Abstract
Background
The safety of living liver donors is the paramount priority of liver transplantation surgeons. The liver has an effective regeneration capacity. The regeneration rate of the liver remnant in living liver donors provides much information useful in liver surgery. The outcome of the remnant liver after hepatectomy can be affected by many different perioperative factors.
Material/Methods
A total of 46 patients were enrolled in the study. Retrospective clinical data, including preoperative and postoperative early and late computed tomography liver volumetry measurements, estimated resection volumes, resected liver weights, and postoperative laboratory values, were statistically evaluated according to the liver resection type.
Results
No significant difference was detected in age, sex, calculated and computed tomography estimated total liver volume, intraoperative Hb decrease, postoperative complications, or postoperative portal vein flow rate. Postoperative liver enlargement rates were significant higher in the right hemihepatectomy (RHH) group than in the left lateral sectionectomy (LLS) group. The size of the liver remnant or graft has a major effect on regeneration rate. Postoperative biliary leakage did not have any significant effect on liver regeneration. No post-hepatectomy liver failure was detected among the liver donors.
Conclusions
Liver hypertrophy depends on the extent of liver resection. The cause of volume decrease in the LLS group after hepatectomy in our series appears to be the gradual atrophy of liver segment 4. RHH and LLS surgeries differ from each other in terms of resected liver volume, as well as inflammatory activity, and the latter appears to affect liver regeneration.
MeSH Keywords: Liver Regeneration, Liver Transplantation, Living Donors, Magnetic Resonance Imaging, Organ Size, Spiral Cone-Beam Computed Tomography
Background
Liver transplantation success depends mainly on the liver graft. Without a suitable graft for the recipient, liver transplantation is impossible to perform. The small cadaveric donor pool is still a major problem, so living related liver transplantation is performed nearly 2 to 3 times more often than cadaveric liver transplantation in Turkey [1]. The safety of living liver donors is the paramount priority of liver transplantation surgeons. In contrast of other organs, the liver has a very effective regeneration capacity [2]. This allows transplant surgeons to take a part of the liver of a healthy person and transplant it to the recipient, who otherwise will not be able to survive. However, living liver donor hepatectomy is still a challenging operation that needs meticulous preoperative evaluation and selection of a liver donor, as well as excellent surgical skills, which directly affects the donor and the recipient. The regeneration rate of the liver remnant in living liver donors provides much information useful in successful liver surgery. The outcome of the remnant liver after hepatectomy for liver donation can be affected by many different perioperative factors. The present study aimed to determine the major factors affecting the hepatic regeneration rate after liver donor surgery in our series, with the goal of improving the overall safety of living liver donors.
Material and Methods
From January 2012 to July 2017, 78 patients underwent hepatectomy as living liver donors in the Hepatopancreatobiliary (HPB) Surgery and Liver Transplantation Unit of the Istanbul Faculty of Medicine, Istanbul University. The medical records of the 78 patients were reviewed retrospectively. We excluded 32 patients because of missing data such as late postoperative abdominal computed tomography (CT) or magnetic resonance (MR) scans. Thus, a total of 46 patients were selected. Donors with suitable liver volume (resection rate ≤75%) were selected from among healthy relatives of recipients. All preoperative biochemical and laboratory values, as well as blood counts, had to be within normal limits. Viral serology also had to be negative. No systemic illnesses of donor candidates were allowed for liver donation. Normal liver biopsy results (without intermediate or high grades of steatosis) and BMI lower than 30 kg/m2 were also criteria for donor selection. Donor operations were all performed by the same surgical team using an ultrasonic surgical dissector-aspirator system during liver transection without using inflow occlusion (Pringle’s) maneuver. According to our routine protocol, we performed postoperative 1st day Doppler evaluation of remnant liver vasculature and postoperative 7th day and postoperative late (>30 days) tri-phasic abdominal CT imaging in all liver donors.
