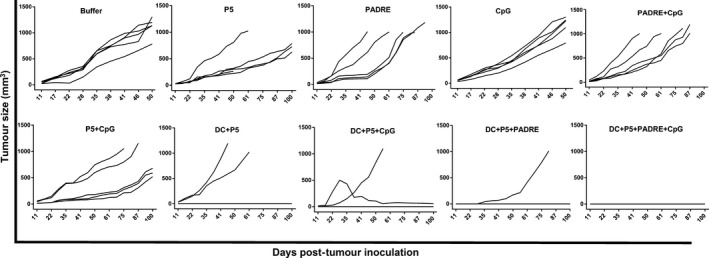
Results of the formulations on TUBO tumour growth in mice. Two weeks after the final boosting dose, TUBO cells were injected into mice (n = 5). Tumours were measured and volumes recorded weekly. The values are means. Mice were monitored for 100 days. Data were analysed by the two‐way anova test and statistical significances were designated as follows: Ns: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Figure 7.
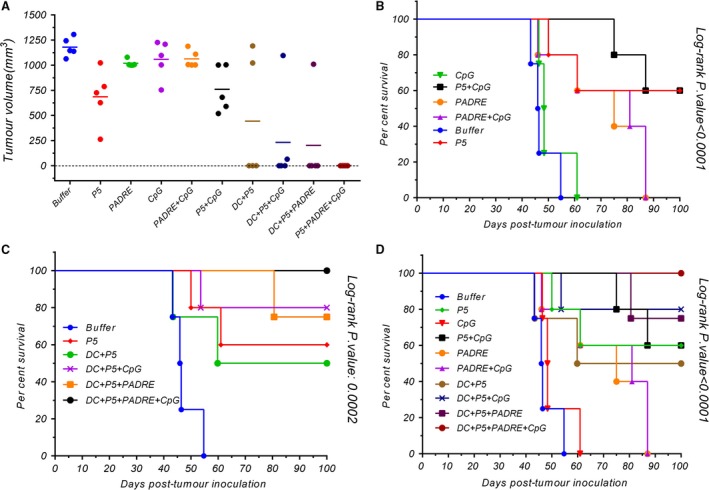
The tumour mass scale based on tumour size in inoculated mice on their last survival days (A), and their survival (B, C and D) were monitored by the multiple comparison log‐rank (Mantel–Cox) test. Statistical significances were designated as follows: ns: P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
The DC + P5 + PADRE + CpG formulation completely inhibited tumour growth. All the mice vaccinated with this formulation were tumour‐free throughout the study, while no mice immunized with peptides alone that survived until day 100 were tumour‐free.
Table 1 displays the different formulations and their corresponding times to reach end‐points (TTE), percentages of tumour growth delay (%TGD) and median survival times (MST). Median survival times were undefined in groups in which most or all the mice survived the duration of the experiment (Table 1).
Table 1.
Beneficial effect of each formulation in immunized mice
| Groups | TTE (days ± S.D.)a | %TGDb | MST(day)c |
|---|---|---|---|
| Buffer | 47.59 ± 4.97 | – | 46.18 |
| P5 | 77.11 ± 26.65 | 29.01 | Undefined |
| PADRE | 92.36 ± 11.33 | 20.88 | 75 |
| CpG | 50.98 ± 6.69 | 6.66 | 48.3214 |
| PADRE + CpG | 69.96 ± 17.73 | 21.76 | 81 |
| P5 + CpG | 92.36 ± 11.33 | 40.74 | Undefined |
| DC + P5 | 75.80 ± 12.96 | 37.22 | 64.39 |
| DC + P5 + CpG | 90.72 ± 6.89 | 47.55 | Undefined |
| DC + P5 + PADRE | 95.16 ± 0.00 | 49.99 | Undefined |
| DC + P5 + PADRE + CpG | 100.00 ± 0.00 | 52.41 | Undefined |
Time to reach end‐point.
