Abstract
Bladder cancer (BC) is one of the most common cancers worldwide with a high progression rate and poor prognosis. The Hippo signalling pathway is a conserved pathway that plays a crucial role in cellular proliferation, differentiation and apoptosis. Furthermore, dysregulation and/or malfunction of the Hippo pathway is common in various human tumours, including BC. In this review, an overview of the Hippo pathway in BC and other cancers is presented. We focus on recent data regarding the Hippo pathway, its network and the regulation of the downstream co‐effectors YAP1/TAZ. The core components of the Hippo pathway, which induce BC stemness acquisition, metastasis and chemoresistance, will be emphasized. Additional research on the Hippo pathway will advance our understanding of the mechanism of BC as well as the development and progression of other cancers and may be exploited therapeutically.
Keywords: bladder cancer, Hippo pathway, YAP1, TAZ, dysregulation, therapeutic target
| • Introduction |
| • The Hippo pathway |
| ‐ Overview of the Hippo signalling pathway |
| ‐ The network of upstream signals of the Hippo pathway |
| ‐ Regulation of YAP1 and TAZ |
| Extrinsic regulators |
| Cell contact and morphology |
| Extrinsic stress signals |
| G protein‐coupled receptors (GPCRs) |
| Intrinsic regulators |
| E proteins |
| Cell cycle |
| Other signalling pathways |
| • The Hippo pathway and its role in cancers |
| ‐ Clinical correlation between the upstream Hippo pathway and human cancers |
| ‐ YAP1/TAZ are key co‐effectors of the Hippo pathway in human cancers |
| • Deregulation of the Hippo pathway and its role in bladder cancer |
| ‐ The Hippo pathway in urinary tract development |
| ‐ The role of the Hippo signalling pathway in bladder cancer |
| • Conclusions |
| • Future perspectives |
| • Acknowledgements |
| • Conflict of interests |
Introduction
BC is the fourth most commonly diagnosed cancer in males. The incidence of BC is about 4 times higher in men than in women 1. More than 70% of patients who have BC are newly diagnosed with non‐muscle‐invasive disease 2. However, after undergoing transurethral resection of the bladder tumour (TURBT) followed by intravesical chemotherapy (22%) or biological therapy with bacillus Calmette‐Guerin (29%) 3, up to 50–70% of cases will experience relapse, and approximately 10–20% will invade into the muscularis propria layer (T2 or greater) 2. For cases with muscle‐invasive disease, treatment options are limited. Cystectomy and chemotherapy combined with radiation are two common options; the long‐term prognosis is poor, however, with a 5‐year survival rate of 47%. For all stages combined, BC patients can expect survival rates of 77% at 5 years and 70% at 10 years 1. High recurrence (range, 50–90%) 4, 5 and progression rates 2 are major obstacles to the treatment of BC. Identifying new therapeutic targets of BC is essential to developing further effective treatment.
The targeting of signalling pathways for cancer treatment has increased in the last decades. However, more therapeutic targets for BC are needed 6. The Hippo signalling pathway, which functions in organ size control, stem cell pluripotency and regeneration 7, has been found to be dysregulated in various human cancers 8, 9, 10, 11, 12, 13. Furthermore, a set of studies have demonstrated the dysregulation of the Hippo pathway in BC 14, 15. This emergence of the Hippo pathway in BC progression may aid in identifying new pharmaceutical targets for BC management. In this review, we first discuss several studies on the roles of the Hippo signalling pathway in embryonic development and human tumours. Then, we detail the various mechanisms of the Hippo pathway in human tumours and in bladder tumours in particular.
The Hippo pathway
Overview of the Hippo signalling pathway
The Hippo signalling pathway, also known as the MST1/2‐WW45‐LATS1/2 signalling pathway, is an important regulator of tissue homeostasis, cell growth and organ size 16, 17. It was initially identified in the fruit fly Drosophila in the search for genes essential for cell proliferation, organ growth and decreased apoptosis 18, 19. A specific set of kinases are its key components, including Warts (Wts), Salvador (Sav) and Hippo (Hpo) 18, 20, 21. These genes function as tumour suppressors in Drosophila, wherein mutation of these genes leads to dysregulation of cell proliferation and apoptosis (Fig. 1) 22. Furthermore, these key components of the signalling pathway are highly conserved in most eukaryotes, from flies to mammals. Deregulation of this pathway, especially mutation of its key components, can activate several oncogenes in cancer cells in various human cancers 8, 9, 10, 11, 12, 23.
Figure 1.
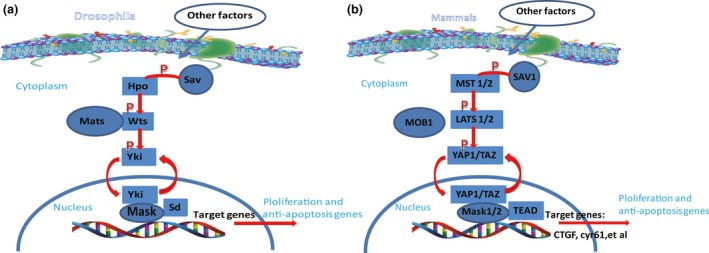
The Hippo signalling pathway in Drosophila and mammals. (A) The Drosophila Hippo pathway. In Drosophila, when Yki is relieved from inhibition through phosphorylation‐dependent or phosphorylation‐independent mechanisms, its nuclear translocation then drives target gene expression to regulate cellular proliferation and apoptosis. The phosphorylation mechanism depends on the core kinase cascade including Hpo, Wts, Sav and Mats. (B) The mammalian Hippo pathway. In mammals, YAP1 and TAZ localize to the nucleus to interact with TEAD, driving target gene expression to regulate cellular proliferation and apoptosis. After phosphorylation, MST1/2 in turn phosphorylates LATS1/2, facilitated by scaffold proteins SAV1 and MOB1. MOB1 also phosphorylates and activates LATS1/2. Activated LATS1/2 phosphorylate YAP1 and TAZ. YAP1 interacts with Mask1/2 to form complex.
The network of upstream signals of the Hippo pathway
Little is known of the exact mechanisms of the Wts, Sav and Hpo kinases, but cellular polarity may be involved, as these components localize to adherent junctions of polarized epithelial cells 24. Wts mutations lead to dysregulated tissue proliferation 18, 25, whereas Hpo and Sav determine the survival and apoptosis of cells 21, 26, 27. This signalling cascade is conserved from flies to mammals, resulting in a similar network in mammals, including two homologues of Hpo (MST1/2), one homologue of Sav (SAV1), two homologues of Wts (LATS1/2), and two homologues of Yki (YAP1 and its paralog TAZ) 28. Both YAP1 and TAZ are critical transcriptional co‐activators and downstream effectors of the pathway 29, Activation and inactivation of the Hippo pathway depend on activation and inactivation of the kinase cascade. First, RASSF1‐A, a member of the RAS association domain family (RASSF), is involved in the translocation of MST1 to mitochondria. After the stimulation of stress elicits K‐RAS, RASSF1‐A binds to MST1/2 and results in their activation 30, 31. Then, MST1 and MST2 form a complex together with SAV1, facilitating the interaction with LATS1/2 32. This interaction between MST1/2 and LATS1/2 depends on a sequential phosphorylation process. Through suppression of protein phosphatase 2A, MST1/2 are dephosphorylated and stimulate LATS1/2 activation 33, 34, 35, 36. Recently, MOB1 has been shown to play a role in this inactivation 34. In turn, LATS1/2 regulate the interaction between YAP1/TAZ and some important transcriptional target partners, such as the TEA domain‐containing sequence‐specific transcription factors SMAD and RUNX, by regulating the phosphorylation of YAP1 and TAZ. TAZ has been reported to bind to YAP1 37, thereby, exerting their functions on the transcription of various target genes 38, 39. After phosphorylation, YAP1 is retained in the cytosol in a depressed state 38, 40, 41. New research has demonstrated that the multiple ankyrin repeats single KH domain (Mask) is required for the transcriptional output of Yki in Drosophila and YAP1 in mammals 42, 43. Mask is conserved in mammals with two homologues, Mask1 (also known as ANKHD1) and Mask2 (also known as ANKRD17)44, 45.The full activity of Yki or the YAP1/TEAD complex is dependent on the expression of Mask or its mammalian homologue, Mask1. After Mask1 knockdown, YAP1 target genes were substantially suppressed although non‐target genes were not affected 42. (Fig. 1)
Regulation of YAP1 and TAZ
In addition to the key components of the Hippo pathway, several other intrinsic and extrinsic regulators of YAP1/TAZ have been observed (Fig. 2).