From the medical records, we collected the following data on the clinical profile of the patients: age, sex, height, weight, hepatectomy type, intraoperative hemoglobin (Hb) decrease (as an indicator of surgical bleeding), preoperative CT-determined total liver volume (preCT-TLV), preoperative CT-determined resected liver volume (preCT-resV), resected liver weight or volume (specific gravity of normal liver tissue is about 1.0 g/cm3, so the volume is taken as similar to the weight), calculated postoperative remnant liver volume (RLV) and percentage (RLV%), postoperative 7th day CT-determined total liver volume (p7-CT-TLV), late postoperative CT-determined total liver volume (lateCT-TLV), and postoperative 1st day portal flow rate according to Doppler measurement. We also recorded the following early and late postoperative (1st and 7th day) laboratory values: platelet (PLT) count, lactate dehydrogenase (LDH), creatinine (CREA), alanine aminotransaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyltransferase (GGT), total bilirubin (TBIL), international normalized ratio (INR), C-reactive protein (CRP), length of postoperative hospitalization, and postoperative complications days). From data in the patient record, were calculated the following: body mass index (BMI), body surface area (BSA) according to DuBois formula, total liver volume (TLV) according to the formula of Vauthey et al., postoperative 7th day liver enlargement rate (between post-hepatectomy and postoperative 7th day abdominal imaging) (P7LER), postoperative total liver enlargement rate (between post-hepatectomy and last postoperative abdominal imaging) (PTLER), postoperative 7-day liver enlargement in ml (P7ml), postoperative 7-day liver enlargement in ml/day (P7ml/day), and postoperative total liver enlargement in ml/day (PTLEml/day). We have calculated: RLV=preCT-TLV–preCT-resV, P7LER=[(p7-CT-TLV–RLV)/RLV]×100, PTLER=[(lateCT-TLV–RLV)/RLV]×100, P7ml=(p7-CT-TLV–RLV), P7ml/day=(p7-CT-TLV–RLV)/7, and the PTLEml/day=(lateCT-TLV–RLV)/n {where n is used to infer total number of postoperative days till the last abdominal imaging}.
We decided to use the preCT-resV rather than the resected liver weight in calculation of enlargement rates because we believe that CT-gained valuables should not be mixed with the weight-measurements after hepatectomy in order to estimate the liver enlargement rates [4–6]. We used CT-MR volumetry software (ExtemePACS 2016 Ankara, Turkey, licensed to Istanbul University) for liver volume determination. All postoperative morbidities were recorded and graded according to the Clavien-Dindo classification. Postoperative liver failure was defined according to the International Study Group of Liver Surgery (ISGLS) grading. The study protocol was approved by the Ethics Committee of Istanbul University Istanbul Medical Faculty (2017/1132).
Statistical evaluation
Data analysis was performed with SPSS 21.0 statistics software (SPSS Inc., Chicago, IL, USA, Licensed to Istanbul University). Mean P7LER, PTLER, P7ml, P7ml/day, PTLEml/day, median days of hospitalization, standard deviations, and frequencies were calculated. The differences between groups were analyzed with the t test for categorical variables and the Mann-Whitney U test for continuous variables. We used the Kruskal-Wallis test and Spearman’s or Pearson’s correlation analysis to compare the liver enlargement rates, type of hepatectomy, length of hospitalization, complication rate, biliary leakage rate, and Doppler-measured portal vein flow rate after hepatectomy. The correlation between TLV and preCT-TLV and between preCT-resV and resected liver weight was evaluated through calculation of intraclass correlation coefficient (ICC). An overall 5% Type-I error level was used to infer statistical significance with a confidence interval of 95%.
Results
A total of 46 patients (17 [37%] females and 29 [63%] males) were included into our study. The median age was 31 years (range, 18 to 58). The median BMI was 24 kg/m2 (range, 18 to 29). After excluding some donors because they were outside the parameters of our study design, only 46 patients remained, who underwent right hepatectomy or left lateral sectionectomy for liver donation. Therefore, we established right hemihepatectomy (RHH) and left lateral sectionectomy (LLS) donor groups. The RHH group consisted of 21 (46%) patients and the LLS group consisted of 25 (54%) patients. Detailed characteristics of donors are given in Table 1. The preoperative total blood count levels and biochemical values were all within normal limits in all of the liver donors. There were no statistically significant differences in sex, age, BMI, height, weight, TLV, preCT-TLV, postoperative portal flow rate, morbidities, biliary leakage rate, length of hospitalization, or intraoperative Hb decrease between the RHH and LLS groups (p<0.05). TBIL1 (p=0.000), CRP1 (0.005), INR1 (p=0.000), ALT7 (p=0.045), TBIL7 (p=0.000), CRP7 (p=0.050), INR7 (p=0.000), PLT7 (p=0.001), and Prio values (p=0.0004) were found to be significantly different between RHH and LLS groups. Because we decided to use the preCT-TLV and preCT-resV rather than calculated-TLV according to the Vauthey et al. formula, and the resected liver weight after hepatectomy in order to calculate the postoperative liver enlargement rates, we compared them using ICC values for reliability. ICC values revealed that using preCT-resV and preCT-TLV is a reliable option {[preCT-TLV and calculated-TLV: Cronbach’s Alpha =0.778; Single Measures ICC=0.636; Average Measures ICC=0.778] and [preCT-resV and resected liver weight: Cronbach’s Alpha=0.965; Single Measures ICC=0.933; Average Measures ICC=0.965]}.
