Abstract
Background:
Both Mitofusin 2 (Mfn2) and pelvic organ prolapse (POP) are related to aging. The aim of the present study was to investigate the variations of Mfn2 expression in the uterosacral ligaments of patients with and/or without POP and their correlations with the expression of procollagen.
Methods:
Fibroblasts were cultured using tissue specimens that were harvested from the uterosacral ligaments of POP and non-POP (NPOP) patients (n = 10 for each group) from September 2016 to December 2016. The Cell Counting Kit-8 (CCK-8) assay was used to compare the differences in cell proliferation between the two groups. Relative quantitative reverse transcription-polymerase chain reaction and Western blotting assays were employed to assess the differences in the mRNA and protein expression levels of Mfn2 and procollagen 1A1/1A2/3A1 between the two groups. The changes in procollagen expression were assessed following the downregulation of Mfn2 in the POP group using RNAi. The data were assessed with independent sample t-test or general linear model univariate analysis using the SPSS 13.0 software.
Results:
The results from CCK-8 assay indicated that cell viability in the POP group was significantly lower compared with that of the NPOP group (td5, 7, 9, 11= −5.925, −6.851, −9.129, and −9.661, respectively, all P < 0.001, from D5 to D11). The mRNA and protein expression levels of Mfn2 in the cultured fibroblasts of the POP group were significantly higher compared with those of the NPOP group (mRNA: t = 2.425, P = 0.032; protein: t = 2.392, P = 0.037, respectively), whereas only the expression levels of procollagen 1A1/1A2/3A1 were significantly higher in the NPOP group (mRNA: t = −2.165, P1A1 = 0.041; t = −2.741, P1A2 = 0.026; t = −2.147, P3A1 = 0.045, respectively; protein: t = −2.418, P1A1 = 0.029; t = −2.405, P1A2 = 0.033; t = −2.470, P3A1 = 0.012, respectively). The expression levels of procollagen in the POP group increased following the downregulation of Mfn2.
Conclusions:
The proliferation rate and cell viability of the fibroblasts in the POP group were significantly lower compared with those in the NPOP group. In the POP fibroblasts, Mfn2 expression was increased, while procollagen expression was decreased.
Keywords: Cell Culture, Mitofusin 2, Pelvic Organ Prolapse, Procollagen
INTRODUCTION
The pathophysiological mechanisms of pelvic organ prolapse (POP) remain unknown. Previous studies have focused on the investigation of the extracellular matrix (ECM) as the main cause for the development of POP.[1,2,3] For example, changes in the content and/or metabolism of collagen fibers have been previously monitored. Although the majority of the studies mainly compared the quantitative and morphological changes of collagen fibers and/or detected the impact of collagenase upon collagen metabolism, the pathogenesis of POP in terms of the variations in the synthesis and secretion of procollagen in fibroblasts have not been explored. Similarly, the proliferation and the induction of apoptosis of the fibroblasts in POP have not been investigated to date.
Mitofusin 2 (Mfn2) is a transmembrane protein embedded in the mitochondria that mediates mitochondrial fusion and plays an important role in the maintenance of the morphology and function of the mitochondria.[4,5] In addition to the aforementioned functions, Mfn2 is a cytoplasmic protein that is involved in the oxidative stress response,[5] signaling transduction,[6,7] the regulation of cell proliferation and apoptosis,[8] and various other biological processes. Therefore, the change in the expression of Mfn2 in the fibroblasts derived from pelvic floor tissues was used as a marker in the present study to add insight to the pathogenesis of POP.
The previous study (in vivo study) conducted by our group demonstrated the isolation of fibroblasts from the uterosacral ligament tissues of POP and non-POP (NPOP) patients by laser capture microdissection.[9] The expression levels of Type I and III procollagen proteins were significantly lower in the POP group compared with the NPOP group, whereas the opposite was noted for the expression of Mfn2, and the previous results were published in European Journal of Obstetrics, Gynecology, and Reproductive Biology in 2014. Based on the previous findings, the present study aimed to verify the results derived by the in vitro experiments and to further explore the impact of Mfn2 on the proliferation, induction of apoptosis, and further biological functions of the human uterosacral ligament fibroblasts. The study further aimed to provide conclusive evidence regarding the role of Mfn2 in the occurrence and progression of POP.
METHODS
Materials
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethics Committee of the hospital (No: 2016 [1173]). Informed written consent was obtained from all patients or their guardians for all study participants before their enrollment in this study.
Study subjects
Uterosacral ligament tissues were obtained from ten cases of Stage III–IV POP patients (postmenopausal) who underwent hysterectomy at the Department of Obstetrics and Gynecology of Peking University First Hospital from September 2016 to December 2016. NPOP patients who accepted hysterectomy due to a benign gynecological disease (n = 10) were enrolled as the control group [Table 1]. The following endpoints were measured in the control group, namely, matched age, body mass index, age of first delivery, number of deliveries, and postmenopausal duration. The POP Quantification System (POP-Q)[10] was used to grade the POPs. None of the patients received hormonal therapy during the 3 months before the surgery, and the participants were free of a history of urogenital tract infections, vaginal surgeries, and diseases that affected collagen metabolism. All the operations were conducted by the same surgical team that consisted of experienced doctors. Specimens were taken from the uterosacral ligament 1 cm beside the cervix.
Table 1.