Figure 2.
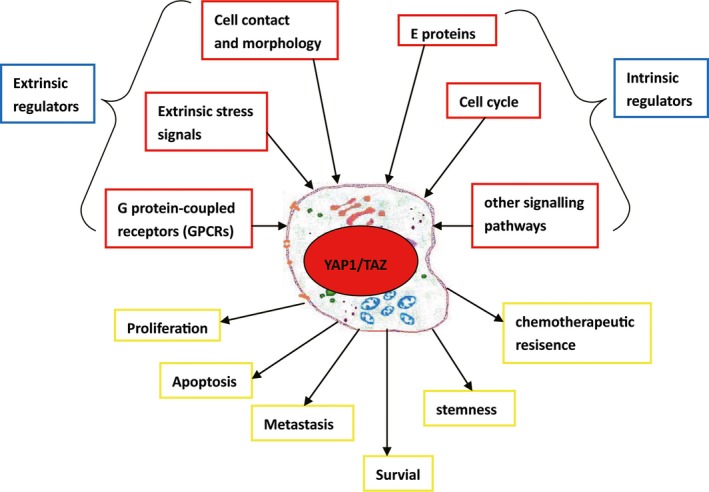
Schematic overview of YAP1/TAZ regulation and function in tumorigenesis.
Extrinsic regulators
Cell contact and morphology
Some environmental cues affect YAP1/TAZ activity. For example, in epithelial cells, apical signalling modulates YAP1/TAZ expression through the canonical Crumbs/CRB‐Hippo/MST‐Warts/LATS kinase cascade. When cells differentiate an apical membrane domain, YAP1/TAZ are phosphorylated and inhibited. Although contact occurs between the cells’ extracellular matrixes (ECMs) and basal membrane domains, these two effectors are stimulated 46. The stiffness or elasticity of the ECM has a dramatic effect on F‐actin bundles 47. In Drosophila cells, Yorkie activation is positively associated with F‐actin expression 48. In mammalian cells, the maintenance of YAP1/TAZ activity requires a stable role of F‐actin contractility 49. Recent research found that cell morphology can regulate YAP1 nuclear localization. Piezo1, a channel that mediates calcium currents, is crucial for YAP1 nuclear localization through the regulation of cytoskeletal tension 50.
Extrinsic stress signals
Considering that the most important role of YAP1/TAZ is to promote cell proliferation and survival 51, a set of extrinsic stress signals, such as endoplasmic reticulum stress, energy stress and hypoxia, has been observed to regulate the Hippo signal pathway. Carbohydrates are the main energy source for cell metabolism. Deran et al. have observed that YAP1 and TAZ phosphorylation is rapidly induced by energy stress caused by glucose deprivation 52. Lack of glucose stimulates AMPK activation, which affects the interaction between TEAD and YAP1/TAZ 53. In contrast to oxidative stress, hypoxia seems to affect the interaction between LATS and YAP1/TAZ. Hyperactivity of SIAH2 caused by hypoxia inhibits LATS in a xenograft animal model 54.
G protein‐coupled receptors (GPCRs)
Extracellular molecules, such as growth factors and hormones, have been hypothesized to regulate the Hippo signalling pathway so as to control homeostasis. Furthermore, it has been demonstrated that regulation of the Hippo signalling pathway by GPCRs is indeed a common response of cells to hormonal cues 55, 56, 57, 58. GPCRs, along with Rho GTPase and the actin cytoskeleton, can promote or suppress Hippo signalling pathway activity. Whether this results in positive or negative regulation depends on the class of G protein involved. For instance, Gα12/13‐ and Gαq/11‐coupled GPCRs promote YAP1 and TAZ activity by regulating the actions of Rho‐GTPases, whereas, Gαs‐coupled GPCRs inactivate YAP1 by increasing the activities of LATs 28, 59.
Intrinsic regulators
Despite these extrinsic regulators, intrinsic transcriptional regulators and signalling pathways are still crucial mediators of the Hippo pathway.
E proteins
E proteins are members of the basic helix‐loop‐helix (bHLH) family that mediate cell proliferation, differentiation and commitment in many tissues 60, 61. After binding to the E‐box sequence (CANNTG), E proteins can inhibit ID protein expression. Furthermore, either elevated expression of E proteins or loss of ID proteins can promote Hippo signalling 62. Previous studies have demonstrated that the interaction between E and I proteins can affect various transcriptional factors associated with the Hippo pathway, such as SMAD, TEAD, PAX, HTH and TBX5. These regulators are required for the phosphorylation of YAP1/TAZ and are involved in reducing the activation of the Hippo pathway 39, 63, 64, 65, 66.
Cell cycle
LATS1/2 are not only critical components of the Hippo pathway, but they are also regarded as regulators of the cell cycle (G1/S, G2/M and mitosis) 67. Furthermore, the cell cycle can influence the activities of YAP1/TAZ. For instance, during the G2‐M phase, YAP1 and TAZ are phosphorylated at multiple sites by CDK1, which increases cell migration and invasion ability 68.
Other signalling pathways
The Hippo signalling pathway is involved in cross‐talk with a number of other signalling pathways, such as the Notch 69, transforming growth factor β (TGF‐β) 70 and Wnt/β‐catenin pathways 71, 72. Among these signalling pathways, most previous studies have focused on the interaction between the Hippo signalling pathway and the Wnt/β‐catenin signalling pathway (Wnt pathway) owing to their obvious roles in tumorigenesis. The Wnt pathway plays critical roles in almost every aspect of embryonic development as well as in homeostasis in various adult tissues. Its germline mutations are associated with a set of human cancers 73. This cross‐interaction was originally reported in 2010 37. In the cytoplasm, both YAP1 and TAZ can directly interact with β‐catenin and suppress β‐catenin nuclear translocation 74, whereas in the nucleus, YAP1 can cooperate with the Wnt pathway to enhance tumorigenicity75, 76. In the cytoplasm, TAZ interacts with CK1δ/ε and DVL and thereby inhibits the WNT3α‐induced phosphorylation of DVL2. Consequently, the Wnt/β‐catenin signalling pathway is inhibited. Meanwhile, the cytoplasmic accumulation of TAZ can also interact with MST and LATS to inhibit Wnt/β‐catenin pathway‐mediated reporter activity 77. Furthermore, an inhibitory role of the Hippo pathway on the Wnt/β‐catenin pathway in heart development has been demonstrated. Chromatin immunoprecipitation (ChIP) assay results have shown that YAP1‐TEAD and β‐catenin‐TCF/LEF cooperatively regulate some target genes, such as SOX2 and SNAIL2 in heart development 78. In the nucleus, previous studies have demonstrated that the tumorigenicity of deregulated Wnt signalling is dependent on at least two distinct transcriptional complexes: β‐catenin‐YAP1‐TBX5 and β‐catenin‐TCF4 76. Furthermore, the co‐localization of YAP and β‐catenin in the nucleus has been observed in several colorectal cancer cell lines 75.
