Abstract
Background:
Antiplatelet therapy (APT) was prevalently being used in the prevention of vascular disease, but the influence of prior APT on the prognosis of patients with intracerebral hemorrhage (ICH) remains controversial. This meta-analysis was to explore the effects of prior APT on the prognosis of patients with primary ICH.
Methods:
PubMed and Embase were searched to identify the eligible studies. The studies comparing the mortality of ICH patients with or without prior APT were included. The quality of these studies was evaluated by the Newcastle–Ottawa quality assessment scale. The adjusted or unadjusted odds ratio (OR) for mortality between ICH patients with and without prior APT were pooled with 95% confidence interval (95% CI) as the effect of this meta-analysis.
Results:
Twenty-two studies fulfilled the inclusion criteria and exhibited high qualities. The pooled OR was 1.37 (95% CI: 1.13–1.66, P = 0.001) for univariate analysis and 1.41 (95% CI: 1.05–1.90, P = 0.024) for multivariate analysis. The meta-regression indicated that for each 1-day increase in the time of assessment, the adjusted OR for the mortality of APT patients decreased by 0.0049 (95% CI: 0.0006–0.0091, P = 0.026) as compared to non-APT patients.
Conclusion:
Prior APT was associated with high mortality in patients with ICH that might be attributed primarily to its strong effect on early time.
Keywords: Antiplatelet Therapy, Intracerebral Hemorrhage, Meta-analysis, Outcome
INTRODUCTION
Intracerebral hemorrhage (ICH) is the second most common type of stroke, with 24.6/100,000 person/year incidences; a 40.1% per month risk of mortality has been noted.[1,2] Prognosis of ICH was associated with different factors including ictus to emergency department arrival time, prior antiplatelet therapy (APT), age, and Glasgow Coma Scale (GCS).[3,4,5] APT was prevalently being used in the primary and secondary prevention of vascular disease, and more than 25% of the patients with spontaneous ICH were undergoing APT.[6] Although aspirin therapy was confirmed to be beneficial in preventing myocardial infarction and ischemic stroke, it also increased the incidence of ICH.[7] Moreover, the influence of prior APT on the prognosis of patients with ICH remains controversial. According to previous studies, prior APT predicted high fatality rate and poor independent functions.[8,9,10,11,12] In contrast, other studies found that prior APT was not associated with the prognosis of ICH.[6,13,14,15,16,17] Although a previous meta-analysis revealed that prior APT was associated with increased mortality, it did not explore the relationship between mortality and time of onset of symptoms.[18] However, it must be emphasized that this relationship is crucial to the protocol of platelet transfusion for reversing the influence of APT on platelet activity that might be related to the prognosis of ICH. A prospective cohort study suggested that the final effect of antithrombotics on 3-month mortality in ICH patients was primarily attributed to its strong effect on early-stage mortality.[8] Furthermore, another prospective cohort study confirmed that platelet transfusion within 6 or 12 h of symptoms onset was associated with smaller hemorrhage size and improved odds of independence at 3 months as compared to that after 12 h for prior APT patients with ICH.[19]
The present meta-analysis explored the hypothesis that prior APT was associated with high mortality in patients with ICH and that the phenomenon was mainly attributed to its strong effect on early mortality necessitating further investigation.
METHODS
Search strategy
This work has been performed according to PRISMA guidelines (http://www.prisma-statement.org). PubMed and Embase were searched using the following search terms: (outcome or dependence or morbidity or death or survival or mortality) AND (clopidogrel or aspirin or antiplatelet or antithrombotic or antithrombosis or “acetylsalicylic acid”) AND (“cerebral hemorrhage” or “intracerebral hemorrhage” or “intracranial hemorrhage” or “hemorrhagic stroke”). The search was limited from the inception up to March 4, 2017, and of English language. We also acquired relevant articles from the reference lists of original papers.
Inclusion and exclusion criteria
This meta-analysis included the studies that fulfilled the following inclusion criteria: (1) Cohort studies concerning the prognosis of primary ICH, (2) ICH was confirmed by computed tomography or magnetic resonance imaging, (3) Prior APT is one of the influencing factors for analysis, and (4) The adjusted or unadjusted odds ratio (OR) with respect to mortality between ICH patients with and without prior APT could be acquired directly or by calculation.
The exclusion criteria were as follows: (1) The studies included subarachnoid hemorrhage or patients with secondary ICH due to trauma, tumor, ruptured aneurysm, or arteriovenous malformations, (2) The APT or non-APT patients who also underwent prior anticoagulation therapy (ACT) and could not be differentiated although complete information was obtained and utilized from the articles, and (3) Repeated studies and articles without original data.
Two of the authors scanned and selected the studies independently, and any controversies were resolved by discussion.
Quality assessment
Newcastle–Ottawa quality assessment scale was used to assess the quality of the study.[20] According to the protocol, a study was evaluated on three parts of selection, comparability, and outcome, containing eight items, and the overall scores ranging from 0 to 9. For comparability, the study acquired 1 point whether it was adjusted for age and previous morbidity or P > 0.05 for age and previous morbidity among APT and non-APT groups; reportedly, the age and previous morbidity were important confounding factors of ICH outcome. Another point was given when one study was adjusted for some other confounding factors. The high-quality study had an overall score ≥5.