Table 1.
Characteristics of donors.
| Right hemihepatectomy | Left lateral sectionectomy | ||
|---|---|---|---|
| Age | 33.67 (±11.425) | 31.72 (±7.850) | p=0.513 |
| Sex (male: female) | 13:8 | 17:8 | n.s. |
| BMI kg/m2 | 23.38 (±2.729) | 24.40 (±2.799) | p=0.219 |
| TLV ml | 1422 (±221) | 1511 (±244) | p=0.202 |
| Preop Ct Volumetry ml | 1369 (±234) | 1505 (±226) | p=0.052 |
| Remnant Liver Volume % | 35 (27–50) | 81 (63–88) | Median (range) |
| Preop est. Ct Res. V ml | 885 (515–1189) | 284 (151–557) | Median (range) |
| Resected liver weight g | 770 (428–1110) | 274 (146–470) | Median (range) |
| Postop0 liver V ml | 484 (302–790) | 1222 (948–1764) | Median (range) |
| Postop7 liver V ml | 900 (683–1193) | 1470 (1047–1846) | Median(range) |
| PostopLate liver V ml | 1004 (754–1277) | 1363 (957–2029) | Median (range) |
| Postop1 LDH IU/l | 398 (±145) | 469 (±262) | p=0.255 |
| Postop1 CREA mg/dl | 0.67 (±0.17) | 0.65 (±0.14) | p=0.627 |
| Postop1 TBIL mg/dl | 2.02 (±0.62) | 1.14 (±0.61) | p=0.000 |
| Postop1 ALP IU/l | 61 (±18) | 58 (±25) | p=0.622 |
| Postop1 AST IU/l | 195 (±124) | 219 (±160) | p=0.577 |
| Postop1 ALT IU/l | 229 (±178) | 313 (±245) | p=0.184 |
| Postop1 GGT U/l | 30 (±27) | 25 (±18) | p=0.461 |
| Postop1 INR | 1.4 (±0.16) | 1.17 (±0.07) | p=0.000 |
| Postop1 CRP mg/dl | 25 (±14) | 43 (±25) | p=0.007 |
| Postop1 PLT ×103/mm3 | 206 (±42) | 211 (±46) | p=0.655 |
| Postop1 PVFlow cm/sec | 39.3 (±14.6) | 37.7 (±13.9) | p=0.697 |
| Hb decrease g/dl | 0.7 (±0.6) | 0.7 (±0.6) | p=0.764 |
| Postop7 LDH IU/l | 308 (±164) | 269 (±93) | p=0.339 |
| Postop7 CREA mg/dl | 0.60 (±0.12) | 0.7 (±0.15) | p=0.476 |
| Postop7 TBIL mg/dl | 0.78 (±0.46) | 0.27 (±0.12) | p=0.000 |
| Postop7 ALP IU/l | 89 (±38) | 100 (±60) | p=0.461 |
| Postop7 AST IU/l | 58 (±28) | 47 (±32) | p=0.240 |
| Postop7 ALT IU/l | 89 (±31) | 115 (±53) | p=0.045 |
| Postop7 GGT U/l | 124 (±118) | 81 (±49) | p=0.127 |
| Postop7 INR | 1.2 (±0.16) | 1 (±0.80) | p=0.000 |
| Postop7 CRP mg/dl | 27.78 (±27.00) | 45.92 (±33.99) | p=0.050 |
| Postop7 PLT ×103/mm3 mean (SD) | 195 (±61) | 260 (±58) | p=0.001 |
n.s. – not significant; BMI – body mass index; BSA – body surface area; TLV – total liver volume; Preop – preoperative; Ct – computed tomography; n.c. – not suitable to compare; est. – estimated; V – volume; Postop0 liver V – Liver volume just after resection; Postop7 liver V – 7th day postoperative liver volume; PostopLate liver V – Liver volume calculation with a late postoperative Ct-scanning; Postop1 – 1th postoperative day; LDH – lactate dehydrogenase; CREA – creatinine; ALT – alanin aminotransaminase; AST – aspartate aminotrasnferase; ALP – alkaline phosphatase; GGT – gamma-glutamyltransferase; TBIL – total bilirubin; INR – international normalized ratio; CRP – C-reactive protein; PLT – platelet count; PVF – portal vein flow; Hb – hemoglobin; Postop7 – 7th postoperative day; SD – standart deviation.
