Abstract
Aims
To compare intraperitoneal (IP) to subcutaneous (SC) insulin delivery in an artificial pancreas (AP).
Research design and methods
Ten adults with type 1 diabetes participated in a non-randomized, non-blinded sequential AP study using the same SC glucose sensing and Zone Model Predictive Control (ZMPC) algorithm adjusted for insulin clearance. On first admission, subjects underwent closed-loop control with SC delivery of a fast-acting insulin analogue for 24 hours. Following implantation of a DiaPort IP insulin delivery system, the identical 24-hour trial was performed with IP regular insulin delivery. The clinical protocol included 3 unannounced meals with 70, 40 and 70 g carbohydrate, respectively. Primary endpoint was time spent with blood glucose (BG) in the range of 80 to 140 mg/dL (4.4–7.7 mmol/L).
Results
Percent of time spent within the 80 to 140 mg/dL range was significantly higher for IP delivery than for SC delivery: 39.8 ± 7.6 vs 25.6 ± 13.1 (P = .03). Mean BG (mg/dL) and percent of time spent within the broader 70 to 180 mg/dL range were also significantly better for IP insulin: 151.0 ± 11.0 vs 190.0 ± 31.0 (P = .004) and 65.7 ± 9.2 vs 43.9 ± 14.7 (P = .001), respectively. Superiority of glucose control with IP insulin came from the reduced time spent in hyperglycaemia (>180 mg/dL: 32.4 ± 8.9 vs 53.5 ± 17.4, P = .014; >250 mg/dL: 5.9 ± 5.6 vs 23.0 ± 11.3, P = .0004). Higher daily doses of insulin (IU) were delivered with the IP route (43.7 ± 0.1 vs 32.3 ± 0.1, P < .001) with no increased percent time spent <70 mg/dL (IP: 2.5 ± 2.9 vs SC: 4.1 ± 5.3, P = .42).
Conclusions
Glycaemic regulation with fully-automated AP delivering IP insulin was superior to that with SC insulin delivery. This pilot study provides proof-of-concept for an AP system combining a ZMPC algorithm with IP insulin delivery.
Keywords: artificial pancreas, CGM, closed-loop, CSII, DiaPort, hyperglycaemia, hypoglycaemia intraperitoneal, insulin pump, model predictive control, type 1 diabetes
1 | INTRODUCTION
Clinical evaluations have shown that artificial pancreas (AP) systems using subcutaneous (SC) insulin delivery and SC glucose sensing with model-predictive control (MPC) algorithms are safe and effective.1 However, controlling hyperglycaemia after meals remains an open challenge.2 While subcutaneous (SC) insulin delivery is convenient and minimally invasive, it introduces delays to insulin action and clearance3 that make tight control difficult. The hurdles to tight glycaemic control caused by these delays are so significant that many studies have added meal announcement with full or partial bolus triggered by the patient to improve postprandial glucose control,4–7 thereby trading-off autonomy for performance.
Intraperitoneal (IP) insulin delivery could address this major challenge and is a promising alternative to the conventional subcutaneous route. Delivering insulin to the intraperitoneal space results in faster pharmacokinetics/pharmacodynamics8,9; hence, it could be easier for an AP controller to quickly respond to glycaemic disturbances. Preliminary studies of closed-loop control using implanted insulin pumps have shown that this type of system is clinically feasible.10–12
In addition to improved pharmacokinetics and pharmacodynamics, IP delivery also better mimics physiological insulin delivery by providing a higher insulin concentration in the portal system than in the peripheral system. There is some evidence to show that this difference results in better insulin/glucagon balance and glycaemic variability.13,14 Some recent studies15,16 indicate that prolonged use of IP infusion does not adversely affect insulin-like growth-factor-1 (IGF-1) concentrations, as observed with prolonged SC use.17 The use of intraperitoneal insulin delivery also reduces frequency and severity of hypoglycaemic episodes.18,19
In this paper, we present a sequential study to compare closed-loop zone MPC using the DiaPort IP insulin delivery system with the traditional SC insulin delivery method during a 24-hour in-clinic protocol. Our objective is to verify the hypothesis that closed-loop control with IP insulin delivery significantly outperforms SC delivery in terms of glycaemic regulation and expedited reaction to meal-related disturbances.
2 | RESEARCH DESIGN AND METHODS
2.1 | Subjects
Subjects aged 18 to 65 years, with an indication for the DiaPort system, were recruited for the study. Indications for the DiaPort system included: poor glucose control under SC insulin delivery as demonstrated by sustained HbA1c > 8% and/or high blood glucose (BG) variability, including recurrent hypoglycaemic events according to self-monitoring of blood glucose data and oral reports despite intensified insulin therapy. Additional inclusion criteria were diagnosis of type 1 diabetes (T1D) for more than 1 year, proper mental status and cognition, affiliation or beneficiary of a social medical insurance and signed informed consent. Exclusion criteria were unwillingness to perform repeated glucose checks, to consume standardized meals and/or to take insulin as directed; evidence of a cardiovascular event during the previous 6 months; non-stabilized retinopathy; clinically significant screening laboratory abnormalities; pregnancy, breast feeding or intention of becoming pregnant; presence of a medical condition requiring the use of an acetaminophen-containing medication that could not be withheld during the study; active involvement or enrolment in another clinical trial, or having been part of a study within 30 days; and persons deprived of freedom, adults protected by law or vulnerable persons.