Data extraction
The baseline data with respect to the name of the study (first author, year of publication), study type (prospective or retrospective), participants’ age (group APT, group non-APT), time after symptom onset upon admission, and adjusted factors in logistic regression including APT were extracted directly from the articles. The sample size and percentage of prior APT were obtained by calculation after the patients with prior ACT or ACT + APT were excluded. The data on when the mortality was reported at different time points from the earliest to that at 3 months were all extracted to compare the mortality at an early time, 3 months, and the period from early time to 3 months. Two of the authors extracted the corresponding data independently, and any controversies were resolved by discussion.
Statistical analysis
We pooled the adjusted OR or unadjusted OR with 95% confidence interval (95% CI) as the effect size of this meta-analysis. The data of mortality were extracted at two or more different time points. When the OR of all the studies was pooled, we selected the time point of 3 months initially, or else, the latest time point was selected. Since the latest time is proximal to 3 months, it can decrease the heterogeneity due to the different times of assessment. The heterogeneity was evaluated using I2 and P value based on Chi-square test. I2 ≤ 50% or P ≥ 0.1 did not demonstrate a significant heterogeneity. A fixed-effects model (Mantel–Haenszel method) was used. I2 > 50% or P < 0.1 indicated a significant heterogeneity, and therefore, a random-effects model (DerSimonian and Laird method) was applied. Moreover, the sensitivity analysis was utilized to identify the sources of heterogeneity and evaluate the stability of this meta-analysis. A funnel plot was drawn to assess the publication bias. Then, the Egger's method judged the publication bias when P < 0.05. STATA statistical version 12.0 (Stata Corporation, College Station, Texas, USA) was used for the data analyses.
RESULTS
Study selection and characteristics
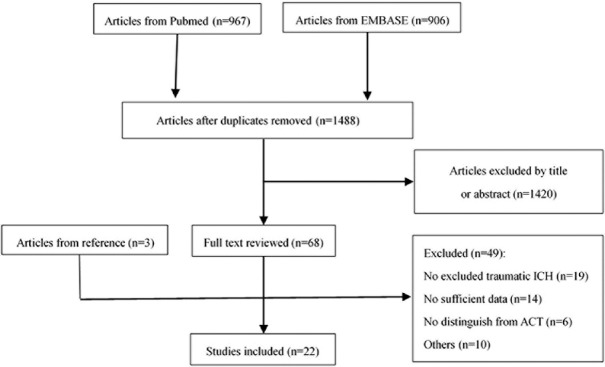
As shown in Figure 1, a total of 1488 articles were identified from PubMed and Embase after removal of duplicate studies. Only 68 articles were retained after screening the title and abstract, and another three abstracted articles were obtained from the corresponding studies’ references. Finally, 22 studies were included for this meta-analysis after scanning the full text and excluding 49 studies among the 71 articles. The characteristics and score for quality assessment of these 22 studies were shown in Table 1. Notably, the meta-analysis by Thompson et al.[18] was not included in these 22 articles, and it encompassed the appropriate unpublished data that were used for discussion.
Figure 1.
Schematic representation of literature search for this meta-analysis. ICH: Intracerebral hemorrhage; ACT: Anticoagulation therapy.
Table 1.
Characteristics and quality assessment of the included studies
| Study | Year | Type | Sample size | Mean age (APT group), years | Mean age (non-APT group), years | Prior APT (%) | Time of assessment | Time of symptom onset upon admission | Adjusted factors | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Camps-Renom et al.[13] | 2017 | Prospective | 223 | 77.3 | 70.1 | 33.2 | 3 months | 24 h | Age; NIHSS, Systolic blood pressure and HV at admission | 9 |
| Roquer et al.[8] | 2017 | Prospective | 440 | 80.0* | 74.0* | 33.4 | 24 h 3 months | NA | Age; previous mRS score | 8 |
| Stein et al.[6] | 2016 | Retrospective | 6555 | 77.2 | 70.1 | 30.9 | In hospital | NA | Age; initial GCS; the presence of IVH; pre-ICH disability | 9 |
| Yang et al.[21] | 2014 | Retrospective | 333 | 65.4 | 57.5 | 20.4 | In hospital | 48 h | NA | 7 |
| Mansouri et al.[9] | 2013 | Prospective | 120 | – | – | 38.3 | 30 days 90 days | 48 h | Age; sex; BMI | 7 |
| Chen et al.[22] | 2013 | Retrospective | 1927 | 68.4 | 61.5 | 12.0 | 1 month 3 months | NA | Age; initial GCS; HV; IVH; infratentorial ICH; hypertension; advanced CKD | 7 |
| Balci et al.[23] | 2012 | Prospective | 337 | 70.1 | 67.2 | 14.2 | In hospital | 12 h | NA | 7 |
| Romero López et al.[24] | 2012 | Retrospective | 146 | 79.0 | 72.0 | 21.9 | In hospital | 12 h | NA | 7 |
| Kuramatsu et al.[25] | 2012 | Retrospective | 210 | 72.2 | 67.9 | 39.5 | In hospital 3 months | NA | Age; ICH score; HCE | 7 |
| Moussouttas et al.[26] | 2010 | Retrospective | 70 | 72.0 | 64.0 | 24.3 | In hospital | 6 h | NA | 6 |