Tumour growth delay (in comparison with buffer group).
Median survival time.
Dendritic cells loaded with P5 + PADRE + CpG inhibited tumour growth completely (TGE = 52%) and increased mice survival times (TTE = 100) more effectively than controls. Mice vaccinated with DCs pulsed with P5 + PADRE with or without CpG had longer life spans than control and peptides alone or with CpG (Fig. 7B–D). The in vivo study demonstrates that DC + P5 + PADRE + CpG had a powerful prophylactic effect on tumour growth, which correlated with mice survival (Fig. 6).
Discussion
In this study, we used a long multi‐epitope peptide, a CD4+ epitope peptide, an adjuvant and DCs as an appropriate delivery system to induce immune responses. As we expected, when the P5 HER‐2/neu‐derived peptide was loaded onto DCs, tumoural immune responses were greater, both in vitro and in vivo, than when free peptide formulations were used. Moreover, PADRE peptide, as a CD4+ T cell activator, remarkably improved the responses generated by P5 peptide loaded DCs, and CpG increased the immune responses.
Peptide vaccines in cancer immunotherapy have been shown to have some advantages 25; however, they also have limitations 17. One limitation is the specificity of peptide epitopes for binding to MHC molecules. To overcome to this problem, we used the P5 peptide, which are 21 amino acids long. Because of its size, P5 contains several epitopes that can activate more T cell colonies and enhance immune responses 30. Our finding is consistent with an earlier study that showed that the HPV16 synthetic long peptide (HPV16‐SLP) as a vaccine in therapy of patients with advanced or recurrent HPV16‐induced gynaecological carcinoma induced CD4+ and CD8+ T cell responses against HPV16 31.
The second limitation is in finding the optimum delivery system for antigens. Dendritic cells are among the best for this purpose and mature DCs have an important role in the induction of strong immune responses.
Several studies have shown that DC maturation is one of the most important steps in vaccination protocols. Mature DCs are more potent than immature ones in eliciting antitumour responses, likely due to their ability to present TAAs on MHC molecules and up‐regulate co‐stimulatory molecules 32. The possible reason why pulsed DCs worked well in vivo is because of their immigration to lymph nodes. It has been shown that migration of antigen‐loaded DCs to lymph nodes is related to their maturation 33. In our study, cultured, peptide‐pulsed DCs grown in the presence of GM‐CSF and IL‐4 induced greater protection against TUBO tumours than a free peptide vaccine. In support of our results, previous studies demonstrated that GM‐CSF is necessary for DC differentiation and function 34, 35, increases the immune response via DC activation and increases their migration to lymph nodes 36. Provenge is also a DC vaccine that contains autologous DCs pulsed with a fusion protein consisting of prostatic acid phosphatase (PAP) and GM‐CSF. PAP is a peptide expressed on 95% of human prostate, brain, testicular, spleen and heart cells 37. In our study, mature DCs induced immune responses in all in vivo and in vitro tests. Previous studies reported that mature or differentiated DCs are the only APCs capable of activating naïve CD8+T lymphocytes in vivo 38, 39 due to their ability to present exogenous antigens, such as TAA, on MHC‐I 7, 40, 41, 42. In a clinical study de Vries and colleagues compared the capacity of mature and immature DCs to induce immune responses in stage IV melanoma patients. They observed tumour regression in patients receiving mature DCs due to expression of stimulatory molecules, particularly when TAAs with low immunogenicity were presented 43. In another study, autologous DCs, when fused with the allogeneic colorectal carcinoma cell line COLM‐6, induced both CD4+ and CD8+ T cells and CTL responses that break down autologous colorectal carcinoma cells 2. In another study, monocyte‐derived human DCs pulsed with purified full‐length wt‐P53 stimulated P53‐specific CTLs in vitro 4.
Using a delivery system targeting dendritic cells can act as an effective strategy in cancer vaccine.