The Hippo pathway and its role in cancers
Clinical correlation between the upstream Hippo pathway and human cancers
Numerous retrospective analyses of tumour specimens have demonstrated a significant association between aberrant expression of Hippo pathway components and cancer clinical stages (Table 1). For instance, MST1/2 and LATS1/2, the upstream kinases of the Hippo pathway, function as tumour suppressors in multiple human cancers 79. In three different murine syngeneic tumour models (B16, SCC7 and 4T1), knockout of LATS1/2 in tumour cells inhibits proliferation. Mechanistically, LATS1/2‐null tumour cells secrete nucleic acid‐rich extracellular vesicles, which induce a type I interferon response via the Toll‐like receptor MYD88/TRIF pathway, thus improving tumour immunogenicity 80. MST1/2 expression is explicitly correlated with increased clinical stage in gastrointestinal cancers 81, 82, 83, 84, 85. Mask1/2 play a crucial role in various human cancers. Mask1 is critical in prostate cancer, myeloma and leukaemia 86, 87, 88, whereas elevated Mask2 expression has been observed in BC 89.
Table 1.
Dysregulated Hippo pathway components in human tumours
| Hippo pathway component | Cancer type | Role in human tumours | Reference |
|---|---|---|---|
| MST1/2 | Gastric cancer | Invasion, metastasis, higher clinical stage, and poorer prognosis | 10, 79, 80, 81, 82, 83, 84, 85, 133 |
| Colorectal cancer | |||
| Hepatocellular cancer | |||
| Breast cancer | |||
| Gastric cancer | |||
| LATS1/2 | Prostate cancer | Proliferation, metastasis, increased clinical stage, reduced overall survival, and recurrence‐free survival | 79, 80, 82, 84, 130, 133 |
| Renal cancer | |||
| Non‐small lung cancer | |||
| Colorectal cancer | |||
| Gastric cancer | |||
| Bladder cancer | |||
| Mask1/2 | Prostate cancer | Proliferation, migration | 86, 87, 88, 89 |
| Myeloma | |||
| Leukaemia | |||
| Bladder cancer | |||
| YAP1 | Bladder cancer | Proliferation, invasion, metastasis, higher clinical stage, reduced overall survival, metastasis‐free survival, and chemotherapy resistance | 12, 14, 15, 89, 134, 136 |
| Gastric cancer | 8, 84, 124, 132 | ||
| Colorectal cancer | 75, 82, 125, 126 | ||
| Squamous cell carcinoma | 116 | ||
| Non‐small cell lung cancer | 9, 92, 119 | ||
| Ovarian cancer | 11, 85 | ||
| Uveal melanoma | 93 | ||
| Endometrial cancer | 94 | ||
| Hepatocellular cancer | 69, 95, 107, 109, 110 | ||
| Pancreatic ductal adenocarcinoma | 68, 96 | ||
| Cholangiocarcinoma | 91 | ||
| Head and neck cancer | 90 | ||
| Breast cancer | 58, 115 | ||
| Malignant mesothelioma | 70 | ||
| Prostate cancer | 86 | ||
| Endometrial cancer | 94 | ||
| Medulloblastomas | 114 | ||
| Meningiomas | 139 | ||
| TAZ | Hepatocellular cancer | Proliferation, invasion, metastasis, higher clinical stage, shorter overall survival, disease recurrence, poor prognosis and chemotherapy resistance | 97, 107 |
| Retinoblastoma | 102 | ||
| Gastric cancer | 84, 123 | ||
| Colon cancer | 82, 101 | ||
| Oral cancer | 98 | ||
| Ovarian cancer | 104 | ||
| Endometrial cancer | 94, 103 | ||
| Osteosarcoma | 105 | ||
| Non‐small cell lung cancer | 100, 117, 119 | ||
| Breast cancer | 40, 58, 111, 138 | ||
| Tongue squamous cell carcinoma | 99 | ||
| lioma | 106 | ||
| Bladder cancer | 136 |
YAP1/TAZ are key co‐effectors of the Hippo pathway in human cancers
As the most important effectors, YAP1 and its closely related paralog TAZ act as oncogenes in various human cancers. Up‐regulation of YAP1 has been observed in gastric cancer, colorectal cancer, squamous cell carcinoma (SCC), non‐small cell lung cancer (NSCLC), ovarian cancer, uveal melanoma, endometrial cancer, hepatocellular cancer (HCC), pancreatic ductal adenocarcinoma, cholangiocarcinoma, and head and neck cancer 9, 82, 84, 90, 91, 92, 93, 94, 95, 96. Meanwhile, overexpression of TAZ is observed in HCC, retinoblastoma, gastric cancer, colon cancer, NSCLC, ovarian cancer, endometrial cancer, osteosarcoma, glioma and oral cancer 84, 94, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106.
In liver cancer, several studies have demonstrated that elevated expression of YAP1/TAZ is associated with higher pathological grades and poor clinical differentiation 97, 107. In transgenic mice, YAP1 overexpression results in hepatomegaly and liver cancers similar to human HCC 108. By interacting with β‐catenin, hydrodynamic transfection of YAP1 promotes liver tumour development 109. In line with this, up‐regulation of YAP1/TAZ in a normal human liver cell line, MHIA, endows tumorigenic properties 110.
Among a database of breast cancer patients, a significant correlation was observed between high pathological grade, metastatic proclivity, carcinoma stemness and poor outcome 111, 112, 113. Breast cancer originates in the epithelial cells of the mammary gland, and YAP1 can promote epithelial‐mesenchymal transition (EMT) and proliferation in breast cancer cell lines 114. In a mouse model, YAP1/TAZ cooperate with Her2, Polyoma‐middle T and Wnt1 to induce breast cancer development 115.
In NSCLC, significant correlations have been demonstrated between up‐regulation of YAP1/TAZ and malignant features (high histological grade, late TNM stage and poor prognosis) 116, 117. By binding with OCT4 through its WW domain, YAP1 promotes SOX2 activity and thus leads to maintenance of tumour stemness 118. Furthermore, after knockdown of the oncogene KRASG12D, non‐metastatic tumours in LAC mice display weaker YAP1/TAZ staining compared with that in metastatic samples 119, 120.
