Abstract
Purpose:
The aim of this study is to assess the current antibiotic prophylaxis practice patterns for cataract surgery in India.
Methods:
This was a questionnaire-based E-survey carried out at a tertiary eye care center in India. An E-mail invitation to complete an online 20 point questionnaire survey was sent to all members of the All India Ophthalmological Society with valid E-mail addresses using a digital E-mail service. Duplicate entries were prevented.
Results:
Out of 1228 total respondents (8.2%) who completed the survey 38% reported using routine intracameral (IC) antibiotic prophylaxis. Another 7% place antibiotics in the irrigating solution. Of those using IC antibiotic prophylaxis, 91% adopted this practice within the past 2 years; 92% are using moxifloxacin with 56% using a commercially available moxifloxacin formulation. Those predominantly performing phacoemulsification (43% vs. 25% performing mostly manual small incision cataract surgery, P < 0.001) and more than 500 cataract surgeries annually (45% vs. 33%, P < 0.001) reported greater use of IC moxifloxacin. Self-reported endophthalmitis rates were statistically significantly greater in those not using IC antibiotics (0.045% vs. 0.036, P = 0.04). Although a majority of respondents believe that IC antibiotics are an important option (54%) and that it is important to have a commercially available solution (68%), many believe that other antibiotic prophylaxis methods are sufficient (31%).
Conclusion:
IC antibiotic prophylaxis for cataract surgery has sharply increased in India. In contrast to the West, intraocular moxifloxacin, which is commercially available in India, is preferred by the vast majority of users.
Keywords: Antibiotics, cataract surgery, endophthalmitis, India, intracameral, survey
Endophthalmitis following cataract surgery is a devastating complication and significant time and expense are spent on a wide array of preventive measures.
The use of intraocular antibiotic prophylaxis following cataract surgery has been increasing following the publication of numerous large retrospective studies and the multicenter randomized controlled trial sponsored by the European Society of Cataract and Refractive Surgeons (ESCRS) which support the efficacy of intracameral (IC) cefuroxime.[1,2,3,4,5,6,7,8,9,10] According to two American Society of Cataract and Refractive Surgery (ASCRS) member surveys, the percentage of surgeons using routine intraocular antibiotic prophylaxis increased from 30% in 2007 to 50% in 2014.[11,12]
There are currently more than 15,000 active cataract surgeons in India who perform approximately 6 million cataract surgeries annually.[13] In contrast to the West, manual small incision extracapsular cataract surgery (MSICS) accounts for a higher percentage of procedures in India due to a greater prevalence of indigent patients. Another important difference in India is the commercial availability of single-use IC moxifloxacin formulations (Auromox, Aurolab, India and 4-Quin, Entod pharmaceuticals, India). With these significant differences in mind, we surveyed ophthalmologist members of the All India Ophthalmological Society (AIOS) regarding current antibiotic prophylaxis practice patterns for cataract surgery.
Methods
The study was approved by the institutional ethics committee of the National Institute of Ophthalmology, Pune, India. The survey was conducted according to the guidelines of the Checklist for Reporting Results of Internet e-Surveys.[14]
An e-mail invitation to complete this online open survey was sent to all AIOS members with valid E-mail addresses using a digital E-mail service (https://mailchimp.com). The online survey was open for 4 weeks, and nonresponders were sent up to two E-mail reminders during this period. The survey was conducted using Survey Monkey (https://www.surveymonkey.com/) whose links recorded the IP addresses of the respondents and assigned a unique respondent ID to each client computer. As a result, duplicate entries were avoided by preventing users from completing the survey more than one time. In addition, duplicate database entries with the same user ID were eliminated before analysis to ensure a single entry from each respondent. Respondents were clearly informed that their participation was voluntary and that their responses would be kept anonymous and used only for data analysis.
The 20 point questionnaire was based on the previously published ASCRS member survey on antibiotic prophylaxis practice patterns.[10,11] Three questions were modified from the original survey to capture the respondent's geographic location (north, south, east, west, or central India), their predominant cataract surgical methodology (i.e. proportion of phacoemulsification (phaco), MSICS, and large incision, extracapsular cataract surgery with sutures) and whether they use IC antibiotic preparations that are commercially available in India.