| Stead et al.[27] | 2010 | Retrospective | 178 | 76.0* | 66.0* | 44.9 | 7 days 30 days | NA | NA | 7 |
| Sansing et al.[28] | 2009 | Prospective | 282 | 71* | 63* | 24.8 | 90 days | 6 h | Age; initial ICH volume; initial GCS; presence of IVH; infratentorial ICH | 7 |
| Toyoda et al.[10] | 2009 | Retrospective | 918 | 71.0 | 65.0 | 19.6 | 3 weeks | 24 h | Age; sex; confounders that showed P≤0.1 on univariate analysis | 9 |
| Creutzfeldt et al.[11] | 2009 | Retrospective | 315 | 70.0 | 62.0 | 21.6 | In hospital | NA | Age; initial GCS; initial heart rate; and so on | 6 |
| Hanger et al.[14] | 2008 | Retrospective | 223 | 75.7 | 69.9 | 40.8 | 7 days 14 days 28 days | NA | Diabetes; Intraventricular spread; HV; hemorrhage location | 7 |
| Karlikaya et al.[29] | 2006 | Retrospective | 664 | 67.1 | 65.8 | 6.0 | 3 weeks | NA | NA | 7 |
| Saloheimo et al.[30] | 2006 | Retrospective | 182 | 71.6 | 65.6 | 24.2 | 4 days 3 months | NA | Age; sex; ICH score on admission; diabetes | 7 |
| Foerch et al.[15] | 2006 | Prospective | 1483 | 75.0 | 70.0 | 29.7 | In hospital | NA | Age; prehospital mRS | 8 |
| Roquer et al.[12] | 2005 | Prospective | 194 | – | – | 24.2 | 30 days | NA | Age; glucose; HV; ventricular extension; GSS | 7 |
| Rosand et al.[16] | 2004 | Prospective | 311 | – | – | 37.3 | 3 months | NA | Age; sex; coronary artery disease; diabetes mellitus; lobar ICH; warfarin use | 6 |
| Nilsson et al.[31] | 2002 | Prospective | 338 | – | – | 21.9 | 30 days | NA | NA | 7 |
| Wong[17] | 1999 | Prospective | 783 | – | – | 4.3 | In hospital | NA | Age; sex; hypertension; diabetes; smoking habit; atrial fibrillation; ischemic heart disease; previous cerebrovascular disease; valvular heart disease | 7 |
*These studies reported median age. NA: Not available; NIHSS: National Institute of Health Stroke Scale; HV: Hemorrhage volume; mRS: Modified Rankin Scale; GCS: Glasgow Coma Scale; GSS: Glasgow Scale Score; IVH: Intraventricular hemorrhage; BMI: Body mass index; CKD: Chronic kidney disease; HCE: Hypercholesterolemia; APT: Antiplatelet therapy; NOS: Newcastle–Ottawa quality assessment scale; ICH: Intracerebral hemorrhage.
Pooled odds ratio for mortality of all studies
The overall 22 studies contained 16,232 patients, and adjusted OR could be acquired from 11 studies concerning 11,565 patients. As shown in Figure 2a, the pooled unadjusted OR of these 22 studies, using the random-effects model, was 1.37 (95% CI: 1.13–1.66, P = 0.001). However, a significant degree of heterogeneity was detected (I2 = 69.1%, P < 0.0001). The pooled adjusted OR of these 11 studies could be observed in Figure 2b; OR = 1.41 (95% CI: 1.05–1.90, P = 0.024); however, a significant degree of heterogeneity was observed (I2 = 69.3%, P < 0.0001).
Figure 2.
(a) Forest plot showing unadjusted OR for mortality. (b) Forest plot showing adjusted OR for mortality. (c) Forest plot showing unadjusted OR for mortality based on the time of symptom onset. (d) Forest plot showing unadjusted OR for mortality of a duration from the first assessment until 30 or 90 days. OR: Odds ratio.
To explore the source of heterogeneity, we conducted a subgroup analysis according to the study type (prospective or retrospective). Among the studies of unadjusted OR, the pooled OR was 1.42 (95% CI: 1.05–1.93, P = 0.023; I2 = 68.1%, P < 0.0001) for prospective studies and 1.32 (95% CI: 1.02–1.72, P = 0.034; I2 = 67.2%, P < 0.0001) for retrospective studies. Among the studies of adjusted OR, the pooled OR was 1.33 (95% CI: 0.95–1.86, P = 0.092; I2 = 45.2%, P = 0.081) for prospective studies and 1.57 (95% CI: 0.81–3.07, P = 0.182; I2 = 84.0%, P < 0.0001) for retrospective studies. No significant improvement on heterogeneity was observed. Therefore, a sensitivity analysis was conducted to explore the source of heterogeneity; the study by Stein et al.[6] exhibited a significant impact on heterogeneity and the value of pooled effect both in unadjusted or adjusted OR. A rather unfavorable result for APT patients was noted with pooled unadjusted OR = 1.40 (95% CI: 1.14–1.73; I2 = 64.0%) and pooled adjusted OR = 1.53 (95% CI: 1.13–2.08; I2 = 52.6%) when the study by Stein et al.[6] was excluded. However, the pooled adjusted OR was 1.28 (95% CI: 0.97–1.68; I2 = 58.4%), 1.35 (95% CI: 1.00–1.82; I2 = 68.8%), and 1.34 (95% CI: 1.00–1.81; I2 = 68.6%), when the studies by Toyoda et al.,[10] Creutzfeldt et al.,[11] or Roquer et al.[12] were excluded, respectively.