Comparison of clinical data between patients of the POP and NPOP (control) groups
| Parameter | NPOP (n = 10) | POP (n = 10) | t | P |
|---|---|---|---|---|
| Age (years) | 59.87 ± 7.10 | 60.19 ± 6.83 | 0.169 | 0.886 |
| BMI (kg/m2) | 24.62 ± 3.08 | 25.72 ± 2.46 | 1.455 | 0.152 |
| Age of first delivery (years) | 25.83 ± 3.49 | 25.32 ± 2.85 | 0.584 | 0.562 |
| Number of deliveries | 2.13 ± 0.82 | 2.26 ± 0.99 | 0.501 | 0.618 |
| Postmenopausal duration (years) | 8.61 ± 6.79 | 9.32 ± 7.01 | 0.374 | 0.710 |
BMI: Body mass index; POP: Pelvic organ prolapse; NPOP: Non-POP.
Main reagents and instruments
The primers for Mfn2, the reference gene β-actin, and procollagen 1A1/1A2/3A1 were synthesized by Shanghai Sangon Biotech (China). Dulbecco's Modified Eagles Medium with High Glucose (SH30022.01B, HyClone) was purchased from GE Healthcare (USA). Dimethyl sulfoxide (DMSO, A3672) was purchased from APPLICHEM (Germany), whereas Trizol RNA extraction reagent, the reverse transcription kit, and the polymerase chain reaction (PCR) kit were purchased from ABI (USA). The Western Blotting Detection Kit was from GE Healthcare (USA) and the fetal bovine serum (10099-141) was from Gibco (USA). Penicillin/streptomycin mixture (KGY002-100), trypsin digestion solution (KGY001), and trypsin-EDTA digestion solution (KGY0012) were purchased from Nanjing KeyGen BioTECH (China). The anti-Mfn2 monoclonal antibody was purchased from Abcom (USA, ab56889), whereas anti-procollagen 1A1/1A2/3A1 monoclonal antibodies and anti-β-actin (TA-09) monoclonal antibody were from Santa Cruz (USA, 1A1: sc-133179; 1A2: sc-166572; 3A1: sc-166333). Mouse antihuman vimentin monoclonal antibody (ZM-0260), mouse antihuman cytokeratin (CK-19) monoclonal antibody, and horseradish peroxidase (HRP)-labeled goat antimouse IgG (ZB-2305) were purchased from Beijing Zhongshan Golden Bridge Biological Technology (China). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Japan). Both double chain DNAoligo and lentiviral expression vector of RNAi were provided by Shanghai Genechem (China).
Primer design
The primers of the target and the internal reference genes were designed based on the primer design principles. The GenBank database was used as a reference. The primers were synthesized by Shanghai Sangon Biotech [Table 2].
Table 2.
Primers sequences of the study
| Name | Sequence | Product length (bp) |
|---|---|---|
| Procollagen 1A1 | Upstream: 5’-CGAGGGCCAAGACGAAGA-3’ Downstream: 5’-CACGTCTCGGTCATGGTACCT-3’ |
73 |
| Procollagen 1A2 | Upstream: 5’-TGGATACGCGGACTTTGTTG-3’ Downstream: 5’-GGCTGGGCCCTTTCTTACAG-3’ |
92 |
| Procollagen 3A1 | Upstream: 5’-TCGCCCTCCTAATGGTCAAG-3’ Downstream: 5’-GGTCACCATTTCTCCCAGGAA-3’ |
80 |
| Mitofusin 2 | Upstream: 5’-CATCAGCTACACTGGCTCCAACT-3’ Downstream: 5’-GATGAGCAAAGGTCCCAGACA-3’ |
65 |
| β-actin | Upstream: 5’-CACGGCTGCTTCCAGCTC-3’ Downstream: 5’-CACAGGACTCCATGCCCAG-3’ |
135 |
The RNAi sequence of Mfn2 and the negative control sequence were as follows: ACTTTGTCACTGCCAAGAA, GTTCTCCGAACGTGTCACGT.
Primary culture and passage of fibroblasts derived from tissue specimens
During hysterectomy, fresh sterile uterosacral ligament tissues with a size of approximately 0.5 cm × 0.5 cm × 0.5 cm were harvested and then cut into pieces with a diameter smaller than 0.1 cm for the primary culture. Following 1–2 weeks of culture, the cells were observed under the microscope and passaged every 2–3 days.
Immunohistochemical identification of fibroblasts (SP method)
Following adhesion to the slides, the cells were incubated with primary antibodies (anti-vimentin and anti-CK-19 antibodies) in a 4°C humidified incubator overnight, while phosphate-buffered saline solution was used for the blank control. The following morning, the cells were incubated with the secondary antibodies (biotin-labeled goat antimouse antibodies) and with the HRP-labeled streptavidin conjugate in a 37°C humidified incubator. Subsequently, the cells were stained using diaminobenzidine, restained with hematoxylin, and washed with running tap water for counterstaining. The slides were dehydrated with gradient ethanol, cleared with xylene twice, mounted with neutral gum, and finally observed under the microscope.
Detection of fibroblast proliferation (Cell Counting Kit-8 assay)
A standard curve based on the optical density (OD) value that corresponded to cell number was developed. A total of six wells were inoculated with 100 μl of cell suspension at a concentration of 4 × 104 cell/ml per well. The cells were subsequently cultured under the same culturing conditions utilized in the standard curve experiment and incubated with CCK-8 solution for 2 h. The OD values of the cells were measured using a microplate reader at 450 nm. The cell counts were calculated based on the standard curve. The measurements were conducted in a new 96-well plate and six different time points were recorded as described above.
Real-time, fluorescence-based quantitative polymerase chain reaction for the detection of the mRNA expression levels of mitofusin 2 and procollagen
Total RNA was extracted from the cells that were cultured in the 60 mm dishes on day 11. Following reverse transcription to cDNA, quantitative PCR was conducted to detect the target and internal reference genes using the primers listed in Table 2. The relative quantification of the expression levels of the target and internal reference genes was estimated using the 2−ΔCT method.