Statistical analysis
All data were exported from the survey monkey website in continuous mode as Comma-separated values (CSV) files, and duplicate responses were identified using the unique respondent identifier and excluded. Similarly, respondents not answering all of the last five questions were taken as incomplete responses and were excluded. All continuous variables were described as mean ± standard deviation or median with interquartile range (IQR), whereas categorical variables were presented as proportions. Differences in IC antibiotic administration rates were compared across five different geographical regions of India using the Chi-square test. Respondents were divided into high- and low-volume groups based on the number of cataract surgeries performed annually (greater or less than 500 cases/year) and differences in IC antibiotic usage rates among these 2 groups were compared using the Chi-square test. Respondents were also divided into three groups according to the surgical mix: predominantly MSICS surgeons (>75% MSICS), predominantly phaco surgeons (>75% phaco) and those performing balanced proportions of both methods. Differences among these 3 groups were also analyzed using the Chi-square test. Differences in self-reported endophthalmitis rates were compared between those using and not using IC antibiotics. Finally, a logistic regression analysis was performed with the intent to identify factors that influenced routine adoption of IC antibiotics. All data were analyzed using STATA 12.0 I/c software package (Forth worth, Texas, USA). All values of P < 0.05 were considered to be statistically significant.
Results
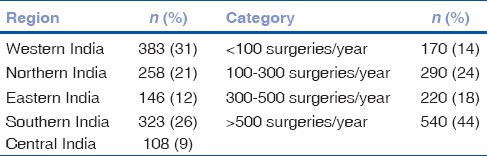
The survey link was e-mailed to 15,041 potential respondents and was conducted between July 15, and August 15, 2017. A total of 1228 respondents (8.2%) completed the survey. We did not identify any duplicate responses from individual respondents. A total of 467 respondents (38%) reported using IC antibiotics routinely after cataract surgery. The geographic distribution of our respondents is shown in Table 1. There were no significant regional differences in the IC antibiotic adoption rates (P = 0.20). Table 1 also shows the breakdown of respondents according to annual surgical volume. A statistically greater proportion of high volume surgeons (>500 cases/year) reported using IC antibiotics (45%) compared to lower volume surgeons (<500 cases/year) (33%) (P < 0.001). In terms of surgical case mix, 205 surgeons (17%) predominantly perform MSICS, 715 (58%) predominantly perform phaco and 308 (25%) performed both phaco and MSICS relatively equally. Significantly fewer of those predominantly performing MSICS use routine IC antibiotic prophylaxis compared to those predominantly performing phaco (25% vs. 43%, P < 0.001).
Table 1.
Geographic distribution and self-reported volume of annual cataract surgery
More than 90% of respondents (n = 1109) use perioperative topical antibiotics for cataract surgery; of these, 73% (806/1109) preferred moxifloxacin and 12% (130/1109) of them preferred gatifloxacin. When using preoperative topical antibiotics, 37% (456/1228) started them 3 days before surgery, 37% (459/1228) started them on the day before surgery, and 10% (122/1228) of respondents started them on arrival at the surgical center. Nearly 94% of respondents (1157/1228) use topical povidone-iodine immediately before surgery. Almost half of the surgeons instil antibiotics at the end of the surgery and 98% of surgeons use topical antibiotics postoperatively [Table 2].
Table 2.
Route of antibiotic used at the end of surgery and pattern of topical antibiotics used postoperatively (respondents could select more than one antibiotic route at the conclusion of surgery)

Overall, 45% (547/1228) of respondents routinely employ intraocular antibiotic prophylaxis. Of these, 85% (467/547) directly inject the antibiotic intracamerally and 15% (80/547) add the antibiotic to the irrigation solution. The antibiotic agent and source used for intraocular prophylaxis are shown in Table 3. Of those using IC antibiotic prophylaxis, 92% are using moxifloxacin, with 56% using a commercially available formulation. Similarly, moxifloxacin was the most common drug (40%) used in the irrigating solution as well. Of the 467 respondents using IC antibiotics, 427 (91%) reported starting this practice within the past 2 years.
Table 3.
Source and route of intraocular antibiotic administration

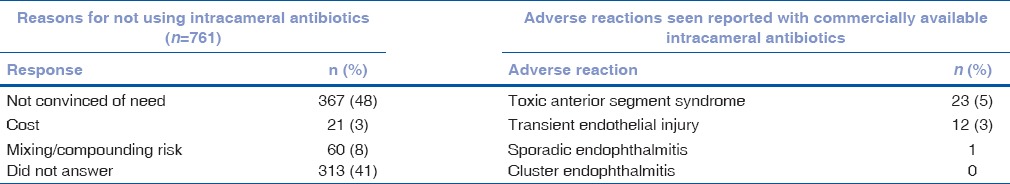
When asked whether IC antibiotics were important for endophthalmitis prophylaxis, 18% were not sure, and nearly one-third believed that it is an important option, but that other antibiotic prophylaxis methods are sufficient [Table 4]. However, the majority of respondents (68%) believe that it is important to have a commercially approved drug for IC use [Table 4]. Among those using IC antibiotics, most use commercially available moxifloxacin formulated for intraocular use and 86% combine it with topical antibiotic postoperatively [Table 5]. The most common reason that respondents gave for not using intraocular antibiotics was being unconvinced of the need (48%), with 41% not providing a reason.