In a new study, Thi Tran and colleges showed the B subunit of the Shiga toxin (STxB) can be used as a vector that targets dendritic cells. This vector after coupling to different tumour antigens induces specific CD8+ T cell responses. This research group finding also mentioned that STxB is effective in human dendritic cell cross‐presentation 44. Another important issue is the role of CD4+ T cells in CD8+ T cell activation and function. Due to their key role in the development of activated and memory CD8+ T cells 15, design of a procedure for CD4+ T cell production in immunotherapy could be very useful. Our previous study indicated that PADRE plus long peptides improve immune responses 45; therefore, in the current study, we used PADRE as a CD4+ T cell activator plus P5 to enhance specific antitumour responses. Loading PADRE plus P5 onto DCs significantly boosted the immune response. This result is consistent with other work that showed that Th cells play a critical role in CTL induction 46. One explanation for this result is that addition of PADRE causes IL‐2 secretion by CD4+ Th‐APCs 47. D'Souza et al. showed IL‐2 can enhance expansion efficacy of CTLs 48. Also, transferred CD4+ Th cells can promote CD8+ CTL expansion and function and this assistance is mediated by IL‐2 49, 50. Another possible reason is due to the costimulatory role of CD80. Umeshappa et al. reported that CD80 costimulatory signalling on CD4+ Th‐APCs affects CD8+ CTL responses 47. In addition, interaction between CD28 and CD80 has been shown to be necessary for T cell proliferation and activation 51.
Although not well characterized, CD4+ Th cells have been shown to inhibit tumour growth 52. They also have an indirect effect via strengthening CD8+ cell responses and attracting and activating macrophages and NK cells 53, 54. Schuurhuis et al. showed that CD4+‐dependent inhibition of tumour growth in the absence of CD8+ cells may also have a tumour‐protective effect 55. Our data demonstrate that PADRE increases the CD4+ Th1 subset population, and as a consequence, the immune response against TUBO tumour cells was greater than that of other groups and control.
Wang et al. showed that primary CD8+ T cell responses to presented antigens in vivo via peptide‐pulsed DCs depend on CD4+ T cell assistance 56. In our study, loading PADRE onto DCs resulted in a significantly greater immune response than PADRE plus P5 peptide without DCs. In fact, simultaneous use of DCs and PADRE enhanced the effect of each of them. The reason may be due to the CD40‐CD40L interaction, which has an important role in CD4+ Th‐mediating function for CD8+ T cell responses. DCs present antigens and activate CD4+ T cells via CD40 costimulatory molecules. When DCs stimulate CD4+ cells, these cells also activate DCs via CD40L and cause DCs to induce CD8+ responses 57, 58, 59. Furthermore, when a potent inflammatory stimulus is not present, DCs must interact with CD4+ T cells to induce strong CD8+ T cell responses 58.
Another problem with peptide vaccines, which we tried to overcome, is the need for a suitable adjuvant that is able to stimulate immune responses in general. CpG‐ODN has been demonstrated to be an effective adjuvant both in vitro and in vivo 60, 61, 62. This synthetic, short, single‐stranded oligonucleotide activates Th1 responses via interaction with TLR9, and due to inhibition of Th2 responses by Th1 cytokines, including IFN‐γ and IL‐12; this motif is useful in cancer immunotherapy 63. Although in our study the immune responses were not significantly different in the presence or absence of CpG, it could increase the immune response against tumour growth. CpG‐ODN also has a direct stimulation effect on activation and maturation of DCs and macrophages, which result in the production of Th1‐like cytokines 64 seen in our study.
Another critical consideration in immunotherapy is the vaccine dosage. We achieved an immune response with a much lower concentration of peptides on our DC‐treated mice than on peptide‐alone‐treated mice (0.5 μg/mouse versus 100 μg/mouse), which could be important in pharmaceutical marketing. One possible reason for the high peptide concentration requirement is that in the latter, the peptides may be removed by other cells, such as macrophages, which are not able to initiate immune responses and activate naïve CD8+ T cells. Another possible reason is that removal of peptides by DCs in vivo may cause tolerance instead of immune stimulation because the DCs may remain immature and unable to present antigens 65.