In gastric cancer, deregulation of the Hippo signalling pathway is significantly correlated with initiation, development and distant metastasis of gastric cancer84. Elevated expression of YAP1 mRNA and YAP1 protein levels both in the nucleus and the cytoplasm was originally observed in high‐grade or metastatic gastric cancer samples 121. The up‐regulation of YAP1 can promote RAF/MEK/ERK pathway activities and thus enhance the expression of c‐FOS in gastric cancer cells 8. Furthermore, RUNX2, a Runt box domain DNA‐binding transcription factor, interacts with YAP1 to inhibit p21 expression, increasing oncogenic properties 122. Similarly, high expression of TAZ has been observed in human gastric cancer 123. Following the disruption of the interaction between TAZ and TEADs, the proliferation of gastric cells is inhibited both in vivo and in vitro 124.
Elevated expression of YAP1 has been observed among cases in four databases of colorectal cancer patients 125. In line with this finding, associations between YAP1/TAZ overexpression and poor prognosis and drug resistance have also been reported 126. Deregulation of the Wnt/β‐catenin signalling pathway is commonly observed in colorectal cancer, which is significantly correlated with the Hippo pathway 127. Among 36 colorectal cancer specimens, up to 86% scored positively for YAP1 and β‐catenin expression 75. In HCT 116 and advanced colorectal cell lines, activation of Wnt/β‐catenin is dependent on endonuclear YAP1 expression 75.
Above all, the key co‐effectors YAP1/TAZ are responsible for various key attributes of many different human cancers. YAP1/TAZ function in tumour cell proliferation, survival, metastasis and stemness (Fig. 2).
Deregulation of the Hippo pathway and its role in bladder cancer
The Hippo pathway in urinary tract development
The most important step in urinary tract development is the movement of the ureter from its initial branch point on the nephric duct (ND) to its final insertion site in the cloaca (primitive bladder and urethra) 128. Proteins in the Hippo signalling pathway, especially YAP1 and TAZ, play an essential role in urinary tract development. After silencing of YAP1 in the ND, most newborn mice die within 24 hrs owing to bladder absence or kidney anomalies 129. Furthermore, YAP1 is essential in the progress of the ureter from ND insertion to the bladder and the development of bladder. YAP1 deletion also results in an abnormal junction between the ureter and bladder 129. These studies highlight the crucial role of the Hippo pathway in urinary tract development.
The role of the Hippo signalling pathway in bladder cancer
Deregulation of the Hippo pathway is significantly correlated with the initiation, development and metastasis of BC (Table 2) 14. MST1/2 and LATS1, the most upstream proteins in the Hippo signalling pathway, act as tumour suppressors of human cancers. Down‐regulation of LATS1 and MST1/2 has been demonstrated in human BC 130, 131. LATS1 mRNA levels were remarkably low in 12 urinary BC specimens from Egyptian patients130. Another tumour suppressor, Runt‐related transcription factor 3 (RUNX3), is also an conserved component of this signalling pathway 131, 132. The interactions among RUNX3, MST1/2 and SAV1 are very complicated. SAV1 initially promotes the interaction between RUNX3 and MST2. In turn, MST2 re‐enhances the activation of SAV1 and RUNX3. Finally, activation of these three components inhibits cell proliferation. After RUNX3 knockdown using siRNA, MST1/2‐mediated cell death was abolished 131, 133. The TEAD‐YAP1 complex is crucial for YAP1 function in various cancers. Research has demonstrated that RUNX3 abrogates the ability of TEAD to bind DNA and thus deregulates TEAD‐YAP activity 132. Recently, a novel cofactor of the TEAD‐YAP complex, named Mask1/2, was identified. Elevated YAP1 expression is able to enhance expression of the target genes (CTGF, cyr61,et al.) and promote BC cell growth and migration, whereas Mask2 knockdown suppresses these genes 89.
Table 2.
Summary of clinical correlations between dysregulated Hippo pathway components and bladder cancer
| Hippo pathway component | Role in bladder cancer development | References |
|---|---|---|
| MST1/2 | MST1/2 and RUNX3 collaborate and mediate BC cell death | 132, 133, 134 |
| RUNX3 | Complicated interaction among MST1/2, RUNX3 and SAV1 deregulate the YAP‐TEAD activity and is crucial in BC cell proliferation and apoptosis | |
| LATS1 | Remarkably low level in BC tissues | 131 |
| Alterations of single base pairs in this gene are observed | ||
| Mask2 | Mask 2 is required for YAP‐induced BC cell growth and migration | 89 |
| YAP1 | Elevated YAP1 expression significantly associates with poor clinicopathologic stage and adverse patient survival | 14, 15 |
| Further, YAP1 expression is inversely correlated with chemotherapy sensitivity | 142 | |
| TAZ | TAZ together with YAP1 protect KLF5 from degradation in BC | 137 |
| Knockdown of KLF5 induces BC cell apoptosis |
As the key downstream effector, YAP1 and its paralog TAZ also play crucial roles in human BC. YAP1 mRNA and YAP1 protein levels were first observed to be dramatically up‐regulated in urothelial carcinoma of the bladder, especially in high‐grade and metastatic samples 14. Furthermore, this study also provided evidence that YAP1 can act as an biomarker for BC because of the significant correlation between elevated YAP1 expression and adverse patient survival. Interestingly, another study observed that nuclear YAP1 and cytoplasmic pYAP1 levels are lower in BC tissues compared to those of normal urothelial tissues 134. I have also explored the mechanism of YAP1 in BC 15. In my opinion, total YAP1 expression is up‐regulated in bladder tumours. After being phosphorylated by LATS, pYAP1 remains in the cytoplasm. Only unphosphorylated YAP1 translocates into the nucleus and functions as an oncogene 135. This can explain differences in the expression of YAP1 and pYAP1 in the cytoplasms and nuclei of carcinoma cells. The co‐partner of YAP1, TAZ, is also activated in BC. In BC, KLF5 acts as an oncogene that promotes cell proliferation; YAP1/TAZ are capable of preventing KLF5 protein degradation 136.
Previous studies have demonstrated that YAP1/TAZ play crucial roles in cancer stem cells 137. Indeed, carcinoma cells with activated YAP1/TAZ are resistant to chemotherapeutic drugs. In a set of human tumours including breast cancer, meningiomas and lung cancer, YAP1/TAZ are capable of maintaining cancer cell stemness and protecting carcinoma cells from chemotherapeutic drugs 116, 138, 139, 140. In BC, platinum‐based chemotherapy is required for treatment of muscle‐invasive BC patients in the perioperative period. In urothelial carcinoma patient‐derived xenograft models, YAP1 expression is inversely correlated with cisplatin sensitivity. Furthermore, in vitro experiments found that DNA damage is not efficiently repaired in YAP1 knock‐down cells. Furthermore, in YAP1‐silenced cells, a significant increase in cell death was observed after cisplatin treatment 141. A more thorough understanding of the mechanisms leading to YAP1 activation during the acquisition of drug resistance would be helpful in developing new treatment strategies.
Furthermore, a set of essential oncogenes in BC can be regulated by the Hippo pathway. P53 142 and c‐Myc 143 are significantly associated with BC progression. YAP1 is also a cofactor of p73, a member of the p53 tumour suppressor family 144; upon DNA damage, p73 interacts with YAP1 through its PPPY motif 145. A recent study revealed a unique positive auto‐regulatory feedback loop underlying the interaction between YAP1 and c‐Myc in liver cancer 146. EMT has been identified as a crucial event in the pathogenesis of BC 147 and is also mediated by YAP1 148. Recently, the long non‐coding RNA H19 (lncRNA H19) has been regarded as an important biomarker in BC 149. We have previously explored the correlation between YAP1and H19 in BC 15.