2.2 | Study design
The purpose of this study was to compare glycaemic control by a closed-loop AP using intraperitoneal insulin delivery vs subcutaneous insulin delivery. Each subject participated in two 24-hour admissions in a non-randomized, non-blinded sequential design. Because of the impossibility of returning to SC insulin delivery after the IP insulin infusion has started, in light of the risk of IP catheter occlusion, the subcutaneous admission needed to be performed before implantation of the DiaPort system; thus, all subjects underwent the 2 study admissions in the same order. The first admission evaluated the closed-loop system using SC fast-acting insulin analogue (Humalog, Eli-Lilly, Indianapolis, Indiana) delivery. The second admission, to evaluate the closed-loop system with IP regular insulin delivery, took place 4 to 20 weeks following implantation of the DiaPort system. This waiting period allowed the subject to recover from surgery and acclimate to the new delivery route after specific pre- and post-implantation training before testing the closed-loop system. An overview of the study is provided online in Supporting Information (Figure S1).
2.3 | DiaPort system
The DiaPort system (Second Generation, Roche Diagnostics, Mannheim, Germany) which is surgically implanted is composed of a catheter located in the peritoneal space and fixed to a metallic body attached to the skin.20 A membrane and polyester felt aim to prevent skin infections. Patient skin grows on the felt and works as a barrier. The port, which sticks up 2 to 3 mm from the skin, can connect to the Accu-chek Spirit Combo pump (Roche Diagnostics, Mannheim, Germany) which delivers the insulin. Infused insulin through the DiaPort is a regular U-100 insulin solution (Insuman Infusat, Sanofi, Paris, France). Fast-acting insulin analogues are not usable in the DiaPort catheter because of their physical instability in this environment, which prompts insulin aggregation in the catheter lumen.20
2.4 | Procedures
The study protocol was approved by the Comité de Protection des Personnes Sud-Méditerranée IV, Montpellier, France and was registered in ClinicalTrails.gov under NCT01555788. Screening visits were conducted to verify that inclusion and exclusion criteria were satisfied and to obtain informed consent. Subjects attended the clinical centre for an outpatient visit 48 to 72 hours before each admission for sensor insertion. This visit also allowed assessment of whether subjects presented with major glucose variability that would affect closed-loop glucose control and to record the following parameters: 24-hour basal rate, correction factor (blood glucose change according to 1 unit of insulin delivery), insulin-to-carbohydrate ratio (amount of insulin needed to cover an oral intake of 10 g carbohydrates), total daily insulin dose, which will serve as inputs for the MPC controller. On each investigation day, subjects were admitted to the clinical research centre and the closed-loop protocol began approximately 2 hours following admission. The subjects remained on closed-loop control for 24 hours before the system was disconnected and they were discharged from the clinical centre. The overall timeline of admissions over 24 hours is provided in Figure S2.
2.5 | Meals
The trial included 3 meals: a 70 g-carbohydrate (CHO) dinner at ~7:00 PM, a 40 g-CHO breakfast at ~8:00 AM and a 70 g-CHO lunch at ~1:00 PM. Although well standardized for the study, these CHO amounts were close to those common to the patients in daily life. The meals were unannounced and no meal bolus was provided; all insulin delivery was in reaction to detected increases in blood glucose concentration by the CGM unit.
2.6 | Closed-loop system
The closed-loop algorithm adopted to control insulin delivery in this study was Zone Model Predictive Control (ZMPC).21 This control strategy uses a model of insulin action22 to calculate the optimal insulin dose necessary to drive the measured glucose concentration within a desired zone of 80 to 140 mg/dL (4.4–7.7 mmol/L). The details of the design and in-silico evaluation of the controller used in this study for IP insulin delivery can be found in the literature23; note that this model is a first approximation of IP delivery and was fitted on clinical data by modifying the transfer function poles to reflect the rapidity of the IP dynamics.
The platform for the controller was the Artificial Pancreas System (APS) operating on a laptop computer.24 Subjects wore 2 Dexcom Seven Plus CGM systems (Dexcom, San Diego, California); however, only 1 sensor was used during the study while the second was scheduled as backup in case of failure of the first. In both admissions, insulin was delivered by an Accu-chek Spirit Combo pump; for the SC phase of the study, infused insulin was a fast-acting insulin analogue whereas, for the IP phase, infused insulin through the DiaPort system was regular insulin. The APS received a CGM measurement every 5 minutes; the controller then computed the optimal insulin dose and transmitted the amount to be delivered via Bluetooth to the pump.
