Abstract
This study describes an investigation into secondary metabolites that are produced by a marine red alga, Symphyocladia latiuscula, which was collected from coastal waters off Qingdao, China. A combination of normal, reversed phase, and gel chromatography was used to isolate six citric acid derived natural products, aconitates A–F (1–6), together with two known and ten new polybrominated phenols, symphyocladins C/D (7a/b), and symphyocladins H–Q (8a/b, 9a/b and 10–15), respectively. Structure elucidation was achieved by detailed spectroscopic (including X-ray crystallographic) analysis. We propose a plausible and convergent biosynthetic pathway involving a key quinone methide intermediate, linking aconitates and symphyocladins.
Keywords: marine red alga, Symphyocladia latiuscula, bromophenols, symphyocladins, aconitates
1. Introduction
Historically, natural products have inspired the development of many pharmaceuticals and agrochemicals, which, have in turn, played an important role in improving human and animal health and agricultural productivity, enhancing the quality of life for communities across the globe [1]. One of the defining characteristics of natural products is their structure diversity, which can encompass complex carbocyclic and heterocyclic scaffolds, annotated with a wide array of functional groups and stereochemical features. As such, even a limited set of biosynthetic precursors can deliver remarkable chemical diversity. Illustrative of this phenomenon are bromophenols from marine red algae (Rhodophyta) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. For example, the red alga Symphyocladia latiuscula (Harvey) Yamada has been reported to produce a diverse array of bromophenols elaborated by sulfoxides, sulphones, sulfates, glutamines, pyrrolidin-2-ones, ureas, diketopiperazines, and aconitic acids, with biological properties spanning antibacterial [11,12], antifungal [10,11,12,13,14], antiviral [15], anticancer [16], free radical scavenging [9,17,18], aldose reductase inhibitory [19], and Taq DNA polymerase inhibitory activities [20]. S. latiuscula bromophenols typically contain at least one 2,3,6-tribromo-4,5-dihydroxybenzyl moiety, as consistent with a highly conserved biosynthetic pathway. This report described our efforts to further elaborate the chemical diversity of S. latiuscula.
2. Results and Discussion
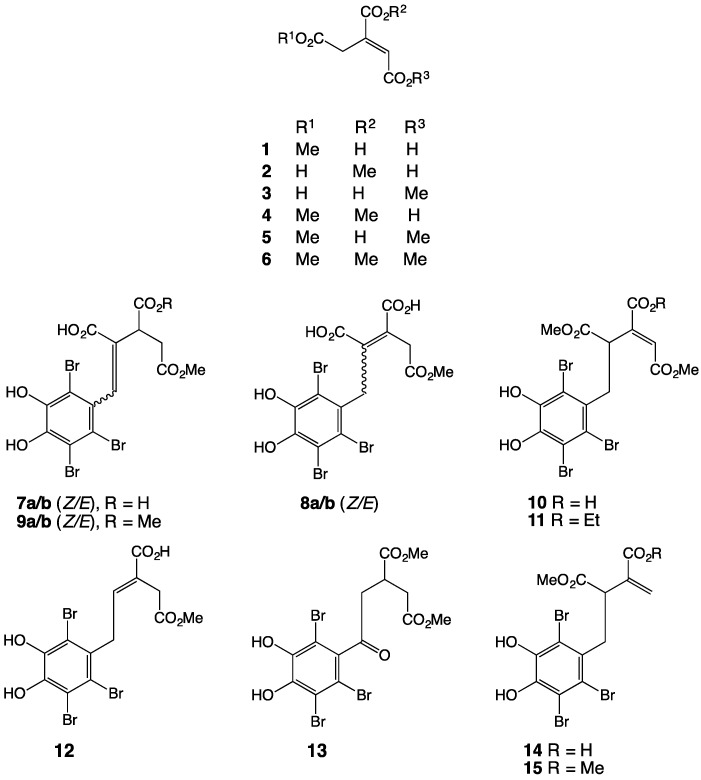
The EtOAc extract of a Chinese collection of S. latiuscula was concentrated in vacuo and subjected to a sequence of normal, reversed phase, and gel chromatography, with HPLC-MS analysis being used to prioritize fractions of interest. Following this strategy, we isolated and characterized six citric acid derived natural products, aconitates A–F (1–6), together with two known and ten new polybrominated phenol adducts, symphyocladins C/D (7a/b), and symphyocladins H–Q (8a/b, 9a/b and 10–15), respectively (Figure 1). A spectroscopic analysis approach (see Table 1, Table 2, Table 3, Table 4 and Table 5) to the structure elucidation of all of these metabolites is summarized below.
Figure 1.
S. latiuscula metabolites 1–15.
Table 1.
1H NMR Data for Compounds 7a/b–9a/b (600 MHz).
| Position | 7a a | 7b a | 8a b | 8b b | 9a c | 9b c |
|---|---|---|---|---|---|---|
| 3 | 3.74, m | 3.74, m | 3.50, m | 3.50, m | ||
| 4a | 3.17, m | 3.17, m | 3.65, s | 3.19, s | 2.98, dd e | 2.976, dd e |
| 4b | 2.490, dd d | 2.489, dd d | 2.41, dd f | 2.40, dd f | ||
| 5-OCH3 | 3.55, s | 3.55, s | 3.71, s | 3.51, s | 3.52, s | 3.52, s |
| 6-OCH3 | 3.541, s | 3.535, s | ||||
| 7′ | 7.544, s | 7.538, s | 4.22, s | 4.34, s | 7.391, s | 7.388, s |
a acetone-d6, b methanol-d4, c DMSO-d6, d J = 16.8, 7.8 Hz, e J = 16.8, 10.8 Hz, f J = 16.8, 3.0 Hz.
