Abstract
Background:
Tisseel (Baxter International Inc., Westlake Village, CA, USA), a fibrin sealant, was originally devised to strengthen repairs of spinal cerebrospinal fluid (CSF) fistulas. Here, we evaluated how Tisseel correlated with hemostasis (e.g., defined as reduced postoperative drainage, time to drain removal, length of stay (LOS), and postoperative transfusion requirements) in 58 patients undergoing 2–3 vs. 79 patients having 4–6 level lumbar laminectomies.
Methods:
We assessed how Tisseel correlated with hemostasis in 58 patients undergoing 2–3 level laminectomies/stenosis (with 48 herniated discs and 20 synovial cysts, 1 degenerative spondylolisthesis) vs. 79 having 4–6 level laminectomies/stenosis (with 39 lumbar discs, 45 synovial cysts, and 26 degenerative spondylolisthesis).
Results:
Following 2–3 level laminectomies, the average drainage on postoperative day 1 was 87.26 cc, and on day 2 was 59.62 cc; most drains were removed and the majority of patients were discharged on postoperative day 2, requiring no transfusions. After 4–6 level decompressions, greater postoperative drainage was observed on postoperative days 1 (e.g., 156.63 cc), and 2 (115.8 cc), and many were continued for 3 (85.7 cc; 44 patients), and 4 postoperative days (93.6: 6 patients) respectively. Drains were typically removed and patients were discharged on postoperative days 3 and 4, with just 6 requiring transfusions. Notably, there were four CSF fistulas for patients undergoing 4–6 level laminectomies; one had a large disc hernation in conjunction with postoperative scare, while three had massive calcified synovial cysts extending to/through the dura.
Conclusions:
Utilizing Tisseel as a hemostatic allowed us to quantitate hemostasis (the average postoperative drainage, time to drain removal, LOS, and postoperative transfusion requirements) for those undergoing 2–3 level laminectomies vs. 4–6 level procedures with large subsets also exhibiting herniated discs, synovial cysts, and degenerative spondylolisthesis.
Keywords: Discs, hemostatic agent, lumbar spine surgery, no CSF fistulas, no fusions length of stay, stenosis, timing drain removal, Tisseel, transfusion requirements
INTRODUCTION
Tisseel (Baxter International Inc., Westlake Village, CA, USA), a fibrin sealant, was originally introduced to strengthen repairs of cerebrospinal fluid (CSF) fistulas (traumatic and deliberate) resulting from lumbar spinal surgery. However, Tisseel is increasingly being utilized in the absence of CSF fistulas as a hemostatic agent for lumbar surgery, with hemostasis variably defined by the amount of postoperative drainage, time to drain removal, length of stay (LOS), and postoperative transfusion requirements. Here we quantitated the comparative impact of Tisseel on hemostasis for patients undergoing 2–3 vs. 4–6 level laminectomies for stenosis with varying frequencies (e.g. with/without) of disc herniations, synovial cysts, and degenerative spondylolisthesis (DS).
