Abstract
OBJECTIVES
To determine the extent of a group A streptococcus (GAS) cluster (2 residents with invasive GAS (invasive case-patients), 2 carriers) caused by a single strain (T antigen type 2 and M protein gene subtype 2.0 (T2, emm 2.0)), evaluate factors contributing to transmission, and provide recommendations for disease control.
DESIGN
Cross-sectional analysis and retrospective review.
SETTING
Skilled nursing facility (SNF).
PARTICIPANTS
SNF residents and staff.
MEASUREMENTS
The initial cluster was identified through laboratory notification and screening of SNF residents with wounds. Laboratory and SNF administrative records were subsequently reviewed to identify additional residents with GAS, oropharyngeal and wound (if present) swabs were collected from SNF staff and residents to examine GAS colonization, staff were surveyed to assess infection control practices and risk factors for GAS colonization, epidemiologic links between case-patients and persons colonized with GAS were determined, and facility infection control practices were assessed.
RESULTS
No additional invasive case-patients were identified. Oropharyngeal swabs obtained from all 167 SNF residents were negative; one wound swab grew GAS that was the same as the outbreak strain (T2, emm 2.0). The outbreak strain was not identified in any of the 162 staff members. One of six staff members diagnosed with GAS pharyngitis worked while ill and had direct contact with invasive case-patients within a few weeks before their onset of symptoms. Additional minor breaches in infection control were noted.
CONCLUSION
Sick healthcare workers may have introduced GAS into the SNF, with propagation by infection control lapses. “Presenteeism,” or working while ill, may introduce and transmit GAS to vulnerable in SNF populations. Identification of an invasive GAS case-patient should trigger a prompt response by facilities to prevent further transmission and workplace culture, and policies should be in place to discourage presenteeism in healthcare settings.
Keywords: Group A Streptococcus, skilled nursing facility, infection control, outbreak, “presenteeism”
Group A streptococcus (GAS) can cause serious, life-threatening infections, particularly in persons with chronic medical conditions or advanced age.1–6 Such risk factors are common in residents of nursing facilities, where GAS outbreaks have been associated with prolonged outbreaks and high case-fatality ratios.3,7 On March 3, 2015, the South Carolina Department of Health and Environmental Control (SC DHEC) sent the Centers for Diseases Control and Prevention (CDC) Streptococcus Laboratory four GAS isolates from cultures (two blood, two wound) collected from four residents of one skilled nursing facility (SNF), a 190-bed for-profit facility providing short-term therapy and skilled nursing in addition to custodial care for long-term residents. Blood cultures were obtained from two SNF residents hospitalized in February 2015. Two GAS-positive wound cultures were collected during screening of all open wounds of SNF residents that was conducted approximately 1 week after the second resident was identified with invasive GAS; two residents with GAS cultured from their wounds had no evidence of invasive skin infection. When all four isolates were confirmed to be T antigen type 2 and M protein gene subtype 2.0 (T2, emm 2.0), SC DHEC requested CDC assistance. The objectives of the subsequent investigation were to determine the extent of the outbreak, evaluate infection control and wound care practices for breaches that may have contributed to transmission, and provide recommendations to halt further GAS spread.
METHODS
Case Definitions
Invasive GAS case-patients were defined as SNF residents or staff from whom GAS was cultured from a normally sterile site (e.g., blood). Noninvasive case-patients were defined as those from whom GAS was isolated from a nonsterile site (e.g., wound, throat) with signs and symptoms consistent with GAS infection. Carriers were defined as asymptomatic persons from whom GAS was isolated from a nonsterile site. Possible case-patients were defined as those who reported an illness consistent with GAS infection (e.g., pharyngitis) without confirmatory laboratory tests.
Case Finding
The following records were reviewed to identify additional cases: GAS culture results collected from September 1, 2014, to March 20, 2015, from two laboratories that process resident specimens; SNF resident infection logs from January 1 to March 31, 2015; and employee absentee logs (January 1–March 11, 2015) to identify any staff members with sick leave potentially associated with GAS infections (e.g., those reporting upper respiratory infections or pharyngitis). A self-administered survey was also distributed to all staff members, including night shift and part-time staff, who worked primarily in resident care areas (e.g., certified nursing assistants (CNAs), registered nurses (RNs), licensed practical nurses (LPNs), physicians, housekeeping staff, physical therapists). The survey included questions about occupation, duration of employment, type of resident contact, location of work, hand hygiene practices, and signs and symptoms associated with GAS infection (sore throat; rash, open wound, or skin infection; fever, cough, or other respiratory infections) from January 1, 2015, through the day of survey administration (March 19–24).
