Abstract
Purpose:
To study the effect of capsular bag irrigation of trypan blue dye (0.06%) on posterior capsular opacification (PCO) in eyes undergoing phacoemulsification.
Methods:
This was a randomized, trial conducted at a tertiary eye care center in central India. The study included 50 patients (100 eyes) with senile cataracts who were scheduled for phacoemulsification and intraocular lens (IOL) implantation and were willing to undergo bilateral cataract surgery. One eye of each patient was randomized to one of two groups. The dye group received 0.2 ml of trypan blue injected in the capsular bag after cortical cleanup under air. The control group (other eye of the same patient) received 0.2 ml of balanced salt solution injected in a similar manner. PCO in the central 3 mm area of IOL optic was analyzed by a masked observer using an evaluation of PCO software computer analysis system at 6, 12, 24, and 36 months.
Results:
The average age of patients was 62.05 ± 6.22 in the dye group and 64.92 ± 7.16 years in the control group. The mean PCO score at 6 months was significantly lower in the dye group (0.10 ± 0.15) than in the control group (0.22 ± 0.30). There were no significant differences in the PCO scores between the two groups from 12 to 36 months. At the end of 3 years, eight eyes in the dye group and seven in the control group required YAG capsulotomy (P = 0.21).
Conclusion:
Capsular bag irrigation of trypan blue dye decreased the PCO score at 6 months, but it had no effect at 36 months.
Keywords: Phacoemulsification, posterior capsular opacification, trypan blue dye
A delayed postoperative complication of cataract surgery is posterior capsular opacification (PCO). Advances in the cataract surgical procedure and IOL design have significantly reduced the PCO rate. Effective hydrodissection, cortical removal, sealed capsular irrigation, square edge optics of the IOL, and ultraviolet treatment of lens epithelial cells (LECs) are a few methods to prevent PCO. Trypan blue is a vital dye that has been shown to reduce the density and viability of LECs when used on the outer surface of the anterior capsule.[1,2,3] Sharma and Panwarinjected trypan blue into the capsular bag after hydrodissection and studied the PCO rate in a follow-up period of 1 year.[4] The long-term results of the effect of trypan blue dye on preventing PCO are not available in the literature.
The purpose of the present study was to report the effect of trypan blue dye irrigation on the causative factor of PCO, i.e., LECs in a follow-up period of 3 years. Trypan blue was injected into the capsular bag after cortical wash under air. The control group had an equivalent amount of balanced salt solution (BSS) injected into the capsular bag in a similar manner.
Methods
Sample size
The sample size in each group was 50 eyes to find a difference of 25% (saline group 35% and trypan blue group 10%), with 80% power and a significance level of 5%.
Patient selection and study design
The present study adhered to the tenets of the Declaration of Helsinki, and the medical ethics committee of the hospital gave ethical approval. Informed consent was obtained from all participants.
This prospective, comparative, randomized, and interventional case series included 100 patients with senile cataracts who were scheduled for phacoemulsification and intraocular lens (IOL) implantation during June–December 2013. Patients with operable cataracts at our outpatient department of Government Medical College who were willing to undergo bilateral cataract surgery in the near future (duration of 1 month) were included in the study. The exclusion criteria were glaucoma, pseudoexfoliation, uveitis, previous intraocular surgeries, subluxated cataracts, diabetes, trauma, monocular vision, poor pupillary dilation, and an age <40 years. The intraoperative exclusion criteria were preexisting posterior capsular opacity or plaque and posterior capsular rent. Preoperative assessment included the best-corrected visual acuity, slit-lamp examination, intraocular pressure, retinal evaluation, and A-scan biometry for IOL power calculation.
One eye of each patient was randomized to one of two groups. The simple randomization method by toss method was used. Heads were assigned to the intervention group (dye group) and tails to the control group (BSS group). The dye group received 0.2 ml of trypan blue (0.06%) injected in the capsular bag after cortical cleanup under air. The control group (other eye of the same patient) received 0.2 ml of BSS injected in a similar manner.