MATERIALS AND METHODS
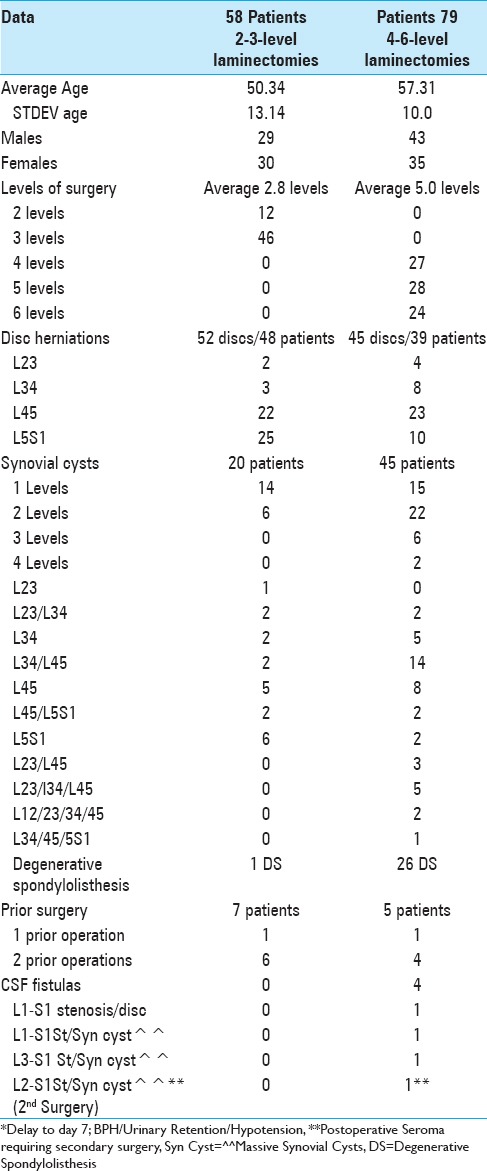
We prospectively followed 58 patients undergoing 2–3 level laminectomies/stenosis and 79 patients undergoing 3–6 level laminectomies/stenosis [Table 1]. The 2–3 level procedures addressed more herniated discs (48 patients), fewer synovial cysts (20 patients), and only rarely degenerative spondylolisthesis (1 patient). Alternatively, the 4-6 level laminectomies/stenosis included more patients with degenerative spondylolisthesis (26 patients), fewer disc herniations (39 patients), but many more synovial cysts (45 patients: often multilevel and/or multifocal). All laminectomies were performed utilizing somatosensory evoked potential (SEP) and electromyographic monitoring. They were performed by the same surgeon, utilizing the operating microscope, and undercutting the lateral gutters to preserve facet joints, thus avoiding the need for fusions.
Table 1.
Tisseel used for hemostasis in patients undergoing 2-3 (58 patients) vs. 4–6-level (79 patients) lumbar laminectomies
RESULTS
Postoperative drainage/other variables
Data for 2–3-level laminectomies
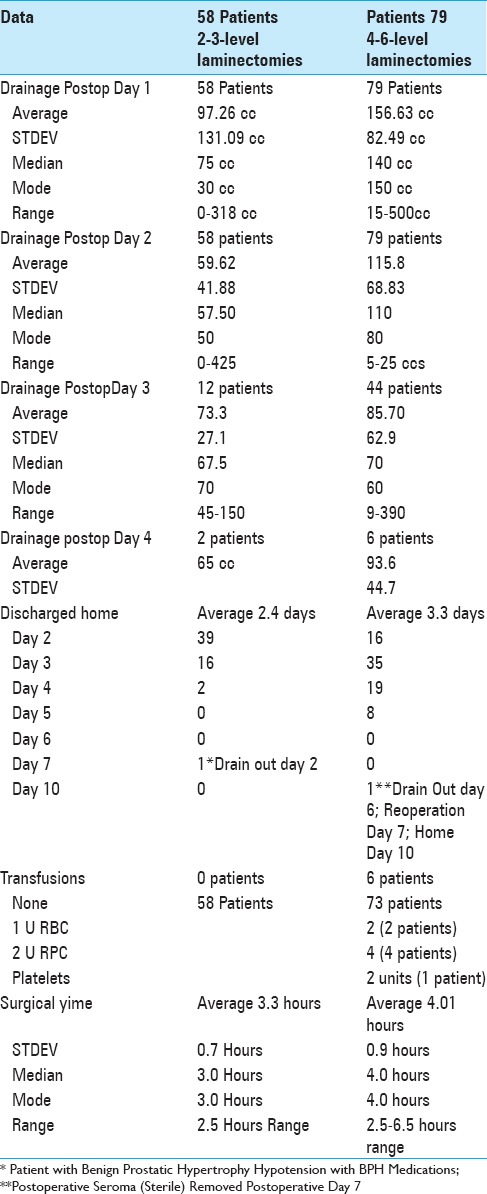
The average operative times for 58 patients undergoing 2–3 level laminectomies was 3.3 h (STDEV 0.7 h) [Table 2]. For 2–3 level decompressions, the average postoperative drainage on days 1–4 was 97.26 cc (day 1:58 patients), 59.62 cc (day 2:58 patients), 73.3 (day) 3:12 patients), and 65 cc (day 4; 2 patients) respectively. Drains were removed and patients were largely discharged on postoperative days 2 (39 patient), 3 (16 patients), and 4 (2 patients). Only one patient, whose drain was removed on postoperative day 2 was discharged on postoperative day 7 due to persistent hypotension associated with benign prostatic hypertrophy medications.