Table 2.
13C NMR Data for Compounds 7a/b–9a/b (150 MHz).
| Position | 7a a | 7b a | 8a b | 8b b | 9a c | 9b c |
|---|---|---|---|---|---|---|
| 1 | 167.18, C | 167.18, C | 170.3, C | 166.5, C | 166.8, C | 166.8, C |
| 2 | 135.46, C | 135.34, C | 145.0, C | 144.4, C | 133.5, C | 133.5, C |
| 3 | 41.39, CH | 41.37, CH | 127.4, C | 137.8, C | 40.2, CH | 40.2, CH |
| 4 | 35.40, CH2 | 35.35, CH2 | 35.0, CH2 | 29.5, CH2 | 33.9, CH2 | 33.9, CH2 |
| 5 | 172.80, C | 172.76, C | 168.7, C | 166.8, C | 171.5, C | 171.5, C |
| 6 | 172.54, C | 172.49, C | 172.4, C | 169.0, C | 171.5, C | 171.5, C |
| 5-OCH3 | 51.87, CH3 | 51.83, CH3 | 52.8, CH3 | 53.1, CH3 | 52.0, CH3 | 52.0, CH3 |
| 6-OCH3 | 51.6, CH3 | 51.6, CH3 | ||||
| 1′ | 129.79, C | 129.76, C | 128.8, C | 127.9, C | 128.07, C | 128.07, C |
| 2′ | 115.38, C | 115.23, C | 118.9, C | 118.4, C | 113.9, C | 113.9, C |
| 3′ | 113.98, C | 113.63, C | 114.8, C | 114.2, C | 113.7, C | 113.6, C |
| 4′ | 144.17, C | 144.10, C | 146.2, C | 145.5, C | 143.9, C | 143.8, C |
| 5′ | 145.36, C | 145.32, C | 145.6, C | 144.8, C | 145.2, C | 145.0, C |
| 6′ | 110.71, C | 110.67, C | 114.7, C | 114.3, C | 110.7, C | 110.6, C |
| 7′ | 141.52, C | 141.48, C | 41.1, CH | 35.9, CH | 140.7, C | 140.7, C |
a acetone-d6, b methanol-d4, c DMSO-d6.
Table 3.
1H NMR Data for Compounds 10–11 (600 MHz).
| Position | 10 a δH, m (J in Hz) | 11 b δH, m (J in Hz) |
|---|---|---|
| 2 | 4.98, dd (11.4, 3.0) | 4.98, dd (11.4, 3.0) |
| 4 | 6.76, s | 6.73, s |
| 1-OCH3 | 3.66, s | 3.70, s |
| 5-OCH3 | 3.44, s | 3.45, s |
| 6-OCH2CH3 | 4.26, br q (7.2) | |
| 6-OCH2CH3 | 1.31, t (7.2) | |
| 7′a | 3.87, dd (14.4, 3.0) | 3.81, dd (14.4, 3.0) |
| 7′b | 3.61, dd (14.4, 11.4) | 3.56, dd (14.4, 11.4) |
a acetone-d6, b methanol-d4.
Table 4.
1H NMR Data for Compounds 12–15 (600 MHz).
| Position | 12 a δH, m (J in Hz) | 13 b δH, m (J in Hz) | 14 b δH, m (J in Hz) | 15 a δH, m (J in Hz) |
|---|---|---|---|---|
| 2a | 6.79, br t (6.6) | 3.35, m | 3.75, dd (9.0, 6.0) | 3.79, dd (9.6, 5.4) |
| 2b | 3.27, dd (20.4, 7.8) | |||
| 3 | 3.34, m | |||
| 4a | 3.60, br s | 2.86, dd (17.4, 7.2) | 6.22. d (1.2) | 6.17, d (1.2) |
| 4b | 2.73, dd (17.4, 6.0) | 5.44, br s | 5.52, s | |
| 1-OCH3 | 3.64, s | 3.62, s | ||
| 5-OCH3 | 3.66, s | 3.68, s | ||
| 6-OCH3 | 3.71, s | 3.71, s | ||
| 7′a | 4.05, s | 3.65, dd (13.8, 6.0) | 3.69, dd (14.4, 5.4) | |
| 7′b | 3.54, dd (13.8, 9.0) | 3.56, dd (14.4, 9.6) |
a acetone-d6, b methanol-d4.
Table 5.