Surgical technique
A single surgeon (R.J.) performed all the surgeries. Preoperative dilatation of the pupil was achieved using a combination of 0.8% tropicamide and 5% phenylephrine. Patients underwent operations with 0.5% topical proparacaine hydrochloride drops instilled twice 10 min before the surgical procedure and supplemented by a 0.5 ml subconjunctival injection of 2% lignocaine hydrochloride at the beginning of surgery. A 20-gauge side port incision was created on the appropriate side, as required. Viscoelastic material (2% hydroxypropyl methylcellulose, Appavisc, Appasamy Ocular Devices, Puducherry, India) was injected through the side port with a 23-gauge blunt tip cannula. A 2.8 mm clear corneal temporal incision was made. Continuous curvilinear capsulorhexis (CCC) was completed using capsulorhexis forceps under viscoelastic pressure. The size of the rhexis was kept at approximately 5.5 mm. Hydrodissection was performed with BSS. The nucleus was managed using the direct chop method. The settings for the nucleus chop were power 90% (linear), vacuum 350 mmHg, and aspiration flow rate 34 cc/min. The parameters were the same for all cases and were not changed until the last fragment was emulsified. Phacoemulsification was performed in the capsular bag. Thorough cortical cleanup was accomplished with an irrigation and aspiration probe. In the dye group, 0.2 ml of trypan blue dye (Marketed by BLUE DYE, Indoco remedies limited, Mumbai, India; Manufactured by Care group, India) was injected under air through the two side ports (0.1 ml each), which were 180 degrees apart. A period of 45 s was allowed for the diffusion of the dye in the bag. The dye was then washed with irrigation and aspiration [Video 1]. Viscoelastic material was injected into the anterior chamber (AC). A single-piece hydrophilic lens (6-mm optic diameter, overall length 12.5 mm, biconvex optic design, and square edge) was implanted in the capsular bag (Acryfold, Appasamy Ocular Devices, Puducherry, India). A thorough AC wash was used to clear viscoelastic material. Stromal hydration of the side port and main incision was completed with BSS. In the control group, all steps were the same except; in place of trypan blue, 0.2 ml of BSS was injected under the air.
Postoperative follow-up period
Patients were followed at 1 and 7 days and at 1, 6, 12, 24, and 36 months. The corrected distance visual acuity and slit-lamp examination were evaluated at every visit. PCO was assessed by an independent observer. Central 3 mm area of IOL optic was considered for the evaluation of PCO (EPCO). Retroillumination slit-lamp images (Imaging system-990 5X Elite, CSO, Italy) were obtained at 6, 12, 24, and 36 months’ visits after full mydriasis. Images were imported into the EPCO software computer analysis system. The data about the diameter of IOL and the central 3 mm diameter area were entered in the software. Capsulorhexis margin was defined. The PCO density was scored on a scale from 0 to 4 (0 = none visible, 1 = minimal wrinkling of posterior capsule with fine layer of LECs, 2 = mild honeycomb PCO; thicker layer LECs with dense fibrosis, 3 = classic Elschnig pearls; very thick layer of LECs, 4 = severe opacity with darkening effect) and then multiplied by the fractional area involved [Figs. 1 and 2] to obtain the PCO score.[5]
Figure 1.
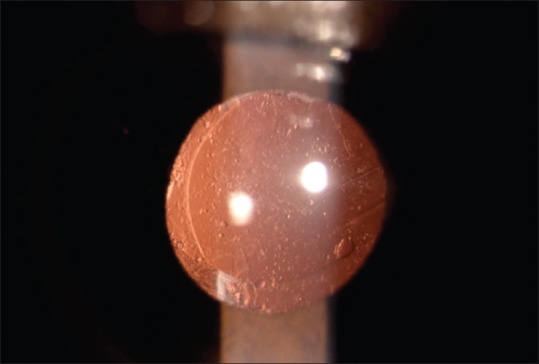
Retroillumination image of the posterior capsular opacification
Figure 2.
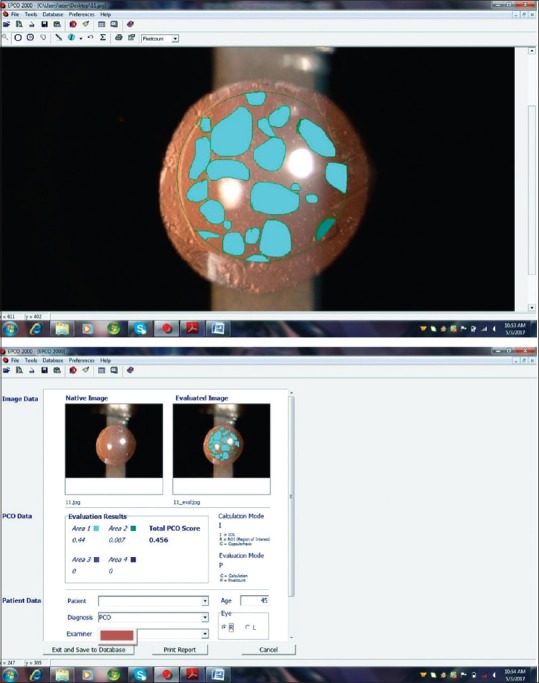
Calculation of evaluation of posterior capsular opacification score
PCO Score = ∑(% area × PCO Grade [0–4]).