Table 2.
Tisseel's impact as a hemostatic on postoperative drainage and length of stay
Data for 4–6-level laminectomies
For the 79 patients undergoing 4–6 level laminectomies, the average operative time was 4.0 h (STDEV 0.9) [Table 2]. The mean postoperative drainage on postoperative days 1–4 averaged: 156.63 cc (day 1: 79 patients), 115.8 cc (day 2: 79 patients), 85.7 cc (day 3: 44 patients), and 93.6 cc (day 4: 6 patients) [Table 2]. Note, for those undergoing 4–6 level laminectomies, discharges were performed on postoperative days 2–5; day 2 (16 patients), day 3 (35 patients), day 4 (19 patients), and day 5 (18 patients). Only one patient who developed a postoperative sterile seroma with persistent drainage, required secondary surgery on postoperative day 7 (still in the hospital); he was discharged home on postoperative day 10.
Postoperative cerebrospinal fluid fistulas
Correlation of intraoperative cerebrospinal fluid fistulas with massive calcified synovial cysts extending to/through the dura
Intraoperative traumatic CSF fistulas were only observed in four patients undergoing the more extensive 4–6 level laminectomies/stenosis. This correlated with a higher incidence (45 cases) of one to four level synovial cysts observed amongst these 79 patients. One patient developed a CSF fistula following an L1-S1 laminectomy for stenosis and excision of a massive disc herniation; this patient had prior surgery, and the fistula was largely attributed to postoperative scar. The other three CSF fistulas occurred during decompressions/removals of massive calcified synovial cysts extending to/through the dura resected during L1-S1, L2-S1, and L3-S1 laminectomies. Notably, although 20 of 58 patients undergoing 2-3 level laminectomies had single/multiple synovial cysts, none developed intraoperative CSF fistulas [Table 1].
Lack of correlation of cerebrospinal fluid fistulas with postoperative scar
Prior surgery did not appear to uniquely contribute to CSF fistulas during secondary or tertiary surgery for patients undergoing 2–3 or 4–6 level laminectomies/stenosis [Table 1]. In fact, although seven of 58 patients undergoing 2–3 level laminectomies had prior surgery (e.g., 1 with one prior, 6 with two prior operation), none developed new intraoperative fistulas. For 5 of 79 patients undergoing 4-6 level laminectomies (1 with one prior, and 4 with two prior operations), only one of the four with a single prior operation developed a CSF fistula.