13C NMR Data for Compounds 10–15 (150 MHz).
| Position | 10 a δC, Type | 11 b δC, Type | 12 a δC, Type | 13 b δC, Type | 14 b δC, Type | 15 a δC, Type |
|---|---|---|---|---|---|---|
| 1 | 171.9, C | 173.5, C | 174.5, C | 172.4, C | ||
| 2 | 43.0, C | 43.7, C | 141.3, C | 44.9, CH2 | 48.8, CH | 48.2, CH |
| 3 | 142.4, C | 142.4, C | 128.1, C | 37.4, CH | 139.3, C | 138.3, C |
| 4 | 130.5, CH | 131.3, CH | 33.2, CH2 | 35.6, CH2 | 129.1, CH2 | 128.7, CH2 |
| 5 | 165.8, C | 166.7, C | 171.3, C | 173.9, C | ||
| 6 | 167.0, C | 167.0, C | 167.9, C | 175.5, C | 169.2, C | 166.6, C |
| 1-OCH3 | 52.3, CH3 | 52.9, CH3 | 52.8, CH3 | 52.3, CH3 | ||
| 5-OCH3 | 52.1, CH3 | 52.5, CH3 | 52.0, CH3 | 52.5, CH3 | ||
| 6-OCH3 | 52.8, CH3 | 52.3, CH3 | ||||
| 6-OCH2CH3 | 63.2, C | |||||
| 6-OCH2CH3 | 14.5, CH3 | |||||
| 1′ | 130.7, C | 130.6, C | 131.0, C | 136.1, C | 131.4, C | 131.3, C |
| 2′ | 118.5, C | 118.8, C | 117.3, C | 114.5, C | 118.3, C | 118.0, C |
| 3′ | 113.7, C | 114.4, C | 114.0, C | 110.5, C | 114.5, C | 113.9, C |
| 4′ | 144.1, C | 145.1, C | 144.4, C | 147.3, C | 145.0, C | 144.0, C |
| 5′ | 143.8, C | 144.8, C | 144.3, C | 145.3, C | 144.8, C | 143.9, C |
| 6′ | 114.3, C | 114.8, C | 113.0, C | 106.3, C | 114.3, C | 113.8, C |
| 7′ | 39.0, CH2 | 39.4, CH2 | 39.0, CH2 | 202.2, C | 39.4, CH2 | 39.1, CH2 |
a acetone-d6, b methanol-d4.
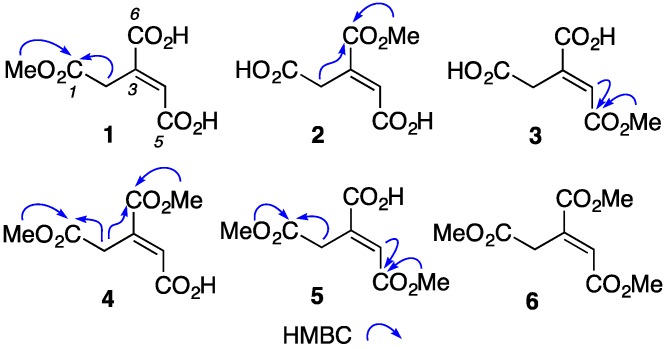
HRESI(+)MS measurements confirmed that 1 (C7H8O6, Δmmu +0.2), 2 (C7H8O6, Δmmu +0.1) and 3 (C7H8O6, Δmmu +0.1) were isomeric, while analysis of the one-dimensional (1D) and two-dimensional (2D) NMR (methanol-d4) data (Figures S8–S13, Tables S2 and S3) suggested they were mono methyl esters of E-aconitic acid, for which we attribute the trivial names aconitates A–C. Assignment of E ∆3,4 configurations were inferred from diagnostic chemical shifts for H-4 and C-2 in 1 (δH 6.92; δC 33.8), 2 (δH 6.89; δC 33.9), and 3 (δH 6.91; δC 33.9), when compared to the authentic standards for E (δH 6.90; δC 33.8) and Z (δH 6.26; δC 40.2) aconitic acid (Figures S1–S7, Table S1). HMBC correlations permitted assignment of the methyl ester regiochemistry across 1–3 with correlations from (i) the OMe (δH 3.67) and H2-2 (δH 3.89) to C-1 (δC 172.7) confirming a C-1 CO2Me in aconitate A (1), (ii) the OMe (δH 3.81) and H2-2 (δH 3.89) to C-6 (δC 168.4) confirming a C-6 CO2Me in aconitate B (2), and (iii) the OMe (δH 3.77) and H-4 (δH 6.91) to C-5 (δC 167.5) confirming a C-5 CO2Me in aconitate C (3) (Figure 2).
Figure 2.
Diagnostic 2D NMR (methanol-d4) correlations for aconitates A–F (1–6).
HRESI(+)MS measurements suggested that 4 (C8H10O6, Δmmu +0.2) and 5 (C8H10O6, Δmmu +0.2) were isomeric dimethyl esters, and 6 (C9H12O6, Δmmu +0.1) was a trimethyl ester, of aconitic acid. Analysis of the NMR (methanol-d4) data for 4–6 (Figures S14–S19, Tables S3 and S4) confirmed these assignments, with E ∆3,4 configurations being inferred from diagnostic chemical shifts for H-4 and C-2 in aconitate D (4) (δH 6.91; δC 33.8), aconitate E (5) (δH 6.92; δC 33.9) and aconitate F (6) (δH 6.92; δC 33.8), and HMBC correlations permitting the assignment of the dimethyl ester regiochemistry across 4 and 5. For example, correlations from an OMe (δH 3.67) and H2-2 (δH 3.91) to C-1 (δC 172.5), and from an OMe (δH 3.80) and H2-2 to C-6 (δC 168.2), confirmed the presence of C-1 CO2Me and C-6 CO2Me moieties in 4, whereas correlations from an OMe (δH 3.67) and H2-2 (δH 3.91) to C-1 (δC 172.5), and from an OMe (δH 3.76) and H-4 (δH 6.92) to C-5 (δC 167.5), confirmed C-1 CO2Me and C-5 CO2Me moieties in 5 (Figure 2).