At the end of year 3, the number of patients requiring YAG capsulotomy for PCO was noted in the two groups.
Statistical analysis
Preoperative and postoperative observations were entered into an Excel sheet. The paired t-test was used to analyze the results of the two groups. Differences were considered significant when the P value was less than 0.005. χ2 analysis was performed to compare the YAG capsulotomy rate between two groups.
Results
The average age of patients was 62.05 (±6.22) years in the trypan blue group and 64.92 (±7.16) years in the control group. There were 22 male and 28 female. An equal number of eyes (n = 50) were randomized into two groups. The PCO score in two groups is depicted in Table 1. The mean PCO score at 6 months was significantly lower in the dye group (0.10 ± 0.15) than in the control group (0.22 ± 0.30). There were no significant differences in the PCO scores between the 2 groups from 12 to 36 months. Posterior capsular dye staining occurred in two eyes [Fig. 3] and an IOL stain in one eye [Fig. 4], which cleared in 2 days.
Table 1.
Mean posterior capsular opacification scores in the 2 groups (central 3 mm)
Figure 3.

Posterior capsular dye staining
Figure 4.
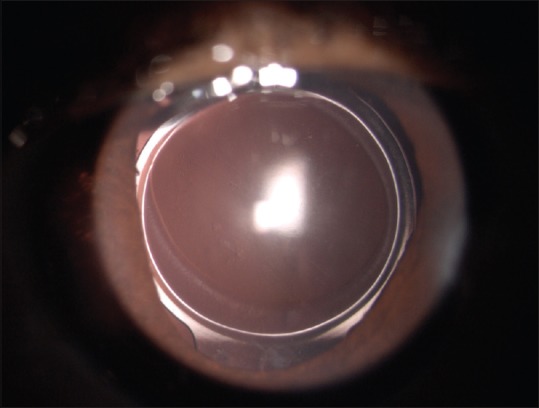
Intraocular lens staining
One patient in the dye group (2%) and two patients (4%) in the control group had partial optic cover of rim of CCC. None of them developed PCO.
At the end of 3 years, 8 eyes (16%) in the dye group and 7 eyes (14%) in the control group required YAG capsulotomy for PCO (P = 0.21).
The best-corrected visual acuity at the end of the study in the dye group was 0.03 logMAR and in the control group was 0.04 logMAR (P = 0.31). No morphological difference in the appearance of the PCO was observed in the two groups.
Discussion
PCO is a common complication after extracapsular cataract surgery. It has been demonstrated that PCO is caused by proliferation, migration, and the epithelial–mesenchymal transition of LECs present in the equatorial area of the capsular bag.[6]
There have been efforts to reduce the incidence of PCO by various means.[7] Use of trypan blue dye is one approach.[1,4] Trypan blue dye selectively stains the basement membrane.[8] It is used to stain the anterior capsule for creating CCC in patients with white or dense cataracts. It has no known toxicity at the concentration approved by the Food and Drug Administration, USA.[9] An in vitro study has shown that trypan blue has little toxicity for LECs irrespective of the dye concentration.[3] However, an in vivo study has shown decreased density and viability of LECs when the dye was used at a concentration of 0.0125% to stain the anterior capsule.[1] The concentration of dye used in our study was 0.06%. This dye concentration has been approved by the FDA, USA. Sharma and Panwar in their study on the initial postoperative PCO results used 0.1% dye that was subcapsularly injected after cortical cleaving hydrodissection.[4] We injected the dye into the capsular bag under air after a thorough cortical wash. This is the reason for keeping the dye concentration 0.06%. The advantage of injecting the dye under air is that the dye diffuses in the capsular bag so that it reaches the LECs at a sufficient concentration. As the air bubble was occupying the entire AC covering the side port and the main incision, gentle placement of the cannula at the side port was sufficient to inject the dye. In the event of a shallow AC due to leakage of an air bubble, one needs to exit the AC and inject an air bubble again. Another advantage was it did not stain the endothelium. Due to nonavailability of specular microscope in the setup, we could not measure corneal endothelial cell count before and after the surgical procedure. However, long-term study by Norn has shown that trypan blue dye does not cause endothelial damage.[10] An exposure period of 45 s was allowed for the diffusion of dye into the capsular bag. Period of 45 s was decided as the concentration of dye used was 0.06%, compared to 0.1% by Sharma and Panwar.[4] Care was taken not to damage the iris and posterior capsule during dye injection. Trypan blue dye has been used to stain the posterior capsule when performing posterior capsulorhexis.[11,12] An exposure time of 30 s was allowed to pass for the staining of the posterior capsule. We had two options for the injection of dye into the capsular bag. One option was air, and another was under viscoelastic. A pilot study performed by us on the injection of dye under viscoelastic did not cause diffusion of dye in the capsular bag. The dye remained in the AC mixed with viscoelastic material. The injection of dye beneath the viscoelastic was risky as one needs to be closer to the posterior capsule for the injection. Therefore, we choose the technique of injecting dye under air.
