Abstract
Endophthalmitis following intraocular surgery is a disastrous complication and can lead to poor visual outcomes and loss of globe integrity. It should be differentiated from toxic anterior segment syndrome (TASS) where management differs drastically. This article presents basic knowledge about postoperative endophthalmitis and describes nine different real–world scenarios ranging from TASS to milder forms of endophthalmitis responding to intravitreal antibiotics alone and more complicated forms associated with corneal involvement, fungal endophthalmitis and cases requiring intraocular lens removal, radical vitrectomy with hyaloid peeling, base dissection, and silicone oil. A case-based approach is followed where practical considerations have been adopted with each case such that it facilitates the readers’ ability to apply theoretical knowledge to real-life clinical situations.
Keywords: Cataract, endophthalmitis, infection, postoperative
Postoperative endophthalmitis is the most devastating complication after intraocular surgery, which is commonly associated with a poor prognosis.[1] Postoperative endophthalmitis can occur following any penetrating ocular surgery; however, 90% of postoperative endophthalmitis occurs following cataract surgery, because cataract surgery is most frequently performed globally.[2] Fortunately, postoperative endophthalmitis occurs rarely clinical occurrence, but it often causes severe visual impairment or even the loss of an eye.[3] It is often difficult to apply theoretical knowledge to clinical scenarios, especially when faced with a difficult situation like endophthalmitis. In this article, we present a basic overview of endophthalmitis and various case studies spanning all possible scenarios of postoperative endophthalmitis and discuss practical approaches to manage the different scenarios presented.
Incidence of Endophthalmitis
Worldwide, the reported incidence of postoperative endophthalmitis is 0.04%–4%. Postcataract surgery incidence is 0.265% (more with clear corneal incision), postkeratoplasty is 0.382%, and postvitrectomy is 0.05%. The incidence of bleb-associated infection is 0.2%–9.6%. This range in the incidence of infection appears to be consistent across numerous patient populations from all over the world.[4,5] In a study of 10-year incidence of endophthalmitis rate at Bascom Palmer Eye Institute (1984–1994),[6] the incidence of postcataract surgery endophthalmitis was 0.09%. In a meta-analysis by Taban et al.,[7] the overall incidence rate of postoperative endophthalmitis was 0.128% from 1963 to 2003. However, the incidence of postoperative endophthalmitis has changed over time and has increased to 0.265%/year over the last few decades, which coincides temporally with the development of self-sealing clear corneal incisions. Several retrospective, comparative, and case-controlled studies have found a significantly higher endophthalmitis rate associated with clear corneal incisions compared to sclera tunnel incisions.[8,9,10,11] Recently, Nagaki et al.[12] reported a statistically increased risk with clear corneal incisions (0.29%) compared to sclerocorneal incisions (0.05%).
Although rare, it is potentially the most feared and devastating complication of intraocular procedures and can lead to a permanent, complete loss of vision. Endophthalmitis has been associated with severe visual loss in 20% of patients. A cluster of endophthalmitis cases may force a temporary shutdown of the operation theatre.
Types of Endophthalmitis
Infectious endophthalmitis is classified by the events leading to the infection and by the timing of the clinical diagnosis. The broad categories include postoperative endophthalmitis (acute-onset, chronic or delayed-onset, conjunctival filtering-bleb associated), posttraumatic endophthalmitis, and endogenous endophthalmitis. Miscellaneous categories include cases associated with microbial keratitis, intravitreal injections, or suture removal. These categories are important in predicting the most frequent causative organisms and in guiding therapeutic decisions before microbiologic confirmation of the clinical diagnosis [Table 1].
Table 1.