Colonization Study
Residents and staff were swabbed to determine the prevalence of GAS colonization and to identify unrecognized sources for ongoing transmission. Oropharyngeal swabs were obtained from staff members who worked in resident care areas and all residents present at the SNF on March 19, 2015. Wounds from residents with open skin lesions were swabbed. Swabs were sent to the CDC Streptococcus Laboratory in Atlanta for culture. If GAS was isolated, the isolate underwent antibiotic susceptibility testing and typing for T antigen and emm.8
Epidemiological Investigation and Infection Control Assessment
Potential epidemiological links between GAS case-patients and carriers were looked for by reviewing medical records and room assignments of GAS culture-positive residents and the work schedule of staff who reported being ill with streptococcal pharyngitis between January 19 and March 19, 2015 (2 weeks before hospitalization of the first case until date of colonization assessment).
To assess infection control (IC) practices, SNF IC policies were reviewed, and staff members were interviewed. IC practices (including hand hygiene, wound care, and transmission-based precautions) of nurses on all four units performing patient care were also observed using CDC hand hygiene and wound care assessment tools.9 The observer arrived unannounced to shadow day-shift staff members completing their duties. Staff members were aware of the purpose of the observation but were not aware of the specific practices being observed.
Ethics
These activities were part of a public health response and were determined to be “nonresearch.” Therefore, CDC institutional review board review was not required.
RESULTS
Case Characteristics and Case Finding
Descriptive epidemiology of the four residents from whom GAS was initially isolated (two invasive case-patients, two carriers) is shown in Table 1 (Residents 1–4). The first invasive case-patient (Resident 1) died while hospitalized for GAS bacteremic pneumonia. The second invasive case-patient (Resident 2) developed GAS bacteremia after being hospitalized for percutaneous endoscopic gastrostomy replacement. Resident 2 died 2 weeks after being discharged back to the SNF; it is unclear whether death was related to GAS infection. Review of laboratory records and resident infection log from the investigation did not identify any additional invasive or noninvasive GAS cases in SNF residents.
Table 1.
Descriptive Epidemiology of Group A Streptococcus (GAS) Case-Patients or Carriers of the Outbreak Strain
| Resident | Age | Sex | Unit | GAS Classification | Culture Date | Specimen Type | Estimated Onset of Infection | Risk Factors for GAS Infection | Skin Breakdown |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 87 | Female | Aa (P) | Invasive | February 2 | Sputum, blood | January 29 (respiratory symptoms) | CHD, CKD, corticosteroid use (from January 31) | Skin breakdown from fall |
| 2 | 71 | Male | Aa and Bc (B: D) | Invasive | February 8 | Blood | February 6b | Sacral decubitus ulcer, tracheostomy, PEG, CVA, hypertension | Stage 2 and 3 sacral decubitus ulcer, tracheostomy, PEG |
| 3 | 65 | Male | A (D) | Carrier | February 12 | Wound | NAd | DM, PVD (after BKA) CHD, CKD | Left BKA stump, right foot (heel, sole) |
| 4 | 72 | Male | A (D) | Carrier | February 12 | Wound | NAd | DM, PVD (after BKA) CVA, CKD | Left foot (heel) |
| 5 | 85 | Female | A (P) | Carrier | March 19 | Wound | NAd | Obesity hypoventilation syndrome | Stage 3 buttock decubitus ulcer |
Residents 1 and 2 were both in unit A January 1–16, 2015.
Warm, erythematous percutaneous endoscopic gastrostomy (PEG) site noted on February 6.
Resident 2 was transferred to different rooms in Units A and B during the stay at the skilled nursing facility (SNF) and was hospitalized from January 17 to 29 for pneumonia due to methicillin-resistant Staphylococcus aureus and February 6 to 12 for PEG replacement. GAS bacteremia was identified after PEG replacement.
GAS from Residents 3 and 4 were detected from a colonization assessment conducted by the SNF and from Resident 5 from the assessment by the investigation team.