The injection of dye under air did cause posterior capsular or IOL staining. Postoperative follow-up showed staining of the posterior capsule in two eyes and an IOL stain in one eye. Staining took two days to clear. Permanent staining of hydrogel lenses has been reported at a dye concentration of 0.1%.[13] None of the patients had blue or dark vision during the follow-up period. No other dye-related complications were seen in any of the patients.
The long-term results of the injection of dye to prevent PCO are not available in the literature. Our study shows that the PCO score at 6 months is significantly lower in the dye group than in the control group. However, from 6 months onward, there were no significant differences in the PCO scores between the two groups. Sharma and Panwar, in their study on PCO prevention by trypan blue dye, have shown that the PCO scores at 6 and 12 months were significantly lower in the dye group than in the control group.[4] The concentration of dye used in their study was 0.1% compared with 0.06% in our study.
They have also shown that at the end of 12 months, 2 eyes in the trypan blue group and 6 eyes in the control group required Nd: YAG laser capsulotomy for significant central PCO.[4] In our study, at the end of 36 months, 8 eyes (16%) in the dye group and 7 eyes (14%) in the control group required YAG capsulotomy for significant PCO (P = 0.21), causing decreased visual acuity. Drop in the visual acuity of 2-Snellen's line was considered for the ND: YAG capsulotomy. This finding suggests that the dye might have no permanent toxicity effect on the LECs. It also indicates that a single dose of the dye might not permanently prevent PCO formation. Using a high concentration of dye in the capsular bag for a long contact period may prevent PCO formation. However, the deleterious effects of the dye on the AC structures and endothelium cannot be denied. What concentration of the dye and length of time of contact are ideal for preventing PCO merits further research.
Hydrophilic IOL has a high PCO rate compared with hydrophobic IOL.[14,15] Hydrophobic implantation would have defeated the purpose of this study. Therefore, we preferred the hydrophilic IOL in our study. On the contrary, the use of dye in patients undergoing hydrophobic IOL implantation would have delayed PCO formation in these lenses. The present study was carried out in a rural setup. Cost was a limiting factor to use hydrophobic lenses.
One patient in the dye group (2%) and two patients (4%) in the control group had partial optic cover of the anterior capsule. Partial overlap of the rim of anterior capsule over the optic of IOL leads to incomplete capsular bend formation creating a gap between optic of IOL and posterior capsule through which LECs migrate over the posterior capsule causing PCO.[16,17,18] Vasavada et al. have shown no significant difference in the development of PCO in patients with partial versus total optic cover of anterior capsule 5 years postoperative in patients implanted with single-piece hydrophobic IOL.[19] In our study, hydrophilic IOL with square edge was used. This could be the reason for no occurrence of PCO in the study group having partial anterior capsular rim cover over the optic of IOL.
There was no significant difference in the best-corrected visual acuity at the end of the study between the two groups (dye group was 0.03 logMAR and control group was 0.04 logMAR P = 0.31), which supports the findings by Sharma and Panwar.[4] There were no changes in the morphology of PCO development in the two groups.
Conclusion
Our study shows that the injection of trypan blue dye under air in a capsular bag is not a difficult technique. It does prevent early PCO; however, the long-term result shows no effect on preventing PCO.