Subgroup analysis according to time
Reportedly, the difference of 3-month mortality between APT and non-APT patients was mainly due to the high death rate in APT patients in the first 24 h after ICH; a significant difference from 24 h to 3 months was not observed between both groups of patients.[8] Therefore, we performed a series of subgroup analysis based on the time of assessment [Table 2]. Another subgroup analysis based on the time of onset of symptoms upon admission [Figure 2c] revealed a pooled OR within 6 h, 12 h, 24 h, and 48 h that was 0.81 (95% CI: 0.42–1.59), 1.29 (95% CI: 0.69–2.40), 1.80 (95% CI: 1.31–2.48), and 3.62 (95% CI: 2.16–6.06), respectively. Seven studies evaluated the mortality at more than 1 time point, and thus, we calculated the OR of mortality for a duration from the first assessment until 30 or 90 days, followed by a corresponding subgroup analysis. Stead et al.[27] was from 7 days to 30 days, Hanger et al.[14] from 7 days to 28 days. Studies by Roquer et al.,[8] Mansouri et al.,[9] Chen et al.,[22] Kuramatsu et al.[25] and Saloheimo et al.[30] were separated from 1 day, 30 days, 30 days, discharge, or 4–90 days, respectively. As shown in Figure 2d, the pooled OR for mortality from early time point to 30 and 90 days was 2.40 (95% CI: 0.96–6.00) and 1.74 (95% CI: 1.26–2.39), respectively.
Table 2.
Subgroup analysis for mortality according to the time of assessment
| Subgroup | n* | Pooled OR (95% CI) | I2 (%) | Subgroup (including data from Thompson et al.) | n* | Pooled OR (95% CI) | I2 (%) |
|---|---|---|---|---|---|---|---|
| All studies | 11 | 1.41 (1.05–1.90) | 69.3 | All studies | 28 | 1.19 (1.08–1.31) | 48.3 |
| 1–7 days | 2 | 1.99 (1.20–3.31) | 36.5 | 1–7 days | 2 | 1.99 (1.20–3.31) | 36.5 |
| 21–30 days | 3 | 2.25 (1.48–3.41) | 29.5 | 21–30 days | 7 | 1.86 (1.44–2.41) | 28.2 |
| 90 days | 4 | 1.42 (1.05–1.92) | 17.4 | 90 days | 13 | 1.33 (1.14–1.56) | 2.1 |
| Discharge | 4 | 1.05 (0.73–1.51) | 63.9 | Discharge | 8 | 0.98 (0.86–1.12) | 39.4 |
*Number of including studies. OR: Odds ratio; CI: Confidence interval.
Meta-regression for time of assessment
To explore the hypothesis that the difference in 3-month mortality between APT and non-APT patients was mainly due to the high death rate in APT patients early after ICH, we conducted a meta-regression based on the time of assessment. The time point of discharge was excluded due to its variability. As shown in Figure 3a, 7 studies containing 10 time points of assessment for adjusted OR were included, and the meta-regression showed that for each day increase in the time of evaluation, the adjusted OR for the mortality of APT patients decreased by 0.0048 as compared to the non-APT patients (95% CI: −0.0024–0.0119; P = 0.165). In addition, Figure 3b indicated that for each day increase in the time of assessment, the adjusted OR for the mortality of APT patients decreased by 0.0049 as compared to the non-APT patients (95% CI: 0.0006–0.0091; P = 0.026), when the unpublished data from Thompson et al.,[18] containing 13 additional time points, were included.
Figure 3.
