Abstract
Glycosaminoglycan Chemical Exchange Saturation Transfer (gagCEST) is an important molecular MRI methodology developed to assess changes in cartilage GAG concentrations. The correction for B0 field inhomogeneity is technically crucial in gagCEST imaging. This study evaluates the accuracy of the B0 estimation determined by the dual gradient echo method and the effect on gagCEST measurements. The results were compared with those from the commonly used z-spectrum method. Eleven knee patients and three healthy volunteers were scanned. Dual gradient echo B0 maps with different ΔTE values (1, 2, 4, 6, 8, and 10 ms) were acquired. The asymmetry of the magnetization transfer ratio at 1 ppm offset referred to the bulk water frequency, MTRasym(1 ppm), was used to quantify cartilage GAG levels. The B0 shifts for all knee patients using the z-spectrum and dual gradient echo methods are strongly correlated for all ΔTE values used (r = 0.997 to 0.786, corresponding to ΔTE = 10 to 1 ms). The corrected MTRasym(1 ppm) values using the z-spectrum method (1.34% ± 0.74%) highly agree only with those using the dual gradient echo methods with ΔTE = 10 ms (1.72% ± 0.80%; r = 0.924) and 8 ms (1.50% ± 0.82%; r = 0.712). The dual gradient echo method with longer ΔTE values (more than 6 ms) has an excellent correlation with the z-spectrum method for gagCEST imaging at 3T.
Keywords: chemical exchange saturation transfer, B0 field inhomogeneity, glycosaminoglycan, knee osteoarthritis, dual gradient echo method
Introduction
Osteoarthritis (OA) is one of the leading causes of chronic disability and characterized by the gradual breakdown and eventual loss of joint cartilage (1–3). This disease is a significant public health problem. Using prevalence estimates, the National Arthritis Data Workgroup reported that OA affects nearly 27 million Americans or 12.1% of the adult population in 2005 (4,5). While there are no recent estimates of medical costs for OA, 2003 US medical expenditures attributable to arthritis and other rheumatic conditions aggregated $81 billion, of which OA is likely to account for a large proportion (6).
X-ray can visualize OA changes of the bone, but it has limited ability to assess soft-tissue involvement. CT is better at providing assessment of soft-tissue and osseous changes, but is not very sensitive for evaluating the extent and severity of OA (7). Magnetic resonance imaging (MRI) has been advancing over the past two decades with the development of cartilage-specific sequences and is unique for assessing meniscal and ligamentous disease related to OA, providing clinicians with more information regarding the health and status of the cartilage in situ (8–11). In addition to the commonly used morphological sequences, such as proton density-, T1- and T2-weighted, multiple advanced MRI imaging methods, including T1ρ (12,13) and 23Na MRI (14,15), have been developed to quantify regional variations in cartilage within its micro-architecture. Chemical exchange saturation transfer (CEST) has been developed as a molecular MRI methodology which detects endogenous macromolecules indirectly through chemical exchange and cross-relaxation with bulk water protons (16–21). The extent of contrast depends on the concentration of available macromolecules. gagCEST-MRI appears to be capable of assessing glycosaminoglycans (GAGs) to detect early articular cartilage degeneration and glycosaminoglycan concentration changes intervertebral disc (22–26). OA was reported to be associated with a decrease in GAG concentration before the observable joint space thinning or morphological changes in the cartilage matrix (Outerbridge grade II or more) (27,28). Thus, gagCEST-MRI can be performed as a useful tool to identify the concentration change of the biochemical components in-vivo and provide complementary information to the routine MR imaging contrasts.
The gagCEST-MRI technique is based on the asymmetry in the z-spectrum between the 1 ppm offset (referenced to the bulk water frequency) where GAG hydroxyl protons resonate and the reference site at −1 ppm. Although the magnetic field inhomogeneity is a common problem for CEST imaging (29,30), the 1 ppm site where the gagCEST effect appears is much closer to the water resonance (0 ppm) than other targeted frequencies, such as 3.5 ppm where the amide proton transfer (APT) is assessed (31–37). The small chemical shift difference makes it harder to distinguish the CEST effect from direct water saturation. The frequency shift even as small as 0.1 ppm in the z-spectrum is able to cause a dramatic change in the quantification of the GAG concentration. A B0 correction is always necessary in the image post-processing stage. The conventional approach for this is to acquire saturation images over a range of frequency offsets with a fine frequency interval (29,30). Then, the whole z-spectrum is assessed by the high order polynomial fit and the B0 map is obtained by looking for the lowest signal intensity in the spectrum (z-spectrum method). However, this scan requires a fairly long scan time (1–2 min) due to the necessary sweep of the RF saturation frequency offsets. On the other hand, it is well known that a B0 map can be acquired with the gradient echo method (38). This scan requires much shorter time (usually less than 1 min) and is easy to be implemented on clinical scanners. The gradient echo method has been used in CEST imaging by several researchers (39–41). Zhao et al. has compared the gradient echo field-mapping method and the z-spectrum-based water saturation shift referencing method in APT imaging of human brain tumor at 3T, showing the comparable MTR asymmetry results (40). Dula et al. evaluated the high order polynomial fit and gradient echo B0 mapping approaches in CEST imaging of brain at 7T (41). However, no previous study has been performed to quantitatively evaluate the performance of the gradient echo B0 mapping methods with different echo times (TEs) and their effects on the gagCEST imaging. The purpose of this study is to examine and improve B0 field inhomogeneity corrections in knee gagCEST imaging using the dual gradient echo B0 mapping method. The widely used z-spectrum method was used as a standard reference in the study.