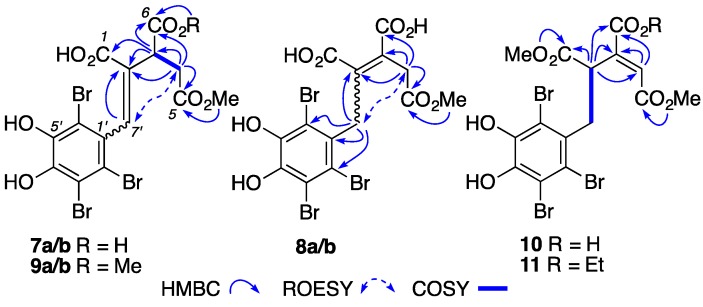
HRESI(+)MS measurements confirmed that 7a/b (C14H11Br3O8, Δmmu +0.5) and 8a/b (C14H11Br3O8, ∆mmu +0.4) were isomeric, and suggested that 9a/b (C15H13Br3O8, Δmmu +0.4) and 10 (C15H13Br3O8, Δmmu +0.5) were CH2 homologues, and 11 (C17H17Br3O8, Δmmu +0.6) was a CH2CH2 homologue of 7a/b and 8a/b. Analysis of the NMR (acetone-d6) data for 7a/b (Figures S20 and S21, Table 1, Table 2 and Table S5) confirmed them as symphyocladins C/D, first reported in 2012 from S. latiuscula as an inseparable mixture of Z/E ∆2,7′ isomers [13]. Analysis of the NMR (methanol-d4) data for symphyocladins H/I (8a/b) (Figures S22–S27, Table 1, Table 2 and Table S6) revealed ∆2,3 and C-5 CO2Me moieties, as evidenced by diagnostic HMBC correlations (Figure 3). Significantly, these data also revealed an interconverting mixture of E/Z ∆2,3 isomers, in which the minor Z isomer, symphyocladin H (8a), as evidenced by a ROESY correlation between H2-4 and H2-7′ (Figure 3), was in equilibrium with the major E isomer, symphyocladin I (8b). Further analysis of this NMR data revealed chemical shift differences diagnostic for ∆2,3 geometric isomers; H2-4 (E δH 3.65, δC 35.0; Z δH 3.19, δC 29.5), C-1 (E δC 170.3; Z δC 166.5), C-3 (E δC 127.4; Z δC 137.8), and C-5 CO2Me (E δH 3.71; Z δH 3.51). Remarkably, the NMR (acetonitrile-d3) data for 8a/b revealed a single Z isomer 8a, as evidenced by simplified spectra, a ROESY correlation between H2-4 and H2-7′, and diagnostic chemical shifts (Figures S28 and S29, Table S7). We speculate that in aprotic solvents (i.e., acetonitrile-d3), hydrogen bonding between adjacent CO2H moieties exclusively favors the lower energy Z ∆2,3 isomer. By contrast, in protic solvents (i.e., methanol-d4), the disruption of this hydrogen bonding favor equilibration to an E/Z ∆2,3 mixture dominated by the less sterically constrained E isomer. This observation highlights the critical importance that NMR solvents can play in the analysis and structure elucidation of natural products.
Figure 3.
Diagnostic 2D NMR correlations for symphyocladins C/D (7a/b), H/I (8a/b), J/K (9a/b) and L–M (10–11) (see Tables and Supporting Information for NMR solvents).
Analysis of the NMR (DMSO-d6) data for symphyocladins J/K (9a/b) (Figures S30 and S31, Table 1, Table 2 and Table S8) identified an inseparable mixture of C-6 CO2Me homologues of 7a/b and 8a/b, as evidenced by spectroscopic comparisons and diagnostic HMBC correlations from the additional CO2Me resonances to C-6 (Figure 3). In this instance, as hydrogen bonding does not stabilize double bond isomers, the Z/E mixture prevails even in an aprotic solvent (i.e., DMSO-d6). Analysis of the NMR (acetone-d6) data for symphyocladin L (10) (Figures S32 and S33, Table 3, Table 5 and Table S9) revealed a ∆3,4 isomer and 1-CO2Me homologue of 7a/b and 8a/b, as evidenced by COSY correlations between H-2 and H2-7′, and diagnostic HMBC correlations positioning both C-1 CO2Me and C-5 CO2Me (Figure 3). The structure of 10 inclusive of an E ∆3,4 configuration and its racemic nature were confirmed by single crystal X-ray analysis with the compound crystallizing in a centrosymmetric space group (Figures S34 and S35). Analysis of the NMR (methanol-d4) data for symphyocladin M (11) (Figures S36 and S37, Table 3, Table 5 and Table S10) revealed it as a C-6 CO2Et homologue of 10, as evidenced by spectroscopic comparisons and an HMBC correlation from the CO2Et moiety to C-6.
