Abstract
Cataract surgery has undergone many changes with the size of incision progressively decreasing over time with an incision of 12.0 mm for intracapsular cataract extraction to 2.2–2.8 mm in phacoemulsification. However, phacoemulsification due to high cost and equipment maintenance cannot be employed widely in developing countries. Manual small-incision cataract surgery (MSICS) offers similar advantages with the merits of wider applicability, less time consuming, a shorter learning curve, and lower cost. MSICS can be performed in high-volume setups due to fast technique. Here, we review the various techniques, safety and efficacy of MSICS, and its progress and utility in developing and underdeveloped countries.
Keywords: Astigmatism, cataract, manual small-incision cataract surgery
Quality of vision and early rehabilitation are two of the clinical parameters that determine the success of cataract surgery. Advances in surgical techniques, instrumentation, and pharmacologic agents have contributed to a revolution in this field, making cataract surgery almost risk free. The most exciting innovation in cataract surgery in the 20th century is the technique of phacoemulsification, introduced by Kelman in 1967.[1] This heralded the beginning of a revolution in the field of cataract surgery known as “small-incision cataract surgery” (SICS) where machine refinements and state-of-the-art technology have taken phacoemulsification to new heights. Other innovative minds to the likes of Fry,[2] Kansas and Sax,[3] Rozakis,[4] Blumenthal et al., and Anis have achieved similar results without the attendant technology[5,6] in the form of manual SICS (MSICS).
The size of incision of a cataract surgery has progressively decreased over time with an incision of 12.0 mm for intracapsular cataract extraction and 10.0 mm for extracapsular cataract extraction (ECCE) to MSICS of 6–7 mm and for phacoemulsification approximately 2.2–2.8 mm. A smaller incision gives distinct advantages to the patient and surgeon, both in the form of early rehabilitation, better intraocular pressure control, and low or negligible postoperative astigmatism and complications. Basic technique of MSICS has taken a sutureless and self-sealing incision into consideration.
Here we review the safety and efficacy of MSICS and its progress and utility in developing and underdeveloped countries.
Modifications of Manual Small-Incision Cataract Surgery Technique
Since the development of MSICS, there has been many changes and modifications in the technique of MSICS. Some of the important and major modifications in MSICS cataract surgery are as follows:
Mininuc technique
In 1987, Blumenthal and Moissiev described the use of anterior chamber maintainer (ACM) in ECCE along with a reduction in incision size which keeps the eye in a normotensive state throughout the surgery.[7] A 6.5–7 mm superior straight scleral tunnel incision was made 2 mm posterior to limbus. Two side ports at 6 and 9 o’clock positions were created and a self-retaining anterior chamber (AC) maintainer was placed and continuous curvilinear capsulorhexis (CCC) was done. Hydrodissection, nucleus manipulation, aspiration of cortex, and dialing of intraocular lens (IOL) in the bag were performed after which the ACM was removed.
Ruit technique
In 1999, Ruit et al. described a new technique for MSICS.[8] A 6.5–7 mm temporal scleral tunnel with a straight incision was created 2 mm from the limbus. A side point incision was made and V-shaped capsulotomy (capsulorhexis) was done. After hydrodissection, nucleus was delivered by viscoexpression. The remaining cortex was aspirated and IOL was implanted in the capsular bag.
Malik's technique
Malik et al. modified the MSICS technique to prevent corneal endothelial cell loss.[9] Malik described a simple maneuver to protect this nonforgiving layer by continuous infusion of 2% hydroxymethyl cellulose through the AC maintainer by an assistant during nuclear delivery. This can be combined with any method of SICS. This procedure is helpful in preventing endothelial cell loss by learning surgeon also.
Double-nylon loop for manual small-incision cataract surgery
Kosakarn developed a technique of manual phacofragmentation called double-nylon loop.[10] By this technique, the lens is divided into three pieces so that the incision can be small, 4.0–5.0 mm, and sutureless, and it allows implantation of a foldable IOL. The double-nylon loop is made from 4-0 nylon inserted through a 20-gauge blunt tip needle and can be reused several times. This technique is less expensive and suitable for use in developing countries.
Steps of Manual Small-Incision Cataract Surgery
After putting a superior rectus bridle suture, a conjunctival flap is made for the proper exposure of sclera.
Incision site and configuration
A one-third to half-thickness scleral incision is made about 1–1.5 mm behind the limbus. The size of the incision varies depending on the hardness and size of the nucleus, usually it varies from 5.5 to 7.5 mm.
Site of incision
Superior
Temporal
Superotemporal
Superior (12 o’clock) incision
Colvard et al. in 1980 created the scleral pocket incision in 12 o’clock position by making a partial-thickness groove in the sclera about 2 mm behind the limbus and then made tunnel extending anteriorly.[11]
Temporal incision
The orientation of the microscope and the surgeon are shifted toward the temporal side of the eye to be operated. For temporal incision, the lamellar dissection into cornea should be more anterior to get a better valve effect of self-sealing incision. The temporal incision has advantages over superior incision such as lesser surgically induced astigmatism and better exposure in deep-set eyes. However, it leads to increased chances of postoperative endophthalmitis compared to superior incision due to lack of the protection by the superior lid, and direct exposure to surroundings.
Superotemporal
Some surgeons prefer superotemporal incision.
Configuration of incision
Straight incision
Chevron or inverted V-shaped incision
Frown incision
Blumenthal side cuts.