Classification of endophthalmitis (most frequent organisms in various clinical settings)

Etiology
Results in the ESCRS postoperative endophthalmitis study, the endophthalmitis vitrectomy study,[4] and other studies assessing the causative organism demonstrate that Gram-positive organisms account for 90% or more of pathogens isolated in culture-positive cases of postoperative endophthalmitis following cataract surgery, with coagulase-negative Staphylococci (i.e., Staphylococcus epidermidis) and Staphylococcus aureus representing the leading causes.[12,13,14,15,16,17,18] However, in India, Gram-negative organisms and fungi are important in etiology.[19,20,21]
It is important to differentiate infective endophthalmitis from sterile postoperative inflammation [Table 2]. Toxic anterior segment syndrome (TASS) is an acute postoperative inflammatory reaction in which a noninfectious substance enters the anterior segment and induces toxic damage to the intraocular tissues. Almost all cases occur after uneventful cataract surgery. In TASS most develop symptoms within 12–24, with decrease in visual acuity, corneal edema extending from limbus to limbus, moderate-to-severe anterior chamber (AC) reaction with cells, flare, hypopyon and fibrin, pupil may be dilated and nonreactive and intraocular pressure may be normal or raised.
Table 2.
Differences between toxic anterior segment syndrome and infective endophthalmitis
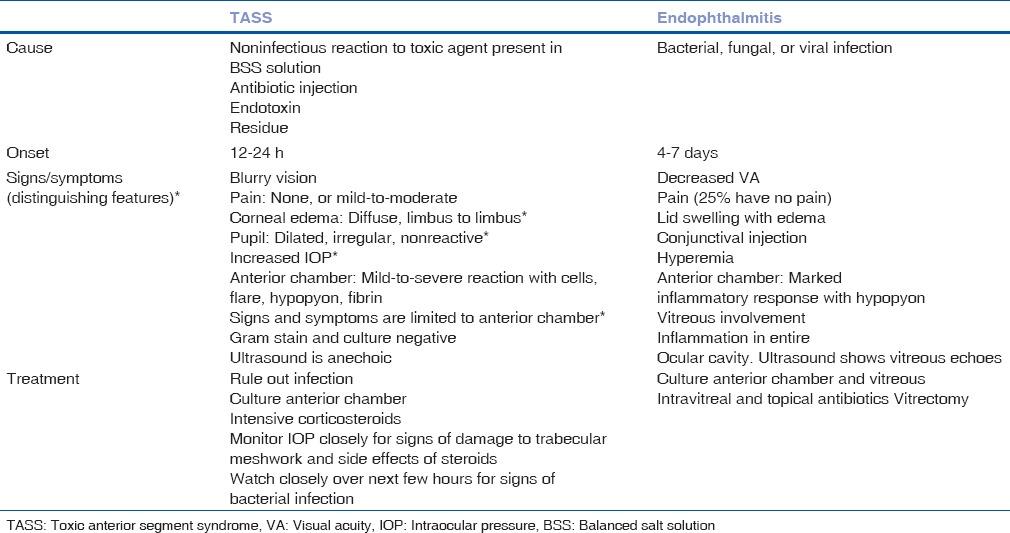
Patient symptoms indicative of endophthalmitis include ocular pain, diminished vision, and headache. Although pain is an important symptom, it is not universal and is not seen in 25% cases. Postoperative endophthalmitis may be acute or have delayed onset. Differentiation is important as the management and prognosis of TASS is significantly different. Delay in diagnosis leads to delay in initiating appropriate treatment. Acute endophthalmitis should be suspected when there is pain and increased in AC reaction on slit lamp examination on the first postoperative day. Decreased glow on distant direct ophthalmoscopy has high sensitivity but low specificity on the first postoperative day for diagnosing acute infectious endophthalmitis. On subsequent postoperative days, decrease in vision following initial improvement along with pain should immediately raise the index of suspicion toward endophthalmitis. The presence of exudates in vitreous on indirect ophthalmoscopy is 100% specific. The presence of a hypopyon along with vitreous exudates is usually diagnostic of endophthalmitis. If there is no hypopyon, role of distant direct ophthalmoscopy, slit lamp examination, indirect ophthalmoscopy, and ultrasound B scan is very important in deciding surgical intervention, and rule out other causes like masquerade secondary to cortex and nucleus drop.
Clinical Evaluation
Slit lamp examination helps to see mobility of the pupil and wound integrity and infiltrates of any. Evaluation of the cataract surgical wound is essential and that is the portal of entry for organisms causing endophthalmitis, and many cases may be related to suture removal. In cases with poorly dilating pupils, significant AC reaction (> +++) without hypopyon and best-corrected visual acuity (BCVA) better than 6/60, sterile reaction should be considered, especially if within 24 h of surgery, and treatment started with intravenous (IV) bolus steroids and intensive topical steroids and antibiotics. However, if BCVA is <6/60, endophthalmitis should be considered and patient should be administered intravitreal antibiotics. An ultrasonography B scan may aid in the diagnosis with nondilating pupils and severe AC reaction by demonstrating vitreous echoes, particularly involving the posterior vitreous cortex. The presence of vitreous exudates on clinical evaluation clinches the diagnosis of endophthalmitis.