In addition to all these, combination therapy against HER2/neu can be applied using monoclonal antibodies together to enhance immune response and survival rate. To reach this goal, Linch SN and colleges demonstrated dual anti‐aOX40 (anti‐CD134)/aCTLA‐4 (anti‐cytotoxic T lymphocyte‐associated protein 4)mAb in combination with anti‐DEC‐205 (dendritic and epithelial cells, 205 kD)–HER2. They analysed that following this immunotherapy, Th2‐cytokine generation by CD4+ was reduced and IFN‐γ production by CD4+ and CD8+ was enhanced. The combination therapy was associated with tumour‐free survival, gathering of effector T cells in tumour site, stimulated as well as reversed T cell anergy resulting in tumour regression 66.
One of the goals of our study was to address some of the limitations of present cancer vaccines. The use and strengthening of the immune system via DCs and CD4+ T cell activation, which are suppressed by cancer cells in many cases, can be an effective strategy in cancer immunotherapy.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgements
We would like to acknowledge Immunology Center at University of Mashhad Medical Science for providing financial support and facilities and equipment for this research (Grant number: 921575).
Contributor Information
Seyed Amir Jalali, Email: jalalia@sbmu.ac.ir.
Mahmoud Reza Jaafari, Email: Jafarimr@mums.ac.ir.
References
- 1. Wei H, Wang S, Zhang D, et al Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009; 15: 4612–21. [DOI] [PubMed] [Google Scholar]
- 2. Koido S, Hara E, Homma S, et al Dendritic cells fused with allogeneic colorectal cancer cell line present multiple colorectal cancer‐specific antigens and induce antitumor immunity against autologous tumor cells. Clin Cancer Res. 2005; 11: 7891–900. [DOI] [PubMed] [Google Scholar]
- 3. Muth S, Schütze K, Schild H, et al Release of dendritic cells from cognate CD4 + T‐cell recognition results in impaired peripheral tolerance and fatal cytotoxic T‐cell mediated autoimmunity. Proc Natl Acad Sci. 2012; 109: 9059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tokunaga N, Murakami T, Endo Y, et al Human monocyte‐derived dendritic cells pulsed with wild‐type p53 protein efficiently induce CTLs against p53 overexpressing human cancer cells. Clin Cancer Res. 2005; 11: 1312–8. [PubMed] [Google Scholar]
- 5. Shariat S, Badiee A, Jalali SA, et al P5 HER2/neu‐derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014; 355: 54–60. [DOI] [PubMed] [Google Scholar]
- 6. Corradin G, Levitskaya J. Priming of CD8+T cell responses to liver stage malaria parasite antigens. Front Immunol. 2014; 5: 527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nierkens S, Tel J, Janssen E, et al Antigen cross‐presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. 2013; 34: 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vogelsang P, Karlsen M, Brun JG, et al Altered phenotype and Stat1 expression in Toll‐like receptor 7/8 stimulated monocyte‐derived dendritic cells from patients with primary Sjögren's syndrome. Arthritis Res Ther. 2014; 16: R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonehill A, Van Nuffel AM, Corthals J, et al Single‐step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res. 2009; 15: 3366–75. [DOI] [PubMed] [Google Scholar]
- 10. Heuzé ML, Vargas P, Chabaud M, et al Migration of dendritic cells: physical principles, molecular mechanisms, and functional implications. Immunol Rev. 2013; 256: 240–54. [DOI] [PubMed] [Google Scholar]
- 11. Mailliard RB, Son Y‐I, Redlinger R, et al Dendritic cells mediate NK cell help for Th1 and CTL responses: two‐signal requirement for the induction of NK cell helper function. J Immunol. 2003; 171: 2366–73. [DOI] [PubMed] [Google Scholar]