Straight incision
Colvard et al. in 1980 was the first surgeon to move the cataract incision to sclera.[11] Straight incision is a straight line incision about 2 mm away from limbus, the two extreme points of which are secured in the sclera, and the inferior edge directly adjacent to these end point cannot sag, thereby limiting the induced astigmatism. McFarland in 1989 was the one who introduced an incision architecture that is self-sealing.[12]
Chevron's inverted V-shaped incision
In 1990, Pallin described a chevron-shaped incision.[13] The incision is given in the form of inverted V, the apex of which is near the limbus and the limbs are away from it. It is difficult to make, but it induces least astigmatism.
Frown incision
In 1991, Singer introduced frown incision, where each end of the incision is further away from the limbus and produced less astigmatism but slightly more than Chevron's incision.[14] It was a modified pocket incision curved opposite to the limbus [Fig. 1].
Figure 1.
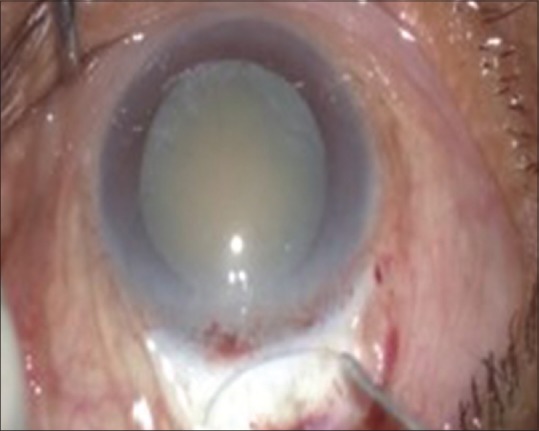
Frown-shaped incision
Blumenthal side cuts
Blumenthal et al. in 1993 devised larger pocket tunnel with minimally induced astigmatism called the Blumenthal side cuts.[15] The incision has a straight line and two oblique cuts at its two ends.
Sclerocorneal tunnel
After making an incision, a proper sclerocorneal tunnel is made with the help of crescent blade and then an entry is made into the AC [Figs. 2 and 3]. It is a triplanar self-sealing tunnel and does not require any suturing. The tunnel should be of one third to half of the width of the thickness of sclera extending 1–1.5 mm into the cornea, improper depth can lead to complications such as premature entry and button holing. Scleral pockets should be made for the smooth delivery of the nucleus. Anterior chamber is formed with viscoelastic substance and capsulotomy is done.
Figure 2.
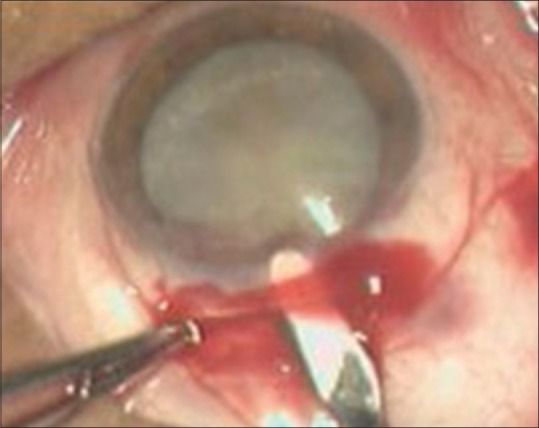
Tunnel formation
Figure 3.

Anterior chamber entry
Capsulotomy
One of the important steps of cataract surgery is to make an opening in the anterior capsule to remove cataractous lens. Trypan blue dye can be used to stain the anterior capsule for better visualization.
Three types of capsular openings are commonly used in MSICS as follows:
Continuous curvilinear capsulorhexis
Can-opener capsulotomy
Envelope (linear) capsulotomy.
Continuous curvilinear capsulorhexis (CCC)
In 1984, Gimbel and Neuhann simultaneously from different parts of the world developed a new technique of anterior capsulotomy. CCC is done using a 26-gauge cystitome or Utrata's forceps. A small medial opening is made in the anterior capsule and a small circular flap is raised. This flap is then directed clockwise or counterclockwise to make a complete circular rhexis and the final tear is outside to inside. CCC technique was coined from the three original names – continuous tear capsulotomy by Gimbel and Neuhann, capsulorhexis by Neuhann, and circular capsulotomy by Kimiya Shimizu in 1987.[16,17] Nucleus prolapse from the bag is a little difficult through capsulorhexis as it is smaller than the nucleus [Fig. 4].
Figure 4.
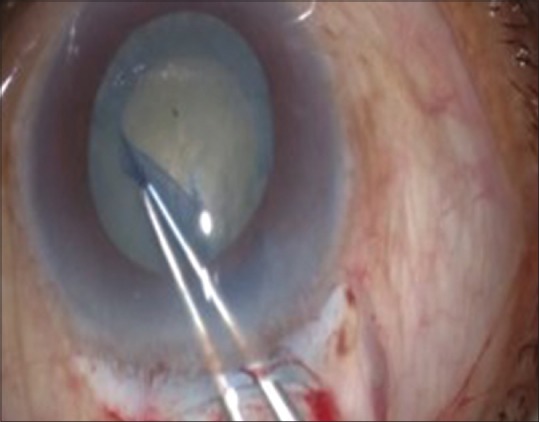
Capsulorhexis
The can-opener capsulotomy
Multiple small tears are connected to create large, central opening. It is easiest to prolapse the nucleus in AC through this capsulotomy, especially in cases of large nucleus as in brown cataract and cataracta nigra, because one end of the nucleus elevates without performing rotation of the nucleus. Here, IOL is placed in ciliary sulcus.