P = private room; CHD = chronic heart disease; CKD = chronic kidney disease; D = double occupancy; CVA = cerebrovascular accident; BKA = below-knee amputation; DM = diabetes mellitus; PVD = peripheral vascular disease.
Colonization Study
One oropharyngeal swab was collected from each of the 167 residents at the facility and 49 wound swabs from 33 residents with wounds (33/167, 19.8%); two wound cultures from one resident grew GAS (Table 1; Resident 5). This resident had been negative for GAS during the first swabbing that SNF staff conducted in February. These and the four isolates previously collected from the residents (Residents 1–4) were all T2, emm2.0.
From March 19 to 24, an oropharyngeal swab was collected from each of the 162 staff members; GAS was isolated from two members (1%). None of the staff members disclosed presence of skin lesions on the day of the survey. The T and emm types differed (T12, emm12; T1, emm1.25) from that of the GAS cases and colonized residents. A 10-day course of cephalexin10 was given to the three carriers identified through the survey; the resident was placed on contact precaution, and staff members did not report to work during the first 24 hours of treatment. Repeat cultures were all negative.
All five residents meeting the case definitions (two invasive case-patients, three carriers) were located in two adjacent units, A and B.
Survey of SNF Staff
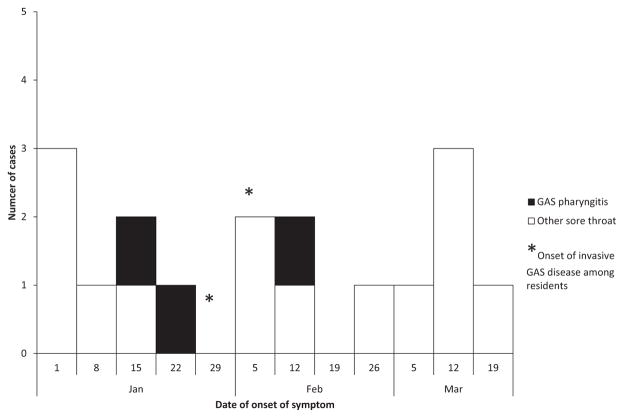
Self-reported illnesses related to GAS infection are summarized in Table 2. Of 34 staff with sore throats, six reported being diagnosed with GAS pharyngitis. Four of the six (1 RN, 1 LPN, 4 CNAs) had less than 1 year of employment at the SNF. Laboratory results confirming GAS pharyngitis were not available for five of the six who reported being diagnosed with GAS pharyngitis. All six individuals reported receiving antibiotics; four received treatment appropriate for GAS pharyngitis (e.g., penicillin, amoxicillin, clindamycin). The epidemic curve for 14 staff members whose date of onset of sore throat was available, including three of the six diagnosed with GAS pharyngitis, is presented in Figure 1.
Table 2.
Self-Reported Group A Streptococcus (GAS)-Related Illness in the Staff Survey, March 19–24, 2015 (n = 162)
| Symptoms or Conditions | Staff Members Who Reported Having Symptoms Anytime Since January 1, 2015 | Staff Members Who Reported Missing Work for Illness (of Those with Symptoms) |
|---|---|---|
| n (%) | ||
| Respiratory infection (e.g., fever, cough) | 39 (24.1) | 21 (53.9) |
| Sore throat | 34 (21.0) | 14 (41.2) |
| GAS pharyngitisa | 6 (3.7) | 5 (83.3) |
| Rash | 8 (4.9) | 1 (12.5) |
| Respiratory infection (e.g., fever, cough) or sore throat | 54 (33.3) | 25 (46.3) |
GAS pharyngitis was asked about only if the staff member reported sore throat in the survey.
Figure 1.
Epidemic curve of onset of sore throat among surveyed staff members in the staff survey, January 1–March 24, 2015, including those reporting provider-diagnosed group A streptococcal (GAS) pharyngitis (n = 14), with date of onset available is shown. Two provider-diagnosed GAS pharyngitis cases occurred before the onset of invasive GAS case-patients, as shown in the epidemic curve (*). GAS pharyngitis indicates staff members with provider-diagnosed GAS pharyngitis.