Table 4.
Responses for importance of intracameral antibiotics and need for Drugs Controller General of India approved commercially available intracameral antibiotic

Table 5.
Responses regarding usage, formulation, and preparation of intracameral antibiotics (n=467)
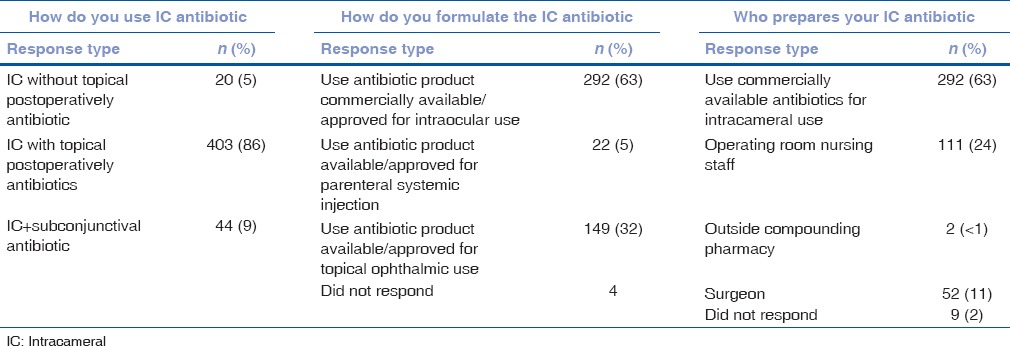
Table 6 shows the number of respondents who have seen adverse reactions to intraocular antibiotic prophylaxis. A total of 141 surgeons (11%) reported using IC antibiotics prepared directly from systemic preparations, either currently or in the past. Of these, 120 had no complications, 13 (9.2%) reported having had TASS (vs. 5% in commercially available IC antibiotics, P = 0.03), and 8 (6%) reported seeing cases with transient endothelial injury (vs. 3% in commercially available IC antibiotics, P = 0.05).
Table 6.
Reasons for not using intracameral antibiotics and adverse reactions seen with use of intracameral antibiotics
Using multivariable logistic regression to analyze differences according to surgical volume and method, surgeons performing >500 cases/year were almost twice as likely to inject IC antibiotics compared to those performing <500 cases/year (odds ratio = 1.71, 95% of confidence interval = 1.3–2.2, P < 0.001). Endophthalmitis rates were reported by 1011 surgeons (mean = 4.1 ± 7.8, median = 1, IQR = 1–5 cases, range = 0–80). Respondents not using IC antibiotics reported significantly higher endophthalmitis rates on average (4.4 ± 8.2 cases, median = 2, IQR = 1–5 cases) compared to those using IC antibiotics (3.63 ± 7.2 cases, median = 1, IQR = 0–4 cases) (P = 0.04). Fifteen surgeons (1.2%) reported an endophthalmitis rate of more than 50 cases/10,000 surgeries (>0.5%). Eleven of these did not use IC antibiotics.
Discussion
Lalitha et al. recent review reported that endophthalmitis rates in India have ranged between 0.04%–0.15% during the past two decades.[13] This is in line with other reported rates internationally,[6,15,16,17] but based on the extremely high-volume of cataract surgery performed in India, Lalitha et al. estimated that there may be as many as 4800 Indian endophthalmitis cases annually.
Use of routine IC antibiotic prophylaxis is increasing in many countries, and the choice of antibiotic agent varies geographically, depending on drug availability.[10,18] Cefuroxime is commercially available in the UK (Ximaract, Bausch and Lomb, UK Limited) and other European countries (Apokam, Thea). A survey of ESCRS surgeons published in 2014[19] showed that 74% usually or always used an IC antibiotic and 82% of them used cefuroxime. In the 2014 ASCRS survey, among respondents using intraocular antibiotic prophylaxis, vancomycin was the most common agent (37% overall and 52% of the American respondents). Moxifloxacin was used by 33% of those employing intraocular antibiotics and cefuroxime by 26%.[12]
Of the 15,041 Indian ophthalmologists to whom the questionnaire was sent, we found that 38% of 1228 who responded, routinely inject an IC antibiotic after cataract surgery. An additional 7% reported adding antibiotics in the irrigating solution. This distribution and the overall rate of 45% using intraocular prophylaxis are similar to results from the 2014 ASCRS survey.[12] We found that higher volume surgeons were more likely to use IC antibiotics and that those injecting IC antibiotics reported a significantly lower rate of endophthalmitis (0.036%) compared to those that do not (0.045%) (P = 0.04).