The envelope (linear) capsulotomy
This technique is preferred over can-opener technique in cases where CCC is difficult. It can be applied in cases of Morgagnian, intumescent, or hypermature cataracts. A horizontal slit is made in the anterior capsule which allows removal of the lens substance and posterior chamber IOL (PCIOL) implantation. IOL can be placed in the bag in majority of the cases. After implantation of IOL, the central anterior capsule over the optic zone is removed. The preferred technique in MSICS is a larger capsulorhexis >5.5–6.0 mm, unlike phaco where 5.25 mm is the desired goal. In hypermature cataracts, can-opener capsulotomy is safer, as prolapse of nucleus in AC becomes easier. Zonules are weak, so there are chances of zonular dialysis while prolapsing the nucleus in the AC. Envelope technique is also an option for MSICS.
After the capsulotomy, the entry into the AC is extended and hydrodissection is done [Figs. 5 and 6].
Figure 5.
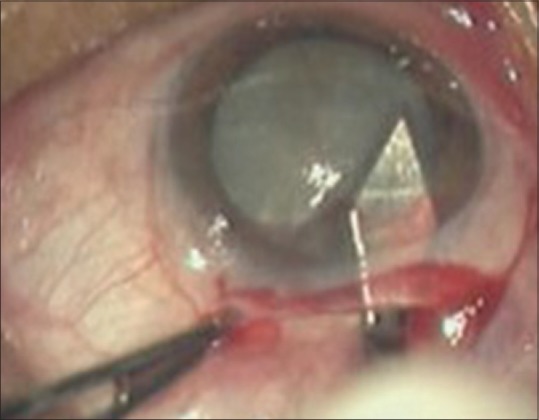
Extension of entry into anterior chamber
Figure 6.
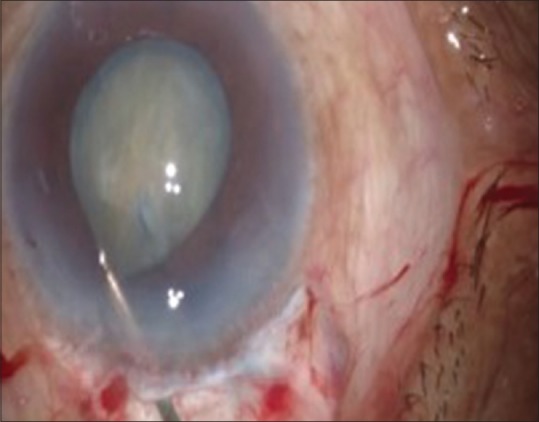
Hydrodissection
Hydroprocedures
Hydroprocedures were first described by Blumenthal and the term hydroprocedures was coined by Faust. Hydroprocedures separate various layers of the cataractous lens – nucleus, epinucleus, and cortex from the capsule. This helps in rotation of nucleus from its bag into the AC. Balanced salt solution (BSS) or Ringer's lactate solution is used.
Hydroprocedures comprise hydrodissection and hydrodelineation.
Hydrodissection
It refers to the almost complete dissection of the corticonuclear mass from the lens capsule. The cannula tip guides about 1 mm behind the rhexis margin in the subcapsular plane. A small amount of fluid is injected with a jerk to produce a fluid wave after slightly tenting the anterior capsule. Intermittent gentle tapping releases the fluid collected behind the nucleus. The fluid wave traverses the whole lens as it separates the cortex from the posterior capsule. This procedure is repeated in all the four quadrants.
Hydrodelineation
It refers to the separation of epinucleus from the nucleus by a fluid wave between the two, with the aim of debulking the nucleus. The cannula is introduced into the cortex and a small amount of fluid is injected in a jerky pulsed dose. A golden ring is seen under the microscope when the fluid goes around the nucleus. It is particularly advantageous in posterior polar cataract.
Nucleus Delivery/Management
Nucleus management consists of two important steps. The first one is getting the nucleus out of the capsular bag; it can be done either with the hydrodissection cannula or with the help of Sinskey hook. Second is the delivery of nucleus out of AC without injury to corneal endothelium. The nucleus may be delivered out of the AC by hydroexpression, viscoexpression, vectis-assisted delivery, sandwich technique, or the fish hook technique [Figs. 7 and 8].
Figure 7.
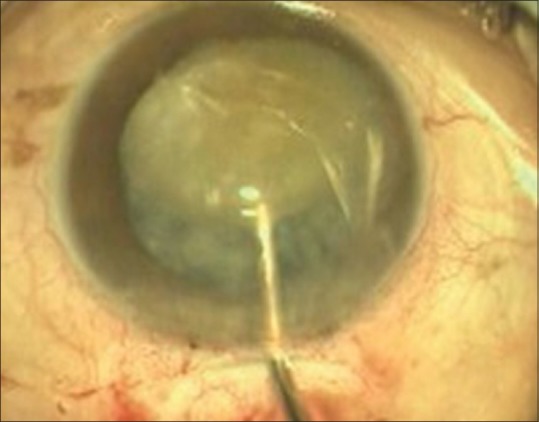
Nucleus rotation in anterior chamber
Figure 8.