Conclusions
The Hippo signalling pathway is an evolutionarily conserved regulator of cell proliferation, apoptosis, organ growth and tissue homeostasis. The function of the Hippo signalling pathway is regulated by a set of intrinsic and extrinsic regulators and also involves cross‐talk with multiple other signalling pathways. Most components of the Hippo pathway, especially the key downstream effectors YAP1/TAZ, act as crucial regulators in various human cancers. In BC, deregulation of the Hippo signalling pathway is correlated with clinicopathological characteristics and prognoses. The Hippo signalling pathway has an essential effect on the proliferation, metastasis and drug resistance of BC. We therefore suggest that the Hippo signalling pathway could be a potential source of functional biomarkers and new therapeutic targets in BC, as well as in many other cancers.
Future perspectives
Much more research needs to be done on various aspects of BC. First, the Hippo signalling pathway, which has already received much attention, demands greater investigation in the field of oncology. Furthermore, the upstream components of the Hippo signalling pathway (other than YAP1/TAZ) in particular require further research and may be important in tumour development. Second, in addition to its effects on cell proliferation, metastasis and chemotherapeutic drug resistance, the effects of the Hippo pathway on lymphangiogenesis, autophagy, angiogenesis and the Warburg effect in BC cells should also be defined. Third, the detailed mechanisms or other factors that are involved in BC processes should be explored further, even though some oncogenes and signalling pathways have already been confirmed to cooperate with the Hippo signalling pathway in BC progression, as multiple intrinsic and extrinsic regulators can affect the activities of the Hippo pathway. It is not clear why so many different factors join to activate the same signalling pathway. A possible explanation is that there are distinct molecular gatekeepers that must be bypassed. Some factors ensure the activity of entry‐level pathway effectors, whereas others inhibit their functions. Thus, different combinations of regulators participate in regulating the signalling pathway in cancer progression. Furthermore, under some specific conditions, YAP1 may switch from an oncogene to an anti‐oncogene. The mechanisms of YAP1 and phosphorylated YAP1 activity in the cytoplasm and nucleus require further investigation. Finally, and most importantly, the Hippo signalling pathway is involved in the development of chemotherapeutic resistance. Specific blockers or antagonists that specifically act on certain components of the Hippo signalling pathway with few side effects should be developed to translate basic research findings into clinical applications. The currently available agents that primarily act on the Hippo pathway are not fully satisfactory as some have limited effects and some are not specific, causing various adverse clinical effects. The development of new drugs that act on the Hippo signalling pathway is urgently needed, although large‐scale studies should be developed before clinical applications are implemented in BC patients.
Above all, the use of proteins the Hippo signalling pathway as diagnostic, prognostic or therapeutic targets for BC is recommended. Although some progress has been achieved in this area, more work remains to be carried out, especially regarding the development of new agents for BC treatment.
Conflict of interests
The authors confirm that there are no conflicts of interest.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (no. 2016YFC0105107). SL and ZW drafted the outline of the manuscript. HZ and XC conducted the literature review. JX assessed the articles and wrote the manuscript. MZ revised the manuscript. All authors have read and approved the final version of this manuscript.
Contributor Information
Zhiliang Weng, Email: wengzl10@163.com.
Shi Li, Email: bubuxiong1989@outlook.com.
References
- 1. Miller KD, Siegel RL, Lin CC, et al Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016; 66: 271–89. [DOI] [PubMed] [Google Scholar]
- 2. Feldman AS, Banyard J, Wu CL, et al Cystatin B as a tissue and urinary biomarker of bladder cancer recurrence and disease progression. Clin Cancer Res. 2009; 15: 1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKellar DP, Nelson H. American college of surgeons commission on cancer rapid quality reporting system: from quality measurement to quality improvement. Bull Am Coll Surg. 2013; 98: 52–3. [PubMed] [Google Scholar]
- 4. Heney NM, Ahmed S, Flanagan MJ, et al Superficial bladder cancer: progression and recurrence. J Urol. 1983; 130: 1083–6. [DOI] [PubMed] [Google Scholar]
- 5. Lutzeyer W, Rubben H, Dahm H. Prognostic parameters in superficial bladder cancer: an analysis of 315 cases. J Urol. 1982; 127: 250–2. [DOI] [PubMed] [Google Scholar]
- 6. Sonpavde G, Jones BS, Bellmunt J, et al Future directions and targeted therapies in bladder cancer. Hematol Oncol Clin North Am. 2015; 29: 361–76, x. [DOI] [PubMed] [Google Scholar]
- 7. Wang SP, Wang LH. Disease implication of hyper‐Hippo signalling. Open Biol. 2016; 6: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang W, Tong JH, Chan AW, et al Yes‐associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011; 17: 2130–9. [DOI] [PubMed] [Google Scholar]
- 9. Wang Y, Dong Q, Zhang Q, et al Overexpression of yes‐associated protein contributes to progression and poor prognosis of non‐small‐cell lung cancer. Cancer Sci. 2010; 101: 1279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou D, Conrad C, Xia F, et al Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009; 16: 425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, George J, Deb S, et al The Hippo pathway transcriptional co‐activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011; 30: 2810–22. [DOI] [PubMed] [Google Scholar]
- 12. Liu JY, Li YH, Lin HX, et al Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013; 13: 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016; 29: 783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu JY, Li YH, Lin HX, et al Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer. 2013; 13: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li S, Yu Z, Chen SS, et al The YAP1 oncogene contributes to bladder cancer cell proliferation and migration by regulating the H19 long noncoding RNA. Urol Oncol. 2015; 33: e1–10. [DOI] [PubMed] [Google Scholar]
- 16. Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008; 13: 188–92. [DOI] [PubMed] [Google Scholar]
- 17. Zhao B, Li L, Lei Q, et al The Hippo‐YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010; 24: 862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Justice RW, Zilian O, Woods DF, et al The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995; 9: 534–46. [DOI] [PubMed] [Google Scholar]
- 19. Watson KL. Drosophila warts–tumor suppressor and member of the myotonic dystrophy protein kinase family. BioEssays. 1995; 17: 673–6. [DOI] [PubMed] [Google Scholar]
- 20. Tapon N, Harvey KF, Bell DW, et al salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002; 110: 467–78. [DOI] [PubMed] [Google Scholar]
- 21. Wu S, Huang J, Dong J, et al hippo encodes a Ste‐20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003; 114: 445–56. [DOI] [PubMed] [Google Scholar]
- 22. Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor‐suppressor genes in Drosophila. Annu Rev Genet. 2006; 40: 335–61. [DOI] [PubMed] [Google Scholar]
- 23. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 24. Bergstralh DT, St Johnston D. Epithelial cell polarity: what flies can teach us about cancer. Essays Biochem. 2012; 53: 129–40. [DOI] [PubMed] [Google Scholar]