Video Available on: www.ijo.in
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nanavaty MA, Johar K, Sivasankaran MA, Vasavada AR, Praveen MR, Zetterström C. Effect of trypan blue staining on the density and viability of lens epithelial cells in white cataract. J Cataract Refract Surg. 2006;32:1483–8. doi: 10.1016/j.jcrs.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Portes AL, Almeida AC, Allodi S, Monteiro ML, Miguel NC. Trypan blue staining for capsulorhexis: Ultrastructural effect on lens epithelial cells and capsules. J Cataract Refract Surg. 2010;36:582–7. doi: 10.1016/j.jcrs.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Melendez RF, Kumar N, Maswadi SM, Zaslow K, Glickmank RD. Photodynamic actions of indocyanine green and trypan blue on human lens epithelial cells in vitro . Am J Ophthalmol. 2005;140:132–4. doi: 10.1016/j.ajo.2004.12.086. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Panwar M. Trypan blue injection into the capsular bag during phacoemulsification: Initial postoperative posterior capsule opacification results. J Cataract Refract Surg. 2013;39:699–704. doi: 10.1016/j.jcrs.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Tetz MR, Nimsgern C. Posterior capsule opacification. Part 2: Clinical findings. J Cataract Refract Surg. 1999;25:1662–74. doi: 10.1016/s0886-3350(99)00259-x. [DOI] [PubMed] [Google Scholar]
- 6.Gotoh N, Perdue NR, Matsushima H, Sage EH, Yan Q, Clark JI. An in vitro model of posterior capsular opacity: SPARC and TGF-beta2 minimize epithelial-to-mesenchymal transition in lens epithelium. Invest Ophthalmol Vis Sci. 2007;48:4679–87. doi: 10.1167/iovs.07-0091. [DOI] [PubMed] [Google Scholar]
- 7.Pandey SK, Apple DJ, Werner L, Maloof AJ, Milverton EJ. Posterior capsule opacification: A review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol. 2004;52:99–112. [PubMed] [Google Scholar]
- 8.Singh AJ, Sarodia UA, Brown L, Jagjivan R, Sampath R. A histological analysis of lens capsules stained with trypan blue for capsulorrhexis in phacoemulsification cataract surgery. Eye (Lond) 2003;17:567–70. doi: 10.1038/sj.eye.6700440. [DOI] [PubMed] [Google Scholar]
- 9.Melles GR, de Waard PW, Pameyer JH, Houdijn Beekhuis W. Trypan blue capsule staining to visualize the capsulorhexis in cataract surgery. J Cataract Refract Surg. 1999;25:7–9. doi: 10.1016/s0886-3350(99)80004-2. [DOI] [PubMed] [Google Scholar]
- 10.Norn MS. Per operative trypan blue vital staining of corneal endothelium. Eight years’ follow up. Acta Ophthalmol (Copenh) 1980;58:550–5. doi: 10.1111/j.1755-3768.1980.tb08296.x. [DOI] [PubMed] [Google Scholar]
- 11.Lotfy A, Abdelrahman A. Trypan blue-assisted posterior capsulorhexis in pediatric cataract surgery. Clin Ophthalmol. 2017;24(11):219–222. doi: 10.2147/OPTH.S123150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saini JS, Jain AK, Sukhija J, Gupta P, Saroha V. Anterior and posterior capsulorhexis in pediatric cataract surgery with or without trypan blue dye: Randomized prospective clinical study. J Cataract Refract Surg. 2003;29:1733–7. doi: 10.1016/s0886-3350(03)00229-3. [DOI] [PubMed] [Google Scholar]
- 13.Werner L, Apple DJ, Crema AS, Izak AM, Pandey SK, Trivedi RH, et al. Permanent blue discoloration of hydrogel intraocular lens by intraoperative trypan blue. J Cataract Refract Surg. 2002;28:1278–86. doi: 10.1016/s0886-3350(02)01207-5. [DOI] [PubMed] [Google Scholar]
- 14.Kugelberg M, Wejde G, Jayaram H, Zetterström C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens: One-year follow-up. J Cataract Refract Surg. 2006;32:1627–31. doi: 10.1016/j.jcrs.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Beltrame G, Salvetat ML, Chizzolini M, Driussi GB, Busatto P, Di Giorgio G, et al. Posterior capsule opacification and Nd:YAG capsulotomy rates after implantation of silicone, hydrogel and soft acrylic intraocular lenses: A two-year follow-up study. Eur J Ophthalmol. 2002;12:388–94. doi: 10.1177/112067210201200508. [DOI] [PubMed] [Google Scholar]
- 16.Nishi O, Nishi K, Akura J. Speed of capsular bend formation at the optic edge of acrylic, silicone, and poly(methyl methacrylate) lenses. J Cataract Refract Surg. 2002;28:431–7. doi: 10.1016/s0886-3350(01)01094-x. [DOI] [PubMed] [Google Scholar]
- 17.Nishi O, Nishi K, Sakanishi K. Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthalmic Surg Lasers. 1998;29:587–94. [PubMed] [Google Scholar]
- 18.Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg. 2000;26:1543–9. doi: 10.1016/s0886-3350(00)00426-0. [DOI] [PubMed] [Google Scholar]
- 19.Vasavada AR, Praveen MR, Shah GD, Johar K, Sankaranarayanan R. A prospective evaluation of posterior capsule opacification in eyes with posterior capsule plaque - A case-control study. Asia Pac J Ophthalmol (Phila) 2017;6:13–20. doi: 10.1097/APO.0000000000000199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