Adoption of IC antibiotic prophylaxis was statistically lower among surgeons predominantly using MSICS, compared to those predominantly performing phaco. Although it requires a larger sutureless incision than phaco, MSICS is very cost effective and efficacious for advanced, mature cataracts. MSICS is therefore often used for charitable eye surgery in resource-poor societies and is more commonly employed in India relative to other large countries. Recent large studies from the Aravind Eye Care System (AECS) reported a slightly higher endophthalmitis rate with MSICS relative to phaco, but that that IC moxifloxacin reduced the MSICS endophthalmitis rate by 3-fold.[20,21]
One striking finding from our survey is that moxifloxacin may account for more than 90% of IC antibiotic use in India. This may reflect the commercial availability of IC moxifloxacin from two Indian pharmaceutical companies. However, 33% of respondents using intraocular antibiotic prophylaxis were injecting moxifloxacin taken from a topical bottle, compared to 56% who used a commercial IC moxifloxacin solution. IC cefuroxime is also commercially available in India (Entod pharmaceuticals, India) but was used by only 6% of surgeons employing intraocular antibiotic prophylaxis.
Although topical povidone-iodine and perioperative topical antibiotics are used by more than 90% of respondents, the majority are not using IC antibiotic prophylaxis. As with the ASCRS survey, the most common reason was being unconvinced of the need (48% compared to 65% in the ASCRS survey). However, compared to the ASCRS survey, cost (3% vs. 19%) and mixing risk (8% vs. 49%) were cited much less frequently by Indian ophthalmologists, which reflects the commercial availability of intraocular antibiotic solutions at reasonable costs in India. Although a majority of respondents believe that IC antibiotics are an important option (54%), and that it is important to have a commercially available solution (68%), many believe that other antibiotic prophylaxis methods are sufficient (31%).
One of the moxifloxacin formulations commercially available in India (Auromox) was used in the largest study published to date of IC moxifloxacin prophylaxis.[21] This 29 months study conducted within the AECS network of hospitals, compared 314,638 consecutive eyes that received IC moxifloxacin to 302,815 eyes that did not, and found a 3.5-fold overall reduction in endophthalmitis rate (3-fold for MSICS and 6-fold for phaco). Because the study was published immediately before our survey in June 2017, it is unclear how many of our respondents were aware of these results. More recently, AECS reported endophthalmitis rates in 1,087,907 consecutive eyes performed during the 51 months starting in January 2013. The endophthalmitis rate was 0.02% in the 555,550 eyes that received IC moxifloxacin prophylaxis, compared to 0.07% in the 532,357 eyes that did not.[21] The study respondents reported TASS and transient endothelial injury in about 5% of eyes and attributed this to the IC antibiotics they used. In contrast, Haripriya et al. did not report any such events in their study. Results from an online survey may not be comparable to a clinical study as the latter is conducted in a more controlled setting with the same drug being used. However, it is likely that there is a possibility of Toxic Anterior Segment Syndrome after using IC antibiotics routinely, as evidenced from our responses. Commercially available antibiotics appear to be relatively safer than IC antibiotics compounded from systemic preparations. Further studies are necessary to understand the adverse effect profile of IC antibiotics. In addition, results from this survey should be interpreted with caution because <10% of Indian ophthalmologists responded to the survey and results, although representative, may not be generalizable to the entire country.
Conclusion
Our survey suggests that IC antibiotic prophylaxis in India is rising sharply. A total of 38% of respondents now use IC antibiotics, with 91% having commenced this within the past 2 years. As more ophthalmologists become aware of recently published results with an IC moxifloxacin solution that is commercially available in India, it is possible that IC antibiotic adoption will further increase for both phaco and MSICS in India.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the inputs from Dr. Sabyasachi Sengupta's Research Academy for Manuscript editing. We thank Dr S.B. Kelkar, Director of National Institute of Ophthalmology, Pune for his valuable inputs.