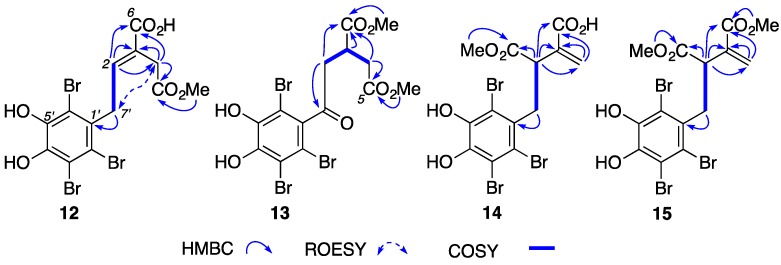
HRESI(+)MS measurements suggested that 12 (C13H11Br3O6, Δmmu +0.5) was a decarboxy analogue of 7a/b and 8a/b; 13 (C14H13Br3O7, Δmmu +0.5) was a dihydro oxidized methyl ester of 12; 14 (C13H11Br3O6, Δmmu +0.5) was a decarboxymethyl analogue of 10; and, 15 (C14H13Br3O6, Δmmu +0.5) was a CH2 homologue of 14. Comparison of the NMR (methanol-d4) data for symphyocladin N (12) (Figures S38 and S39, Table 4, Table 5 and Table S11) with that for 8a/b revealed the key difference as replacement of the C-1 CO2H moiety in 8a/b with an H-2 olefinic methine (δH 6.79) coupled to H2-7′ (δH 4.05). The presence of a C-5 CO2Me moiety in 12 was evident from an HMBC correlation from the OMe (δH 3.66) to C-5 (δC 171.3), while an E Δ2,3 configuration was confirmed by a ROESY correlation between H2-4 (δH 3.60) and H2-7′ (Figure 4). Analysis of the NMR (methanol-d4) data for symphyocladin O (13) (Figures S40 and S41, Table 4, Table 5, and Table S12) revealed it to be a saturated oxidized analogue of 12, as evidenced by COSY correlations between a diastereotopic H2-2 (δH 3.35/3.27), through H-3 (δH 3.34) to a diastereotopic H2-4 (δH 2.86/2.73). Likewise, replacement of the C-7′ sp3 methylene in 12 (δC 39.0) with a carbonyl resonance in 13 (δC 202.2) was evidence of a 7-oxo moiety. Diagnostic HMBC correlations also established the presence of incorporated C-5 CO2Me (δH 3.68) and C-6 CO2Me (δH 3.71) moieties (see Figure 3).
Figure 4.
Diagnostic 2D NMR correlations for symphyocladins N–Q (12–15) (see Table 4 and Table 5 and Supporting Information for NMR solvents).
Comparison of the NMR (methanol-d4) data for symphyocladin P (14) (Figures S42 and S43, Table 4, Table 5 and Table S13) with that for 10 revealed the key difference as replacement of the C-5 CO2Me moiety in 10 with a diastereotopic H2-4 olefinic methylene (δH 6.22/5.44). Comparison of the NMR (acetone-d6) data for symphyocladin Q (15) (Figures S44 and S45, Table 4, Table 5 and Table S14) with that for 14 revealed the key difference as an additional resonance, attributed to a C-6 CO2Me moiety (δH 3.71). Structure assignments for 14 and 15 were further supported by diagnostic 2D NMR correlations (Figure 4).
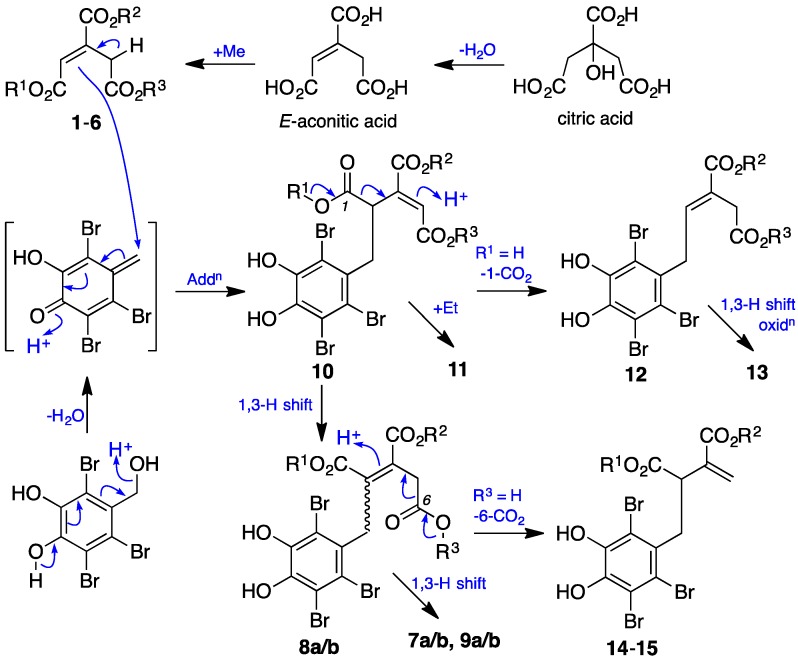
Structural similarities across 1–15 suggest a highly conserved biosynthesis. Building on this observation, we propose a biosynthetic relationship (Figure 5), in which the aconitates A–F (1–6) are viewed as mono, di, and tri methyl esters of the precursor E-aconitic acid, itself a dehydration product of citric acid. Likewise, metabolites 7–15 can be viewed as adducts between aconitates and an intermediate quinone methide that is generated from 2,3,6-tribromo-4,5-dihydroxybenzyl alcohol, further elaborated by a combination of 1,3-hydride shifts, decarboxylations and oxidations. Although 7a/b, 9a/b, 10–11, and 13 incorporate a single chiral sp3 center, as they do not exhibit measurable optical rotations they are presumed to be racemic, as confirmed for 10 by X-ray crystallography. The absence of double bond migrations (i.e., racemization) during isolation and handling suggests that this racemic character is a function of achiral adduct addition. The proposed biosynthetic relationship informs a possible biomimetic synthesis of 7–15, although, in our hands, synthetic 2,3,6-tribromo-4,5-dihydroxybenzyl alcohol proved stable to both acid and base conditions indicative of a requirement to activate the benzyl alcohol moiety to effect dehydration and the formation of a quinone methide.