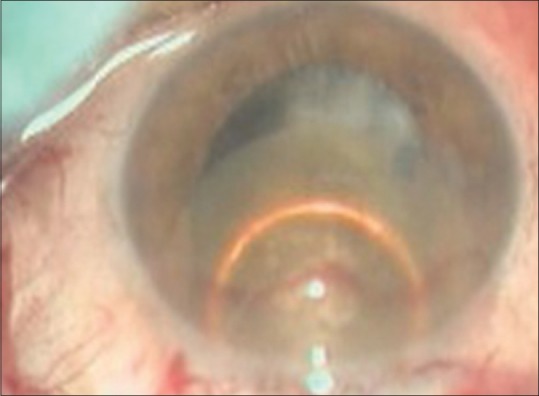
Nucleus delivery using vectis
Hydroexpression
-
(a)
Blumenthal technique: Blumenthal and Moisseiv in 1987 used ACM for the safe delivery of nucleus from AC without endothelial cell loss[7]
-
(b)
Brierley reported successful hydroexpression of the nucleus performed while performing SICS[18]
-
(c)
Friedburg et al.: The technique of “viscosurgically assisted hydro-jet irrigation of lens nucleus” was performed in 100 eyes. A bent cannula was used and a jet stream of fluid was created to separate the nucleus from the cortex and pressing the nucleus out of the bag.
Viscoexpression
Corydon and Thim in 1991 used a specially designed bent cannula for hydro- and viscoexpression of the nucleus through a continuous capsular capsulorhexis.[19] Thim et al. evaluated the technique of nucleus delivery in cadaver eyes with visco- and hydroexpression and concluded that viscoexpression is a safer and easier technique of nucleus delivery.[20]
Bellucci et al. demonstrated the results of viscoexpression in 142 eyes, concluding that a large capsulorhexis is the best way for nucleus delivery.[21] The success rate was 93% with low inflammation rate.
In a technique by Korynta, viscoexpression was performed in 369 cases and the complications were reported in 8.1% cases only. Relaxing capsular incision was required in 17.1% cases.
In a technique by Burton and Pickering, SICS was performed using a limbal incision and nucleus was delivered successfully by viscoexpression in 87.7% cases.[22]
Sandwich technique
Bayramlar et al. in a study of 37 eyes performed the technique of nucleus delivery.[23] The endonucleus was moved into the AC and extracted by sandwiching it between the irrigating vectis and iris spatula. Posterior capsule rupture, vitreous loss, and transient corneal edema were complications of the procedure.
Modified fish hook technique
Hennig et al. performed MSICS in 500 eyes.[24] Nucleus was delivered by fish hook technique using a needle tip hook. The best-corrected visual acuity was 6/18 or better in 96.2% cases at 6 weeks and after 1 year (95.9%). Six weeks postoperatively, 85.5% of eyes had against the rule astigmatism with a mean induced cylinder of 1.41 D (standard deviation: 0.8).
Use of anterior chamber maintainer
Blumenthal reported and advised the use of continuous positive pressure in the AC using ACM with good surgical results.[25]
Chawla and Adams used the ACM in cataract surgery in 258 cases and found that the use of ACM increased surgical control of the AC depth and maintained the position of the posterior capsule.[26] They concluded that it was a safer procedure and used less viscoelastic agents.
Wright et al. reported the results of a prospective clinical trial of 46 eyes undergoing cataract surgery using the ACM without viscoelastic agents.[27] Nearly 70% of cases had unaided visual acuity of 6/12 or better at 3-month follow-up. The main central and superior endothelial cell losses at 12 months after surgery were 20% and 25%, respectively.
Sharma et al. also reported good results in cases of SICS with IOL implant using ACM.[28]
Manual Phaco Fracture
Bartov et al. described a technique for planned manual ECCE.[29] They applied a modified mininuc ECCE technique in which the scleral tunnel was made wide enough to allow a nucleus of any size to settle in the tunnel. An inverted V-shaped incision of 5.0 mm is made in which the exposed part of the nucleus lodge in the sclera tunnel is manually picked and fragmented until it is small enough to be removed through the incision.
Kansas and Sax described a new technique in which the lens nucleus is manually split into pieces.[3] Kansas trisector and Kansas vectis were utilized for nucleus splitting, fragments were done, and then viscoexpressed through a smaller incision.
Hepşen et al. performed SICS in 59 eyes of 54 patients and nucleus was managed by phacotrisection technique.[30] The endonucleus was prolapsed into the AC and trisector using triangular trisector and solid vectis placed anteriorly and posteriorly, respectively. The best-corrected visual acuity of 20/40 or better was achieved in 83% of cases and of 20/25 or better in 47%. The most frequent intraoperative complication was transient corneal edema (54% cases).
Two Sinskey Method
Rao and Lam performed MSICS using a simple technique of employing two Sinskey hooks for nucleus extraction from the capsular bag.[31] The two hooks are introduced through separate paracentesis entry. The left-sided hook engages the nucleus under the capsulorhexis and lifts it at the superior pole toward the wound after rotating it. The right-hand side hook is placed underneath the elevated superior pole of the nucleus to keep it above the margin of the capsulorhexis and to prevent it from falling back into the bag when the first hook is retracted.
After the nucleus delivery, the remaining cortical matter is washed with the irrigation aspiration Simcoe cannula [Fig. 9].
Figure 9.
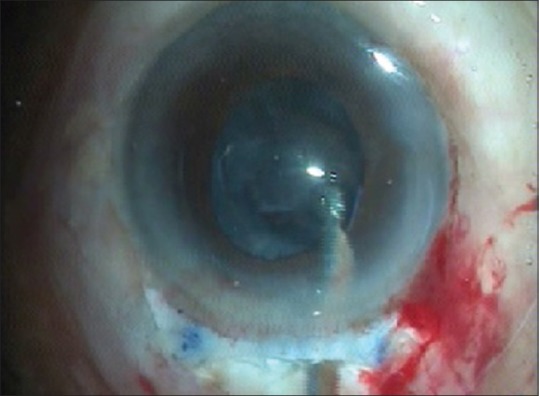
Cortical matter aspiration
Intraocular Lens Implantation
Following MSICS, it is rational to use a cost-effective rigid IOL as the incision size is already 6–7 mm wide. However, foldable IOLs can also be implanted in SICS, if cost is not a limiting factor and incision size is <5 mm [Fig. 10].