Case Situations and Studies
Case 1: Toxic anterior segment syndrome
On postoperative day 1, there is significant AC reaction with 2–3+ cells, no hypopyon and good fundal glow [Fig. 1]. There may be a dilemma of whether to initiate intravitreal antibiotics or intensive topical + systemic steroids. In view of BCVA better than 6/60, this may be treated as TASS with topical and IV steroids but requires close follow-up.
Figure 1.
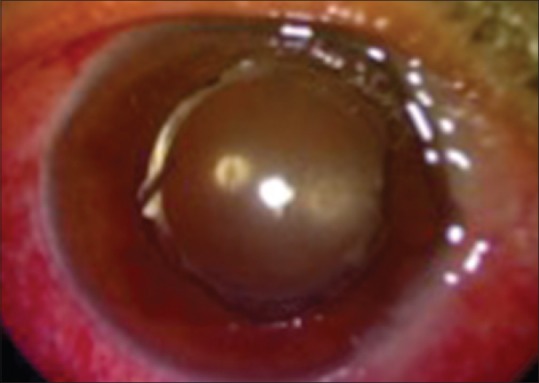
Toxic anterior segment syndrome in the immediate postoperative period
Case 2: Toxic anterior segment syndrome
On postoperative day 1, BCVA was found to be 6/24 with unusual postoperative AC reaction and good fundal glow [Fig. 2a and b]. The patient was treated with intensive steroids following which, BCVA improved to 6/18 on day 2, with significantly less AC reaction, and much clearer fundus details [Fig. 2c and d]. On day 14, same patient was noted to have BCVA of 6/6, with quiet AC, and normal appearance of fundus [Fig. 2e and f].
Figure 2.
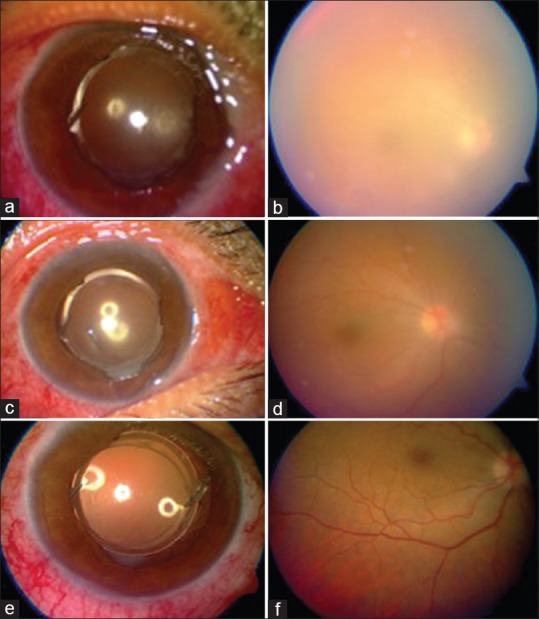
Anterior segment (a) and fundus involvement (b) in a case of severe toxic anterior segment syndrome. Serial images on day 2 (c and d) and day 14 (e and f) show improvement
Case 3: Acute endophthalmitis
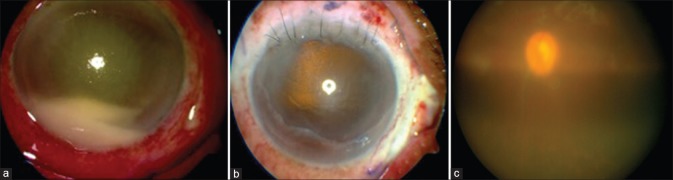
Postphacoemulsification surgery day 4, the patient presented with BCVA of hand motion, with hypopyon [Fig. 3a] and no fundal glow. The patient was diagnosed with acute postoperative endophthalmitis and treated with intravitreal vancomycin + ceftazidime and topical medical management. On postoperative day 3, the BCVA was finger counting at ½ M with the resolution of the hypopyon [Fig. 3b] and on postoperative day 6, BCVA was 3/60 with improving AC reaction [Fig. 3c]. At postoperative 2 weeks, BCVA was 6/24 with relative clear AC and vitreous [Fig. 3d and e] and at 3 weeks, BCVA had improved to 6/6 with clear view of the fundus [Fig. 3f].