Figure 5.
A plausible biosynthetic relationship linking 1–15.
3. Materials and Methods
General Experimental Procedures. Specific optical rotations ([α]D) were measured on a polarimeter in a 100 × 2 mm cell at 22 °C. NMR spectra were obtained on a Bruker Avance DRX600 or DRX500 spectrometers, in the solvents indicated and referenced to residual 1H and 13C signals in deuterated solvents. Electrospray ionization mass spectra (ESIMS) were acquired using an Agilent 1100 Series separations module equipped with an Agilent 1100 Series LC/MSD mass detector in both positive and negative ion modes. High-resolution ESIMS measurements were obtained on a Bruker micrOTOF mass spectrometer by direct infusion in MeCN at 3 mL/min using sodium formate clusters as an internal calibrant. HPLC was performed using an Agilent 1100 Series separations module equipped with Agilent 1100 Series diode array and/or multiple wavelength detectors and Agilent 1100 Series fraction collector, controlled using ChemStation Rev.B02.01 and Purify version A.1.2 software.
Algal material. Symphyocladia latiuscula was collected on the coast of Qingdao, Shandong Province, China, in May 2004. The specimen identification was verified by Dr. Kui-Shuang Shao (Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China). A voucher specimen (No. 2004X16) was deposited at the Herbarium of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China.
Extraction and isolation. The air-dried red alga Symphyocladia latiuscula (4.3 kg) was extracted with 95% EtOH at room temperature (3 × 72 h). After the solvent was removed under reduced pressure at <40 °C, a dark residue (610 g) was obtained. The residue was partitioned between EtOAc and H2O, and the EtOAc-soluble partition (320 g) was chromatographed over silica gel, eluting with a gradient of 0–100% Me2CO/petroleum ether to yield 85 fractions (F1–F85) (see Supporting Information Scheme S1 for the fractionation scheme). Fraction F54 was further fractionated over Sephadex LH-20 using CHCl3–MeOH (2:1) to afford 21 fractions.
The sixth fraction from Sephadex LH-20 chromatography was fractionated on an ODS column, eluted by a stepwise gradient (0–100% MeOH/H2O) to afford 11 fractions. The third fraction was subjected to HPLC separation (Zorbax Eclipse XDB-C8, 5 μm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 10 to 80% MeCN/H2O over 15 min, with isocratic 0.01% TFA modifier) to yield 1–6; the sixth fraction was subjected to HPLC separation (Zorbax SB-C18, 5 μm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 30 to 75% MeCN/H2O over 14 min, with isocratic 0.01% TFA modifier) to yield 8a/b and 9a/b; and the seventh fraction was subjected to HPLC separation (Zorbax Eclipse XDB-C8, 5 μm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 40 to 55% MeCN/H2O over 20 min, with isocratic 0.01% TFA modifier) to yield 11 and 13.
The seventh fraction from Sephadex LH-20 chromatography was subjected to HPLC fractionation (Zorbax SB-C18, 5 um, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 30 to 80% MeCN/H2O over 14 min, with isocratic 0.01% TFA modifier) to yield 10.
The eighth fraction from Sephadex LH-20 chromatography was fractionated on an ODS column, eluted with a stepwise gradient (0–100% MeOH/H2O) to afford 11 fractions; the sixth fraction was subjected to HPLC fractionation (Zorbax SB-C18, 5 δm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 35 to 50% MeCN/H2O over 15 min, with isocratic 0.01% TFA modifier) to yield 14; the seventh fraction was subjected to HPLC fractionation (Zorbax SB-C18, 5 μm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 50 to 60% MeCN/H2O over 12 min, with isocratic 0.01% TFA modifier) to yield 12 and 15.
The ninth fraction from Sephadex LH-20 chromatography was fractionated on an ODS column, eluted with a stepwise gradient (0–100% MeOH/H2O) to afford 11 fractions; the third fraction was subjected to HPLC fractionation (Zorbax SB-C18, 5 μm, 250 × 9.4 mm column, 3.0 mL/min, gradient elution from 30 to 80% MeCN/H2O over 20 min, with isocratic 0.01% TFA modifier) to yield 7a/b.