- 25. Xu T, Wang W, Zhang S, et al Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995; 121: 1053–63. [DOI] [PubMed] [Google Scholar]
- 26. Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell‐cycle exit in Drosophila. Nat Cell Biol. 2003; 5: 921–7. [DOI] [PubMed] [Google Scholar]
- 27. Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003; 114: 457–67. [DOI] [PubMed] [Google Scholar]
- 28. Yu F‐X, Zhao B, Panupinthu N, et al Regulation of the Hippo‐YAP Pathway by G‐Protein‐Coupled receptor signaling. Cell. 2012; 150: 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gandhirajan RK, Jain M, Walla B, et al Cysteine S‐Glutathionylation promotes stability and activation of the Hippo downstream effector transcriptional co‐activator with PDZ‐binding motif (TAZ). J Biol Chem. 2016; 291: 11596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Del Re Dominic P, Matsuda T, Zhai P, et al Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl‐xL. Mol Cell. 2014; 54: 639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Neill E, Rushworth L, Baccarini M, et al Role of the kinase MST2 in suppression of apoptosis by the proto‐oncogene product Raf‐1. Science. 2004; 306: 2267–70. [DOI] [PubMed] [Google Scholar]
- 32. Romano D, Matallanas D, Weitsman G, et al Proapoptotic kinase MST2 coordinates signaling crosstalk between RASSF1A, Raf‐1, and Akt. Cancer Res. 2010; 70: 1195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoa L, Kulaberoglu Y, Gundogdu R, et al The characterisation of LATS2 kinase regulation in Hippo‐YAP signalling. Cell Signal. 2016; 28: 488–97. [DOI] [PubMed] [Google Scholar]
- 34. Ni L, Zheng Y, Hara M, et al Structural basis for Mob1‐dependent activation of the core Mst‐Lats kinase cascade in Hippo signaling. Genes Dev. 2015; 29: 1416–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo C, Zhang X, Pfeifer GP. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20‐like kinases MST1 and MST2. J Biol Chem. 2011; 286: 6253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ribeiro PS, Josué F, Wepf A, et al Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell. 2010; 39: 521–34. [DOI] [PubMed] [Google Scholar]
- 37. Varelas X, Miller BW, Sopko R, et al The Hippo pathway regulates Wnt/beta‐catenin signaling. Dev Cell. 2010; 18: 579–91. [DOI] [PubMed] [Google Scholar]
- 38. Zhang H, Liu CY, Zha ZY, et al TEAD transcription factors mediate the function of TAZ in cell growth and epithelial‐mesenchymal transition. J Biol Chem. 2009; 284: 13355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao B, Ye X, Yu J, et al TEAD mediates YAP‐dependent gene induction and growth control. Genes Dev. 2008; 22: 1962–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lai D, Ho KC, Hao Y, et al Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Can Res. 2011; 71: 2728–38. [DOI] [PubMed] [Google Scholar]
- 41. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016; 30: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sansores‐Garcia L, Atkins M, Moya IM, et al Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr Biol. 2013; 23: 229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Enderle L, McNeill H. Hippo gains weight: added insights and complexity to pathway control. Sci Signal. 2013; 6: re7. [DOI] [PubMed] [Google Scholar]
- 44. Smith RK, Carroll PM, Allard JD, et al MASK, a large ankyrin repeat and KH domain‐containing protein involved in Drosophila receptor tyrosine kinase signaling. Development. 2002; 129: 71–82. [DOI] [PubMed] [Google Scholar]
- 45. Poulin F, Brueschke A, Sonenberg N. Gene fusion and overlapping reading frames in the mammalian genes for 4E‐BP3 and MASK. J Biol Chem. 2003; 278: 52290–7. [DOI] [PubMed] [Google Scholar]
- 46. Elbediwy A, Vincentmistiaen ZI, Thompson BJ. YAP and TAZ in epithelial stem cells: a sensor for cell polarity, mechanical forces and tissue damage. BioEssays. 2016; 38: 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lecuit T, Yap AS. E‐cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015; 17: 533–9. [DOI] [PubMed] [Google Scholar]
- 48. Fernández BG, Gaspar P, Bráspereira C, et al Actin‐Capping Protein and the Hippo pathway regulate F‐actin and tissue growth in Drosophila. Development. 2011; 138: 2337–46. [DOI] [PubMed] [Google Scholar]
- 49. Dupont S, Morsut L, Aragona M, et al Role of YAP/TAZ in mechanotransduction. Nature. 2011; 474: 179–83. [DOI] [PubMed] [Google Scholar]
- 50. Pathak MM, Nourse JL, Tran T, et al Stretch‐activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA. 2014; 111: 16148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dong J, Feldmann G, Huang J, et al Elucidation of a universal size‐control mechanism in Drosophila and mammals. Cell. 2007; 130: 1120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DeRan M, Yang J, Shen CH, et al Energy stress regulates hippo‐YAP signaling involving AMPK‐mediated regulation of angiomotin‐like 1 protein. Cell Rep. 2014; 9: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mo JS, Meng Z, Kim YC, et al Cellular energy stress induces AMPK‐mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015; 17: 500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma B, Chen Y, Chen L, et al Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015; 17: 95–103. [DOI] [PubMed] [Google Scholar]
- 55. Miller E, Yang J, DeRan M, et al Identification of serum‐derived sphingosine‐1‐phosphate as a small molecule regulator of YAP. Chem Biol. 2012; 19: 955–62. [DOI] [PubMed] [Google Scholar]
- 56. Mo JS, Yu FX, Gong R, et al Regulation of the Hippo‐YAP pathway by protease‐activated receptors (PARs). Genes Dev. 2012; 26: 2138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gong R, Hong AW, Plouffe SW, et al Opposing roles of conventional and novel PKC isoforms in Hippo‐YAP pathway regulation. Cell Res. 2015; 25: 985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou X, Wang S, Wang Z, et al Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015; 125: 2123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu FX, Zhang Y, Park HW, et al Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013; 27: 1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Desprez PY, Sumida T, Coppé JP. Helix‐loop‐helix proteins in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2003; 8: 225–39. [DOI] [PubMed] [Google Scholar]
- 61. Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009; 9: 175–84. [DOI] [PubMed] [Google Scholar]
- 62. Wang LH, Baker NE. Salvador‐Warts‐Hippo Pathway in a developmental checkpoint monitoring Helix‐Loop‐Helix Proteins. Dev Cell. 2015; 32: 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009; 23: 2307–19. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alarcón C, Zaromytidou AI, Xi Q, et al Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF‐beta pathways. Cell. 2009; 139: 757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hong JH, Hwang ES, Mcmanus MT, et al TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005; 309: 1074–8. [DOI] [PubMed] [Google Scholar]
- 66. Zhang L, Ren F, Zhang Q, et al The TEAD/TEF Family of Transcription Factor Scalloped Mediates Hippo Signaling in Organ Size Control. Dev Cell. 2008; 14: 377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tao W, Zhang S, Turenchalk GS, et al Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999; 21: 177–81. [DOI] [PubMed] [Google Scholar]