Figure 3.
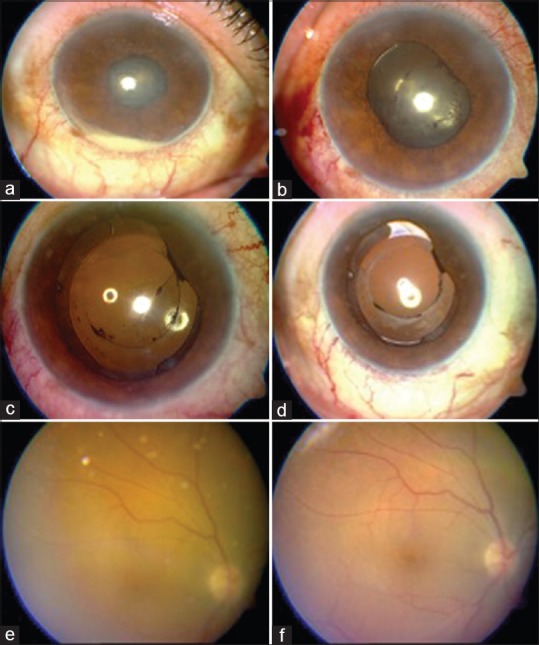
Hypopyon in a case of acute postoperative endophthalmitis (a), resolution of hypopyon on day 3 (b), quieter anterior chamber on day 6 (c), relatively clear anterior chamber and vitreous (d and e) at 2 weeks and clear vitreous at 3 weeks (f)
In situations where there is a partial response to intravitreal antibiotics with decrease or resolution of hypopyon and improvement in fundus glow indicative of reduced vitreous inflammation, but persisting AC reaction (3–4+), further intravitreal antibiotics are not preferred, and conservative medical management is continued. After intravitreal antibiotics, the patient is closely monitored for 24–36 h. If there is no worsening, medical treatment can be continued for 48 h following which decision regarding additional intravitreal antibiotics or surgical intervention is to be taken. If there is worsening, patient has to be taken up for surgical intervention in the form of pars plana vitrectomy (PPV). In situations where there is no response to intravitreal antibiotics or in very severe infection, radical pars planavitrectomy with peeling of hyaloid and base dissection is required. There is no role for core vitrectomy in this situation.
At present, best choice of intravitreal antibiotics is vancomycin (1 mg in 0.1 ml) combined with ceftazidime (2.25 mg in 0.1 ml) in separate syringes. Alternatively, vancomycin may be combined with amikacin (400 μg in 0.1 ml). Topical treatment comprises of ciprofloxacin/gatifloxacin/moxifloxacin 1 hourly or fortified cefazoline + tobramycin1 hourly along with cycloplegics in the form of atropine every six hourly. The topical drug dosage is tailored according to response. Topical steroids are added 1–2 days later. IV ciprofloxacin 200 mg twice daily is required in very severe cases. Oral steroids can be administered as 1–1.5 mg/kg single dose along with oral antibiotics. Oral ciprofloxacin 750 mg twice daily for 7–10 days usually preferred although currently, many clinicians prefer oral gatifloxacin or moxifloxacin.
Case 4: Post-pars plana vitrectomy endophthalmitis
Four days following parsplana vitrectomy, a pseudophakic patient presented with vision of light perception only and, no fundal glow, intense exudates in pupillary area with hypopyon [Fig. 4a]. Patient was taken up for radical PPV with IOL removal and intravitreal antibiotics. On day 9, there was a significant reduction in AC reaction and improved fundus visualization [Fig. 4b and c].
Figure 4.
Pupillary exudates and hypopyon in a case of post pars plana vitrectomy endophthalmitis (a), followed by postoperative picture on day 9 (b and c)
IOL removal during vitrectomy for endophthalmitis may be indicated in very severe endophthalmitis, Propionibacterium acnes endophthalmitis, fungal endophthalmitis, and recurrent endophthalmitis.