2.7 | Safety and monitoring
Throughout the study, a clinician was present to monitor the glucose signal and insulin delivery, as well as to advise the subject on use of the system. Venous blood was drawn every hour except around meals, when it was drawn 15, 10 and 5 minutes prior to the meal and 5, 10, 20, 30, 40, 50, 60, 80, 100 and 120 minutes after the meal. The samples were analysed using a YSI STAT 2300 PLUS (Yellow Springs, Ohio) blood glucose analyser. These venous measurements were used by the clinicians to verify measurements made by the CGM and to provide a safeguard against hypoglycaemia. Note that the YSI measurements were not available to the controller. The total insulin in the serum samples was measured using a radio-immunologic method (bi-insulin immuno-radiometric assay, Schering CIS bio international, Gif sur Yvette, France) in the Nuclear Medicine Department of Montpellier University Hospital.
To prevent hypoglycaemia, the Health Monitoring System (HMS)25 was active throughout each study. In the case of predicted hypoglycaemia, a message was triggered to advise the subject to consume 16 g of oral CHO. The suggestion to treat with oral CHO was accepted or rejected at the discretion of the study physician. The HMS alarms were disabled for 3 subjects during the SC study and 4 subjects during the IP study because of human error. During all studies, the physician monitored the CGM trace and subject status for signs of hypoglycaemia. Patients with YSI plasma glucose less than 60 mg/dL were treated with approximately 15 g of glucose (juice or glucose tablets), and YSI plasma glucose was reassessed every 15 minutes until BG exceeded 70 mg/dL. The study physician also monitored for hyperglycaemia by performing a urine ketone test in cases of YSI plasma glucose exceeding 250 mg/dL. The pump would then be checked for catheter occlusion and to ensure that the algorithm was delivering insulin doses properly.
2.8 | Statistical analysis
No sample size calculation was performed for this pilot study. Because of the higher accuracy and satisfactory temporal resolution of data collection, we performed statistical analysis on the YSI data obtained. All statistical data are presented in a mean ± one standard deviation format unless explicitly stated otherwise. The differences between each metric were compared using non-parametric Wilcoxon rank-sum tests because of the small number of samples. MATLAB 2016a software (Mathworks, Inc., Natick, Massachusetts) was used for all analyses. Note that a P-value <.05 is considered statistically significant.
3 | RESULTS
3.1 | Participants
Ten subjects, whose main individual characteristics are presented in Table 1, completed the SC and IP branches of the study. Of these, 7 subjects were male, 3 were female, and the average age was 49 years with a standard deviation of 11 years. Other statistics include: BMI, 24 ± 4 kg/m2; HbA1c, 7.7% ± 1.0% (61 ± 11 mmol/mol); T1D duration, 29 ± 14 years; insulin pump use, 8.5 ± 7.8 years. Kidney function assessed on plasma creatinine was within normal range. None of the patients presented elevated anti-insulin antibody levels. All subjects underwent the two 24-hour closed-loop admissions. No DiaPort-related adverse events occurred during the study period.
TABLE 1.
Characteristics of study subjects at inclusion
| Overall Mean SD |
Subject ID | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Sex | F | M | F | M | M | M | F | M | M | M | |
| Height [cm] | 167 ± 6 | 160 | 171 | 166 | 162 | 169 | 178 | 156 | 171 | 170 | 170 |
| Weight [kg] | 67 ± 12 | 77 | 94 | 62 | 55 | 63 | 68 | 58 | 54 | 73 | 66 |
| Age [years] | 49 ± 10 | 61 | 44 | 62 | 57 | 36 | 45 | 53 | 30 | 51 | 55 |
| Total daily dose of insulin [U] | 43 ± 23 | 52 | 105 | 32 | 23.1 | 41.6 | 41 | 26.5 | 38 | 31.5 | 38 |
| Correction factor [mg/dL/U] | 55 ± 19 | 50 | 13 | 50 | 80 | 50 | 70 | 66 | 66 | 40 | 70 |
| Carbohydrate/insulin ratio [g CHO/U] | 12 ± 5 | 10 | 4 | 9 | 11 | 18 | 13 | 20 | 18 | 8 | 14 |
| Duration of type 1 diabetes [yrs] | 29 ± 15 | 38 | 25 | 12 | 57 | 25 | 16 | 36 | 28 | 44 | 12 |
3.2 | Glucose control
The percent of time spent in the glucose range of 80 to 140 mg/dL (primary study endpoint) was 39.8% ± 7.6% for IP delivery as compared to 25.6% ± 13.1% for SC delivery. The difference between these results was statistically significant, with a P-value of .03. The percent of time spent in the broader glycaemic control range of 70 to 180 mg/dL, the report of which is recommended for AP trials,26 was 65.7% ± 9.2% for IP delivery and 43.9% ± 14.7% for SC delivery, that is, significantly higher with IP insulin (P = .001). The mean BG levels between IP and SC phases were also significantly different, in favour of IP insulin: 151 ± 11 vs 190 ± 31 mg/dL (P = .004).