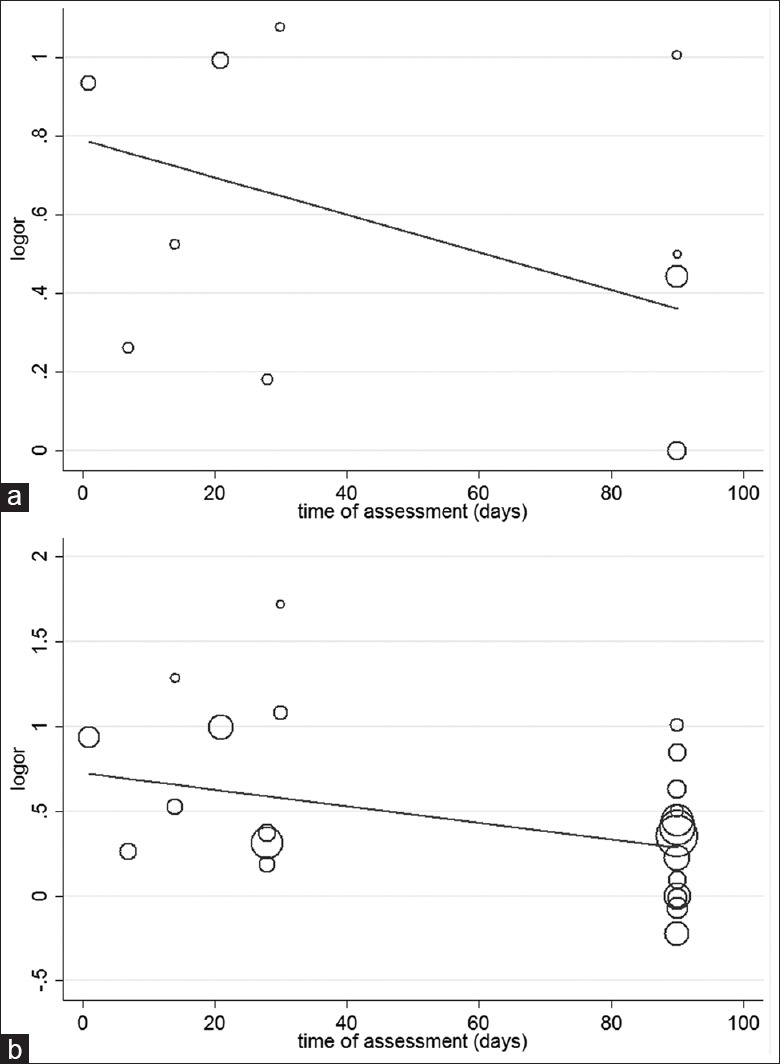
(a) Meta-regression for the time of assessment; there were 7 studies containing 10 time points. (b) Meta-regression for the time of assessment. Overall, 23 time points were present when studies by Thompson et al. were included.
Publication bias
The funnel plots for the overall 22 studies were found to be approximately symmetrical [Figure 4]. Furthermore, the Egger's test showed P = 0.13, which was consistent with that from the funnel plots.
Figure 4.
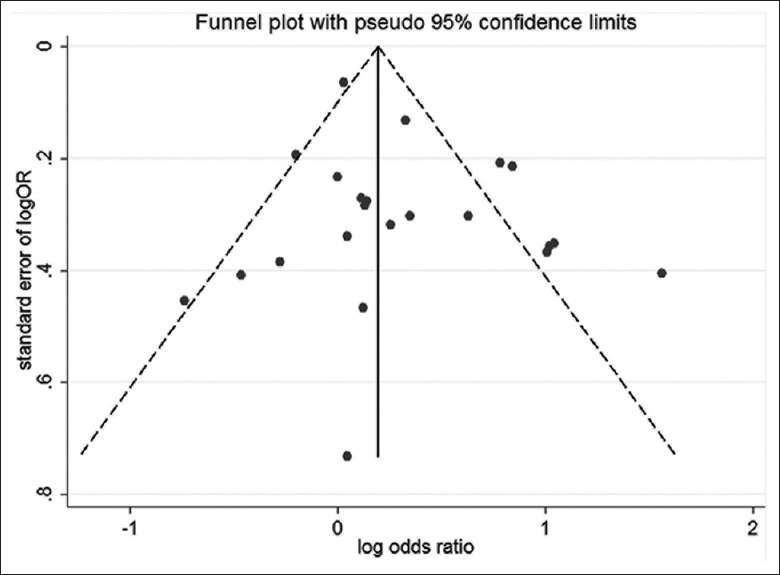
Funnel plots illustrating the publication bias.
DISCUSSION
The present meta-analysis was conducted to explore the association between mortality and prior-ICH APT. Although a similar meta-analysis has been performed previously, it did not focus on the association between mortality and time of symptom onset. However, Lovelock and Rothwell speculated that prior-ICH APT might cause a large early hematoma expansion, thereby leading to a high early mortality.[32] Moreover, another prospective cohort study found that the effect of antithrombotic pretreatment on mortality from 24 h to 3 months was insignificant, suggesting that the final effect of antithrombotic pretreatment on 3-month mortality remained significant primarily due to its strong effect on very early mortality.[8] In addition, recent clinical studies have been published on this topic. Thus, we decided to perform this meta-analysis focusing on the association between mortality and time of symptom onset.
We selected mortality as the primary outcome, and pooled both unadjusted OR and adjusted OR. Both these effect sizes showed a significant increase in patients with prior-ICH APT as compared to patients without APT, thereby suggesting that prior APT played an unfavorable role in the prognosis of ICH. Herein, morbidity was susceptible to the researchers’ subjectivity and various assessment criteria; on the other hand, since sufficient data were lacking, we did not conduct an analysis of morbidity. As shown in Table 2, similar results were acquired when corresponding data from Thompson et al.[18] were included.
Significant heterogeneity for the mortality existed in both the pooled unadjusted OR and adjusted OR. A significant improvement was not observed in the subgroup analysis according to the study type (prospective or retrospective). In addition to prior APT, the prognosis of ICH was found to be associated with different factors including ictus to emergency department arrival time, age, and GCS, all of which could be the confounding factors and the resource of heterogeneity for the unadjusted OR.[3,4,5] The heterogeneity of adjusted OR could be ascribed to the different adjusted factors in the multivariate analysis of each study.