Case 5: Endophthalmitis with corneal abscess
On postoperative day 4, patient presented with vision of light perception and was found to have infiltration with corneal abscess at the site of cataract surgical wound and a yellow fundal glow [Fig. 5a and b]. The patient was taken up for immediate radical vitrectomy with silicone oil injection. Postoperatively, wound infection was treated with intensive topical moxifloxacin. At 4 weeks, there was resolution of the corneal abscess, clear view of the fundus under silicone oil and BCVA was maintained at 6/24 [Fig. 5c and d].
Figure 5.
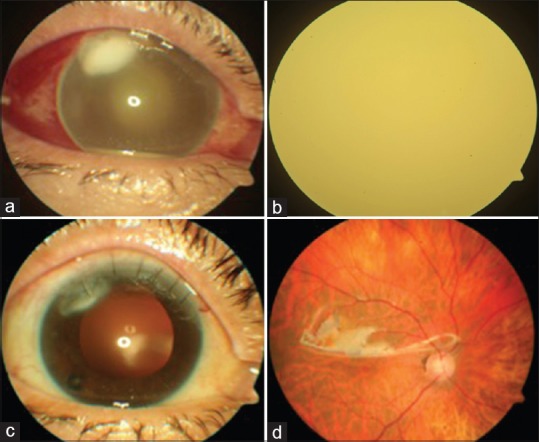
Case of corneal abscess (a) and endophthalmitis (b) followed by postoperative resolution (c and d)
Silicone oil is indicated in cases with severe vitritis and exudates over the retinal surface, appearance of atrophic retina or occurrence of iatrogenic breaks intraoperative. The anti-infective role of silicone oil is debated; however, its inert nature is postulated to assist in the resolution of infection.
Case 6: Fungal endophthalmitis
Patient presented with hand motion vision, relatively quiet AC with streak hypopyon [Fig. 6a] and yellowish vitreous exudates in the form of cotton balls [Fig. 6b]. The patient underwent radical vitrectomy with posterior hyaloid peeling and removal of subhyaloid pus pockets. Postoperative improvement was seen on postoperative day 1 [Fig. 6c] and media was clear on day 21 [Fig. 6d].
Figure 6.
Hypopyon and cotton ball vitreous exudates in a case of fungal endophthalmitis (a and b) followed by resolution postvitrectomy (c and d)
Chronic endophthalmitis is typically caused by P. acnes. It runs a chronic course with multiple recurrences. Usual intravitreal antibiotic injections are not of much help. Treatment options include “In the bag,” vancomycin 1 mg/0.1 ml, PPV + partial capsulectomy, and PPV + total capsulectomy + IOL explantation.
Case 7: Chronic endophthalmitis
Patient experienced recurrent attacks of uveitis with posterior capsular plaque [Fig. 7a and b] and transient visual improvement with steroids. Vitreous tap confirmed P. acne and patient underwent PPV + partial capsulectomy + in-the-bag vancomycin injection. There was postoperative improvement in inflammation, and BCVA improved from finger counting to 6/60 on day 9 [Fig. 7c]. The BCVA gradually improved to 6/18 on day 27 [Fig. 7d] and 6/9 on day 49 of follow-up [Fig. 7e and f].
Figure 7.