Figure 10.
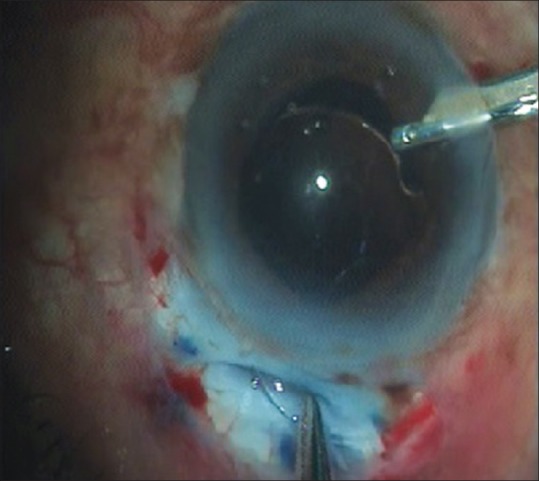
Intraocular lens implantation
In modified Blumenthal technique, the ACM is removed and chamber is filled with viscoelastic material before IOL implantation or alternatively IOL can be implanted with ACM and flow of BSS alone.[7] The IOL is implanted in the bag.
In Ruit technique, incision size was 6.5 mm–7.0 mm and a polymethylmethacrylate lens was implanted in the bag after deepening the AC by injecting air.[8,32] Air was removed by Simcoe cannula and replaced with irrigating fluid. Some modifications have been done by Dhanpal. Additional viscoelastic agent is injected before inserting IOL and it is aspirated with Simcoe cannula.
Kosakarn, after doing double-nylon loop technique, inserted foldable IOL.[10] This technique was safe with good result and few complications. The mean percentage of endothelial cell loss at 1 month was 9.19%.
Astigmatism
This is a well-known fact that corneal incision causes greatest limbal intermediate, while scleral incision causes least astigmatism. Ruit et al. compared surgically induced astigmatism in phaco and SICS at 6 weeks and 6 months postoperatively.[33] At 6-month follow–up, Ruit et al. reported mean astigmatism of 0.7 D for phaco group and 0.88 D for MSICS group. Both these studies showed no significant difference in surgically induced astigmatism in the two techniques. Venkatesh et al. and George et al. reported lesser surgically induced astigmatism in phaco.[34,35]
A prospective Japanese trial which compared 3.2 mm with 5.5 mm MSICS incision found 0.3 D less surgically induced astigmatism with smaller incision.[36] Other MSICS studies reported less surgically induced astigmatism with temporal and superotemporal scleral tunnel incision compared with superior incision.[37,38] This is because temporal incision is less likely to be affected by blinking and gravity.
Muralikrishnan et al. reported 1 D of surgically induced astigmatism with MSICS and phaco.[39] Many studies reported less astigmatism in phaco at 6-week follow-up as compared with MSICS although there is no significant difference in the long term.
Using smaller and tunneled incisions located temporally, the astigmatism caused by MSICS can be reduced to a great extent, thereby improving the uncorrected visual acuity of MSICS in the short term as well.
Complications in Small-Incision Cataract Surgery
Venkatesh in a study of 100 eyes that underwent MSICS with white cataract reported corneal edema with >10 Descemet's folds in 6%, and 7% cases had corneal edema with <10 Descemet's folds.[40] Six cases had mild iritis and three eyes had moderate iritis. Iridodialysis was observed in one eye.
Venkatesh et al. compared the result in 593 cases of SICS with irrigating vectis and reported intraocular complications in 11 (1.9%) cases which included 8 cases with posterior capsular rent (PCR) with or without vitreous prolapse, 76 eyes had complications on 1st postoperative day which included hypopyon in one, severe iritis in 19, and mild iritis in 16 eyes.[41] Thirty-three cases of transient corneal edema and residual cortex in one case were also observed. Vajpayee et al. reported endothelial cell loss of 17.66% ±3.6% in the phacofracture group and 7 cases out of 60 had central corneal edema.[42] Hepşen et al. found posterior capsular rupture with vitreous loss as the most common complication in SICS with phacofracture method.[30]
Bellucci et al. compared the techniques of viscoexpression, irrigating vectis, and nucleus fragmentation in 77, 25, and 40 eyes, respectively.[21] They found that nucleus expression was successful in 68%, nucleus fragmentation in 90%, and viscoexpression in 93% of eyes. Postoperative inflammation was least in the viscoexpression group.
Kothari et al. reported 8.1% incidence of vitreous loss in SICS performed with Blumenthal technique.[43] Sharma et al. reported 3% incidence of posterior capsular opacification (PCO) in SICS performed with Blumenthal technique.[28] They reported that 90% of the patients had good visual activity at the end of 3 months.