PTLER, PTLEml/day, P7LER, P7ml, and P7ml/day values are significantly higher in the RHH group than in the LLS group (Table 2).
Table 2.
Liver enlargement rates and gained volumes.
| RHH | LLS | ||
|---|---|---|---|
| PTLER % median (range) | 109 (21–198) | 7 (−11–53) | p=0.000 |
| PTLEml/daymedian(range) | 14 (4–22) | 1 (−4–22) | p=0.000 |
| P7LER % mean (SD) | 94 (±39) | 22 (±16) | p=0.000 |
| P7ml mean (SD) | 416 (±121) | 246 (±170) | p=0.000 |
| P7ml/day mean (SD) | 60 (±17) | 35 (±24) | p=0.000 |
RHH – right hemihepatectomy; LLS – left lateral sectionectomy; PTLER – postoperative total liver enlargement rate; PTLEml/day – postoperative total liver enlargement in ml/day; P7LER – postoperative 7th day liver enlargement rate; SD – standart deviation; P7ml – postoperative 7 days liver enlargement in ml; P7ml/day – postoperative 7 days liver enlargement in ml/day.
No statistically significant difference was found between males and females in terms of PTLER, PTLEml/day, P7LER, P7ml, and P7ml/day values.
No post-hepatectomy liver failure (PHLF) was detected among liver donors according to ISGLS criteria in RHH and LLS groups after liver resection for living related liver transplantation.
According to Clavien-Dindo classification, a total of 17 patients (37%) developed postoperative complications, including bile leakage, wound complications, controlled low-grade ascites, hemorrhage, subileus, or drug allergy (16 [35%] patients grade I, and 1 [2%] patient grade II) (Table 3). There was no mortality (no mortality was also observed in our donor hepatectomy series since the beginning of our living-donor liver transplant program).
Table 3.
Morbidities after liver resection.
| RHH | LLS | ||
|---|---|---|---|
| Number of patients with morbidities | 8 | 9 | p=0.905 |
| Clavien-Dindo (n; grade) | 8;G I | 8; G I and 1;G II | p=0.905 |
| Biliary leakage n | 4 | 5 | p=0.937 |
| PHLF n | 0 | 0 |
RHH – right hemihepatectomy; LLS – left lateral sectionectomy; n – number of patients; G – grade; PHLF – posthepatectomy liver failure.
There was no statistically significant difference between donors with and without postoperative complications in terms of PTLER, PTLEml/day, P7LER, P7ml, and P7ml/day values (p=0.724, p=0.290, p=0.300, p=0.802, and p=0.392, respectively). Postoperative biliary leakage did not have any statistically significant effect on liver regeneration rates after hepatectomy (PTLER, PTLEml/day, P7LER, P7ml, and P7ml/day, p=0.879, p=0.552, p=0.648, p=0.740, and p=0740, respectively).
Spearman analysis showed a significant inverse relationship between PTLER and RLV% (p=0.000, r=−0.836), RLV (p=0.000, r=−0.820), p7-CT-TLV (p=0.000, r=−0.676), lateCT-TLV (p=0.003, r=−0.423), postoperative 1st day CRP (CRP1) (p=0.027, r=−0.326), postoperative 1st day PLT (p=0.022, r=−0.337), postoperative 7th day ALT (p=0.050, r=−0.291), and postoperative 7th day PLT (PLT2) (p=0.000, r=−0.523), and showed a positive relationship between PTLER and preCT-resV (p=0.000, r=0.756), resected liver weight (p=0.000, r=0.677), postoperative 1st day TBIL (TBIL1) (p=0.001, r=0.486), postoperative 1st day INR (INR1) (p=0.000, r=0.697), postoperative 7th day TBIL (TBIL7) (p=0.000, r=0.658), and postoperative 7th day INR (INR2) (p=0.000, r=0.581).