The tighter glucose control obtained with the IP route was related to significantly less time spent in a hyperglycaemic state. Indeed, the percent of time spent with BG >180 mg/dL and BG >250 mg/dL was significantly higher using SC delivery, with only 32.4% ± 8.9% and 5.9% ± 5.6%, respectively, for IP delivery as compared to 53.5% ± 17.4% and 23.0% ± 11.3 % (P = .0014 and .0004, respectively) for SC delivery. Although not statistically significant (P = .42), it is noteworthy that the mean percent of time spent with BG <70 mg/dL was almost halved for the IP cases compared to the SC cases: 2.5% ± 2.9% vs 4.1% ± 5.3%.
Concretely, the maximum BG measured by YSI during the 24-hour period was 283 ± 30 mg/dL (15.7 ± 1.7 mmol/L) when IP insulin was used and 358 ± 42 mg/dL (19.9 ± 2.3 mmol/L) when SC insulin was used, indicating that IP delivery outperforms SC delivery in terms of hyperglycaemia management, while maintaining lower statistical dispersion among subjects. The maximum BG for each subject was, on average, 75 mg/dL less during the IP admission than during the SC admission, which is a statistically significant difference (P < .01). Additional metrics are provided in Table S1.
Figure 1A clarifies that the percent of time in the euglycaemic range (80–140 mg/dL) is higher for IP delivery, with a marked reduction in the time spent in a hyperglycaemic state (>180 mg/dL) in the overall study, with statistically significant differences. Figure 1B to D illustrates that the rapid dynamics of IP delivery results in efficient reduction of post-meal BG levels; hence, the percent of time >180 mg/dL is lower for IP delivery after all 3 unannounced meals. Additionally, the percent of time in the tight control range of 80 to 140 mg/dL and the clinically safe range of 70 to 180 mg/dL are heightened after each meal. Note that the improvement with IP over SC delivery is statistically significant after the dinner meal. Although there is slightly more time spent in the low BG range of <70 mg/dL after breakfast and dinner with IP delivery, the change is negligible after lunch, probably explained by the higher post-breakfast BG value. Overall, however, as detailed in Figure 1A, the percent of time <70 mg/dL is lower in the case of IP delivery, although not statistically significant. To demonstrate the superiority of IP delivery at night, we refer to Figure 1E. From the box plot, it appears that the percent of time in the tight control range of 80 to 140 mg/dL (IP median, 55.36% vs SC median, 42.26%) and in the wider range of 70–180 mg/dL (IP median, 64.88% vs SC median, 62.49%) are higher for IP delivery than for SC delivery, while the percent of time <70 mg/dL (IP median, 0% vs SC median, 2.38%) is notably reduced. This improvement against hypoglycaemia is not at the cost of hyperglycaemia; the percent of time >180 mg/dL is also reduced (IP median, 1.78% vs SC median, 14.29%; P < .05).
FIGURE 1.
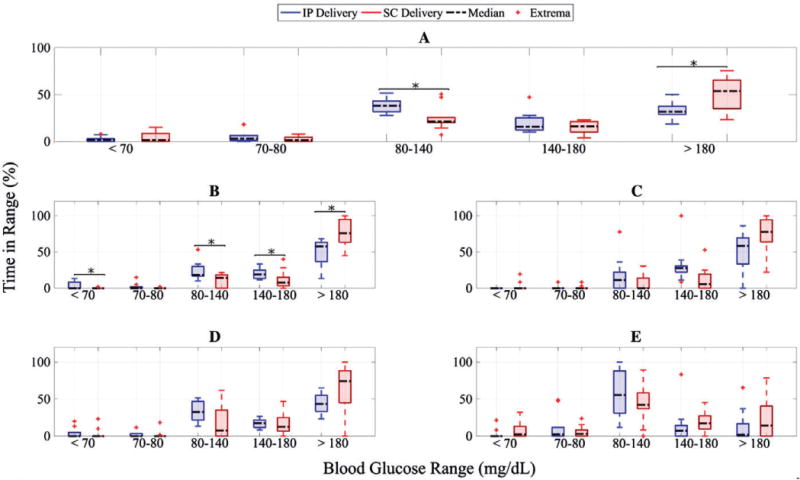
Percent of time in different glycaemic ranges based on YSI measurements during closed-loop control with intraperitoneal (IP) insulin delivery (blue) and subcutaneous (SC) insulin delivery case (red), with statistically significant difference between data shown using black asterisks (“*”). A, Data for the entire study; B, data from start of dinner to 5 hours after dinner; C, data from start of breakfast to 5 hours after breakfast; D, data from start of lunch to 5 hours after lunch; E, data on overnight period
3.3 | Insulin delivery
The temporal variations in BG and delivered insulin over 24 hours are provided in Figure 2. Specifically, we illustrate the median BG and the median delivered insulin on the subplots (A) and (C) for the IP delivery system along with the interquartile range. The corresponding data for the SC system are shown in subplots (B) and (D). We observe that the median BG values computed over all times and all patients for IP delivery are significantly lower than those for SC delivery (IP, 144.7 mg/dL vs SC, 189.7 mg/dL; P < .001), which can be related to the fact that the total insulin delivered (on average for all subjects) is higher via the IP route than via the SC route (IP, 43.66 ± 0.08 U vs SC, 32.29 ± 0.05 U; P < .001). The median and interquartile range (IQR) for the percent time in the 80 to 140 mg/dL range is 38.3 (IQR, 31.7, 43.3) % for IP vs 21.5 (IQR, 20.2, 25.6) % for SC. The control algorithm is more aggressive for IP delivery because it is designed on an approximate model23 that exhibits higher insulin clearance rates.