Venkatesh et al. reported 1.4% incidence of PCR in cases of SICS, whereas Ruit et al. reported none in MSICS.[34,44] In cases of MSICS in brown and black cataract, Venkatesh et al. reported PCR in 2% cases and Gogate encountered PCR in 6% of MSICS cases.[35,45]
Ruit et al. reported Grade I PCO in 26.1% cases of MSICS at 6-month follow-up examination.[44] The incidence of Grade II PCO was 17.4%. George et al. reported postoperative endothelial cell loss of 4.21% in SICS.[35] In Blumenthal technique, Wright et al. reported a mean central and superior cell loss of 16% and 22%, respectively, after 3 months of surgery when only fluid was used.[27] Malik et al. reported a cell loss of 5.5% at 3 months when viscoelastic agent was used for nuclear delivery.[46]
A retrospective observational series conducted at a single eye hospital by Ravindran et al. reported 0.12% incidence of endophthalmitis after MSICS.[47]
Small-Incision Cataract Surgery in Developing Countries
The WHO efforts have brought a dramatic change in cataract surgical volumes. Natchiar et al. have advocated a high-volume, high-quality cataract surgery approach to maximize the productivity of a particular surgeon.[48] Aravind Eye Hospital, Madurai, Tamil Nadu, is utilizing such a high-volume system in South India for more than a decade. Venkatesh et al. published a study on the outcome of 593 cataract surgeries performed in Aravind Eye Hospital by three surgeons utilizing this approach and reported an average surgical time of 3.75 min, i.e., 16–18 cases/hour.[41]
MSICS takes less time than phaco. Ruit et al. and Gogate et al. reported mean surgical time of approximately 15.5 min for phaco and 9 min for MSICS.[33,49] High-volume delivery system reduces the time for a MSICS to <4.5 min.
The cost compared with phaco was much less for SICS, i.e., $17.03 and $15 in studies by Muralikrishnan et al. and Ruit et al., respectively. Gogate et al. reported $42.10 for phaco and $15.37 for SICS.[50,51] MSICS is a more cost-effective and financially viable option for many settings in developing world. Excellent effectiveness makes MSICS as the most appropriate technique for performing high-volume cataract surgeries, especially in developing countries.
Tabin et al. reported that cataract accounts for almost 75% of cases of avoidable blindness in developing countries.[52]
The cataract surgical rate (CSR) represents the number of cataract operations performed annually per 1 million populations. There are significant differences in CSR among different countries. The CSR is usually between 4000 and 6000 in prosperous countries. In the last 20 years, CSR has been significantly raised in India from 1500 to approximately 4000. In Latin America and parts of Asia, the CSR ranges from 500 to 2000/million/year. Surprisingly, the CSR in China is close to 500 at par with some poorer countries of Asia.
Conclusion
MSICS offers similar advantages to phacoemulsification; due to its less surgical time, low cost, and wider applicability, it is more popular in developing and underdeveloped countries, where high-volume surgery is the norm. With a huge amount of avoidable blindness due to cataract prevailing in our country and most of the population not able to afford phacoemulsification, MSICS is an important tool for eliminating it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kelman CD. Phaco-emulsification and aspiration. A report of 500 consecutive cases. Am J Ophthalmol. 1973;75:764–8. doi: 10.1016/0002-9394(73)90878-7. [DOI] [PubMed] [Google Scholar]
- 2.Fry LL. The phaco sandwich technique. Thorofare, N.J: Slack; 1990. pp. 91–110. [Google Scholar]
- 3.Kansas PG, Sax R. Small incision cataract extraction and implantation surgery using a manual phacofragmentation technique. J Cataract Refract Surg. 1988;14:328–30. doi: 10.1016/s0886-3350(88)80127-5. [DOI] [PubMed] [Google Scholar]
- 4.Rozakis GW. Cataract surgery alternative small-incision techniques. Thorofare, N.J: Slack; 1990. pp. 71–110. [Google Scholar]
- 5.Blumenthal M, Ashkenazai I, Assia E, Cahore M. Small incision manual ECCE using selective hydrodissection. Ophthalmic Surg. 1992;23:699–701. [PubMed] [Google Scholar]
- 6.Anis AY. Understanding hydrodissection the term and related procedures. Ocul Surg News. 1990;8:142–3. [Google Scholar]
- 7.Blumenthal M, Moisseiev J. Anterior chamber maintainer for extracapsular cataract extraction and intraocular lens implantation. J Cataract Refract Surg. 1987;13:204–6. doi: 10.1016/s0886-3350(87)80137-2. [DOI] [PubMed] [Google Scholar]
- 8.Ruit S, Tabin GC, Nissman SA, Paudyal G, Gurung R. Low-cost high-volume extracapsular cataract extraction with posterior chamber intraocular lens implantation in Nepal. Ophthalmology. 1999;106:1887–92. doi: 10.1016/S0161-6420(99)90397-4. [DOI] [PubMed] [Google Scholar]
- 9.Malik KP, Goel R. Malik's technique of continuous 2% hydroxymethyl cellulose (HPMC) infusion assisted nuclear delivery in manual SICS. J Ophthalmol. 2016;3:190–1. [Google Scholar]