- 68. Yang S, Zhang L, Purohit V, et al Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation‐dependent manner through LPAR3. Oncotarget. 2015; 6: 36019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tschaharganeh DF, Chen X, Latzko P, et al Yes‐Associated Protein Up‐regulates Jagged‐1 and Activates the NOTCH Pathway in Human Hepatocellular Carcinoma. Gastroenterology. 2013; 144: 1530–U368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujii M, Toyoda T, Nakanishi H, et al TGF‐β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012; 209: 479–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim M, Jho E‐H. Cross‐talk between Wnt/beta‐catenin and Hippo signaling pathways: a brief review. Bmb Rep. 2014; 47: 540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim S, Jho E‐H. Merlin, a regulator of Hippo signaling, regulates Wnt/beta‐catenin signaling. Bmb Rep. 2016; 49: 357–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Clevers H. Wnt/beta‐catenin signaling in development and disease. Cell. 2006; 127: 469–80. [DOI] [PubMed] [Google Scholar]
- 74. Imajo M, Miyatake K, Iimura A, et al A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta‐catenin signalling. EMBO J. 2012; 31: 1109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Konsavage WM, Kyler SL, Rennoll SA, et al Wnt/β‐Catenin signaling regulates yes‐associated protein (YAP) Gene expression in colorectal carcinoma cells. J Biol Chem. 2012; 287: 11730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosenbluh J, Nijhawan D, Cox AG, et al beta‐Catenin‐driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012; 151: 1457–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Makita R, Uchijima Y, Nishiyama K, et al Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol. 2008; 294: F542–53. [DOI] [PubMed] [Google Scholar]
- 78. Heallen T, Zhang M, Wang J, et al Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011; 332: 458–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hay BA, Guo M. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev Cell. 2003; 5: 361–3. [DOI] [PubMed] [Google Scholar]
- 80. Moroishi T, Hayashi T, Pan WW, et al The Hippo Pathway Kinases LATS1/2 suppress cancer immunity. Cell. 2016; 167: 1525–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Karamitopoulou E, Zlobec I, Patsouris E, et al Loss of E‐cadherin independently predicts the lymph node status in colorectal cancer. Pathology. 2011; 43: 133–7. [DOI] [PubMed] [Google Scholar]
- 82. Liang K, Zhou G, Zhang Q, et al Expression of hippo pathway in colorectal cancer. Saudi J Gastroenterol. 2014; 20: 188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lin X, Cai F, Li X, et al Prognostic significance of mammalian sterile 20‐like kinase 1 in breast cancer. Tumor Biol. 2013; 34: 3239–43. [DOI] [PubMed] [Google Scholar]
- 84. Zhou G‐X, Li X‐Y, Zhang Q, et al Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013; 14: 199–205. [DOI] [PubMed] [Google Scholar]
- 85. Yagi H, Asanoma K, Ohgami T, et al GEP oncogene promotes cell proliferation through YAP activation in ovarian cancer. Oncogene. 2016; 35: 4471–80. [DOI] [PubMed] [Google Scholar]
- 86. Machado‐Neto JA, Lazarini M, Favaro P, et al ANKHD1, a novel component of the Hippo signaling pathway, promotes YAP1 activation and cell cycle progression in prostate cancer cells. Exp Cell Res. 2014; 324: 137–45. [DOI] [PubMed] [Google Scholar]
- 87. Dhyani A, Machado‐Neto JA, Favaro P, et al ANKHD1 represses p21 (WAF1/CIP1) promoter and promotes multiple myeloma cell growth. Eur J Cancer. 2015; 51: 252–9. [DOI] [PubMed] [Google Scholar]
- 88. Traina F, Favaro PM, Medina Sde S, et al ANKHD1, ankyrin repeat and KH domain containing 1, is overexpressed in acute leukemias and is associated with SHP2 in K562 cells. Biochem Biophys Acta. 2006; 1762: 828–34. [DOI] [PubMed] [Google Scholar]
- 89. Dong L, Lin F, Wu W, et al Transcriptional cofactor Mask2 is required for YAP‐induced cell growth and migration in bladder cancer cell. J Cancer. 2016; 7: 2132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ge L, Smail M, Meng W, et al Yes‐associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS ONE. 2011; 6: e27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pei T, Li Y, Wang J, et al YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015; 6: 17206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Su L, Ma W, Yuan J, et al Expression of Yes‐associated protein in non‐small cell lung cancer and its relationship with clinical pathological factors. Chin Med J. 2012; 125: 4003–8. [PubMed] [Google Scholar]
- 93. Yoo JH, Shi DS, Grossmann AH, et al ARF6 Is an actionable node that orchestrates oncogenic GNAQ Signaling in Uveal melanoma. Cancer Cell. 2016; 29: 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang C, Gu C, Jeong KJ, et al YAP/TAZ‐Mediated upregulation of GAB2 leads to increased sensitivity to growth factor‐induced activation of the PI3K pathway. Cancer Res. 2017; 77: 1637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cai WY, Lin LY, Hao H, et al Yes‐associated protein/TEA domain family member and hepatocyte nuclear factor 4‐Alpha (HNF4 alpha) repress reciprocally to regulate hepatocarcinogenesis in rats and mice. Hepatology. 2017; 65: 1206–21. [DOI] [PubMed] [Google Scholar]
- 96. Murakami S, Shahbazian D, Surana R, et al Yes‐associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017; 36: 1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Guo Y, Pan Q, Zhang J, et al Functional and clinical evidence that TAZ is a candidate oncogene in hepatocellular carcinoma. J Cell Biochem. 2015; 116: 2465–75. [DOI] [PubMed] [Google Scholar]
- 98. Li Z, Wang Y, Zhu Y, et al The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015; 9: 1091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wei Z, Wang Y, Li Z, et al Overexpression of Hippo pathway effector TAZ in tongue squamous cell carcinoma: correlation with clinicopathological features and patients’ prognosis. J Oral Pathol Med. 2013; 42: 747–54. [DOI] [PubMed] [Google Scholar]
- 100. Xie M, Zhang L, He C‐S, et al Prognostic significance of TAZ expression in resected non‐small cell lung cancer. J Thorac Oncol. 2012; 7: 799–807. [DOI] [PubMed] [Google Scholar]
- 101. Zeng C, Huang L, Zheng Y, et al [Expression of transcriptional coactivator with PDZ‐binding motif (TAZ) in colon cancer tissues and its clinical significance]. Zhonghua wei chang wai ke za zhi. 2015; 18: 1154–7. [PubMed] [Google Scholar]
- 102. Zhang Y, Xue C, Cui H, et al High expression of TAZ indicates a poor prognosis in retinoblastoma. Diagn Pathol. 2015; 10: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhan M, Ikeda JI, Wada N, et al Prognostic significance of a component of the Hippo pathway, TAZ, in human uterine endometrioid adenocarcinoma. Oncol Lett. 2016; 11: 3611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen G, Xie J, Huang P, et al Overexpression of TAZ promotes cell proliferation, migration and epithelial‐mesenchymal transition in ovarian cancer. Oncol Lett. 2016; 12: 1821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ma J, Huang K, Ma Y, et al The TAZ‐miR‐224‐SMAD4 axis promotes tumorigenesis in osteosarcoma. Cell Death Dis. 2017; 8: e2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Qiu X, Jiao J, Li Y, et al Overexpression of FZD7 promotes glioma cell proliferation by upregulating TAZ. Oncotarget. 2016; 7: 85987–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Han S‐X, Bai E, Jin G‐H, et al Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014; 2014: 261365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shen S, Guo X, Yan H, et al A miR‐130a‐YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015; 25: 997–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tao J, Calvisi DF, Ranganathan S, et al Activation of β‐catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014; 147: 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu M, Chan S, Liu A, et al AXL receptor kinase is a mediator of YAP‐dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011; 30: 1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cordenonsi M, Zanconato F, Azzolin L, et al The Hippo transducer TAZ confers cancer stem cell‐related traits on breast cancer cells. Cell. 2011; 147: 759–72. [DOI] [PubMed] [Google Scholar]