- 12. Bürdek M, Spranger S, Wilde S, et al Three‐day dendritic cells for vaccine development: antigen uptake, processing and presentation. J Transl Med. 2010; 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds G, Gibbon J, Pratt A, et al Synovial CD4 + T‐cell‐derived GM‐CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis. 2016; 75: 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gogolák P, Réthi B, Hajas G, et al Targeting dendritic cells for priming cellular immune responses. J Mol Recognit. 2003; 16: 299–317. [DOI] [PubMed] [Google Scholar]
- 15. Wu C‐Y, Monie A, Pang X, et al Improving therapeutic HPV peptide‐based vaccine potency by enhancing CD4 + T help and dendritic cell activation. J Biomed Sci. 2010; 17: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boudreau JE, Bonehill A, Thielemans K, et al Engineering dendritic cells to enhance cancer immunotherapy. Mol Ther. 2011; 19: 841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandermeulen G, Uyttenhove C, De Plaen E, et al Intramuscular electroporation of a P1A‐encoding plasmid vaccine delays P815 mastocytoma growth. Bioelectrochemistry. 2014; 100: 112–8. [DOI] [PubMed] [Google Scholar]
- 18. Ressing ME, Van Driel WJ, Brandt RM, et al Detection of T helper responses, but not of human papillomavirus‐specific cytotoxic T lymphocyte responses, after peptide vaccination of patients with cervical carcinoma. J Immunother. 2000; 23: 255–66. [DOI] [PubMed] [Google Scholar]
- 19. Rosa DS, Tzelepis F, Cunha MG, et al The pan HLA DR‐binding epitope improves adjuvant‐assisted immunization with a recombinant protein containing a malaria vaccine candidate. Immunol Lett. 2004; 92: 259–68. [DOI] [PubMed] [Google Scholar]
- 20. Weber JS, Hua FL, Spears L, et al A phase I trial of an HLA‐A1 restricted MAGE‐3 epitope peptide with incomplete Freund's adjuvant in patients with resected high‐risk melanoma. J Immunother. 1999; 22: 431–40. [DOI] [PubMed] [Google Scholar]
- 21. Kavanagh B, Ko A, Venook A, et al Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007; 30: 762–72. [DOI] [PubMed] [Google Scholar]
- 22. Wilson HL, Dar A, Napper SK, et al Immune mechanisms and therapeutic potential of CpG oligodeoxynucleotides. Int Rev Immunol. 2006; 25: 183–213. [DOI] [PubMed] [Google Scholar]
- 23. Bode C, Zhao G, Steinhagen F, et al CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011; 10: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaafari MR, Badiee A, Khamesipour A, et al The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine. 2007; 25: 6107–17. [DOI] [PubMed] [Google Scholar]
- 25. Jalali SA, Sankian M, Tavakkol‐Afshari J, et al Induction of tumor‐specific immunity by multi‐epitope rat HER2/neu‐derived peptides encapsulated in LPD Nanoparticles. Nanomed Nanotechnol Biol Med. 2012; 8: 692–701. [DOI] [PubMed] [Google Scholar]
- 26. Moisa A, Kolesanova E. Synthetic peptide vaccines. Biomed Chem. 2010; 4: 321–32. [DOI] [PubMed] [Google Scholar]
- 27. Slingluff CL Jr. The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011; 17: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alavizadeh SH, Badiee A, Khamesipour A, et al The role of liposome–protamine–DNA nanoparticles containing CpG oligodeoxynucleotides in the course of infection induced by Leishmania major in BALB/c mice. Exp Parasitol. 2012; 132: 313–9. [DOI] [PubMed] [Google Scholar]
- 29. Mansourian M, Badiee A, Jalali SA, et al Effective induction of anti‐tumor immunity using p5 HER‐2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co‐administrated with CpG‐ODN. Immunol Lett. 2014; 162: 87–93. [DOI] [PubMed] [Google Scholar]