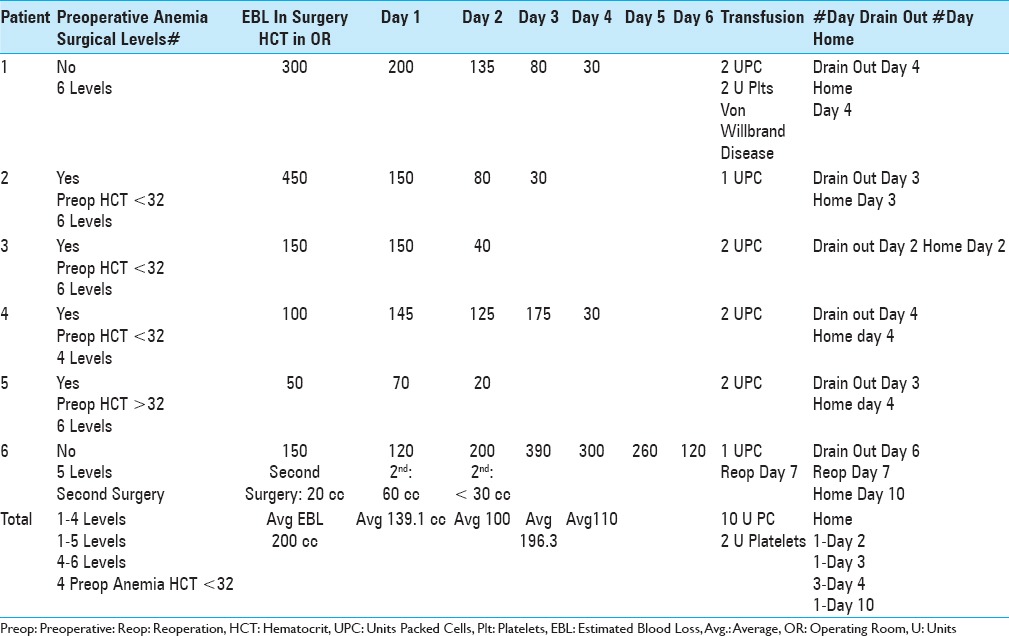
Transfusion requirements: 6 (7.6%) of 79 patients undergoing4-6-level laminectomies
Utilizing Tisseel to facilitate hemostasis, no transfusions were required for the 58 patients undergoing 2–3 level laminectomies, while 6 (7.6%) of the 79 undergoing 4–6 level laminectomies required transfusions [Tables 2 and 3]. Notably, these 6 patients underwent more extensive procedures that incurred increased postoperative drainage, time to drain removal, and therefore, greater blood loss thus requiring postoperative transfusions [Tables 2 and 3]. Four of the six has preoperative anemia (HCT with hydration preoperatively 32). The estimated blood loss (EBL) during surgery ranged from 50 cc to 450 cc, and the volume of postoperative drainage varied. The average postoperative drainage on day 1 (70–150), day 2 (20–200), day 3 (30–390), and day 4 (30-300) were skewed by the patient with a postoperative seroma (drained until day 6, reoperation day 7, and discharged home day 10).
Table 3.
Transfusion requirements in six patients undergoing 4-6-level laminectomies/stenosis
DISCUSSION
Fibrocaps (dry powder fibrin sealant) contributes to spinal hemostasis
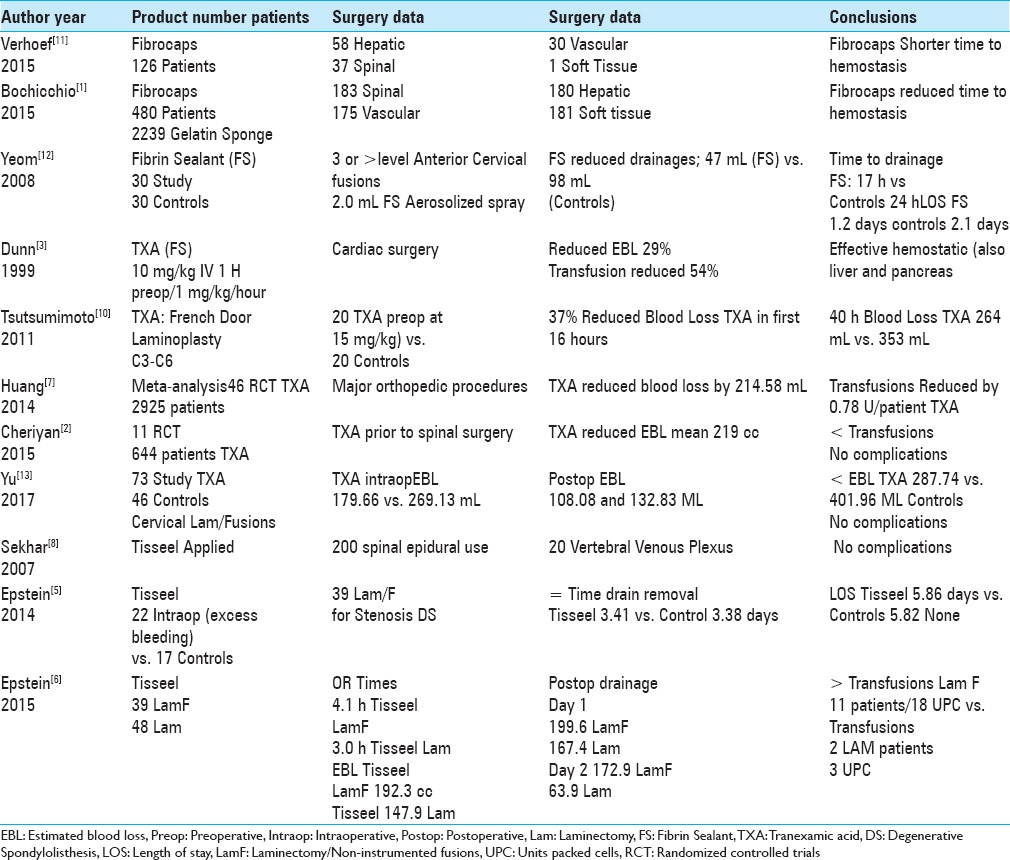
Two studies in 2015 utilized Fibrocaps for epidural spinal hemostasis and other surgical procedures [Table 4].[1,11] Verhoef et al. (2015) employed Fibrocaps, “ready-to-use, dry-powder fibrin sealant containing human plasma-derived thrombin and fibrinogen” in 126 adults undergoing hepatic (N = 58), spinal (N = 37), peripheral vascular (N = 30), and soft tissue procedures (N = 1).[11] Patients were randomized to Fibrocaps: N = 47 vs. sponge alone N = 23, secondary Fibrocaps N = 39, and gelatin sponge vs. sponge alone N = 17. The time to hemostasis was shorter utilizing Fibrocaps. Bochicchio et al. (2015), using Fibrocaps in 480 patients vs. a gelatin sponge alone (239 patients) for patients randomized to multiple surgical procedures [spinal (N = 183), vascular (N = 175), hepatic (N = 180), or soft-tissue (N = 181)].[1] They also found Fibrocaps significantly reduced the time to hemostasis.