Moreover, drug sensitivity, combination APT, and the duration or dosage of APT could influence the outcome of ICH. Different patients exhibit differential drug sensitivities. In a prospective study concerning the prognosis of ICH patients, the activities of daily living (ADL) score of APT-resistant and APT-semi-responsive patients did not vary significantly from non-APT patients; however, the APT-sensitive patients had a poor ADL score.[33] Another prospective study demonstrated that the reduced platelet activity was associated with poor modified Rankin scores at 3 months, which was primarily driven by mortality, although some patients not known to administer the antiplatelet medication exhibited a reduced platelet activity.[34] With respect to combination or single APT, a study found that mortality did not differ between patients pretreated with aspirin and clopidogrel;[8] another study found that combination APT was associated with a poor functional outcome.[18] The duration of APT may also influence the outcome of ICH patients. In the study by de Gea-García et al. and Baharoglu et al., the enrolled patients underwent APT for at least 7 days; however, the influence of duration of APT was not explored further.[35,36] Moreover, other studies did not mention the duration of APT, which might be a part of the resource of heterogeneity.
The resource of heterogeneity was not observed after a sensitivity analysis; however, no statistical significance was noted for pooled adjusted OR when excluding any one of the studies by Toyoda et al.,[10] Creutzfeldt et al.,[11] or Roquer et al.[12] This phenomenon indicated the instability of this meta-analysis.
Another subgroup analysis according to the time of assessment [Table 2] showed a significant improvement in the heterogeneity of adjusted OR except the subgroup according to the time of discharge, which was a variable time point. This result indicated that different time periods were a major source of heterogeneity, thereby directing the investigation on the relationship between mortality and time of symptom onset. If the hypothesis that the effect of prior APT on mortality from early stage to 3 months was insignificant, and the final effect of APT on the 3-month mortality remained significant due to its strong effect on very early mortality was true, then the earlier the time of symptom onset was applied upon admission, the greater the pooled OR. Therefore, a subgroup analysis was conducted according to the time of symptom onset upon admission [Figure 2c], which was not in agreement with our hypothesis. As shown in Figure 2d, the pooled estimate of the APT effect from early time to 30 days was insignificant; however, it was remarkable from early time to 90 days. Given that these two subgroup analyses were based on the unadjusted estimates of the APT effect and only a few studies were included, we conducted a meta-regression to explore the hypothesis. This approach could use the corresponding included data completely; we found that the time of assessment was associated with the adjusted OR for mortality when the study by Thompson et al. was included [Figure 3b]. The platelet life was 7–10 days, with approximately a 10% rate of update daily. Thus, the platelet activity reaches >50% of the initial level and exerts its normal physiological function after ceasing the intake of aspirin for 5 days.[37] Prior APT patients presented with a poor outcome might be related to its effect on inducing hematoma growth,[13] which was related to the platelet activity. The hypothesis that the final effect of antiplatelet on 3-month mortality remained significant mainly due to its strong effect on very early mortality can be explained theoretically as described above.
Nonetheless, clinicians and patients are required to focus on the mortality as well as morbidity since the desired quality of life is important for patients.[38] However, we did not perform a meta-analysis for morbidity due to insufficiency of new data. The language was restricted to only English while performing the literature research. Thus, the publication bias was inevitable, although it was not detected by statistical methods. A significant heterogeneity was observed for mortality, and the sensitivity analysis indicated that this meta-analysis model was unstable. Considering other inevitable factors, a conclusion from the present study should be drawn with caution.
In conclusion, prior APT was associated with high mortality in patients with ICH, which might be attributed mainly to its strong effect on early time. Therefore, APT should be used under stringent indications. Nevertheless, different transfusion time points should be designed while conducting a trial for investigating the platelet transfusion to improve the prognosis of ICH patients with prior APT.