- 30. Amir Jalali S, Parmiani G. Pre‐clinical and clinical aspects of peptide‐based vaccine against human solid tumors. Recent Pat Biotechnol. 2011; 5: 108–17. [DOI] [PubMed] [Google Scholar]
- 31. van Poelgeest MI, Welters MJ, van Esch EM, et al HPV16 synthetic long peptide (HPV16‐SLP) vaccination therapy of patients with advanced or recurrent HPV16‐induced gynecological carcinoma, a phase II trial. J Transl Med. 2013; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001; 106: 255–8. [DOI] [PubMed] [Google Scholar]
- 33. de Vries IJM, Krooshoop DJ, Scharenborg NM, et al Effective migration of antigen‐pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Can Res. 2003; 63: 12–7. [PubMed] [Google Scholar]
- 34. Hill HC, Conway TF, Sabel MS, et al Cancer immunotherapy with interleukin 12 and granulocyte‐macrophage colony‐stimulating factor‐encapsulated microspheres. Can Res. 2002; 62: 7254–63. [PubMed] [Google Scholar]
- 35. Price JD, Tarbell KV. The role of dendritic cell subsets and innate immunity in the pathogenesis of type 1 diabetes and other autoimmune diseases. Front Immunol. 2015; 6: 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura M, Iwahashi M, Nakamori M, et al Dendritic cells genetically engineered to simultaneously express endogenous tumor antigen and granulocyte macrophage colony‐stimulating factor elicit potent therapeutic antitumor immunity. Clin Cancer Res. 2002; 8: 2742–9. [PubMed] [Google Scholar]
- 37. Su Z, Dannull J, Yang BK, et al Telomerase mRNA‐transfected dendritic cells stimulate antigen‐specific CD8 + and CD4 + T cell responses in patients with metastatic prostate cancer. J Immunol. 2005; 174: 3798–807. [DOI] [PubMed] [Google Scholar]
- 38. Benechet AP, Menon M, Khanna KM. Visualizing T cell migration in situ . Front Immunol. 2014; 5: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blair DA, Dustin ML. T cell priming goes through a new phase. Nat Immunol. 2013; 14: 311–2. [DOI] [PubMed] [Google Scholar]
- 40. Dresch C, Leverrier Y, Marvel J, et al Development of antigen cross‐presentation capacity in dendritic cells. Trends Immunol. 2012; 33: 381–8. [DOI] [PubMed] [Google Scholar]
- 41. McDonnell AM, Joost Lesterhuis W, Khong A, et al Restoration of defective cross‐presentation in tumors by gemcitabine. Oncoimmunology. 2015; 4: e1005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhan Y, Xu Y, Lew AM. The regulation of the development and function of dendritic cell subsets by GM‐CSF: more than a hematopoietic growth factor. Mol Immunol. 2012; 52: 30–7. [DOI] [PubMed] [Google Scholar]
- 43. de Vries IJM, Lesterhuis WJ, Scharenborg NM, et al Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003; 9: 5091–100. [PubMed] [Google Scholar]
- 44. Tran T, Diniz MO, Dransart E, et al A therapeutic Her2/neu vaccine targeting dendritic cells preferentially inhibits the growth of low Her2/neu–expressing tumor in HLA‐A2 transgenic mice. Clin Cancer Res. 2016; 22: 4133–44. [DOI] [PubMed] [Google Scholar]
- 45. Ghaffari‐Nazari H, Tavakkol‐Afshari J, Jaafari MR, et al Improving multi‐epitope long peptide vaccine potency by using a strategy that enhances CD4 + T help in BALB/c mice. PLoS One. 2015; 10: e0142563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiang J, Huang H, Liu Y. A new dynamic model of CD8 + T effector cell responses via CD4 + T helper‐antigen‐presenting cells. J Immunol. 2005; 174: 7497–505. [DOI] [PubMed] [Google Scholar]
- 47. Umeshappa CS, Huang H, Xie Y, et al CD4 + Th‐APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4 + and central memory CD8 + T cell responses. J Immunol. 2009; 182: 193–206. [DOI] [PubMed] [Google Scholar]