Protein expression of mitofusin 2 and procollagen in fibroblasts
Total proteins were extracted from 106 cells, and the protein concentration was determined using a protein concentration standard curve. Using a loading volume of 60 μg per well, the proteins were separated by electrophoresis, transferred to a PVDF membrane, stained with Ponceau S and blocked with skimmed milk. Subsequently, the proteins were incubated with anti-Mfn2, anti-procollagen 1A1/1A2/3A1, and anti-β-actin monoclonal antibodies. Following washing of the primary antibodies, the membranes were incubated by HRP-labeled goat antimouse IgG antibody for enhanced chemiluminescence Western blotting detection. The AlphaImager™ Gel Imaging System (Alpha Innotech) was used to scan the grayscale levels of the specific ladders and calculate the grayscale ratios of the target proteins to the β-actin protein.
Transfection experiments
Mfn2 RNAi was conducted in POP fibroblasts, and the cells were divided into three groups. (1) The short hairpin RNA group (sh group) contained POP fibroblasts that were transfected with short hairpin RNA (shRNA)-positive interference lentiviral sequence; (2) The short hairpin RNA-negative control (shNC) group contained POP fibroblasts that were transfected with shRNA negative control lentiviral sequence; (3) The control group: POP fibroblasts in the absence of lentiviral infection. The expression levels of Mfn2 and procollagen in each group were measured following successful transfection.
Statistical analysis
The data measured, namely, the expression levels of the Mfn2 and the procollagen proteins were expressed as a mean ± standard deviation (SD). All the data were analyzed using the SPSS 13.0 software (New York, IBM, USA). If the data were in normal distributions, between-group comparisons were assessed with the independent sample t-test and general linear model-univariate analysis and/or one-way analysis of variance (ANOVA) test; otherwise, between-group comparisons were assessed with Wilcoxon test. A value of P < 0.05 was used to indicate significant differences.
RESULTS
Identification of fibroblasts
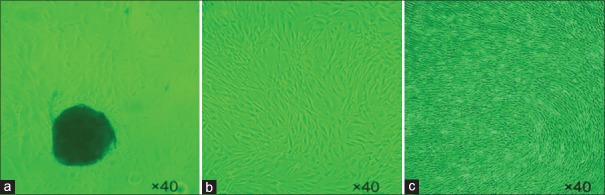
The cell growth was monitored by microscopy following 1–2 weeks of inoculation of the tissues in the culture flask. The cells manifested at a growth zone that surrounded the tissue specimens [Figure 1a]. The cells were gradually elongated and formed a long spindle and/or polygonal shape. Following 7 days, local cell fusions that covered the major part of the flask surface could be observed. At the 20-day period, complete cell fusions were observed. Following passage of the cells, the cell growth was rapid, and the cells that exhibited optimal conditions were highly elongated. The fusion was completed on the 7th day [Figure 1b]. However, the cells would accumulate spirally when cultured continuously [Figure 1c]. Following passage to the third generation, the majority of the cells in the flask exhibited a spindle shape.
Figure 1.
Primary culture of the fibroblasts and their growth and morphology (Fibroblasts observed under ×40 light microscope with no staining). Structures colored in black (a) indicate the tissue specimens; (b) the fusion was completed on the 7th days; (c) the cells would accumulate spirally when cultured continuously.
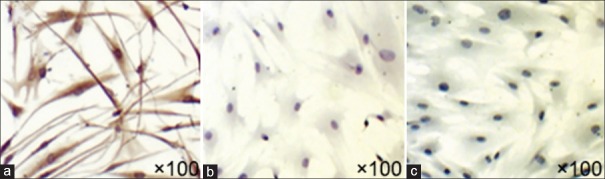
Cell immunohistochemistry indicated that intracellular expression of vimentin was positive (2A), whereas that of the CK-19 was negative (2B), highlighting that these fibroblasts were interstitially derived [Figure 2].
Figure 2.
Fibroblasts which were observed after cell IHC. Fibroblasts observed under ×100 light microscope after IHC, IHC indicated that intracellular expression of vimentin was positive (a), while that of the CK-19 was negative (b), highlighting that these fibroblasts were interstitially derived. (a) Anti-vimentin antibody (primary antibody); (b) anti-CK-19 antibody (primary antibody); (c) blank control (PBS). IHC: Immunohistochemistry.
Determination of proliferation in the fibroblasts of the pelvic organ prolapse and the non-pelvic organ prolapse groups
Following seeding of the fibroblasts that corresponded to the two different groups in separate culture flasks, the differences in the cell proliferation were monitored between a 3- and 5-day time period. The cells were cultured using the same cell density and culturing conditions. The data obtained by microscopical evaluation indicated that fibroblasts in the POP group exhibited lower adhesion numbers, survival rate, and smaller cell size, whereas those in the NPOP group indicated higher cell numbers and faster proliferation rates. The cells in the POP groups were further characterized by a wider, longer, and more elongated morphology with broad intercellular contacts [Figure 3].
Figure 3.