Financial support and sponsorship
This research was supported by grants from the National Natural Science Foundation of China (No. 81171089; 81471201).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–69. doi: 10.1016/S1474-4422(09)70025-0. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76. doi: 10.1016/S1474-4422(09)70340-0. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 3.James ML, Cox M, Xian Y, Smith EE, Bhatt DL, Schulte PJ, et al. Sex and age interactions and differences in outcomes after intracerebral hemorrhage. J Womens Health (Larchmt) 2017;26:380–8. doi: 10.1089/jwh.2016.5849. doi: 10.1089/jwh.2016.5849. [DOI] [PubMed] [Google Scholar]
- 4.Fan JS, Huang HH, Chen YC, Yen DH, Kao WF, Huang MS, et al. Emergency department neurologic deterioration in patients with spontaneous intracerebral hemorrhage: Incidence, predictors, and prognostic significance. Acad Emerg Med. 2012;19:133–8. doi: 10.1111/j.1553-2712.2011.01285.x. doi: 10.1111/j.1553-2712.2011.01285.x. [DOI] [PubMed] [Google Scholar]
- 5.Rost NS, Smith EE, Chang Y, Snider RW, Chanderraj R, Schwab K, et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke. 2008;39:2304–9. doi: 10.1161/STROKEAHA.107.512202. doi: 10.1161/STROKEAHA.107.512202. [DOI] [PubMed] [Google Scholar]
- 6.Stein M, Misselwitz B, Hamann GF, Kolodziej M, Reinges MH, Uhl E, et al. In-hospital mortality after pre-treatment with antiplatelet agents or oral anticoagulants and hematoma evacuation of intracerebral hematomas. J Clin Neurosci. 2016;26:42–5. doi: 10.1016/j.jocn.2015.05.069. doi: 10.1016/j.jocn.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 7.He J, Whelton PK, Vu B, Klag MJ. Aspirin and risk of hemorrhagic stroke: A meta-analysis of randomized controlled trials. JAMA. 1998;280:1930–5. doi: 10.1001/jama.280.22.1930. doi: 10.1001/jama.280.22.1930. [DOI] [PubMed] [Google Scholar]
- 8.Roquer J, Vivanco Hidalgo RM, Ois A, Rodríguez Campello A, Cuadrado Godia E, Giralt Steinhauer E, et al. Antithrombotic pretreatment increases very-early mortality in primary intracerebral hemorrhage. Neurology. 2017;88:885–91. doi: 10.1212/WNL.0000000000003659. doi: 10.1212/WNL.0000000000003659. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri B, Heidari K, Asadollahi S, Nazari M, Assarzadegan F, Amini A, et al. Mortality and functional disability after spontaneous intracranial hemorrhage: The predictive impact of overall admission factors. Neurol Sci. 2013;34:1933–9. doi: 10.1007/s10072-013-1410-0. doi: 10.1007/s10072-013-1410-0. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda K, Yasaka M, Nagata K, Nagao T, Gotoh J, Sakamoto T, et al. Antithrombotic therapy influences location, enlargement, and mortality from intracerebral hemorrhage. The Bleeding with Antithrombotic Therapy (BAT) Retrospective Study. Cerebrovasc Dis. 2009;27:151–9. doi: 10.1159/000177924. doi: 10.1159/000177924. [DOI] [PubMed] [Google Scholar]
- 11.Creutzfeldt CJ, Weinstein JR, Longstreth WT, Jr, Becker KJ, McPharlin TO, Tirschwell DL, et al. Prior antiplatelet therapy, platelet infusion therapy, and outcome after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2009;18:221–8. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.007. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roquer J, Rodríguez Campello A, Gomis M, Ois A, Puente V, Munteis E, et al. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol. 2005;252:412–6. doi: 10.1007/s00415-005-0659-5. doi: 10.1007/s00415-005-0659-5. [DOI] [PubMed] [Google Scholar]
- 13.Camps-Renom P, Alejaldre-Monforte A, Delgado-Mederos R, Martínez-Domeño A, Prats-Sánchez L, Pascual-Goñi E, et al. Does prior antiplatelet therapy influence hematoma volume and hematoma growth following intracerebral hemorrhage? Results from a prospective study and a meta-analysis. Eur J Neurol. 2017;24:302–8. doi: 10.1111/ene.13193. doi: 10.1111/ene.13193. [DOI] [PubMed] [Google Scholar]
- 14.Hanger HC, Fletcher VJ, Wilkinson TJ, Brown AJ, Frampton CM, Sainsbury R, et al. Effect of aspirin and warfarin on early survival after intracerebral haemorrhage. J Neurol. 2008;255:347–52. doi: 10.1007/s00415-008-0650-z. doi: 10.1007/s00415-008-0650-z. [DOI] [PubMed] [Google Scholar]
- 15.Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke. 2006;37:2165–7. doi: 10.1161/01.STR.0000231842.32153.74. doi: 10.1161/01.STR.0000231842.32153.74. [DOI] [PubMed] [Google Scholar]
- 16.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–4. doi: 10.1001/archinte.164.8.880. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 17.Wong KS. Risk factors for early death in acute ischemic stroke and intracerebral hemorrhage: A prospective hospital-based study in Asia. Asian Acute Stroke Advisory Panel. Stroke. 1999;30:2326–30. doi: 10.1161/01.str.30.11.2326. doi.org/10.1161/01.STR.30.11.2326. [DOI] [PubMed] [Google Scholar]