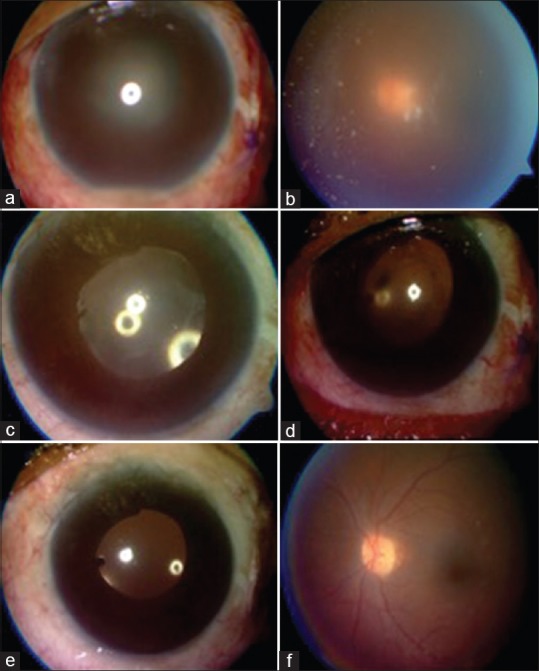
Propionibacterium acnes endophthalmitis (a and b), treated with vitrectomy followed by gradual resolution (c-f)
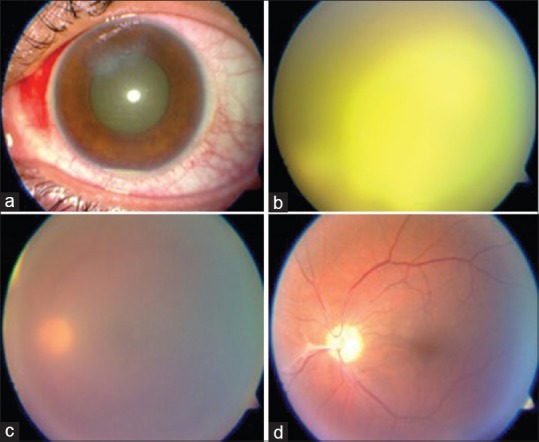
Case 8: Endophthalmitis after intravitreal Avastin
It is extremely severe due to direct inoculation of organism in vitreous [Fig. 8]. Prognosis is very poor, and vitrectomy is frequently required. Lucentis scores over Avastin because of its efficacy and safety. Safety with regard to the preparation of Avastin is always a source of concern as there is no uniform method; multiple pricks are involved during alliquoting. There have been incidents of cluster endophthalmitis with Avastin
Figure 8.
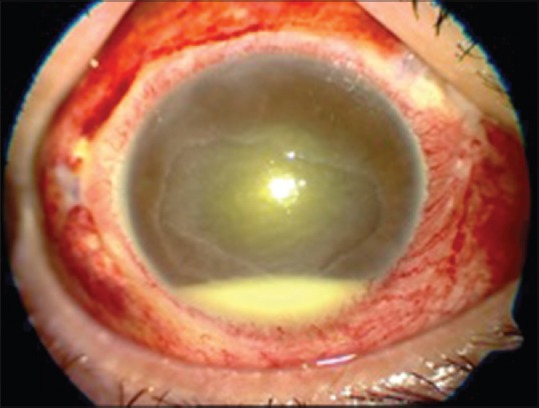
Endophthalmitis after intravitreal Avastin
Case 9: Endophthalmitis with corneal involvement [Fig. 9]
Figure 9.
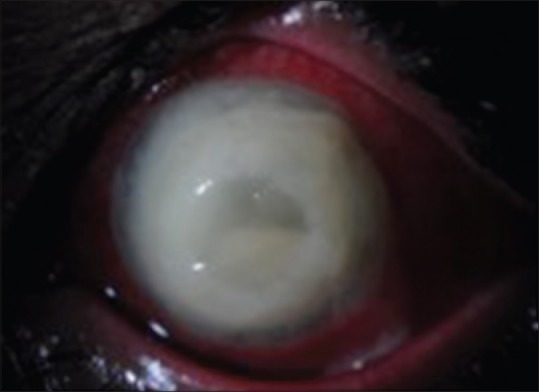
Endophthalmitis with very severe corneal involvement and melting
Prognosis is generally poor in these cases and management requires the help of a corneal surgeon. The treating retinal surgeon has to depend on intravitreal antibiotic injections and intensive topical treatment along with serial ultrasound monitoring. Definitive vitreous surgery is difficult due to poor visualization. If there is no response clinically or on ultrasound, one can try core vitrectomy with a compromised view. Temporary keratoprosthesis combined with vitrectomy and penetrating keratoplasty may be required in the most severe cases. Alternatively, newer procedures such as endoscopic vitrectomy may be tried in such cases.