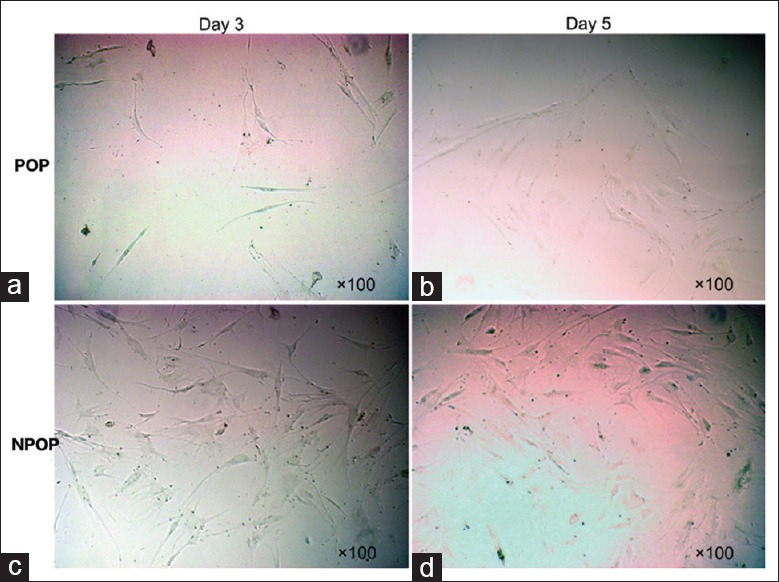
Growth and morphological differences of fibroblasts between POP and NPOP groups. Growth and morphological differences of fibroblasts observed under ×100 light microscope without staining. Fibroblasts in the POP group exhibited lower adhesion numbers, survival rate, and smaller cell size, while those in the NPOP group indicated higher cell numbers and faster proliferation rates. (a) POP cells observed on the 3rd day, (b) POP-cells observed on the 5th day, (c) NPOP-cells observed on the 3rd day, (d) NPOP-cells observed on the 5th day. POP: Pelvic organ prolapse; NPOP: Non-pelvic organ prolapse.
The CCK-8 assay was applied to assess the cellular viability daily, for a total period of 11 days following initial seeding. During the period of 5–11 days following seeding, the cells were in the logarithmic phase of proliferation, whereas the viability of the fibroblasts from the POP group was significantly lower compared with that of the NPOP group (td5, 7, 9, 11= −5.925, −6.851, −9.129, and −9.661, respectively; all P < 0.001, from D5 to D11 [Table 3]).
Table 3.
Comparisons of cell viability of fibroblasts (CCK-8) between POP and NPOP groups at different culturing time points
| Group | Day 1 | Day 3 | Day 5 | Day 7 | Day 9 | Day 11 |
|---|---|---|---|---|---|---|
| POP (n = 10) | 0.183 ± 0.003 | 0.216 ± 0.014 | 0.264 ± 0.015 | 0.410 ± 0.044 | 0.552 ± 0.189 | 0.792 ± 0.278 |
| NPOP (n = 10) | 0.182 ± 0.003 | 0.221 ± 0.022 | 0.332 ± 0.087 | 0.552 ± 0.154 | 1.047 ± 0.375 | 1.404 ± 0.404 |
| t | 1.347 | −1.411 | −5.925 | −6.851 | −9.129 | −9.661 |
| P | 0.181 | 0.161 | <0.001 | <0.001 | <0.001 | <0.001 |
During the period of 5–11 days following seeding the cells were in the logarithmic phase of proliferation, whereas the viability of the fibroblasts from the POP group was significantly lower compared with that of the NPOP group. POP: Pelvic organ prolapse; NPOP: Non-POP; CCK-8: Cell counting kit-8.
The mRNA and protein expression levels of mitofusin 2 were upregulated, while those of procollagen were downregulated in pelvic organ prolapse fibroblasts
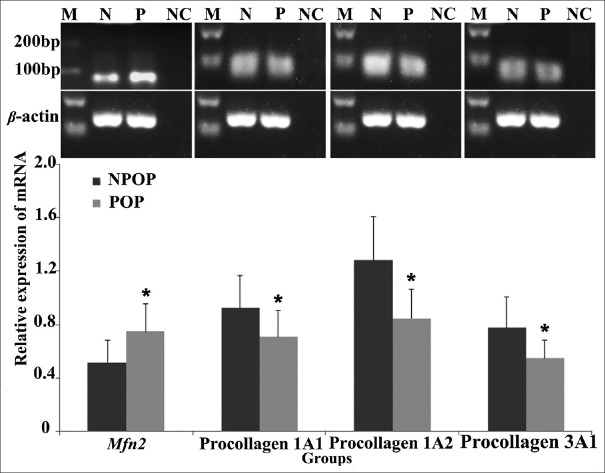
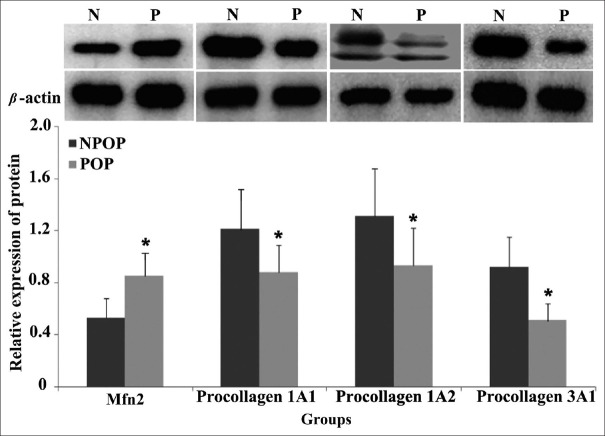
The mRNA and protein expression levels of Mfn2 in the cultured POP fibroblasts were significantly higher compared with those of the NPOP group (mRNA: t = 2.425, P = 0.032; protein: t = 2.392, P = 0.037), whereas the mRNA and protein expression levels of procollagen 1A1/1A2/3A1 were significantly higher in the NPOP compared with the POP groups (mRNA: t = −2.165, P1A1 = 0.041; t = −2.741, P1A2 = 0.026; t = −2.147, P3A1 = 0.045; protein: t = −2.418, P1A1 = 0.029; t = −2.405, P1A2 = 0.033; t = −2.470, P3A1 = 0.012, respectively) [Tables 4, 5 and Figures 4, 5]. These results were consistent with the in vivo results reported in the previous study of our group.