FIGURE 2.
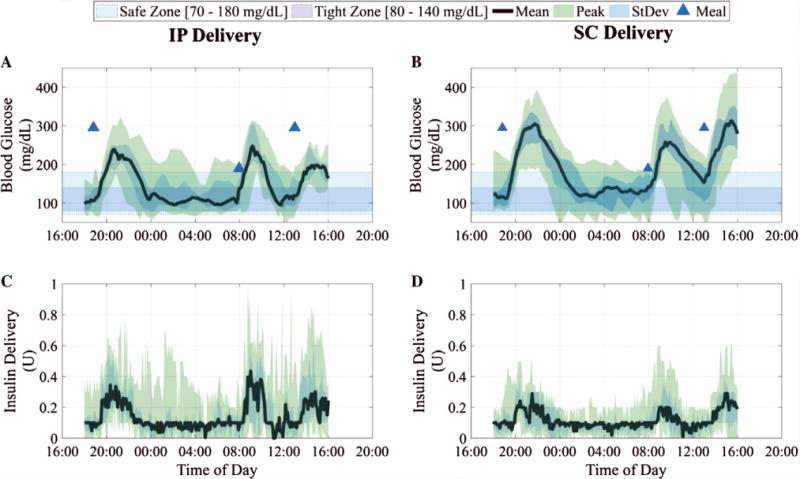
Blood glucose results are summarized in (A) and (B) as median YSI-measured values (continuous thick line), interquartile range (shaded), for closed-loop trials with intraperitoneal (IP) insulin delivery and subcutaneous (SC) insulin delivery, respectively. The closed-loop duration is 22 hours from 6:00 PM. The control objective (80–140 mg/dL) is shown as the blue rectangular band and the safety region (70–180 mg/dL) as the cyan rectangular band. The median insulin delivery from insulin pump for all trials is shown in (C) and (D) for IP delivery and SC delivery, respectively, along with the interquartile range
Figure 3 depicts the cumulative distribution of glucose levels, either as YSI-measured values or CGM values, during the closed-loop trials.26 We note that the mean of data with the IP delivery (darker shade) is shifted to the left of that with SC delivery (lighter shade), and the dispersion around the mean is tighter for IP delivery. This indicates that the percent of time in euglycaemic range for IP delivery is higher than that for SC delivery, with smaller deviations outside of this tight range, in spite of unannounced meals.
FIGURE 3.
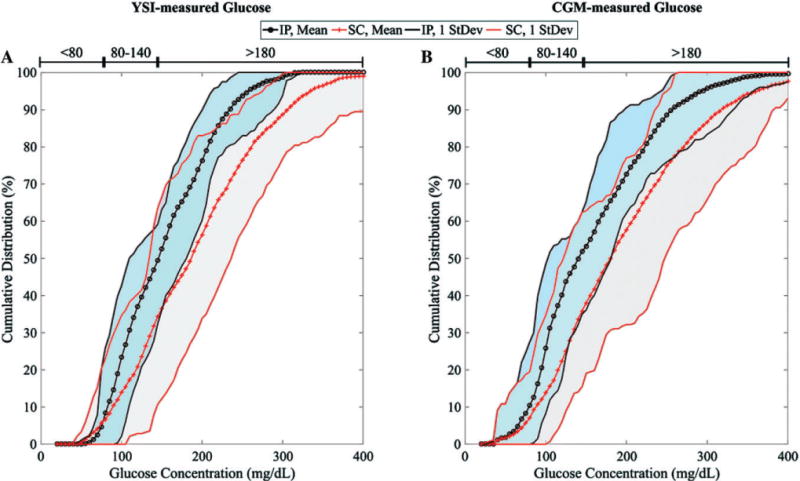
Cumulative distribution of blood glucose values for all subjects during closed-loop trials with IP delivery (dark cyan shaded region) and SC delivery (light gray shaded region) based on (A) YSI-measured values and (B) CGM values. The mean values for the IP delivery case and the SC delivery case are represented by the black dotted and red crossed lines, respectively
3.4 | Safety
The health monitoring system (HMS) algorithm was active during each study. The Health Monitoring System was unintentionally disabled for Subjects 6, 7 and 9 in the SC study and for Subjects 4, 8, 9 and 10 in the IP study; this is explained by human error. During the IP study, there was an average of 4.7 ± 2.7 logged rescue CHO treatments per subject, while during the SC study there were 3.0 ± 3.1 logged treatments per subject (P = .16). The minimum BG was 65 ± 22 mg/dL when SC delivery was used, and was 64 ± 15 mg/dL when IP delivery was used.