There was also a significant inverse relationship between P7LER and RLV%, preCT-TLV, RLV, p7-CT-TLV, lateCT-TLV, CRP1, and PLT7, and a positive relationship between P7LER and preCT-resV, resected liver weight, TBIL1, INR1, TBIL7, and INR7, according to Spearman analysis.
A negative correlation was found between P7ml and RLV%, RLV, CRP1, INR1, and preCT-TLV, PLT7, and a positive correlation was found between P7ml and preCT-resV, resected liver weight, and TBIL7.
P7ml/day values were inversely correlated with RLV%, CRP1, preCT-TLV, and PLT2, and were positively correlated with RLV, preCT-resV, resected liver weight, INR1, and TBIL7.
PTLEml/day values were positively correlated with preCT-resV, resected liver weight, TBIL1, INR1, TBIL7, and INR7, and were negatively correlated with RLV%, RLV, p7-CT-TLV, and PLT7.
No correlation was found between intraoperative Hb decrease and liver enlargement rates.
We created a new and simple parameter, called the “postoperative regeneration-inflammatory ratio” (PRIO), through dividing the PLT1 value by the CRP1 value to help predict the general trend of liver regeneration after hepatectomy (Table 4). PRIO value was found to be positively but weakly correlated with PTLER (p=0.047, r=0.297) and P7LER (p=0.002, r=0.452).
Table 4.
Postoperative regeneration-inflammatory ratio values.
| RHH | LLS | ||
|---|---|---|---|
| Postop1CRP mg/dl | 25 (±15) | 43 (±25) | p=0.005 |
| Postop1PLT ×103/mm3 | 206 (±42) | 211 (±46) | p=0.655 |
| Prio (mean; SD) | 10411 (±5262) | 6118 (±3681) | p=0.003 |
RHH – right hemihepatectomy; LLS – left lateral sectionectomy; Postop1 – 1th postoperative day; CRP – C-reactive protein; PLT – platelet count; Prio – postoperative regeneration-inflammatory ratio (Prio=PLT1/CRP1); SD – standart deviation.
Discussion
The first successful liver transplantation was performed by Thomas Starzl in 1967 in the United States. The first liver transplant in Turkey was performed by Haberal in 1988. The first successful living donor liver transplant in Turkey was performed in 1990 by the same team [1]. Turkey has one of the highest volumes of liver transplantation per population worldwide [1]. This would be impossible in Turkey without living donor procedures because of the very limited number of cadaveric liver grafts due to several reasons. With the rising number of living donor liver transplantations, the safety of liver donors became a high priority in our department. Detailed preoperative evaluation and meticulous selection of donors is a sine qua non for living donor liver transplantation. The regeneration capacity of the liver enables successful transplantation of a partial liver graft [2]. Several molecular pathways were described and reported in detail which expanded the knowledge of liver transplant surgeons as well as HPB surgeons about clinical aspects of liver regeneration [2,7,8].
The statistical evaluation of our 2 groups (RHH and LLS) revealed that they are comparable—no significant difference was detected among age, sex, BMI, TLV, preCT-TLV, intraoperative Hb decrease, postoperative complications, or postoperative portal vein flow rate (Tables 1, 3).