Table 4.
mRNA expression levels of Mfn2 and procollagen in the POP and NPOP fibroblasts
| Group | Mfn2 | Procollagen | ||
|---|---|---|---|---|
| 1A1 | 1A2 | 3A1 | ||
| POP (n = 10) | 0.76 ± 0.21 | 0.70 ± 0.19 | 0.82 ± 0.22 | 0.55 ± 0.13 |
| NPOP (n = 10) | 0.53 ± 0.17 | 0.95 ± 0.25 | 1.26 ± 0.34 | 0.78 ± 0.23 |
| t | 2.425 | −2.165 | −2.741 | −2.147 |
| P | 0.032 | 0.041 | 0.026 | 0.045 |
Compared to the NPOP, in the POP fibroblasts, the mRNA of Mfn2 was increased, while mRNA of procollagen was decreased. POP: Pelvic organ prolapse; NPOP: Non-POP; Mfn2: Mitofusin 2.
Table 5.
Protein expression levels of Mfn2 and procollagen in the POP and NPOP fibroblasts
| Group | Mfn2 | Procollagen | ||
|---|---|---|---|---|
| 1A1 | 1A2 | 3A1 | ||
| POP (n = 10) | 0.84 ± 0.17 | 0.88 ± 0.21 | 0.93 ± 0.29 | 0.54 ± 0.13 |
| NPOP (n = 10) | 0.53 ± 0.15 | 1.20 ± 0.29 | 1.31 ± 0.38 | 0.91 ± 0.23 |
| t | 2.392 | −2.418 | −2.405 | −2.470 |
| P | 0.037 | 0.029 | 0.033 | 0.012 |
Compared to the NPOP, in the POP fibroblasts, the protein expression of Mfn2 was increased, while procollagen expression was decreased. POP: Pelvic organ prolapse; NPOP: Non-POP; Mfn2: Mitofusin 2.
Figure 4.
mRNA expression levels of Mfn2 and procollagen in the POP and NPOP fibroblasts. Compared to the NPOP, the mRNA levels of Mfn2 in POP fibroblasts were upregulated, while mRNA levels of procollagen were downregulated. tMfn2(P:N) = 2.425, t1A1(P:N) = −2.165, t1A2(P:N) = −2.741, t3A1(P:N) = −2.147, respectively; PMfn2(P:N) = 0.032, P1A1(P:N) = 0.041, P1A2(P:N) = 0.026, P3A1(P:N) = 0.045, respectively. The significance was presented as *P < 0.05. M: Marker; N(NPOP): Non-pelvic organ prolapse; P(POP): Pelvic organ prolapse; NC: Negative Control; Mfn2: Mitofusin 2.
Figure 5.
Protein expression levels of Mfn2 and procollagen in the POP and NPOP fibroblasts. Compared to the NPOP, the protein expression levels of Mfn2 in POP fibroblasts were upregulated, while procollagen protein expression levels were downregulated. tMfn2(P:N) = 2.392, t1A1(P:N) = −2.418, t1A2(P:N) = −2.405, t3A1(P:N) = −2.470, respectively; PMfn2(P:N) = 0.037; P1A1(P:N) = 0.029; P1A2(P:N) = 0.033; P3A1(P:N) = 0.012, respectively. The significance was presented as *P < 0.05. N(NPOP): Non-pelvic organ prolapse; P(POP): Pelvic organ prolapse; Mfn2: Mitofusin 2.
The expression of procollagen was upregulated in pelvic organ prolapse fibroblasts through inhibition of the expression of mitofusin 2
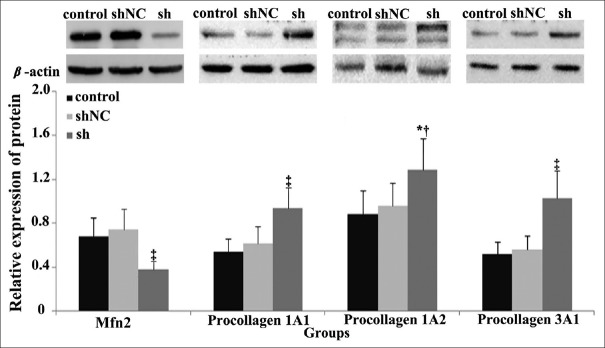
Following 96 h of the initial transfection with Mfn2 lentiviral RNAi in POP fibroblasts, the protein expression levels of Mfn2 were significantly lower than those in the shNC and control groups although no significant difference between negative control (shNC) and control groups (FMfn2(sh-shNC-control)= 26.661, PMfn2(sh:shNC) < 0.001, PMfn2(sh:control) < 0.001, and PMfn2(shNC:control) = 0.838, respectively) was noted. Furthermore, the protein expression levels of the procollagen 1A1/1A2/3A1 in the sh group were significantly higher compared with those of the shNC and the control groups (F1A1(sh-shNC-control) = 18.277, F1A2(sh-shNC-control) = 6.606, and F3A1(sh-shNC-control)= 36.178, respectively, P1A1(sh: shNC)< 0.001, P1A1(sh:control) < 0.001, and P1A1(shNC:control) = 0.445, respectively;P1A2(sh:shNC)= 0.017, P1A2(sh:control)= 0.007, and P1A2(shNC:control)= 0.928, respectively; P3A1(sh:shNC) < 0.001, P3A1(sh:control) < 0.001, and P3A1(shNC:control) = 0.897, respectively). No significant difference was noted between the two control groups. That indicates that the expression levels of procollagen can be improved by the downregulation of the expression levels of Mfn2 in POP fibroblasts [Table 6 and Figure 6].
Table 6.