Aconitate A (1): White solid; NMR (600 MHz, methanol-d4) see Table S2; HRESIMS m/z 189.0396 [M + H]+ (calcd. for C7H8O6 189.0394).
Aconitate B (2): White solid; NMR (600 MHz, methanol-d4) see Table S2; HRESIMS m/z 189.0395 [M + H]+ (calcd. for C7H8O6 189.0394).
Aconitate C (3): White solid; NMR (600 MHz, methanol-d4) see Table S3; HRESIMS m/z 189.0395 [M + H]+ (calcd. for C7H8O6 189.0394).
Aconitate D (4): White solid; NMR (600 MHz, methanol-d4) see Table S3; HRESIMS m/z 203.0552 [M + H]+ (calcd. for C8H11O6, 203.0550)
Aconitate E (5): White solid; NMR (600 MHz, methanol-d4) see Table S4; HRESIMS m/z 203.0551 [M + H]+ (calcd. for C8H11O6, 203.0550).
Aconitate F (6): White solid; NMR (600 MHz, methanol-d4) see Table S4; HRESIMS m/z 217.0708 [M + H]+ (calcd. for C9H13O6, 217.0707).
Symphyocladins C/D (7a/b): Light brown solid; NMR (600 MHz, acetone-d6) see Table 1, Table 2 and Table S5; HRESIMS m/z 544.8082 [M + H]+ (calcd. for C14H12Br3O8, 544.8077).
Symphyocladins H/I (8a/b): Light brown solid; NMR (600 MHz, methanol-d4, acetonitrile-d3) see Table 1, Table 2, Tables S6 and S7; HRESIMS m/z 544.8081 [M + H]+ (calcd. for C14H12Br3O8, 544.8077).
Symphyocladins J/K (9a/b): Light brown solid; NMR (600 MHz, DMSO-d6) see Table 1, Table 2 and Table S8; HRESIMS m/z 558.8237 [M + H]+ (calcd. for C15H14Br3O8, 558.8233).
Symphyocladin L (10): Light brown solid; NMR (600 MHz, acetone-d6) see Table 3, Table 5 and Table S9; HRESIMS m/z 558.8238 [M + H]+ (calcd. for C15H14Br3O8, 558.8233).
Symphyocladin M (11): Light brown solid; NMR (600 MHz, methanol-d4) see Table 3, Table 5 and Table S10; HRESIMS m/z 586.8552 [M + H]+ (calcd. for C17H17Br3O8, 586.8546).
Symphyocladin N (12): Light brown solid; NMR (600 MHz, acetone-d6) see Table 4, Table 5 and Table S11; HRESIMS m/z 500.8184 [M + H]+ (calcd. for C13H12Br3O6, 500.8179).
Symphyocladin O (13): Light brown solid; NMR (600 MHz, methanol-d4) see Table 4, Table 5 and Table S12; HRESIMS m/z 530.8289 [M + H]+ (calcd. for C14H14Br3O7, 530.8284).
Symphyocladin P (14): Light brown solid; NMR (600 MHz, methanol-d4) see Table 4, Table 5 and Table S13; HRESIMS m/z 500.8184 [M + H]+ (calcd. for C13H12Br3O6, 500.8179).
Symphyocladin Q (15): Light brown solid; NMR (600 MHz, acetone-d6) see Table 3, Table 4 and Table S14; HRESIMS m/z 514.8340 [M + H]+ (calcd. for C14H14Br3O6, 514.8335).
X-ray crystallography. X-ray crystallographic data were collected on an Oxford Diffraction Gemini CCD diffractometer with Mo-Kα radiation (0.71073 Å) operating within the range 2 < 2θ < 50°. The sample was cooled to 190 K with an Oxford Cryosystems Desktop Cooler. Data reduction and empirical absorption corrections were performed using CrysAlisPro (Rigaku Oxford Diffraction, Yarnton, Oxfordshire, UK). The structure was solved by Direct Methods and refined with SHELX [21] and all of the calculations and refinements were carried out by WinGX package [22]. All non-H atoms were refined aniostropically. The thermal ellipsoid plot was produced with ORTEP [23] and the unit cell diagram was drawn with PLATON [24]. Crystallographic data including structure factors in CIF format have been deposited with the Cambridge Crystallographic Data Centre (CCDC 1569026).
Acknowledgments
This work was supported in part by the Chinese Government Fundamental Research Funds for the Central Universities, the National Key Research and Development Program of China (2017YFD0201203), and the University of Queensland, Institute for Molecular Bioscience.
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/12/374/s1, Isolation Scheme as well as Tabulated 1D and 2D NMR data and spectra for 1–15, X-ray of compound 10.