Table 4.
Comparison of fibrin sealants, fibrin glues, fibrocaps, transexamic acid, and Tisseel for hemostasis in spine and other surgery
Role of fibrin sealants/fibrin glues in spinal and orthopedic surgery
Multiple studies have documented the efficacy of fibrin sealant/fibrin glue (FS/FG) in promoting postoperative spinal hemostasis [Table 4].[9,12] In 2008, Yeom et al., performed a retrospective analysis of 30 matched-pairs of patients undergoing >or = 3 level anterior cervical fusions; they documented FS contributed to hemostasis using 2.0 mL of fibrin sealant (e.g., fine aerosolized spray) vs. control group (no FS).[12] They found FS reduced total drainage (averaged 47 mL vs. 98 mL), time until drain removal (< or = 20 mL/shift (average 17 h vs. 24 h)), and decreased LOS (average 1.2 days vs. 2.1 days). Complication rates for both groups were comparable: two readmissions from each group within 4 postoperative days for dysphagia, dyspnea, and pneumonia. In 2009, Thoms and Marwin documented the beneficial role of fibrin sealants in limiting the time to hemostasis and perioperative bleeding for knee and hip arthroplasty.[9]
Tranexamic acid (fibrin Sealant) reduces blood loss in spine surgery
Several studies demonstrated how preoperative intravenous administration of tranexamic acid (TXA) (FS) contributed to hemostasis and reduced postoperative blood loss following spinal surgery [Table 4].[2,7,10,13,14]
Tranexamic acid reduces blood loss in cardiac and orthopedic surgery
In 1999, Dunn and Goa defined TXA as a “synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect through the reversible blockade of lysine binding sites on plasminogen molecules [Table 4].”[3] When TXA was administered intravenously (TXA 10 mg/kg initial dose and subsequent infusion of 1 mg/kg/h) prior to cardiac surgery, it reduced blood loss by 29%, and the transfusion requirement by 54%. It was also effective for other operations (e.g., liver and pancreas). Huang et al. (2014) in their meta-analysis of 46 randomized controlled trials (RCTs) identified 2,925 patients undergoing major orthopedic procedures.[7] On average, TXA reduced total blood loss by 408.33 mL, intraoperative blood loss by 125.65 mL, postoperative blood loss by 214.58 mL, and blood transfusions per patient by 0.78 U without an increase in thrombotic risks.[7]
Tranexamic acid reduces blood loss in cervical spine surgery
In 2011, Tsutsumimoto et al. performed 40 consecutive French door cervical laminoplasties (C3-C6); 20 patients received intravenous TXA prior to the incision (15 mg/kg body weight) vs. 20 receiving placebo [Table 4].[10] Despite nearly comparable intraoperative blood loss in both groups, within the first 16 postoperative hours, TXA patients demonstrated a 37% decrease in postoperative blood loss vs. controls (132.0 ± 45.3 vs. 211.0 ± 41.5 mL); at 40 postoperative hours the difference was TXA (264.1 ± 75.1 mL) vs. controls (353.9 ± 60.8 mL). In 2017, Yu et al. (2017) documented TXA effectively promoted hemostasis for 73 patients undergoing cervical laminectomy/lateral mass screw fusion vs. 46 controls; TXA contributed to decreased intraoperative blood loss (179.66 vs. 269.13), postoperative blood loss (108.08 and 132.83), total blood loss (287.74 vs. 401.96), without any major thromboembolic/other complications.[13]