Protein expression levels of procollagen and Mfn2 in POP fibroblasts via Mfn2 inhibition
| Group | Mfn2 | Procollagen | ||
|---|---|---|---|---|
| 1A1 | 1A2 | 3A1 | ||
| Sh (n = 10) | 0.39 ± 0.07 | 0.99 ± 0.17 | 1.23 ± 0.32 | 1.06 ± 0.19 |
| shNC (n = 10) | 0.70 ± 0.14 | 0.67 ± 0.16 | 0.92 ± 0.14 | 0.58 ± 0.12 |
| Control (n = 10) | 0.68 ± 0.09 | 0.57 ± 0.16 | 0.88 ± 0.18 | 0.55 ± 0.13 |
| F | 26.661 | 18.277 | 6.606 | 36.178 |
| Psh-shNC | <0.001 | <0.001 | 0.017 | <0.001 |
| Psh-control | <0.001 | <0.001 | 0.007 | <0.001 |
| PshNC-control | 0.838 | 0.445 | 0.928 | 0.897 |
P<0.05 indicates significant difference, one-way ANOVA test and univariate analysis. The expression levels of Mfn2 were downregulated in sh group, but the expression levels of procollagen were increased in sh group. POP: Pelvic organ prolapse; Mfn2: Mitofusin 2; sh: POP fibroblasts that were transfected with shRNA positive interference lentiviral sequence; shNC: POP fibroblasts that were transfected with shRNA negative control lentiviral sequence; Control: POP fibroblasts in the absence of lentiviral infection; ANOVA: Analysis of variance.
Figure 6.
Protein expression levels of Mfn2 and procollagen in RNAi-transfected fibroblasts. Compared to the control and shNC groups, the protein expression levels of Mfn2 were downregulated in sh group, but the protein expression levels of pro-collagen were upregulated. FMfn2(sh-shNC-control) = 26.661; F1A1(sh-shNC-control) = 18.277; F1A2(sh-shNC-control) = 6.606; F3A1(sh-shNC-control) = 36.178, respectively. PMfn2(sh:shNC) < 0.001, PMfn2(sh:control) < 0.001, PMfn2(shNC:control) = 0.838, respectively; P1A1(sh:shNC) < 0.001, P1A1(sh:control) < 0.001, P1A1(shNC:control) = 0.445, respectively; P1A2(sh:shNC) = 0.017; P1A2(sh:control) = 0.007; P1A2(shNC:control) = 0.928, respectively; P3A1(sh:shNC) < 0.001; P3A1(sh:control) < 0.001; P3A1(shNC:control) = 0.897, respectively. *P < 0.05, †P < 0.01, ‡P < 0.001. sh: POP fibroblasts that were transfected with shRNA positive interference lentiviral sequence; shNC: POP fibroblasts that were transfected with shRNA negative control lentiviral sequence; control: POP fibroblasts in the absence of lentiviral infection. Mfn2: Mitofusin 2; POP: Pelvic organ prolapse.
DISCUSSION
Previous studies that examined the POP mainly focused on the investigation of clinical research[11,12,13,14] or the ECM as the main cause for the development of POP. A limited number of studies have addressed the morphology and cell proliferation of the fibroblasts that are secreted to the ECM. A previous study conducted by our group demonstrated the successful isolation of fibroblasts from the uterosacral ligament tissues of POP and NPOP patients using laser capture microdissection.[9] Abnormal mRNA and protein expression levels of Mfn2 and procollagen (upregulated Mfn2 and downregulated procollagen in the POP group) were noted.[9] In the present study, an in vitro cultured fibroblast model was used to add insight to the pathogenesis of POP. The cells were derived from the uterosacral ligament tissues of POP and NPOP patients and the differences in the expression of Mfn2 and procollagen as well as the differences in the cell proliferation and apoptosis between the two groups were compared. Consistency in the Mfn2 and procollagen expression (POP and NPOP groups) was noted between the in vivo study conducted previously and the in vitro results produced in the current study. The in vitro data further indicated that altered Mfn2 expression resulted in abnormal fibroblast proliferation and function, which might be one of the key factors that contribute to the occurrence and progression of POP.
The comparison between the in vivo and in vitro studies was achieved by the selection of appropriate patients that exhibited the same inclusion criteria. All participants enrolled were postmenopausal females, who complied with the inclusion criteria reported in the in vivo study, whereas the diagnostic criteria for POP were based on the current internationally recognized POP-Q system. In addition, in terms of the enrollment criteria for the control group, only NPOP patients with a matched age, BMI, age of first delivery, number of deliveries, and postmenopausal duration who accepted hysterectomy due to a benign gynecologic disease were enrolled. None of the patients received any estrogenic treatment during the 3 months period before the surgery, and the participants were free of a history of urogenital tract infections, vaginal surgeries, and diseases that affect collagen metabolism. The aforementioned selection criteria eliminated the impact of female hormones and other factors that could interfere with the validation of the in vitro method that utilized hosting fibroblasts. The same surgeon with the same experienced surgical team further ensured the homogeneity of the tissue/cell origin.
Abnormal procollagen synthesis and secretion in the fibroblasts might be an important pathogenetic mechanism of POP. Currently, it is reported that total collagen content is reduced in the pelvic floor tissues in the POP patients including the parametrium and vaginal apex,[3,15] periurethral ligaments, or sacral ligaments,[16] indicating that variations in the ECM collagen content significantly affect the supporting effect caused by these proteins to the pelvic floor tissues. Type I collagen is characterized by potent plasticity and anti-tension ability that enables it to counter continuous tension. Type III collagen notably increases the elasticity and ductility, contributing to the responses to periodic tension. Microfibers that comprised certain packing arrangements of Type I and Type III collagens exhibited an effect upon the tensile properties of connective tissues.[17,18] The present study indicated that the protein expression levels of Type I and Type III procollagens in the fibroblasts obtained from the uterosacral ligament tissues of the POP patients were significantly reduced, which might lead to a declined Type I/III collagen secretion into the ECM. In contrast to these observations, the protein expression levels of both Type I and Type III procollagens in the in vitro cultured POP fibroblasts were lower compared with those of the NPOP fibroblasts, which was consistent with the results observed in the tissue study. Based on the synthetic process of collagens, the declined procollagen production in the fibroblasts will theoretically reduce the syntheses of collagens and collagen fibers in the ECM and result in decreased supporting strength in the pelvic floor tissues.