Author Contributions
X. Xu red alga collection, extractions, compound isolation and spectroscopic data analysis, H.Y. and L.Y. assisted in chemical fractionation, X. Xiao in acquisition of spectroscopic data. P.V.B. carried out X-ray analyses, and P.N. synthetic studies. F.S. acquired and analyzed spectroscopic data. Z.G.K., A.A.S. and R.J.C. analysed spectroscopic data and assembly the Supporting Information. R.J.C. proposed the biosynthesis. R.J.C. and F.S. managed the research, assigned structures, and co-drafted the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Liu M., Hansen P.E., Lin X.K. Bromophenols in marine algae and their bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X., Li X.M., Gao L.X., Cui C.M., Li C.S., Li J., Wang B.G. Extraction and PTP1B inhibitory activity of bromophenols from the marine red alga Symphyocladia latiuscula. Chin. J. Oceanol. Limn. 2011;29:686–690. doi: 10.1007/s00343-011-0136-1. [DOI] [Google Scholar]
- 4.Li K., Li X.M., Gloer J.B., Wang B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food Chem. 2012;135:868–872. doi: 10.1016/j.foodchem.2012.05.117. [DOI] [PubMed] [Google Scholar]
- 5.Lin X.K., Liu M. Bromophenols from marine algae with potential anti-diabetic activities. J. Ocean Univ. China. 2012;11:533–538. doi: 10.1007/s11802-012-2109-1. [DOI] [Google Scholar]
- 6.Olsen E.K., Hansen E., Isaksson J., Andersen J.H. Antioxidant effect of four bromophenols from the red algae Vertebrata lanosa. Planta Med. 2012;78:1146. doi: 10.1055/s-0032-1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javan A.J., Javan M.J., Tehrani Z.A. Theoretical investigation on antioxidant activity of bromophenols from the marine red alga Rhodomela confervoides: H-atom vs. electron transfer mechanism. J. Agric. Food Chem. 2013;61:1534–1541. doi: 10.1021/jf304926m. [DOI] [PubMed] [Google Scholar]
- 8.Olsen E.K., Hansen E., Isaksson J., Andersen J.H. Cellular antioxidant effect of four bromophenols from the red algae, Vertebrata lanosa. Mar. Drugs. 2013;11:2769–2784. doi: 10.3390/md11082769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X., Yin L., Gao L., Gao J., Chen J., Li J., Song F. Two new bromophenols with radical scavenging activity from marine red alga Symphyocladia latiuscula. Mar. Drugs. 2013;11:842–847. doi: 10.3390/md11030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Yin L., Gao J., Gao L., Song F. Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem. Biodivers. 2014;11:807–811. doi: 10.1002/cbdv.201300239. [DOI] [PubMed] [Google Scholar]
- 11.Kurata K., Amiya T. Bis(2,3,6-tribromo-4,5-dihydroxybenzyl) ether from the red alga, Symphyocladia latiuscula. Phytochemistry. 1980;19:141–142. doi: 10.1016/0031-9422(80)85032-1. [DOI] [Google Scholar]
- 12.Xu X., Song F., Fan X., Fang N., Shi J. A novel bromophenol from marine red alga Symphyocladia latiuscula. Chem. Nat. Compd. 2009;45:811–813. doi: 10.1007/s10600-010-9501-0. [DOI] [Google Scholar]
- 13.Xu X., Piggott A.M., Yin L., Capon R.J., Song F. Symphyocladins A–G: Bromophenol adducts from a Chinese marine red alga, Symphyocladia latiuscula. Tetrahedron Lett. 2012;53:2103–2106. doi: 10.1016/j.tetlet.2012.02.044. [DOI] [Google Scholar]
- 14.Xu X., Yin L., Wang Y., Wang S., Song F. A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat. Prod. Res. 2013;27:723–726. doi: 10.1080/14786419.2012.695362. [DOI] [PubMed] [Google Scholar]
- 15.Park H.J., Kurokawa M., Shiraki K., Nakamura N., Choi J.S., Hattori M. Antiviral activity of the marine alga Symphyocladia latiuscula against herpes simplex virus (HSV-1) in vitro and its therapeutic efficacy against HSV-1 infection in mice. Biol. Pharm. Bull. 2005;28:2258–2262. doi: 10.1248/bpb.28.2258. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.H., Park S.E., Hossain M.A., Kim M.Y., Kim M.N., Chung H.Y., Choi J.S., Yoo Y.H., Kim N.D. 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Arch. Pharm. Res. 2007;30:1132–1137. doi: 10.1007/BF02980248. [DOI] [PubMed] [Google Scholar]
- 17.Choi J.S., Park H.J., Jung H.A., Chung H.Y., Jung J.H., Choi W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000;63:1705–1706. doi: 10.1021/np0002278. [DOI] [PubMed] [Google Scholar]
- 18.Duan X.J., Li X.M., Wang B.G. Highly brominated mono-and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007;70:1210–1213. doi: 10.1021/np070061b. [DOI] [PubMed] [Google Scholar]
- 19.Wang W., Okada Y., Shi H.B., Wang Y.Q., Okuyama T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005;68:620–622. doi: 10.1021/np040199j. [DOI] [PubMed] [Google Scholar]
- 20.Jin H.J., Oh M.Y., Jin D.H., Hong Y.K. Identification of a Taq DNA polymerase inhibitor from the red seaweed Symphyocladia latiuscula. J. Environ. Biol. 2008;29:475–478. [PubMed] [Google Scholar]
- 21.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 22.Farrugia L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI) J. Appl. Crystallogr. 1997;30:565. doi: 10.1107/S0021889897003117. [DOI] [Google Scholar]
- 23.Farrugia L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999;32:837–838. doi: 10.1107/S0021889899006020. [DOI] [Google Scholar]
- 24.Spek A.L. PLATON, An integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. Sect A. 1990;46:C34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.