Of the 54 (33%) staff members who reported an episode of sore throat, respiratory infection, or both since January 1, 25 (46%) reported missing work for their illness (median 2 days, range <1–8 days), including five of the six staff members diagnosed with GAS pharyngitis (median 4.5 days, range 3–6 days).
A focused review of the work schedule of the six staff members diagnosed with GAS pharyngitis revealed that two worked primarily in the unit where both case-patients resided (Unit A), and both reported GAS pharyngitis in the 2 to 3 weeks before the illness onset of the two invasive case-patients. One of these staff members had direct patient contact with both invasive case-patients before the onset of the residents’ illnesses. Although both staff members missed work after receiving a diagnosis of GAS pharyngitis, records indicated that the staff member who had direct contact with both case-patients had worked while symptomatic. None of the six who reported GAS pharyngitis had GAS isolated during the colonization study, but all had received antibiotic treatment for their illnesses. The two additional staff members colonized with different GAS strains showed no epidemiological link to the case-patients.
Infection Control
Staff observations revealed that hand hygiene was performed more often after resident contact than before (45 (68.2%) occurrences out of 66 hand hygiene opportunities before resident contact vs 61 (91.0%) occurrences out of 67 opportunities after resident contact). Hand hygiene lapses, including failure to wash hands or apply alcohol-based hand rub, were observed before individuals putting on gloves (7/21 missed hand hygiene opportunities), before and after limited resident contact such as delivering medication or a food tray (10/21 missed hand hygiene opportunities), and after contact with objects in the hallways between resident rooms. In the survey, few staff members (2.6%) reported not using gloves when changing wound dressings or bathing residents.
Nurses (RNs, LPNs) appeared to have better hand hygiene performance than other occupations, and staff members with longer length of employment at the facility (5–10 years) had better hand hygiene performance than those with shorter lengths of employment (<1 year). Staff members with fewer years of experience were also observed to be less familiar with the wound care protocol than more-experienced staff. These individuals made frequent trips back and forth from the wound care supply cart for forgotten items and took items into resident rooms inappropriately, which may have caused cross-contamination of equipment and increased opportunities for GAS transmission. Differences were observed in the cleaning and disinfection method of shared wound care equipment, such as scissors (e.g., soap and water, alcohol, surface disinfectant wipes, multiple combinations of these), suggesting that a standardized cleaning protocol was not effectively implemented. At least one staff member who was known to have GAS pharyngitis before the onset of cases was found to be unfamiliar with wound care procedures.
DISCUSSION
A small but fatal cluster of invasive GAS infections at a SNF is described. The evidence suggests that a sick healthcare worker might have introduced the infection, which was subsequently transmitted to other residents as a result of breaches in infection control practices; two staff members were ill with GAS pharyngitis in the few weeks before the onset of the two invasive case-patients and worked in the units where the two invasive case-patients resided, and one staff member who reported working after illness onset had direct contact with both invasive case-patients. It was not possible to observe or review documentation of staff infection control practices while individuals were actively ill and working, but observation of lapses in hand hygiene and wound care during the public health investigation suggests that breaches were occurring in the weeks before the initial case. The new acquisition of GAS colonization that matched the emm type of the case-patients by a resident who had a previous culture-negative wound specimen further supports ongoing transmission in the facility due to lapses in infection control.
Although lapses in infection control may have propagated transmission within this facility, this investigation highlights a unique aspect of infection control: the role of sick healthcare workers in introducing an outbreak pathogen. “Presenteeism,” the opposite of absenteeism, is a term used to refer to employees working while ill.11,12 Most literature about presenteeism addresses its effect on employee productivity and employer finances,13–17 but in a healthcare setting, this practice can also affect disease transmission.