TBIL1, INR1, TBIL7, INR7, and PRIO levels were significantly higher in the RHH group than in the LLS group, and CRP1, CRP7, PLT7, and ALT7 values were found to be significantly higher in the LLS group than in the RHH group. After the LLS procedure, we detected significantly higher levels of CRP in donors than in RHH. PRIO values were significantly higher in the RHH group. After RHH, liver regeneration was significantly faster than in the LLS group. In the first postoperative week, there is a burst of growth in the remnant liver tissue. Mean values reached 60 ml/day and 35 ml/day in the first postoperative week in the RHH and LLS group, respectively. Mean P7LER values are also impressive in both groups: 94% (RHH) vs. 22% (LLS) (Table 2). Several factors may affect regeneration rates. Spearman analysis showed that P7LER and PTLER have a strong negative correlation with RLV% and have a strong correlation with preCT-resV (Figure 1). Our results show that the size of the liver remnant or graft had the greatest impact on regeneration rate and had quantity similar to that previously reported [2,9–11]. BMI levels below 30 kg/m2 did not affect the regeneration rates after hepatectomy, consistent with Lock et al. [12]. Although Margonis et al. reported a positive impact of early postoperative platelet count on volumetric liver enlargement, we were unable to show any significant difference between donors with PLT1 lower than 150×103/mm3 and normal PLT1 values [13]. Interestingly, we realized after comparison of the PLT7 values with the post-hepatectomy liver regeneration rates (cut-off level taken as 150×103/mm3) that there was a statistically significant inverse correlation between PLT7 values and P7LER (p=0.039), p7ml (p=0.042), and P7ml/day (p=0.041). No significant correlation was detected between PLT7 and PTLER and PTLEml/day. Following RHH, with obviously greater resection volume, postoperative TBIL1, INR1, TBIL7, and INR2 values are higher than post-hepatectomy values after LLS. Liver regeneration after RHH is without doubt faster than after LLS in total as well as in the first week. Remarkable significant higher levels of CRP1, and CRP2 after LLS than RHH revealed that LLS provokes more inflammation than does RHH. The evaluation of our series in terms of postoperative CRP levels showed a moderate to weak negative correlation with the liver regeneration rates, similar to some previous reports [14,15].
Figure 1.
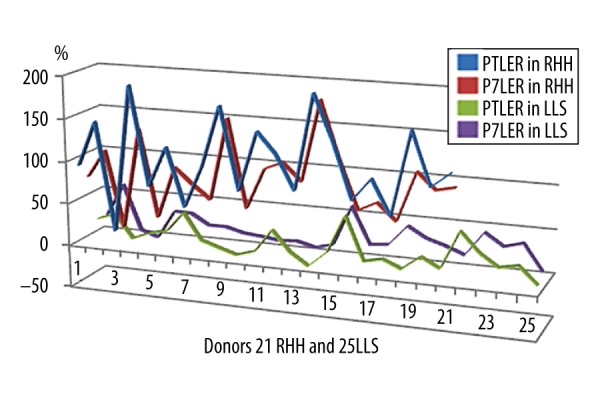
Total and 7th day regeneration rates after right hemihepatectomy and left lateral sectionectomy. RHH – right hemihepatectomy; LLS – left lateral sectionectomy; PTLER – postoperative total liver enlargement rate, P7LER – postoperative 7th day liver enlargement rate.
CT volumetry and TLV are reliable and non-invasive methods to determine the liver volume according to ICC evaluation. CT volumetry is more accurate in left lateral section grafts (Figures 2, 3). RHH and LLS are different surgical resections. Both the amount of resected liver volume and the anatomic location of resected liver segments affect the postoperative regeneration process. Olthoff et al. studied a large series including living–liver donors (right and left hemilivers) and recipients [16]. The liver regeneration rates of right or left liver remnants seem to be similar in their study. The features of enlargement rate of remnant liver after left lateral resection differs from the regeneration of remnant liver after hepatic lobectomies. After removal of left lateral section liver grafts, we observed that 7 out of 25 left lateral section donors (28%) had a low-grade shrinkage of the liver remnant (with negative PTLER values). Atrophy of the remnant liver can have several causes [2]. The cause of volume decrease in the LLS group after hepatectomy in our series seems to be gradual atrophy of liver segment 4. The subgroup analysis of donors with negative PTLER showed no significant difference in terms of TBIL1, CRP1, PLT1, portal vein flow rate, or postoperative complications. Biliary leakage after hepatectomy was reported to be the cause of decreased regeneration rates in some studies [17]. We showed that adequately drained biliary leaks did not deteriorate post-hepatectomy liver regeneration rates in our study. Various experimental studies were published related to acceleration of liver regeneration in the post-hepatectomy period [18–20]. Recently, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS procedure) has become popular as a serious alternative to portal vein ligation and two-stage hepatectomy, but, according to the multicenter trial of Croome et al. [21], the hypertrophy after ALPPS is not unique. We developed a simple “PRIO” index to predict the regeneration rate after hepatectomy. PRIO values were significantly higher in the RHH group. PRIO value was only weakly correlated with PTLER and P7LER, but we think that it is promising and deserves further study.