Prevention and Prophylaxis
Eyelid and ocular surface microflora have been implicated as the source of infection in most cases of postoperative endophthalmitis.[19,20,21] Because bacteria can be cultured from the ocular surface of almost any person, certain risk factors may make patients more susceptible to infection by their ocular surface microflora. Risk factors for endophthalmitis include chronic bacterial blepharitis, active conjunctivitis, infections of the lacrimal drainage system, tear drainage obstruction, contaminated eye drops, contact lens wear, prosthesis in the fellow eye, and active nonocular infections. These conditions may lead to an abnormally elevated population of ocular surface microbes or colonization of the ocular surface by atypical organisms with greater virulence than the normal microflora. Host factors that lower resistance to infection such as chronic immunosuppressive therapy and diabetes mellitus have also been reported to be significant risk factors for postoperative endophthalmitis.
To reduce the incidence of postoperative endophthalmitis, each of the factors implicated in the pathogenesis should be addressed. First, an attempt should be made to decrease or eliminate eyelid and conjunctival microflora both preoperatively and intraoperatively. This goal may be accomplished using preoperative topical antibiotics and topical antiseptic agents. Second, administering subconjunctival antibiotic at the time of surgery should be considered.
Studies evaluating the effectiveness of preoperative administration of antibiotics and povidone-iodine[19,20,21] have reported a significant decrease in conjunctival bacterial colony counts. Topical antibiotics were reported to be most effective in decreasing conjunctival bacterial colony counts when administered 2 h before surgery rather than one or more days before surgery. The combination of topical antibiotics and povidone-iodine was found to sterilize the conjunctiva in more than 80% of treated patients.
Subconjunctival antibiotics are commonly administered after intraocular surgery. The rationale for subconjunctival antibiotic administration at the completion of the ocular procedure is to inhibit growth of bacteria that may gain entry into the eye during the operative procedure. Studies performed evaluating the effectiveness of prophylactic subconjunctival antibiotics in reducing the incidence of postoperative endophthalmitis reported conflicting results. Administering antibiotics in the irrigating fluid for cataract surgery has become a common technique for infection prophylaxis. This technique carries the risks of antibiotic toxicity, cost, and the possibility of the emergence of resistant bacteria.
The various strategies to prevent postoperative endophthalmitis are based on current knowledge regarding the pathogenic mechanisms of postoperative endophthalmitis. Perhaps of greatest importance, the preoperative ocular examination will help to identify the high-risk patient as previously described. In these patients, eyelid and conjunctival cultures can be performed before performing intraocular surgery. Based on the culture results and the overall clinical evaluation, preoperative topical antibiotic treatment may be considered. In patients with eye diseases requiring chronic administration of topical medications, new sterile medications should be provided to the patient before and after intraocular surgery.
On the day of cataract surgery, treating patients with prophylactic topical antibiotics that have activity against organisms commonly causing endophthalmitis can be considered. A thorough surgical prep, which includes lid margins, is performed. Instillation of 5% povidone-iodine on the conjunctiva followed by irrigation with saline is part of the surgical prep. The eyelids and eyelashes can be draped out of the surgical field with a plastic eye drape. A dry surgical field can be maintained when instruments are passed in and out of the eye. Attention to watertight wound closure is a priority, particularly in complicated surgical procedures or in reoperations that tend to have a higher incidence of postoperative wound leak. Vitreous incarceration in the wound should be eliminated by anterior vitrectomy techniques. At the conclusion of surgery, subconjunctival antibiotic injection using a combination of agents effective against the majority of causative Gram-positive and Gram-negative organisms can be considered.
Conclusion
This review article presents a case-based approach to different scenarios of postoperative endophthalmitis ranging from TASS to milder forms of endophthalmitis that respond to intravitreal antibiotic agents alone and then discusses severe forms of endophthalmitis with their practical considerations such as IOL removal, use of silicone oil, fungal endophthalmitis, P. acnes endophthalmitis, and severe infection involving the cornea. A case-based approach enables practical application of theoretical knowledge, and hence, authors have adopted this approach in this review article.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge the assistance of Dr. Sabyasachi Sengupta in manuscript editing.