Tranexamic acid reduces blood loss in spinal surgery overall
In 2014, when Zhang et al. performed a meta-analysis of six RCTs (randomized controlled trials) involving spinal surgery (RCTs; 411 patients), TXA-treated patients exhibited significantly reduced blood loss and transfusion requirements vs. placebo patients, without increasing thromboembolic complications [Table 4].[14] Cheriyan et al. (2015) analyzed 11 RCTs in which 644 patients received TXA prior to spinal surgery; TXA reduced “intraoperative, postoperative, and total blood loss by an average of 219 mL” and reduced transfusion rates without thrombotic complications.[2]
Tisseel's (fibrin sealant) role in spinal hemostasis
Several studies have unsuccessfully utilized Tisseel FS/FG to promote epidural spinal hemostasis [Table 4].[4,5,6,8] Sekhar et al. (2007) successfully utilized Tisseel to facilitate hemostasis in the epidural space (N = 200 patients) and vertebral venous plexus (N = 20 patients) without complications.[8] When Epstein (2014) reviewed the literature regarding the utility of Tisseel and other FS/FG not only to treat CSF fistulas, but also to promote hemostasis in spine surgery; it reduced perioperative bleeding, transfusion requirements, LOS, postoperative scar/radiculitis, and infection (e.g., if impregnated with antibiotics).[4] In 2014, for 39 patients undergoing multilevel laminectomies/1–2 level non-instrumented fusions (LamF) for stenosis/degenerative spondylolisthesis (DS), Epstein also utilized Tisseel in 22 patients demonstrating increased intraoperative bleeding vs. 17 showing no increased intraoperative bleeding (the latter not requiring Tisseel).[5] Here, the addition of tisseel both equalized the time to drain removal (3.41 days vs. 3.38 days), and LOS (5.86 vs. 5.82).[5] Epstein in 2015 then compared two different series of patients receiving Tisseel; 39 underwent average 4.4 level laminectomies/1.3 level non-instrumented fusions (LamF) vs. 48 having average 4.0 level laminectomies (LAM) alone.[6] The LamF patients had (as anticipated) longer average surgical times (4.1 h LamF vs. 3.0 h Lam), greater EBL (192.3 vs. 147.9 cc), more postoperative drainage (day 1; 199.6 vs. 167.4 cc; day 2; 172.9 vs. 63.9 cc), longer LOS (4.6 vs. 2.5 days), and greater transfusion requirements (11 LamF patients; 18 UPC (units of packed cells) vs. 2 Lam patients; 3 UPC). Additionally, here data for the previous 48 patients undergoing average 4.0 level LAM (without non instrumented fusions) were compared with those from this series of 79 patients having 4–6 level LAM (also without non instrumented fusions) (averaging 5.0 level procedures). Although drainage on postoperative day 1 was nearly comparable for both groups, as anticipated, those undergoing 4 vs. 5 level laminectomies had less blood loss on postoperative day 2 (63.9 cc vs. 115.8 cc), shorter LOS (2.5 days vs. 3.3 days), and lower transfusion greater transfusion requirements [2 patients; 4.0 level laminectomies (3 UPC) vs. six patients undergoing 5.0 level laminecotmies (10 UPC; 2 U platelets)]. Of interest, the present series of 58 patients having less extensive 2–3 level laminectomies (average 2.8 levels), exhibited less EBL on postoperative days 1 and 2 (97.6 cc and 59.62 cc), nearly equal LOS (2.4 days), but required no transfusions.