References
- 1.Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32:407–10. doi: 10.1016/j.jcrs.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 2.Endophthalmitis Study Group. European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–88. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 3.García-Sáenz MC, Arias-Puente A, Rodríguez-Caravaca G, Bañuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery ten-year comparative study. J Cataract Refract Surg. 2010;36:203–7. doi: 10.1016/j.jcrs.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Behndig A, Montan P, Stenevi U, Kugelberg M, Lundström M. One million cataract surgeries: Swedish National Cataract Register 1992-2009. J Cataract Refract Surg. 2011;37:1539–45. doi: 10.1016/j.jcrs.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Tan CS, Wong HK, Yang FP. Epidemiology of postoperative endophthalmitis in an Asian population: 11-year incidence and effect of intracameral antibiotic agents. J Cataract Refract Surg. 2012;38:425–30. doi: 10.1016/j.jcrs.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 6.Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish National Study. J Cataract Refract Surg. 2013;39:15–21. doi: 10.1016/j.jcrs.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 7.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39:8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Daien V, Papinaud L, Gillies MC, Domerg C, Nagot N, Lacombe S, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmol. 2016;134:810–6. doi: 10.1001/jamaophthalmol.2016.1351. [DOI] [PubMed] [Google Scholar]
- 9.Creuzot-Garcher C, Benzenine E, Mariet AS, de Lazzer A, Chiquet C, Bron AM, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: A Nationwide Study in France from 2005 to 2014. Ophthalmology. 2016;123:1414–20. doi: 10.1016/j.ophtha.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Haripriya A, Baam ZR, Chang DF. Endophthalmitis prophylaxis for cataract surgery. Asia Pac J Ophthalmol (Phila) 2017;6:324–9. doi: 10.22608/APO.2017200. [DOI] [PubMed] [Google Scholar]
- 11.Chang DF, Braga-Mele R, Mamalis N, Masket S, Miller KM, Nichamin LD, et al. Prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33:1801–5. doi: 10.1016/j.jcrs.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Chang DF, Braga-Mele R, Henderson BA, Mamalis N, Vasavada A. ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2014 ASCRS member survey. J Cataract Refract Surg. 2015;41:1300–5. doi: 10.1016/j.jcrs.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Lalitha P, Sengupta S, Ravindran RD, Sharma S, Joseph J, Ambiya V, et al. A literature review and update on the incidence and microbiology spectrum of postcataract surgery endophthalmitis over past two decades in India. Indian J Ophthalmol. 2017;65:673–7. doi: 10.4103/ijo.IJO_509_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eysenbach G. Improving the quality of web surveys: The checklist for reporting results of internet E-surveys (CHERRIES) J Med Internet Res. 2004;6:e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day AC, Donachie PH, Sparrow JM, Johnston RL. Royal College of Ophthalmologists’ National Ophthalmology Database. The royal college of ophthalmologists’ national ophthalmology database study of cataract surgery: Report 1, visual outcomes and complications. Eye (Lond) 2015;29:552–60. doi: 10.1038/eye.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam KY, Lee JE, Lee JE, Jeung WJ, Park JM, Park JM, et al. Clinical features of infectious endophthalmitis in South Korea: A five-year multicenter study. BMC Infect Dis. 2015;15:177. doi: 10.1186/s12879-015-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao K, Zhu Y, Zhu Z, Wu J, Liu Y, Lu Y, et al. The incidence of postoperative endophthalmitis after cataract surgery in China: A multicenter investigation of 2006-2011. Br J Ophthalmol. 2013;97:1312–7. doi: 10.1136/bjophthalmol-2013-303282. [DOI] [PubMed] [Google Scholar]
- 18.Behndig A, Cochener B, Güell JL, Kodjikian L, Mencucci R, Nuijts RM, et al. Endophthalmitis prophylaxis in cataract surgery: Overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39:1421–31. doi: 10.1016/j.jcrs.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: Update on the ESCRS endophthalmitis study. J Cataract Refract Surg. 2014;40:138–42. doi: 10.1016/j.jcrs.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind eye hospital. Ophthalmology. 2016;123:302–8. doi: 10.1016/j.ophtha.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Haripriya A, Chang DF, Ravindran RD. Endophthalmitis reduction with intracameral moxifloxacin prophylaxis: Analysis of 600 000 surgeries. Ophthalmology. 2017;124:768–75. doi: 10.1016/j.ophtha.2017.01.026. [DOI] [PubMed] [Google Scholar]