Despite many reports of GAS outbreaks in long-term care facilities such as SNFs and nursing homes, only a few studies identified sick healthcare workers as a possible source of infection.18,19 Addressing presenteeism among staff members of long-term care facilities with GAS infection can be challenging. Prevention of presenteeism first requires that staff realize the risk to others while working ill; therefore, presenteeism may be more likely to occur when individuals experience mild illness.20 While symptoms of GAS in otherwise healthy staff members can be mild (e.g., pharyngitis, respiratory symptoms),19,21 this organism can result in invasive disease if transmitted to a vulnerable population such as residents of long-term care facilities,7 where risks for severe GAS infections (e.g., advanced age, crowded living conditions, breaks in the skin, and underlying illnesses),7 are common. Second, previous reports have shown that workers at long-term care facilities may be more susceptible to presenteeism. A Swedish study found that nursing home aides were most prone to presenteeism (65%) compared to all other occupations surveyed, perhaps reflecting this occupation’s low hierarchical position, limited job and financial security,11 and low workload flexibility.20
Factors contributing to presenteeism can vary among different types of healthcare workers, including status within an organization, staffing needs, job and financial security, and expectations or pressure from supervisors.22 Workplace policies could influence staff members’ decision to come to work ill, thus, facility management should consider developing policies that are realistic and that encourage staff to comply with rules against presenteeism. One example is a “paid sick leave” policy, which was demonstrated to be associated with a decrease in communicable disease outbreaks in a study of nursing homes.23 Minimizing requirements to get paid sick leave (e.g., requiring certification from a clinician) may also help discourage presenteeism.22 Given the challenges in preventing presenteeism among staff members who are sick with GAS, these efforts should be coupled with an increased level of awareness by the health facilities to respond promptly whenever an invasive GAS case patient is identified, such as by reviewing IC practices and conducting active surveillance to identify sick staff members or other residents colonized with GAS to prevent further transmission and development of serious disease among other vulnerable individuals.
The results of this investigation are subject to several limitations. First, since we did not have the information on T and emm types of GAS strains from the two staff members who were diagnosed with GAS pharyngitis prior to the resident infections, we were unable to prove that these were the same strain. However, we considered that the epidemiological evidence was strong enough to suggest that GAS was transmitted by infected staff. Second, since the colonization assessment was performed more than a month after the cluster occurred, the results may not be representative of the GAS colonization at the time of the cluster. Last, the lag time in the investigation may have resulted in recall bias in the staff survey.
Despite these limitations, this investigation highlights how staff presenteeism may contribute to transmission of disease, especially in a long-term care facility. Encouraging staff to avoid working while ill should be a priority in all healthcare settings, especially nursing homes and other long-term care facilities, to prevent the introduction of infectious diseases into a vulnerable population. Solutions for presenteeism should include educating staff on the harms of presenteeism, promoting staff identification and reporting of any illness symptoms, and implementing sick leave policies that support staff decisions to avoid working when sick.
Acknowledgments
We would like to thank Lori Goulet, RN, MSN; Vicky Hinz, RN, BSN; Linda Bell, MD; and Dana Giurgiutiu, PhD, MPH, South Carolina Department of Health and Environmental Control; the staff members at the SNF; and Zhongya Li, BS and Deloris Jackson, MS at the Centers for Disease Control and Prevention, for their assistance and hard work.
Footnotes
Conflict of Interest: None.
Author Contributions: All authors: conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the manuscript.
Sponsor’s Role: Not applicable.
References
- 1.Thigpen MC, Thomas DM, Gloss D, et al. Nursing home outbreak of invasive group A streptococcal infections caused by 2 distinct strains. Infect Control Hosp Epidemiol. 2007;28:68–74. doi: 10.1086/508821. [DOI] [PubMed] [Google Scholar]