Figure 2.
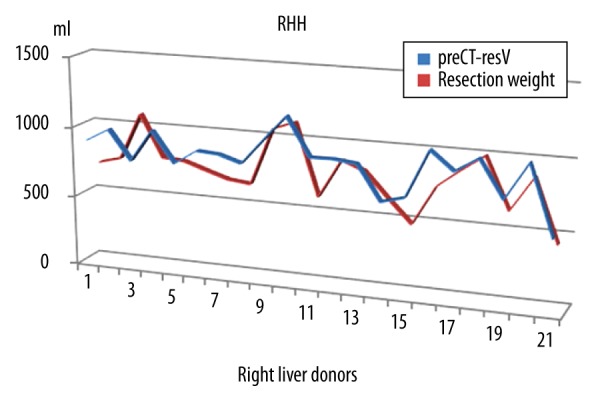
Estimated resection volumes and actual resection weights of right hemiliver donors. preCT-resV – preoperative estimated liver volume found by computed tomography volumetry, RHH – right hemihepatectomy; resection weight – resection volume in ml.
Figure 3.
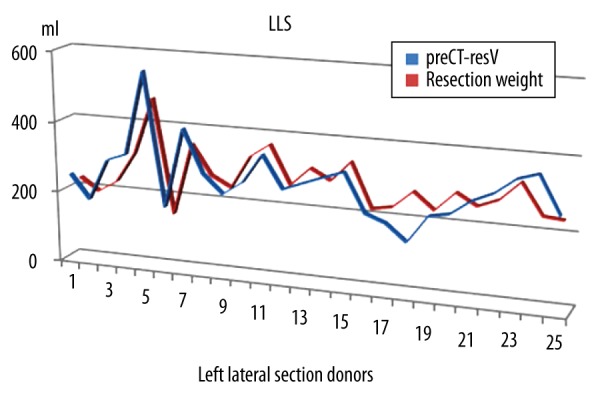
Estimated resection volumes and actual resection weights of left lateral section donors. preCT-resV – preoperative estimated liver volume found by computed tomography volumetry, LLS – left lateral sectionectomy; resection weight – resection volume in ml.
Conclusions
Living-donor liver transplantation requires a healthy donor with a liver suitable for the recipient. Liver regeneration is a critical feature of this sophisticated organ which makes the transplant possible. The regeneration process begins on a molecular basis just after hepatectomy and has an obvious clinical impact on surgical outcome. In our small series, we were able to show results similar to those of several previous reports that liver hypertrophy depends on the extent of liver resection. Postoperative early PLT count was not found to be correlated with the regeneration rates in healthy liver donors. RHH and LLS surgeries differ from each other in terms of resected liver volume, as well as inflammatory activity during liver regeneration. No negative effect of postoperative biliary leakage on regeneration rates was found. Our new inflammatory quotient PRIO was found to be promising for the prediction of regeneration rates after hepatectomy. Living-donor liver transplantation can be performed safely with the help of strict donor selection criteria. Transplant surgeons should make every effort to select the best liver donor, perform the correct surgery in an excellent way, and support the metabolic environment of the remnant liver for optimum regeneration.