- 18.Thompson BB, Béjot Y, Caso V, Castillo J, Christensen H, Flaherty ML, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: A systematic review. Neurology. 2010;75:1333–42. doi: 10.1212/WNL.0b013e3181f735e5. doi: 10.1212/WNL.0b013e3181f735e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidech AM, Liebling SM, Rosenberg NF, Lindholm PF, Bernstein RA, Batjer HH, et al. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocrit Care. 2012;16:82–7. doi: 10.1007/s12028-011-9619-3. doi: 10.1007/s12028-011-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Yang NR, Kim SJ, Seo EK. Spontaneous intracerebral hemorrhage with antiplatelets/anticoagulants/none: A comparison analysis. Acta Neurochir (Wien) 2014;156:1319–25. doi: 10.1007/s00701-014-2080-2. doi: 10.1007/s00701-014-2080-2. [DOI] [PubMed] [Google Scholar]
- 22.Chen YW, Tang SC, Tsai LK, Yeh SJ, Chiou HY, Yip PK, et al. Pre-ICH warfarin use, not antiplatelets, increased case fatality in spontaneous ICH patients. Eur J Neurol. 2013;20:1128–34. doi: 10.1111/j.1468-1331.2012.03847.x. doi: 10.1111/j.1468-1331.2012.03847.x. [DOI] [PubMed] [Google Scholar]
- 23.Balci K, Utku U, Asil T, Celik Y, Tekinaslan I, Ir N, et al. The effect of admission blood pressure on the prognosis of patients with intracerebral hemorrhage that occurred during treatment with aspirin, warfarin, or no drugs. Clin Exp Hypertens. 2012;34:118–24. doi: 10.3109/10641963.2011.601380. doi: 10.3109/10641963.2011.601380. [DOI] [PubMed] [Google Scholar]
- 24.Romero López J, Maciñeiras Montero JL, Fontanillo Fontanillo M, Escriche Jaime D, Moreno Carretero MJ, Corredera García E, et al. Lobar intracerebral haemorrhage: Analysis of a series and characteristics of patients receiving antiplatelet or anticoagulation treatment. Neurologia. 2012;27:387–93. doi: 10.1016/j.nrl.2011.07.011. doi: 10.1016/j.nrl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Kuramatsu JB, Mauer C, Kiphuth IC, Lücking H, Kloska SP, Köhrmann M, et al. Reported antiplatelet use influences long-term outcome independently in deep intracerebral hemorrhage. Neurosurgery. 2012;70:342–50. doi: 10.1227/NEU.0b013e3182311266. doi: 10.1227/NEU.0b013e3182311266. [DOI] [PubMed] [Google Scholar]
- 26.Moussouttas M, Malhotra R, Fernandez L, Maltenfort M, Holowecki M, Delgado J, et al. Role of antiplatelet agents in hematoma expansion during the acute period of intracerebral hemorrhage. Neurocrit Care. 2010;12:24–9. doi: 10.1007/s12028-009-9290-0. doi: 10.1007/s12028-009-9290-0. [DOI] [PubMed] [Google Scholar]
- 27.Stead LG, Jain A, Bellolio MF, Odufuye AO, Dhillon RK, Manivannan V, et al. Effect of anticoagulant and antiplatelet therapy in patients with spontaneous intra-cerebral hemorrhage: Does medication use predict worse outcome? Clin Neurol Neurosurg. 2010;112:275–81. doi: 10.1016/j.clineuro.2009.12.002. doi: 10.1016/j.clineuro.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Sansing LH, Messe SR, Cucchiara BL, Cohen SN, Lyden PD, Kasner SE, et al. Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology. 2009;72:1397–402. doi: 10.1212/01.wnl.0000342709.31341.88. doi: 10.1212/01.wnl.0000342709.31341.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karlikaya G, Varlbas F, Demirkaya M, Orken C, Tireli H. Does prior aspirin use reduce stroke mortality? Neurologist. 2006;12:263–7. doi: 10.1097/01.nrl.0000219637.83981.3c. doi: 10.1097/01.nrl.0000219637.83981.3c. [DOI] [PubMed] [Google Scholar]
- 30.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen ER, Hillbom M, et al. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006;37:129–33. doi: 10.1161/01.STR.0000196991.03618.31. doi: 10.1161/01.STR.0000196991.03618.31. [DOI] [PubMed] [Google Scholar]
- 31.Nilsson OG, Lindgren A, Brandt L, Säveland H. Prediction of death in patients with primary intracerebral hemorrhage: A prospective study of a defined population. J Neurosurg. 2002;97:531–6. doi: 10.3171/jns.2002.97.3.0531. doi: 10.3171/jns.2002.97.3.0531. [DOI] [PubMed] [Google Scholar]
- 32.Lovelock CE, Rothwell PM. Does antiplatelet therapy at the time of intracerebral hemorrhage bode poor outcome? Neurology. 2010;75:1314–5. doi: 10.1212/WNL.0b013e3181f73707. doi: 10.1212/WNL.0b013e3181f73707. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Sun Z, Zhao W, Zhang J, Chen J, Li Y, et al. Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. J Neurosurg. 2013;118:94–103. doi: 10.3171/2012.9.JNS112286. doi: 10.3171/2012.9.JNS112286. [DOI] [PubMed] [Google Scholar]
- 34.Naidech AM, Jovanovic B, Liebling S, Garg RK, Bassin SL, Bendok BR, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–401. doi: 10.1161/STROKEAHA.109.550939. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 35.de Gea-García JH, Fernández-Vivas M, Núñez-Ruiz R, Rubio-Alonso M, Villegas I, Martínez-Fresneda M, et al. Antiplatelet therapies are associated with hematoma enlargement and increased mortality in intracranial hemorrhage. Med Intensiva. 2012;36:548–55. doi: 10.1016/j.medin.2012.01.004. doi: 10.1016/j.medin.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet. 2016;387:2605–13. doi: 10.1016/S0140-6736(16)30392-0. doi: 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 37.FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ, 2nd, Lawson JA, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest. 1983;71:676–88. doi: 10.1172/JCI110814. doi: 10.1172/JCI110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steer CB, Marx GM, Galani E, Harper PG, Khayat D. Quality of life: It's never too late. J Clin Oncol. 2002;20:2915–7. doi: 10.1200/JCO.2002.20.13.2915. doi: 10.1200/JCO.2002.20.13.2915. [DOI] [PubMed] [Google Scholar]