- 2.Rainbow J, Jewell B, Danila RN, et al. Invasive group a streptococcal disease in nursing homes, Minnesota, 1995–2006. Emerg Infect Dis. 2008;14:772–777. doi: 10.3201/eid1405.0704072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dooling KL, Crist MB, Nguyen DB, et al. Investigation of a prolonged Group A streptococcal outbreak among residents of a skilled nursing facility, Georgia, 2009–2012. Clin Infect Dis. 2013;57:1562–1567. doi: 10.1093/cid/cit558. [DOI] [PubMed] [Google Scholar]
- 4.Arnold KE, Schweitzer JL, Wallace B, et al. Tightly clustered outbreak of group A streptococcal disease at a long-term care facility. Infect Control Hosp Epidemiol. 2006;27:1377–1384. doi: 10.1086/508820. [DOI] [PubMed] [Google Scholar]
- 5.Greene CM, Van Beneden CA, Javadi M, et al. Cluster of deaths from group A streptococcus in a long-term care facility—Georgia, 2001. Am J Infect Control. 2005;33:108–113. doi: 10.1016/j.ajic.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Deutscher M, Schillie S, Gould C, et al. Investigation of a group A streptococcal outbreak among residents of a long-term acute care hospital. Clin Infect Dis. 2011;52:988–994. doi: 10.1093/cid/cir084. [DOI] [PubMed] [Google Scholar]
- 7.Jordan HT, Richards CL, Jr, Burton DC, et al. Group A streptococcal disease in long-term care facilities: Descriptive epidemiology and potential control measures. Clin Infect Dis. 2007;45:742–752. doi: 10.1086/520992. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Protocol for emm Typing. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 9.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. Healthcare-Associated Infections (HAIs) Atlanta, GA: Centers for Disease Control and Prevention; 2015. Prevention Toolkits. [Google Scholar]
- 10.Public Health Agency of Canada. Guidelines for the prevention and control of invasive group A streptococcal disease. Can Commun Dis Rep. 2006;32S2:1–26. [Google Scholar]
- 11.Aronsson G, Gustafsson K, Dallner M. Sick but yet at work. An empirical study of sickness presenteeism. J Epidemiol Community Health. 2000;54:502–509. doi: 10.1136/jech.54.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronsson G, Gustafsson K. Sickness presenteeism: Prevalence, attendance-pressure factors, and an outline of a model for research. J Occup Environ Med. 2005;47:958–966. doi: 10.1097/01.jom.0000177219.75677.17. [DOI] [PubMed] [Google Scholar]
- 13.Letvak SA, Ruhm CJ, Gupta SN. Nurses’ presenteeism and its effects on self-reported quality of care and costs. Am J Nurs. 2012;112:30–38. doi: 10.1097/01.NAJ.0000411176.15696.f9. quiz 48, 39. [DOI] [PubMed] [Google Scholar]
- 14.Mattke S, Balakrishnan A, Bergamo G, et al. A review of methods to measure health-related productivity loss. Am J Manage Care. 2007;13:211–217. [PubMed] [Google Scholar]
- 15.Brooks A, Hagen SE, Sathyanarayanan S, et al. Presenteeism: Critical issues. J Occup Environ Med. 2010;52:1055–1067. doi: 10.1097/JOM.0b013e3181f475cc. [DOI] [PubMed] [Google Scholar]
- 16.Goetzel RZ, Long SR, Ozminkowski RJ, et al. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med. 2004;46:398–412. doi: 10.1097/01.jom.0000121151.40413.bd. [DOI] [PubMed] [Google Scholar]
- 17.Schultz AB, Chen CY, Edington DW. The cost and impact of health conditions on presenteeism to employers: A review of the literature. Pharmacoeconomics. 2009;27:365–378. doi: 10.2165/00019053-200927050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Barnham M, Kerby J. Streptococcus pyogenes pneumonia in residential homes: probable spread of infection from the staff. J Hosp Infect. 1981;2:255–257. doi: 10.1016/0195-6701(81)90046-3. [DOI] [PubMed] [Google Scholar]
- 19.Harkness GA, Bentley DW, Mottley M, et al. Streptococcus pyogenes outbreak in a long-term care facility. Am J Infect Control. 1992;20:142–148. doi: 10.1016/s0196-6553(05)80181-6. [DOI] [PubMed] [Google Scholar]
- 20.Krane L, Larsen EL, Nielsen CV, et al. Attitudes towards sickness absence and sickness presenteeism in health and care sectors in Norway and Denmark: A qualitative study. BMC Public Health. 2014;14:880. doi: 10.1186/1471-2458-14-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandler RE, Lee LE, Townes JM, et al. Transmission of group A Streptococcus limited to healthcare workers with exposure in the operating room. Infect Control Hosp Epidemiol. 2006;27:1159–1163. doi: 10.1086/508819. [DOI] [PubMed] [Google Scholar]
- 22.Widera E, Chang A, Chen HL. Presenteeism: A public health hazard. J Gen Intern Med. 2010;25:1244–1247. doi: 10.1007/s11606-010-1422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Birkhead GS, Strogatz DS, et al. Impact of institution size, staffing patterns, and infection control practices on communicable disease outbreaks in New York State nursing homes. Am J Epidemiol. 1996;143:1042–1049. doi: 10.1093/oxfordjournals.aje.a008668. [DOI] [PubMed] [Google Scholar]