Acknowledgements
We would like to thank Prof. Dr. I. Ozden, Prof. Dr. Y. Tekant, Prof. Dr. A. Alper, and our transplant coordinator Zulayin Ilik for their extraordinary support in this project.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Moray G, Arslan G, Haberal M. The history of liver transplantation in Turkey. Exp Clin Transplant. 2014;1:20–23. doi: 10.6002/ect.25liver.l18. [DOI] [PubMed] [Google Scholar]
- 2.de Jonge J, Kim M. Olthoff Liver regeneration: Mechanisms and clinical relevance. In: Jarnagin WR, Allen PJ, Chapman WC, editors. Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas. 6th ed. Philadelphia: Elsevier; 2017. pp. 93–107. [Google Scholar]
- 3.Um EH, Hwang S, Song GW, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg. 2015;19(4):133–38. doi: 10.14701/kjhbps.2015.19.4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dello S, Kele PGS, Porte RJ, et al. Influence of preoperative chemotherapy on CT volumetric liver regeneration following right hemihepatectomy. World J Surg. 2014;38:497–504. doi: 10.1007/s00268-013-2278-0. [DOI] [PubMed] [Google Scholar]
- 5.Shirabe K, Motomura T, Takeishi K, et al. Human early liver regeneration after hepatectomy in patients with hepatocellular carcinoma: Special reference to age. Scand J Surg. 2013;102:101–5. doi: 10.1177/1457496913482250. [DOI] [PubMed] [Google Scholar]
- 6.Kele PG, de Boer M, van der Jagt EJ, et al. Early hepatic regeneration index and completeness of regeneration at 6 months after partial hepatectomy. Br J Surg. 2012;99:1113–19. doi: 10.1002/bjs.8807. [DOI] [PubMed] [Google Scholar]
- 7.Alison MR, Lin WR. Diverse routes to liver regeneration. J Pathol. 2016;238:371–74. doi: 10.1002/path.4667. [DOI] [PubMed] [Google Scholar]
- 8.Silveiraa MRG, Silvab T, Novaes PC, et al. Ex situ regeneration of liver remnants hypothermically preserved for 24 hours. Transplant Proc. 2014;46:1857–61. doi: 10.1016/j.transproceed.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Tralhao JG, Abrantes AM, Hoti E, et al. Hepatectomy and liver regeneration: From experimental research to clinical application. ANZ J Surg. 2014;84:665–71. doi: 10.1111/ans.12201. [DOI] [PubMed] [Google Scholar]
- 10.Gruttadauria S, Pagano D, Liotta R, et al. Liver volume restoration and hepatic microarchitecture in small-for-size syndrome. Ann Transplant. 2015;20:381–89. doi: 10.12659/AOT.894082. [DOI] [PubMed] [Google Scholar]
- 11.Meier M, Andersen KJ, Knudsen AR, et al. Liver regeneration is dependent on the extent of Hepatectomy. J Surg Res. 2016;205:76–84. doi: 10.1016/j.jss.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Lock JF, Malinowski M, Seehofer D, et al. Function and volume recovery after partial hepatectomy: Influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297–304. doi: 10.1007/s00423-012-0972-2. [DOI] [PubMed] [Google Scholar]
- 13.Margonis GA, Amini N, Buettner S, et al. Impact of early postoperative platelet count on volumetric liver gain and perioperative outcomes after major liver resection. Br J Surg. 2016;103:899–907. doi: 10.1002/bjs.10120. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Miyake Y, Umeda Y, et al. Serial changes of serum growth factor levels and liver regeneration after partial hepatectomy in healthy humans. Int J Mol Sci. 2013;14:20877–89. doi: 10.3390/ijms141020877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts KJ, Bharathy KGS, Lodge JPA. Kinetics of liver function tests after a hepatectomy for colorectal liver metastases predict post-operative liver failure as defined by the International Study Group for Liver Surgery. HPB (Oxford) 2013;15:345–51. doi: 10.1111/j.1477-2574.2012.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olthoff KM, Emond JC, Shearon TH, et al. Liver regeneration after living donor transplantation: Adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2015;21:79–88. doi: 10.1002/lt.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lederer A, Seehofer D, Schirmeier A. Postoperative bile leakage inhibits liver regeneration after 70% hepatectomy in rats. J Invest Surg. 2013;26:36–45. doi: 10.3109/08941939.2012.691603. [DOI] [PubMed] [Google Scholar]
- 18.Gul M, Cömert M, Çakmak GK, et al. Effect of erythropoietin on liver regeneration in an experimental model of partial hepatectomy. Int J Surg. 2013;11:59–63. doi: 10.1016/j.ijsu.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Marinić J, Broznić D, Milin Č. Preexposure to olive oil polyphenols extract increases oxidative load and improves liver mass restoration after hepatectomy in mice via stress-sensitive genes. Oxid Med Cell Longev. 2016;2016:9191407. doi: 10.1155/2016/9191407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumer F, Colakoglu MK, Ozdemir Y, et al. Effect of nebivolol on liver regeneration in an experimental 70% partial hepatectomy model. Asian J Surg. 2017;40:375–79. doi: 10.1016/j.asjsur.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Croome KP, Hernandez-Alejandro R, Parker M, et al. Is the liver kinetic growth rate in ALPPS unprecedented when compared with PVE and living donor liver transplant? A multicentre analysis. HPB (Oxford) 2015;17:477–84. doi: 10.1111/hpb.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]