4 | DISCUSSION
In this study, we assessed an AP that combines IP insulin delivery with ZMPC control algorithm and SC glucose sensing, aiming at full automation, for example, with no meal announcement. Use of an IP insulin delivery resulted in superior glucose control as compared to the same AP system using an SC insulin delivery. Indeed, the percent of time spent in the 80 to 140 mg/dL glucose range, which was the primary study endpoint, also considered as the optimal goal of diabetes therapy, as well as the percent of time in the broader 70 to 180 mg/dL range reported in most AP studies were significantly greater and the mean blood glucose level was more frequently in, or closer to, the normal range when insulin was infused intraperitoneally.
If sustained for 2 to 3 months in normal life, this improvement in glucose control with AP using IP insulin could result in lower HbA1c levels. Of note, even in open-loop mode, IP insulin infusion from implantable insulin pumps has been reported as allowing lower HbA1c levels than CSII.27 IP infusion using the DiaPort system was not shown to reduce HbA1c levels significantly as compared with CSII, but occurrence of severe hypoglycaemia was dramatically reduced.19
Improved control with IP insulin delivery resulted from significantly reduced time spent above 180 mg/dL. Of note, we used a ZMPC algorithm in the current study that is close to the control algorithms used in most recent AP studies with SC insulin infusion. The percent of time spent in the 70 to 180 mg/dL glucose range that we obtained in the current study while using SC insulin is similar to that reported in the AP studies using SC insulin with no meal announcement.2 This result supports the effectiveness of our ZMPC algorithm while our patients were included, using criteria of poor glucose control that are not usually met in reported AP studies. Indeed, in the vast majority of reported AP trials with SC insulin infusion, patients who experienced severe hypoglycaemia during the previous months were excluded, whereas, in the present work, most patients reported at least one severe hypoglycaemic episode per month before the study. Hence, the superiority of glucose control in closed-loop mode with IP insulin delivery can be attributed to the uniqueness of this route of infusion.9 As shown by our data on plasma insulin (Figure S3), IP insulin resulted in an earlier post-meal peak than did SC insulin. However, plasma insulin levels were otherwise very similar with SC or IP insulin. This observation can be related to the quicker absorption of IP-infused insulin and the specific distribution of similarly infused insulin that occurs primarily to the liver via the portal vein.8,9 Because of hepatic clearance of IP-delivered insulin at first pass, peripheral plasma insulin levels are not higher, while amounts of infused insulin are higher. However, insulin action on hepatic glucose release can contribute to lower post-meal glucose levels.
Limitations of our study include the non-randomized performance of SC- and IP-infused insulin closed-loop trials. This was related to the indication for the surgical DiaPort implantation, explained by the poor control of diabetes in studied patients while using SC insulin. Hence, it would have been unethical to neutralize IP insulin delivery once the DiaPort had been implanted in order to revert to SC insulin infusion for some time if the randomization would have led to a closed-loop trial with IP insulin first. Moreover, an interruption of IP insulin infusion through the DiaPort catheter would represent a risk of catheter occlusion. Another limitation of our study relates to the non-optimal adaptation of the ZMPC algorithm to IP insulin delivery which can be attributed to the model approximation,23 resulting in excessive aggressiveness of the IP control algorithm. Together with the HMS algorithm which was based on SC insulin action, the aggressiveness of the ZMPC algorithm prompted to a higher amount of rescue carbohydrates in IP insulin trials, as detailed in Table S2. As the modeling effort generates high fidelity approximation of IP dynamics, the control performance with IP insulin delivery is expected to improve further, and the quantity of rescue carbohydrates should reduce. In terms of implementation of AP systems using IP insulin, a current limitation relates to the limited use of intraperitoneal insulin for diabetes therapy.27 Although the interest of this mode of therapy has been highlighted recently for patients who fail in controlling diabetes with SC insulin,28 implantable insulin pumps using the IP route and the DiaPort system remain available only in a few European countries. Of note, the second generation of DiaPort which was used in the present study has recently been reported to be associated with less frequent adverse events20 than that of the first generation.19 However, to know how this second generation of the DiaPort system will compete with implantable insulin pumps for IP insulin delivery requires further studies.29 Altogether, the DiaPort system and the implantable pumps using IP delivery provide similar glucose control. DiaPort systems allow easier management by patients, which is close to CSII, whereas implanted pumps involve constraints related to the need for tighter and more expensive medical follow-up and management. However, infections at the port implantation site remain the main adverse event associated with DiaPort use. Under-delivery events represent the key burden of implantable pumps, although they can be overcome in most cases by rinsing procedures of the pump with NaOH solutions in order to solubilize insulin aggregates.29 Whether the currently developed faster-acting insulin analogues for SC use can mimic the pharmacokinetics and pharmacodynamics of IP-delivered insulin, and then improve glucose control in AP studies, remains to be investigated. Of note, insulin distribution in the body, including a first liver pass, will remain a specificity of IP insulin delivery.