Mfn2 is a novel gene characterized by Chen et al.,[19] which was previously known as hyperplasia suppressor gene. Mfn2 is a vascular smooth muscle cell (VSMC) proliferation suppressor as demonstrated by studies that investigated the proliferation of rat VSMC. The human Mfn2 protein comprises 757 amino acids and possesses two transmembrane domains that span the outer mitochondrial membrane and are scattered along the mitochondrial networks.[20,21,22] Mitochondria are considered the main sites for intracellular oxidative phosphorylation and synthesis of adenosine triphosphate, providing energy supplies for various cellular activities. Previous studies have demonstrated that the main function of Mfn2 was to mediate mitochondrial fusion[4] and regulate mitochondrial morphology.[4,23] This renders mitochondria function as a dynamic network that constantly undergoes fusion and fission.[4,23] However, Mfn2 overexpression affects mitochondrial fusion,[4,24] causing mitochondrial dysfunction that acts as one of the main pro-aging factors.[25] Mitochondria have an independent genetic system, and the accumulation of mitochondrial DNA mutations promotes tissue aging. Therefore, mitochondrial fusion plays a key role in the prevention of mitochondrial DNA mutations.[26,27] Thus, the examination of the expression levels of Mfn2 in the pelvic floor tissues is considered a crucial factor in the investigation of the pathogenesis of POP. The majority of studies to date have focused on tissues such as the skeletal muscle, myocardium, vascular endothelium, and/or smooth muscle cell, while a limited number of studies have reported the roles of fibroblasts in the pelvic floor tissues. Since POP is a disease associated with aging,[28] abnormal Mfn2 expression possibly correlates with the pathogenesis of POP. In the present and the previous studies conducted by our group, the difference in the expression levels of Mfn2 between the POP and NPOP fibroblasts was initially compared. The data indicated an upregulated expression of Mfn2 in the POP fibroblasts that were directly obtained from the uterosacral ligament tissues (previous in vivo study). The same pattern was noted in the cultured POP fibroblasts that originated from the uterosacral ligament tissues (present in vitro study). The increase in the expression of the Mfn2 levels indicated a significant negative correlation with the expression of procollagen. The expression of Mfn2 was downregulated in RNAi-transfected fibroblasts, but the expression of pro-collagen increased. The results indicate that Mfn2 might inhibit the procollagen expression in the fibroblasts, whereas Mfn2 overexpression promotes aging, which might also be responsible for the slow growth of POP fibroblasts.
There were some limitations in the study. For example, this research did not focus on the mitochondrial energy metabolism and mitophagy. The link between Mfn2 and procollagen remains unknown and can be investigated in the future.
In summary, we concluded that lower proliferation rate and viability were observed in the in vitro cultured POP fibroblasts compared with the NPOP fibroblasts. In the POP fibroblasts, Mfn2 expression was upregulated while procollagen expression was downregulated, which was consistent with our previous in vivo results. The results indicate that the genetic properties of the cultured fibroblasts remain unchanged and that this in vitro model is suitable for subsequent studies.
In the present study, when the expression of Mfn2 was downregulated, the expression levels of the procollagen forms were upregulated, and the support of the pelvic floor was enhanced. This might provide the basis for a new therapy of POP.
Financial support and sponsorship
This work was supported by a grant from the National Natural Science Foundation of China (No. 81401185).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Kotova SL, Timashev PS, Guller AE, Shekhter AB, Misurkin PI, Bagratashvili VN, et al. Collagen structure deterioration in the skin of patients with pelvic organ prolapse determined by atomic force microscopy. Microsc Microanal. 2015;21:324–33. doi: 10.1017/S1431927615000148. doi: 10.1017/S143192715000148. [DOI] [PubMed] [Google Scholar]
- 2.Kufaishi H, Alarab M, Drutz H, Lye S, Shynlova O. Static mechanical loading influences the expression of extracellular matrix and cell adhesion proteins in vaginal cells derived from premenopausal women with severe pelvic organ prolapse. Reprod Sci. 2016;23:978–92. doi: 10.1177/1933719115625844. doi: 10.1177/1933719115625844. [DOI] [PubMed] [Google Scholar]
- 3.Liang R, Zong W, Palcsey S, Abramowitch S, Moalli PA. Impact of prolapse meshes on the metabolism of vaginal extracellular matrix in rhesus macaque. Am J Obstet Gynecol. 2015;212:174.e1–7. doi: 10.1016/j.ajog.2014.08.008. doi: 10.1016/j.ajog.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61:683–94. doi: 10.1016/j.molcel.2016.02.022. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Zorzano A, Hernández-Alvarez MI, Sebastián D, Muñoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22:1020–31. doi: 10.1089/ars.2014.6208. doi: 10.1089/ars.2014.6208. [DOI] [PubMed] [Google Scholar]