- 112. Di Agostino S, Sorrentino G, Ingallina E, et al YAP enhances the pro‐proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2016; 17(2): 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zanconato F, Piccolo S. Eradicating tumor drug resistance at its YAP‐biomechanical roots. EMBO J. 2016; 35(5): 459–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fernandez LA, Northcott PA, Dalton J, et al YAP1 is amplified and up‐regulated in hedgehog‐associated medulloblastomas and mediates Sonic hedgehog‐driven neural precursor proliferation. Genes Dev. 2009; 23: 2729–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chen D, Sun Y, Wei Y, et al LIFR is a breast cancer metastasis suppressor upstream of the Hippo‐YAP pathway and a prognostic marker. Nat Med. 2012; 18: 1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cheng H, Zhang Z, Rodriguez‐Barrueco R, et al Functional genomics screen identifies YAP1 as a key determinant to enhance treatment sensitivity in lung cancer cells. Oncotarget. 2016; 7(20): 28976–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Noguchi S, Saito A, Horie M, et al An Integrative Analysis of the Tumorigenic Role of TAZ in Human Non‐Small Cell Lung Cancer. Clin Cancer Res. 2014; 20: 4660–72. [DOI] [PubMed] [Google Scholar]
- 118. Bora‐Singhal N, Nguyen J, Schaal C, et al YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self‐Renewal and Vascular Mimicry of Stem‐Like Cells. Stem Cells. 2015; 33: 1705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lau AN, Curtis SJ, Fillmore CM, et al Tumor‐propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014; 33: 468–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mohseni M, Sun J, Lau A, et al A genetic screen identifies an LKB1–MARK signalling axis controlling the Hippo–YAP pathway. Nat Cell Biol. 2014; 16: 108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gayyed MF. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Journal of Gastrointestinal Cancer. 2006; 37: 103–9. [DOI] [PubMed] [Google Scholar]
- 122. Vitolo MI, Al E. The RUNX2 transcription factor cooperates with the YES‐associated protein, YAP65, to promote cell transformation. Cancer Biol Ther. 2007; 6: 856–63. [DOI] [PubMed] [Google Scholar]
- 123. Yue G, Sun X, Gimenez‐Capitan A, et al TAZ is highly expressed in gastric signet ring cell carcinoma. Biomed Res Int. 2014; 2014: 393064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jiao S, Wang H, Shi Z, et al A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014; 25: 166–80. [DOI] [PubMed] [Google Scholar]
- 125. Lee K, Sook LS, Kim S, et al Significant Association of Oncogene YAP1 with Poor Prognosis and Cetuximab Resistance in Colorectal Cancer Patients. Clin Cancer Res. 2014; 21: 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim DH, Kim SH, Lee OJ, et al Differential expression of Yes‐associated protein and phosphorylated Yes‐associated protein is correlated with expression of Ki‐67 and phospho‐ERK in colorectal adenocarcinoma. Histol Histopathol. 2013; 28: 1483–90. [DOI] [PubMed] [Google Scholar]
- 127. Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015; 21: 212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chia I, Grote D, Marcotte M, et al Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3‐Raldh2‐Ret molecular network in mice. Development. 2011; 138: 2089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Reginensi A, Hoshi M, Boualia SK, et al Yap and Taz are required for Ret‐dependent urinary tract morphogenesis. Development. 2015; 142: 2696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Saadeldin MK, Shawer H, Mostafa A, et al New genetic variants of LATS1 detected in urinary bladder and colon cancer. Front Genet. 2015; 5: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Min B, Kim MK, Zhang JW, et al Identification of RUNX3 as a component of the MST/Hpo signaling pathway. J Cell Physiol. 2012; 227: 839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Qiao Y, Lin SJ, Chen Y, et al RUNX3 is a novel negative regulator of oncogenic TEAD‐YAP complex in gastric cancer. Oncogene. 2015; 35: 2664–74. [DOI] [PubMed] [Google Scholar]
- 133. Li XJ, Park ES, Park MH, et al 3,3’‐Diindolylmethane suppresses the growth of gastric cancer cells via activation of the Hippo signaling pathway. Oncol Rep. 2013; 30: 2419–26. [DOI] [PubMed] [Google Scholar]
- 134. Latz S, Umbach T, Goltz D, et al Cytoplasmatic and Nuclear YAP1 and pYAP1 Staining in Urothelial Bladder Cancer. Urol Int. 2016; 96: 39–45. [DOI] [PubMed] [Google Scholar]
- 135. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013; 13: 246–57. [DOI] [PubMed] [Google Scholar]
- 136. Gao Y, Shi Q, Xu S, et al Curcumin promotes KLF5 proteasome degradation through downregulating YAP/TAZ in bladder cancer cells. Int J Mol Sci. 2014; 15: 15173–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Panciera T, Azzolin L, Fujimura A, et al Induction of Expandable Tissue‐Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell. 2016; 19: 725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Bartucci M, Dattilo R, Moriconi C, et al TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015; 34: 681–90. [DOI] [PubMed] [Google Scholar]
- 139. Baia GS, Caballero OL, Orr BA, et al Yes‐associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012; 10: 904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mao B, Hu F, Cheng J, et al SIRT1 regulates YAP2‐mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014; 33: 1468–74. [DOI] [PubMed] [Google Scholar]
- 141. Ciamporcero E, Shen H, Ramakrishnan S, et al YAP activation protects urothelial cell carcinoma from treatment‐induced DNA damage. Oncogene. 2016; 35: 1541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Esrig D, Elmajian D, Groshen S, et al Accumulation of nuclear P53 and tumor progression in bladder‐cancer. N Engl J Med. 1994; 331: 1259–64. [DOI] [PubMed] [Google Scholar]
- 143. Jeong KC, Kim KT, Seo HH, et al Intravesical instillation of c‐MYC inhibitor KSI‐3716 suppresses orthotopic bladder tumor growth. J Urol. 2014; 191: 510–8. [DOI] [PubMed] [Google Scholar]
- 144. Hoshino M, Qi ML, Yoshimura N, et al Transcriptional repression induces a slowly progressive atypical neuronal death associated with changes of YAP isoforms and p73. J Cell Biol. 2006; 172: 589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Levy D, Adamovich Y, Reuven N, et al The Yes‐associated protein 1 stabilizes p73 by preventing Itch‐mediated ubiquitination of p73. Cell Death Differ. 2007; 14: 743–51. [DOI] [PubMed] [Google Scholar]
- 146. Xiao W, Wang J, Ou C, et al Mutual interaction between YAP and c‐Myc is critical for carcinogenesis in liver cancer. Biochem Biophys Res Commun. 2013; 439: 167–72. [DOI] [PubMed] [Google Scholar]
- 147. Singh R, Ansari JA, Maurya N, et al Epithelial‐to‐mesenchymal transition and its correlation with clinicopathologic features in patients with urothelial carcinoma of the bladder. Clin Genitourin Cancer. 2017; 15: e187–97. [DOI] [PubMed] [Google Scholar]
- 148. Shao DD, Xue W, Krall EB, et al KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014; 158: 171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Luo M, Li ZW, Wang W, et al Long non‐coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E‐cadherin expression. Cancer Lett. 2013; 333: 213–21. [DOI] [PubMed] [Google Scholar]