Meanwhile, it has been established recently that the glucose-sensing dynamics are considerably improved in the intraperitoneal space,30 which can be attributed to physiological advantages that the intraperitoneal space provides. For example, the peritoneal cavity is heavily laden with blood vessels that are unaffected by perturbations in temperature, external fluctuations and reduced blood flow during sensing.31,32 In fact, the authors report that the sensing dynamics in the IP space are twice as fast as those of the subcutaneous space, making it a strong contender in future glucose-sensing technology. Hence, combining IP insulin infusion and IP glucose sensing could provide solutions of problems of delay that have thus far limited the development of fully-automated AP systems.
Supplementary Material
Acknowledgments
We acknowledge Thomas Frei, from Roche, for coordinating the DiaPort clinical procedures and acting as a point of contact at Roche Diagnostics. We would also like to thank and acknowledge product support from Roche Corp. and Dexcom Inc., as well as thank the patients for their participation in the study.
Funding information
This work was supported by JDRF (grant 17-2011-515) and National Institutes of Health (grant, R01DK085628, DP3DK101068)
Footnotes
Conflict of interest
H. C. Z. is currently employed by Verily Diabetes; L. H. is currently employed by Agilent Technologies; J.L. is currently employed by ExxonMobil. E. R. has served as a consultant/advisor for Roche Diagnostics and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author contributions
H. C. Z., E. D. and E. R. helped design the study protocol, contributed to the technology design and ensured the regulatory approval of the CLC system, provided primary technical and clinical support on site during all sessions, analysed the data, and edited the manuscript. J. L. helped construct the controller infrastructure, contributed to the discussion, reviewed and edited the manuscript, and generated figures used in the statistical analysis. A. F., M. J. P. and J. P. conducted the clinical trials, collected the data, and reviewed and edited the manuscript. F. J. D., contributed to the technology design, contributed to the discussion, and reviewed and edited the manuscript. L. H. and A. C. authored the manuscript, contributed to the discussion, generated figures to supplement the ensuing discussion, and performed statistical analysis on the data. F. J. D. edited and reviewed the manuscript. H. C. Z was the principal investigator of this project and is the guarantor of this work; as such, H. C. Z. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ORCID
Eyal Dassau http://orcid.org/0000-0001-5333-6892
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- 1.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59:1795–1805. doi: 10.1007/s00125-016-4022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle FJ, III, Huyett LM, Lee JB, Zisser HC, Dassau E. Closed loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37:1191–1197. doi: 10.2337/dc13-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovorka R. Continuous glucose monitoring and closed-loop systems. Diabet Med. 2006;23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed] [Google Scholar]
- 4.Del Favero S, Place J, Kropff J, et al. Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab. 2015;17:468–476. doi: 10.1111/dom.12440. [DOI] [PubMed] [Google Scholar]
- 5.Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in Type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205–1211. doi: 10.2337/dc14-3073. [DOI] [PubMed] [Google Scholar]
- 6.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: An open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3:17–26. doi: 10.1016/S2213-8587(14)70226-8. [DOI] [PubMed] [Google Scholar]
- 7.Finan DA, Dassau E, Breton MD, et al. Sensitivity of the predictive hypoglycemia minimizer system to the algorithm aggressiveness factor. J Diabetes Sci Technol. 2015;10:104–110. doi: 10.1177/1932296815593292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selam JL, Bergman RN, Raccah D, Jean-Didier N, Lozano J, Charles MA. Determination of portal insulin absorption from peritoneum via novel nonisotopic method. Diabetes. 1990;39:1361–1365. doi: 10.2337/diab.39.11.1361. [DOI] [PubMed] [Google Scholar]
- 9.Nathan DM, Dunn FL, Bruch J, et al. Postprandial insulin profiles with implantable pump therapy may explain decreased frequency of severe hypoglycemia, compared with intensive subcutaneous regimens, in insulin-dependent diabetes mellitus patients. Am J Med. 1996;100:412–417. doi: 10.1016/S0002-9343(97)89516-2. [DOI] [PubMed] [Google Scholar]
- 10.Renard E, Costalat G, Chevassus H, Bringer J. Closed loop insulin delivery using implanted insulin pumps and sensors in type 1 diabetic patients. Diabetes Res Clin Pract. 2006;74:S173–S177. [Google Scholar]
- 11.Renard E. Clinical experience with an implanted closed-loop insulin delivery system. Arq Bras Endocrinol Metabol. 2008;52:349–354. doi: 10.1590/s0004-27302008000200023. [DOI] [PubMed] [Google Scholar]