- 10.Kosakarn P. Double nylon loop for manual small-incision cataract surgery. J Cataract Refract Surg. 2009;35:422–4. doi: 10.1016/j.jcrs.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 11.Colvard DM, Kratz RP, Mazzocco TR, Davidson B. Clinical evaluation of the terry surgical keratometer. J Am Intraocul Implant Soc. 1980;6:249–51. doi: 10.1016/s0146-2776(80)80071-1. [DOI] [PubMed] [Google Scholar]
- 12.McFarland M. Small incision cataract surgery, foldable lenses, one stitch surgery sutureless surgery. Thorofare, N.J: Slack; 1990. pp. 107–16. [Google Scholar]
- 13.Pallin SL. Chevron sutureless closure: A preliminary report. J Cataract Refract Surg. 1991;17(Suppl 1):706–9. doi: 10.1016/s0886-3350(13)80687-6. [DOI] [PubMed] [Google Scholar]
- 14.Singer JA. Frown incision for minimizing induced astigmatism after small incision cataract surgery with rigid optic intraocular lens implantation. J Cataract Refract Surg. 1991;17(Suppl 1):677–88. doi: 10.1016/s0886-3350(13)80683-9. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal M, Ashkenazi I, Fogel R, Assia EI. The gliding nucleus. J Cataract Refract Surg. 1993;19:435–7. doi: 10.1016/s0886-3350(13)80322-7. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16:31–7. doi: 10.1016/s0886-3350(13)80870-x. [DOI] [PubMed] [Google Scholar]
- 17.Gimbel HV, Neuhann TT. Continuous curvilinear capsulorhexis technique. J Cataract Refract Surg. 1991;17:110–1. doi: 10.1016/s0886-3350(13)81001-2. [DOI] [PubMed] [Google Scholar]
- 18.Brierley L. Hydroexpression of the nucleus. J Cataract Refract Surg. 1993;19:666–7. doi: 10.1016/s0886-3350(13)80023-5. [DOI] [PubMed] [Google Scholar]
- 19.Corydon L, Thim K. Continuous circular capsulorhexis and nucleus delivery in planned extracapsular cataract extraction. J Cataract Refract Surg. 1991;17:628–32. doi: 10.1016/s0886-3350(13)81053-x. [DOI] [PubMed] [Google Scholar]
- 20.Thim K, Krag S, Corydon L. Hydroexpression and viscoexpression of the nucleus through a continuous circular capsulorhexis. J Cataract Refract Surg. 1993;19:209–12. doi: 10.1016/s0886-3350(13)80944-3. [DOI] [PubMed] [Google Scholar]
- 21.Bellucci R, Morselli S, Pucci V, Bonomi L. Nucleus viscoexpression compared with other techniques of nucleus removal in extracapsular cataract extraction with capsulorhexis. Ophthalmic Surg. 1994;25:432–7. [PubMed] [Google Scholar]
- 22.Burton RL, Pickering S. Extracapsular cataract surgery using capsulorhexis with viscoexpression via a limbal section. J Cataract Refract Surg. 1995;21:297–301. doi: 10.1016/s0886-3350(13)80136-8. [DOI] [PubMed] [Google Scholar]
- 23.Bayramlar H, Cekiç O, Totan Y. Manual tunnel incision extracapsular cataract extraction using the sandwich technique. J Cataract Refract Surg. 1999;25:312–5. doi: 10.1016/s0886-3350(99)80077-7. [DOI] [PubMed] [Google Scholar]
- 24.Hennig A, Kumar J, Yorston D, Foster A. Sutureless cataract surgery with nucleus extraction: Outcome of a prospective study in Nepal. Br J Ophthalmol. 2003;87:266–70. doi: 10.1136/bjo.87.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumenthal M. Manual ECCE, the present state of the art. Klin Monbl Augenheilkd. 1994;205:266–70. doi: 10.1055/s-2008-1045526. [DOI] [PubMed] [Google Scholar]
- 26.Chawla HB, Adams AD. Use of the anterior chamber maintainer in anterior segment surgery. J Cataract Refract Surg. 1996;22:172–7. doi: 10.1016/s0886-3350(96)80214-8. [DOI] [PubMed] [Google Scholar]
- 27.Wright M, Chawla H, Adams A. Results of small incision extracapsular cataract surgery using the anterior chamber maintainer without viscoelastic. Br J Ophthalmol. 1999;83:71–5. doi: 10.1136/bjo.83.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma T, Dhingra N, Worstmann T. Audit of small-incision cataract surgery using an anterior chamber maintainer. Eye (Lond) 2000;14(Pt 4):646–50. doi: 10.1038/eye.2000.158. [DOI] [PubMed] [Google Scholar]
- 29.Bartov E, Isakov I, Rock T. Nucleus fragmentation in a scleral pocket for small incision extracapsular cataract extraction. J Cataract Refract Surg. 1998;24:160–5. doi: 10.1016/s0886-3350(98)80195-8. [DOI] [PubMed] [Google Scholar]
- 30.Hepşen IF, Cekiç O, Bayramlar H, Totan Y. Small incision extracapsular cataract surgery with manual phacotrisection. J Cataract Refract Surg. 2000;26:1048–51. doi: 10.1016/s0886-3350(99)00464-2. [DOI] [PubMed] [Google Scholar]
- 31.Rao SK, Lam DS. A simple technique for nucleus extraction from the capsular bag in manual small incision cataract surgery. Indian J Ophthalmol. 2005;53:214–5. doi: 10.4103/0301-4738.16693. [DOI] [PubMed] [Google Scholar]
- 32.Ruit S, Paudyal G, Gurung R, Tabin G, Moran D, Brian G, et al. An innovation in developing world cataract surgery: Sutureless extracapsular cataract extraction with intraocular lens implantation. Clin Exp Ophthalmol. 2000;28:274–9. doi: 10.1046/j.1442-9071.2000.00316.x. [DOI] [PubMed] [Google Scholar]