References
- 1.Maguire JI. Postoperative endophthalmitis: Optimal management and the role and timing of vitrectomy surgery. Eye (Lond) 2008;22:1290–300. doi: 10.1038/eye.2008.51. [DOI] [PubMed] [Google Scholar]
- 2.Verbraeken H. Treatment of postoperative endophthalmitis. Ophthalmologica. 1995;209:165–71. doi: 10.1159/000310605. [DOI] [PubMed] [Google Scholar]
- 3.Kresloff MS, Castellarin AA, Zarbin MA. Endophthalmitis. Surv Ophthalmol. 1998;43:193–224. doi: 10.1016/s0039-6257(98)00036-8. [DOI] [PubMed] [Google Scholar]
- 4.Results of the endophthalmitis vitrectomy study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis vitrectomy study group. Arch Ophthalmol. 1995;113:1479–96. [PubMed] [Google Scholar]
- 5.Fintelmann RE, Naseri A. Prophylaxis of postoperative endophthalmitis following cataract surgery: Current status and future directions. Drugs. 2010;70:1395–409. doi: 10.2165/11537950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Endophthalmitis Study Group. European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613–20. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 8.Aaberg TM, Jr, Flynn HW, Jr, Schiffman J, Newton J. Nosocomial acute-onset postoperative endophthalmitis survey A 10-year review of incidence and outcomes. Ophthalmology. 1998;105:1004–10. doi: 10.1016/S0161-6420(98)96000-6. [DOI] [PubMed] [Google Scholar]
- 9.Cooper BA, Holekamp NM, Bohigian G, Thompson PA. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003;136:300–5. doi: 10.1016/s0002-9394(03)00202-2. [DOI] [PubMed] [Google Scholar]
- 10.Lertsumitkul S, Myers PC, O’Rourke MT, Chandra J. Endophthalmitis in the Western Sydney region: A case-control study. Clin Exp Ophthalmol. 2001;29:400–5. doi: 10.1046/j.1442-9071.2001.d01-20.x. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell PJ, Donnenfeld ED, Perry HD. New horizons in fluoroquinolone therapy. Ophthalmol Times. 2002;27:1–15. [Google Scholar]
- 12.Nagaki Y, Hayasaka S, Kadoi C, Matsumoto M, Yanagisawa S, Watanabe K, et al. Bacterial endophthalmitis after small-incision cataract surgery. Effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:20–6. doi: 10.1016/s0886-3350(02)01483-9. [DOI] [PubMed] [Google Scholar]
- 13.Barrow D, McDermott M, Elliot D, Frank R. Acute postoperative endophthalmitis and modern cataract surgery technique [ARVO abstract 1340] Invest Ophthalmol Vis Sci. 2001;42:S24. [Google Scholar]
- 14.Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in the endophthalmitis vitrectomy study. Am J Ophthalmol. 1996;122:1–7. doi: 10.1016/s0002-9394(14)71959-2. [DOI] [PubMed] [Google Scholar]
- 15.Benz MS, Scott IU, Flynn HW, Jr, Unonius N, Miller D. Endophthalmitis isolates and antibiotic sensitivities: A 6-year review of culture-proven cases. Am J Ophthalmol. 2004;137:38–42. doi: 10.1016/s0002-9394(03)00896-1. [DOI] [PubMed] [Google Scholar]
- 16.Recchia FM, Busbee BG, Pearlman RB, Carvalho-Recchia CA, Ho AC. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol. 2005;123:341–6. doi: 10.1001/archopht.123.3.341. [DOI] [PubMed] [Google Scholar]
- 17.Barry P, Cordovés L, Gardner S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis following Cataract Surgery: Data, Dilemmas and Conclusions. Paper Presented at the European Society of Cataract and Refractive Surgeons. Dublin, Ireland: 2013. [Google Scholar]
- 18.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769–75. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 19.Verma L, Venkatesh P, Tewari HK. Management of Endophthalmitis, AIOS CME Series-4. 2000. [Last accessed on 2017 Nov 12]. Available from: http://www.aios.org/cme/cmeseries4.pdf .
- 20.Das T, Dogra MR, Gopal L, Jalali S, Kumar A, Malpani A, et al. Postsurgical endophthalmitis: Diagnosis and management. Indian J Ophthalmol. 1995;43:103–16. [PubMed] [Google Scholar]
- 21.Anand AR, Therese KL, Madhavan HN. Spectrum of aetiological agents of postoperative endophthalmitis and antibiotic susceptibility of bacterial isolates. Indian J Ophthalmol. 2000;48:123–8. [PubMed] [Google Scholar]