- 12.Renard E, Place J, Cantwell M, Chevassus H, Palerm CC. Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery. Diabetes Care. 2010;33:121–127. doi: 10.2337/dc09-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dijk PR, Logtenberg SJJ, Gans ROB, Bilo HJG, Kleefstra N. Intraperitoneal insulin infusion: treatment option for type 1 diabetes resulting in beneficial endocrine effects beyond glycaemia. Clin Endocrinol (Oxf) 2014;81:488–497. doi: 10.1111/cen.12546. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk PR, Groenier KH, DeVries JH, et al. Continuous intraperitoneal insulin infusion versus subcutaneous insulin therapy in the treatment of type 1 diabetes: effects on glycemic variability. Diabetes Technol Ther. 2015;17:379–384. doi: 10.1089/dia.2015.0001. [DOI] [PubMed] [Google Scholar]
- 15.van Dijk PR, Logtenberg SJJ, Chisalita SI, et al. After 6 years of intraperitoneal insulin administration IGF-I concentrations in T1DM patients are at low-normal level. Growth Horm IGF Res. 2015;25:316–319. doi: 10.1016/j.ghir.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Hedman CA, Frystyk J, Lindström T, Oskarsson P, Arnqvist HJ. Intraperitoneal insulin delivery to patients with type 1 diabetes results in higher serum IGF-I bioactivity than continuous subcutaneous insulin infusion. Clin Endocrinol (Oxf) 2014;81:58–62. doi: 10.1111/cen.12296. [DOI] [PubMed] [Google Scholar]
- 17.Boering M, van Dijk PR, Logtenberg SJJ, et al. Effects of intraperitoneal insulin versus subcutaneous insulin administration on sex hormone-binding globulin concentrations in patients with type 1 diabetes mellitus. Endocr Connect. 2016;5:136–142. doi: 10.1530/EC-16-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeandidier N, Selam J-L, Renard E, et al. Decreased severe hypoglycemia frequency during intraperitoneal insulin infusion using programming implantable pumps. Diabetes Care. 1994;19:780. doi: 10.2337/diacare.19.7.780. [DOI] [PubMed] [Google Scholar]
- 19.Liebl A, Hoogma R, Renard E, et al. A reduction in severe hypoglycaemia in type 1 diabetes in a randomized crossover study of continuous intraperitoneal compared with subcutaneous insulin infusion. Diabetes Obes Metab. 2009;11:1001–1008. doi: 10.1111/j.1463-1326.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Verdugo R, Erbach M, Schnell O. A new optimized percutaneous access system for CIPII. J Diabetes Sci Technol. 2017 doi: 10.1177/1932296817694913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosman B, Dassau E, Zisser HC, Jovanovic L, Doyle FJ. Zone model predictive control: a strategy to minimize hyper- and hypoglycemic events. J Diabetes Sci Technol. 2010;4:961–975. doi: 10.1177/193229681000400428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Heusden K, Dassau E, Zisser HC, Seborg DE, Doyle FJ., III Control-relevant models for glucose control using a priori patient characteristics. IEEE Trans Biomed Eng. 2012;59:1839–1849. doi: 10.1109/TBME.2011.2176939. [DOI] [PubMed] [Google Scholar]
- 23.Lee JJ, Dassau E, Zisser H, Doyle FJ., III Design and in silico evaluation of an intraperitoneal–subcutaneous (IP–SC) artificial pancreas. Comput Chem Eng. 2014;70:180–188. doi: 10.1016/j.compchemeng.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey RA, Dassau E, Bevier WC, et al. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther. 2014;16:348–357. doi: 10.1089/dia.2013.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey RA, Dassau E, Zisser H, Seborg DE, Jovanovič L, Doyle FJ., III Design of the health monitoring system for the artificial pancreas: low glucose prediction module. J Diabetes Sci Technol. 2012;6:1345–1354. doi: 10.1177/193229681200600613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus report. Diabetes Care. 2016;39:1175–1179. doi: 10.2337/dc15-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaan N, Teplova A, Stam G, Spaan J, Lucas C. Systematic review: continuous intraperitoneal insulin infusion with implantable insulin pumps for diabetes mellitus. Acta Diabetol. 2014;51:339–351. doi: 10.1007/s00592-014-0557-3. [DOI] [PubMed] [Google Scholar]
- 28.Spaan NA, Teplova AE, Renard E, Spaan JAE. Implantable insulin pumps: an effective option with restricted dissemination. Lancet Diabetes Endocrinol. 2014;2:358–360. doi: 10.1016/S2213-8587(14)70035-X. [DOI] [PubMed] [Google Scholar]
- 29.Renard E. Analysis of “A new optimized percutaneous access system for CIPII. J Diabetes Sci Technol. 2017 doi: 10.1177/1932296817703671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huyett LM, Dassau E, Zisser HC, Doyle FJ., III The impact of glucose sensing dynamics on the closed-loop artificial pancreas. Proc Am Control Conf. 2015;5:5116–5121. [Google Scholar]
- 31.Burnett DR, Huyett LM, Zisser HC, et al. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes. 2014;63:2498–2505. doi: 10.2337/db13-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huyett LM, Dassau E, Zisser HC, Doyle FJ., III Design and evaluation of a robust PID controller for a fully implantable artificial pancreas. Ind Eng Chem Res. 2015;54:10311–10321. doi: 10.1021/acs.iecr.5b01237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