- 48. D'Souza WN, Schluns KS, Masopust D, et al Essential role for IL‐2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol. 2002; 168: 5566–72. [DOI] [PubMed] [Google Scholar]
- 49. Huang H, Hao S, Li F, et al CD4 + Th1 cells promote CD8 + Tc1 cell survival, memory response, tumor localization and therapy by targeted delivery of interleukin 2 via acquired pMHC I complexes. Immunology. 2007; 120: 148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Antony PA, Piccirillo CA, Akpinarli A, et al CD8 + T cell immunity against a tumor/self‐antigen is augmented by CD4 + T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005; 174: 2591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Markosyan N, Chen EP, Evans RA, et al Mammary carcinoma cell derived Cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activity. Breast Cancer Res. 2013; 15: R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Knutson KL, Disis ML. Tumor antigen‐specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005; 54: 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schurmans LR, Diehl L, den Boer AT, et al Rejection of intraocular tumors by CD4 + T cells without induction of phthisis. J Immunol. 2001; 167: 5832–7. [DOI] [PubMed] [Google Scholar]
- 54. Cohen PA, Peng L, Plautz GE, et al CD4 + T cells in adoptive immunotherapy and the indirect mechanism of tumor rejection. Crit Rev Immunol. 2000; 20: 17–56. [PubMed] [Google Scholar]
- 55. Schuurhuis DH, van Montfoort N, Ioan‐Facsinay A, et al Immune complex‐loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006; 176: 4573–80. [DOI] [PubMed] [Google Scholar]
- 56. Wang J‐CE, Livingstone AM. Cutting edge: CD4 + T cell help can be essential for primary CD8 + T cell responses in vivo . J Immunol. 2003; 171: 6339–43. [DOI] [PubMed] [Google Scholar]
- 57. Bennett SR, Carbone FR, Karamalis F, et al Help for cytotoxic‐T‐cell responses is mediated by CD40 signalling. Nature. 1998; 393: 478–80. [DOI] [PubMed] [Google Scholar]
- 58. Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4 + T‐helper and a T‐killer cell. Nature. 1998; 393: 474–8. [DOI] [PubMed] [Google Scholar]
- 59. Schoenberger SP, Toes RE, van der Voort EI, et al T‐cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998; 393: 480–3. [DOI] [PubMed] [Google Scholar]
- 60. Lipford GB, Bauer M, Blank C, et al CpG‐containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997; 27: 2340–4. [DOI] [PubMed] [Google Scholar]
- 61. Krieg AM, Wagner H. Causing a commotion in the blood: immunotherapy progresses from bacteria to bacterial DNA. Immunol Today. 2000; 21: 521–6. [DOI] [PubMed] [Google Scholar]
- 62. Sparwasser T, Koch E‐S, Vabulas RM, et al Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998; 28: 2045–54. [DOI] [PubMed] [Google Scholar]
- 63. Meng W, Yamazaki T, Nishida Y, et al Nuclease‐resistant immunostimulatory phosphodiester CpG oligodeoxynucleotides as human Toll‐like receptor 9 agonists. BMC Biotechnol. 2011; 11: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krieg AM, Davis HL. CpG ODN as a Th1 immune enhancer for prophylactic and therapeutic vaccines. Vaccine Adjuvants: Springer; 2006. pp. 87–110. [Google Scholar]
- 65. Vives‐Pi M, Rodríguez‐Fernández S, Pujol‐Autonell I. How apoptotic β‐cells direct immune response to tolerance or to autoimmune diabetes: a review. Apoptosis. 2015; 20: 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Linch SN, Redmond WL. How do I steer this thing? Using dendritic cell targeted vaccination to more effectively guide the antitumor immune response with combination immunotherapy. J Immunother Cancer. 2016; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]