- 6.Lu Z, Li S, Zhao S, Fa X. Upregulated miR-17 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation and apoptosis by targeting mitofusin 2. Med Sci Monit. 2016;22:3301–8. doi: 10.12659/MSM.900487. doi: 10.12659/MSM.900487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y, Zhou D, Xu X, Zhao X, Huang P, Zhou X, et al. Clinical significance of mitofusin-2 and its signaling pathways in hepatocellular carcinoma. World J Surg Oncol. 2016;14:179. doi: 10.1186/s12957-016-0922-5. doi: 10.1186/s12957-016-0922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen KH, Dasgupta A, Ding J, Indig FE, Ghosh P, Longo DL, et al. Role of mitofusin 2 (Mfn2) in controlling cellular proliferation. FASEB J. 2014;28:382–94. doi: 10.1096/fj.13-230037. doi: 10.1096/fj.13-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen HY, Lu Y, Qi Y, Bai WP, Liao QP. Relationship between the expressions of mitofusin-2 and procollagen in uterosacral ligament fibroblasts of postmenopausal patients with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2014;174:141–5. doi: 10.1016/j.ejogrb.2013.11.024. doi: 10.1016/j.ejogrb.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. doi: 10.1016/S0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 11.Wang SY, Cao TT, Wang RZ, Yang X, Sun XL, Wang JL, et al. Incidence and risk factors of de novo stress urinary incontinence after pelvic floor reconstruction: A Nested Case-Control Study. Chin Med J. 2017;130:678–83. doi: 10.4103/0366-6999.201592. doi: 10.4103/0366-6999.201592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niu K, Lu YX, Shen WJ, Zhang YH, Wang WY. Risk factors for mesh exposure after transvaginal mesh surgery. Chin Med J. 2016;129:1795–9. doi: 10.4103/0366-6999.186631. doi: 10.4103/0366-6999.186631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu L, Chen J, Xu T, Lang JH. Tension-free polypropylene mesh-related surgical repair for pelvic organ prolapse has a good anatomic success rate but a high risk of complications. Chin Med J. 2015;128:295. doi: 10.4103/0366-6999.150088. doi: 10.4103/0366-6999.150088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang S, Zhu L, Zhang L, Sun ZJ, Tao X, Lang JH, et al. Manometric comparison of anorectal function after posterior vaginal compartment repair with and without mesh. Chin Med J. 2015;128:438–42. doi: 10.4103/0366-6999.151065. doi: 10.4103/0366-6999.151065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown BN, Mani D, Nolfi AL, Liang R, Abramowitch SD, Moalli PA, et al. Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am J Obstet Gynecol. 2015;213:668.e1–10. doi: 10.1016/j.ajog.2015.08.002. doi: 10.1016/j.ajog.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon MJ, Kim EJ, Lee M, Kim H, Choi JR, Chae HD, et al. MicroRNA-30d and microRNA-181a regulate HOXA11 expression in the uterosacral ligaments and are overexpressed in pelvic organ prolapse. J Cell Mol Med. 2015;19:501–9. doi: 10.1111/jcmm.12448. doi: 10.1111/jcmm.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulrich D, Edwards SL, Su K, White JF, Ramshaw JA, Jenkin G, et al. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PLoS One. 2014;9:e93172. doi: 10.1371/journal.pone.0093172. doi: 10.1371/journal.pone.0093172.g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T, Sridharan I, Ma Y, Zhu B, Chi N, Kobak W, et al. Identifying distinct nanoscopic features of native collagen fibrils towards early diagnosis of pelvic organ prolapse. Nanomedicine. 2016;12:667–75. doi: 10.1016/j.nano.2015.11.006. doi: 10.1016/j.nano.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Liu N, Zhou A, Tang C, Ma D, Tang J, et al. The role of hypertension-related gene in aortic vascular smooth muscle cells from mice and rats. Chin Med J. 2001;114:833–6. [PubMed] [Google Scholar]
- 20.Lascaratos G, Garway-Heath DF, Willoughby CE, Chau KY, Schapira AH. Mitochondrial dysfunction in glaucoma: Understanding genetic influences. Mitochondrion. 2012;12:202–12. doi: 10.1016/j.mito.2011.11.004. doi: 10.1016/j.mito.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZH, Clark C, Geisbrecht ER. Analysis of mitochondrial structure and function in the Drosophila larval musculature. Mitochondrion. 2016;26:33–42. doi: 10.1016/j.mito.2015.11.005. doi: 10.1016/j.mito.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin EE, Detmer SA, Chan DC. Molecular mechanism of mitochondrial membrane fusion. Biochim Biophys Acta. 2006;1763:482–9. doi: 10.1016/j.bbamcr.2006.02.003. doi: 10.1016/j.bbamcr.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Chan DC. Mitochondrial dynamics – Fusion, fission, movement, and mitophagy – In neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–76. doi: 10.1093/hmg/ddp326. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lou Y, Li R, Liu J, Zhang Y, Zhang X, Jin B, et al. Mitofusin-2 over-expresses and leads to dysregulation of cell cycle and cell invasion in lung adenocarcinoma. Med Oncol. 2015;32:132. doi: 10.1007/s12032-015-0515-0. doi: 10.1007/s12032-015-0515-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, et al. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–83. doi: 10.1074/jbc.M700679200. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Zou X, Feng Z, Luo C, Liu J, Li H, et al. Evidence for association of mitochondrial metabolism alteration with lipid accumulation in aging rats. Exp Gerontol. 2014;56:3–12. doi: 10.1016/j.exger.2014.02.001. doi: 10.1016/j.exger.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Sebastián D, Zorzano A. When MFN2 (mitofusin 2) met autophagy: A new age for old muscles. Autophagy. 2016;12:2250–1. doi: 10.1080/15548627.2016.1215383. doi: 10.1080/15548627.2016.1215383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AA, Eilber KS, Clemens JQ, Wu N, Pashos CL, Anger JT, et al. Trends in management of pelvic organ prolapse among female medicare beneficiaries. Am J Obstet Gynecol. 2015;212:463.e1–8. doi: 10.1016/j.ajog.2014.10.025. doi: 10.1016/j.ajog.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]