CONCLUSIONS
One may utilize the data presented in this study to advise patients undergoing 2-3 and 4-6 level laminectomies regarding their anticipated time to drain removal, LOS, risk of a CSF fistula, and potential requirement for a transfusion. Here, the addition of Tisseel to facilitate hemostasis for 79 patients undergoing the more extensive 4-6 level laminectomies (without non instrumented fusions), correlated with greater postoperative drainage, time to drain removal, LOS, and postoperative transfusion requirements. Alternatively, for those undergoing more restricted 2-3 level laminectomies (without non instrumented fusions), all parameters were appropriately/respetively decreased. Notably, utilizing Tisseel, there were no neurological complications, no infections, and no readmissions for patients in either operative group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
REFERENCES
- 1.Bochicchio GV, Gupta N, Porte RJ, Renkens KL, Pattyn P, Topal B, et al. The FINISH-3 trial: A phase 3, international, randomized, single-blind, controlled trial of topical fibrocaps in intraoperative surgical hemostasis. J Am Coll Surg. 2015;220:70–81. doi: 10.1016/j.jamcollsurg.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Cheriyan T, Maier SP 2nd, Bianco K, Slobodyanyuk K, Rattenni RN, Lafage V, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: A meta-analysis. Spine J. 2015;15:752–61. doi: 10.1016/j.spinee.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Dunn CJ, Goa KL. Tranexamic acid: A review of its use in surgery and other indications. Drugs. 1999;57:1005–32. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 4.Epstein NE. Hemostasis and other benefits of fibrin sealants/glues in spine surgery beyond cerebrospinal fluid leak repairs. Surg Neurol Int. 2014;5(Suppl 7):S304–14. doi: 10.4103/2152-7806.139615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epstein NE. Tisseel utilized as hemostatic in spine surgery impacts time to drain removal and length of stay. Surg Neurol Int. 2014;5(Suppl 7):S354–61. doi: 10.4103/2152-7806.139668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein NE. Tisseel does not reduce postoperative drainage, length of stay, and transfusion requirements for lumbar laminectomy with noninstrumented fusion versus laminectomy alone. Surg Neurol Int. 2015;6(Suppl 4):S172–6. doi: 10.4103/2152-7806.156561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F, Wu D, Ma G, Yin Z, Wang Q. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: A meta-analysis. J Surg Res. 2014;186:318–27. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: Technical note. Neurosurgery. 2007;61(3 Suppl):E51. doi: 10.1227/01.neu.0000289711.95426.50. discussion E51. [DOI] [PubMed] [Google Scholar]
- 9.Thoms RJ, Marwin SE. The role of fibrin sealants in orthopaedic surgery. J Am Acad Orthop Surg. 2009;17:727–36. doi: 10.5435/00124635-200912000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsumimoto T, Shimogata M, Ohta H, Yui M, Yoda I, Misawa H. Tranexamic acid reduces perioperative blood loss in cervical laminoplasty: A prospective randomized study. Spine (Phila Pa 1976) 2011;36:1913–8. doi: 10.1097/BRS.0b013e3181fb3a42. [DOI] [PubMed] [Google Scholar]
- 11.Verhoef C, Singla N, Moneta G, Muir W, Rijken A, Lockstadt H, et al. Fibrocaps for surgical hemostasis: Two randomized, controlled phase II trials. J Surg Res. 2015;194:679–87. doi: 10.1016/j.jss.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Yeom JS, Buchowski JM, Shen HX, Liu G, Bunmaprasert T, Riew KD. Effect of fibrin sealant on drain output and duration of hospitalization after multilevel anterior cervical fusion: A retrospective matched pair analysis. Spine (Phila Pa 1976) 2008;33:E543–7. doi: 10.1097/BRS.0b013e31817c6c9b. [DOI] [PubMed] [Google Scholar]
- 13.Yu CC, Gao WJ, Yang JS, Gu H, Md MZ, Sun K, Hao DJ. Can tranexamic acid reduce blood loss in cervical laminectomy with lateral mass screw fixation and bone grafting: A retrospective observational study. Medicine (Baltimore) 2017;96:e6043. doi: 10.1097/MD.0000000000006043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Wang K, Li FN, Huang X, Li Q, Chen Z, et al. Effectiveness of tranexamic acid in reducing blood loss in spinal surgery: A meta-analysis. BMC Musculoskelet Disord. 2014;15:448. doi: 10.1186/1471-2474-15-448. [DOI] [PMC free article] [PubMed] [Google Scholar]