- 33.Ruit S, Tabin G, Chang D, Bajracharya L, Kline DC, Richheimer W, et al. Aprospective randomized clinical trial of phacoemulsification vs. manual sutureless small-incision extracapsular cataract surgery in Nepal. Am J Ophthalmol. 2007;143:32–8. doi: 10.1016/j.ajo.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Venkatesh R, Tan CS, Sengupta S, Ravindran RD, Krishnan KT, Chang DF, et al. Phacoemulsification versus manual small-incision cataract surgery for white cataract. J Cataract Refract Surg. 2010;36:1849–54. doi: 10.1016/j.jcrs.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 35.George R, Rupauliha P, Sripriya AV, Rajesh PS, Vahan PV, Praveen S, et al. Comparison of endothelial cell loss and surgically induced astigmatism following conventional extracapsular cataract surgery, manual small-incision surgery and phacoemulsification. Ophthalmic Epidemiol. 2005;12:293–7. doi: 10.1080/09286580591005778. [DOI] [PubMed] [Google Scholar]
- 36.Kimura H, Kuroda S, Mizoguchi N, Terauchi H, Matsumura M, Nagata M, et al. Extracapsular cataract extraction with a sutureless incision for dense cataracts. J Cataract Refract Surg. 1999;25:1275–9. doi: 10.1016/s0886-3350(99)00148-0. [DOI] [PubMed] [Google Scholar]
- 37.Gokhale NS, Sawhney S. Reduction in astigmatism in manual small incision cataract surgery through change of incision site. Indian J Ophthalmol. 2005;53:201–3. doi: 10.4103/0301-4738.16684. [DOI] [PubMed] [Google Scholar]
- 38.Reddy B, Raj A, Singh VP. Site of incision and corneal astigmatism in conventional SICS versus phacoemulsification. Ann Ophthalmol (Skokie) 2007;39:209–16. doi: 10.1007/s12009-007-0020-y. [DOI] [PubMed] [Google Scholar]
- 39.Muralikrishnan R, Venkatesh R, Manohar BB. A comparison of the effectiveness and cost effectiveness of three different methods of cataract extraction in relation to the magnitude of post-operative astigmatism. Asia Pac J Ophthalmol. 2003;15:33–40. [Google Scholar]
- 40.Venkatesh R, Das M, Prashanth S, Muralikrishnan R. Manual small incision cataract surgery in eyes with white cataracts. Indian J Ophthalmol. 2005;53:173–6. doi: 10.4103/0301-4738.16675. [DOI] [PubMed] [Google Scholar]
- 41.Venkatesh R, Muralikrishnan R, Balent LC, Prakash SK, Prajna NV. Outcomes of high volume cataract surgeries in a developing country. Br J Ophthalmol. 2005;89:1079–83. doi: 10.1136/bjo.2004.063479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vajpayee RB, Sabarwal S, Sharma N, Angra SK. Phacofracture versus phacoemulsification in eyes with age-related cataract. J Cataract Refract Surg. 1998;24:1252–5. doi: 10.1016/s0886-3350(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 43.Kothari M, Thomas R, Parikh R, Braganza A, Kuriakose T, Muliyil J, et al. The incidence of vitreous loss and visual outcome in patients undergoing cataract surgery in a teaching hospital. Indian J Ophthalmol. 2003;51:45–52. [PubMed] [Google Scholar]
- 44.Ruit S, Tabin G, Chang GF. A prospective randomized clinical trial of phacoemulsification versus manual sutureless small incision extracapsular cataract surgery in Nepal. Am J Ophthalmol. 2007;143:32–8. doi: 10.1016/j.ajo.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 45.Gogate PM, Deshpande M, Wormald RP, Deshpande R, Kulkarni SR. Extracapsular cataract surgery compared with manual small incision cataract surgery in community eye care setting in Western India: A randomised controlled trial. Br J Ophthalmol. 2003;87:667–72. doi: 10.1136/bjo.87.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malik KP, Goel R. Endothelial cell loss in manual small incision cataract surgery. New Delhi: CBS Publishers; 2003. pp. 67–9. [Google Scholar]
- 47.Ravindran RD, Venkatesh R, Chang DF, Sengupta S, Gyatsho J, Talwar B, et al. Incidence of post-cataract endophthalmitis at Aravind eye hospital: Outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg. 2009;35:629–36. doi: 10.1016/j.jcrs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Natchiar G, Robin AL, Thulasiraj RD, Krishnaswamy S. Attacking the backlog of India's curable blind. The Aravind eye hospital model. Arch Ophthalmol. 1994;112:987–93. doi: 10.1001/archopht.1994.01090190135035. [DOI] [PubMed] [Google Scholar]
- 49.Gogate PM, Kulkarni SR, Krishnaiah S, Deshpande RD, Joshi SA, Palimkar A, et al. Safety and efficacy of phacoemulsification compared with manual small-incision cataract surgery by a randomized controlled clinical trial: Six-week results. Ophthalmology. 2005;112:869–74. doi: 10.1016/j.ophtha.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 50.Muralikrishnan R, Venkatesh R, Prajna NV, Frick KD. Economic cost of cataract surgery procedures in an established eye care centre in Southern India. Ophthalmic Epidemiol. 2004;11:369–80. doi: 10.1080/09286580490888762. [DOI] [PubMed] [Google Scholar]
- 51.Gogate P, Deshpande M, Nirmalan PK. Why do phacoemulsification? Manual small-incision cataract surgery is almost as effective, but less expensive. Ophthalmology. 2007;114:965–8. doi: 10.1016/j.ophtha.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 52.Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. 2008;19:55–9. doi: 10.1097/ICU.0b013e3282f154bd. [DOI] [PubMed] [Google Scholar]