Methods
Study Design and Population
Eleven clinical patients (age, 36 ± 9 years) were referred for 3T MR imaging of the knee from July to September 2010. The reason to include clinical patients is to vary the endogenous local environment around the cartilage area among each individual case which helps broaden the observed local B0 field homogeneity range. Bilateral knees were scanned for the volunteers and the single diseased knee was scanned for each patient. Three healthy volunteers (age, 25 ± 3 years) without known knee problems were also enrolled in this study. All volunteers and patients agreed prior to the imaging to participate in this research study. All examinations were performed in accordance with the approval of the Institutional Review Board (IRB).
MR Imaging Protocol
gagCEST-MRI was performed on a 3T whole body MRI system (Achieva, Philips Healthcare, Cleveland, OH) using an 8-channel phased array knee coil. Patients’ ankles were wrapped in concave foam and their knees in pads within the coil in order to stabilize the whole leg. For gagCEST imaging, prior to the regular image readout, a series of RF saturation pulses were applied to saturate the magnetization of protons at different RF irradiation frequencies. The scan comprised of two sets of eight block pulses, with a single pulse duration of 29 ms. A delay of 35 ms was made between the first set of eight block pulses and the second to overcome hardware limitations. The total pre-saturation pulse duration was 464 ms. The average pre-pulse saturation power (B1sat) was 1.4 μT. For the image acquisition, an axial single-slice multi-shot turbo spin echo (TSE) sequence with fat suppression was employed to achieve high resolution proton density weighted images (FOV = 160 × 160 mm2, matrix size = 256 × 256, slice thickness = 4 mm, TR/TE = 1000/7 ms, TSE factor = 12, NSA = 2, SENSE factor = 2). Higher-order shimming was applied. The slice that covered the largest patellofemoral cartilage area was chosen. To comply with the z-spectrum method, the entire z-spectrum was acquired with 33 offsets referenced to the bulk water resonance in intervals of 0.25 ppm from 4 to −4 ppm. One image without saturation pre-pulse was acquired for the signal normalization. Five additional dual gradient echo B0 maps with ΔTE of 1 ms, 2 ms, 4 ms, 8 ms and 10 ms were also acquired in order to perform the B0 frequency correction. Five ΔTEs were tested because, theoretically, the B0 value can be better fitted with a longer evolution time between the two echoes. Gradual increase in ΔTE was tested to see if it helps improve gagCEST evaluation accuracy. The B0 map acquisition time varied with individual ΔTE values, from 15 seconds (ΔTE = 1 ms) to 38 seconds (ΔTE = 10 ms) due to TR value variation, which leads to a total acquisition time of 6 minutes 42 seconds.
Image Post-Processing
Both region of interest (ROI) and pixel based analysis were performed within the patellofemoral cartilage region. All the image analysis was finished using in-house developed software based on the Interactive Data Language (IDL, Exelis Visual Information Solutions, Boulder, CO) environment. Signals acquired with saturation pulses (Ssat) were divided by the one obtained without saturation (S0) for normalization. The normalized values accounting for 33 frequency offsets were fitted to a 12th order polynomial curve using least-squares minimization. Based on generated coefficients, the z-spectrum was created by interpolating the curve into 16001 offsets with an offset resolution of 0.001 ppm for ROI analysis and 1601 offsets with an offset resolution of 0.01 ppm for pixel analysis. B0 corrections were performed using two types of methodologies including the z-spectrum method: assuming the actual water frequency (0 ppm) to be at the frequency with the lowest signal intensity; and the dual gradient echo method: correcting the shift of the actual water frequency with each of the five B0 maps. The z-spectrum curves corrected by both methods were compared. A B0 frequency shift map in the cartilage area was calculated based on the minimum signal intensity of the z-spectrum. It was compared with the five dual gradient echo B0 maps.
To quantitatively assess the GAG concentration, a magnetization transfer ratio asymmetry (MTRasym) at 1 ppm offset referred to bulk water frequency was defined:
where Ssat and S0 are the signals measured with and without the saturation pre-pulse, respectively.
Statistical Analysis
Two kinds of data sets were analyzed: B0 shift values and MTRasym (1 ppm). Each data set was calculated with the z-spectrum method and the dual gradient echo methods with different ΔTEs. The Pearson coefficient was calculated to evaluate the correlation between the two types of methods. Using a correlation coeffecient, the relationships between the two methods were categorized as low (r <0.5), moderate (0.5 < r < 0.7), and strong (r > 0.7). All data were analyzed using Microsoft Excel 2010.
Results
Healthy Volunteer Studies
The average B0 shifts in the patellofemoral compartments (n = 6) from all volunteers are: 0.18 ± 0.09 ppm using the z-spectrum method; 0.18 ± 0.09 ppm using the dual gradient echo method (ΔTE = 10 ms); 0.19 ± 0.10 (ΔTE = 8 ms); 0.18 ± 0.10 (ΔTE = 4 ms); 0.17 ± 0.10 (ΔTE = 2 ms); and 0.18 ± 0.09 (ΔTE = 1 ms). The average MTRasym(1 ppm) values using the z-spectrum method were measured to be 2.10% ± 0.33%; using the dual gradient echo method to be 2.05% ± 0.56% (ΔTE = 10 ms); 1.58% ± 1.02% (ΔTE = 8 ms); 2.24% ± 1.62% (ΔTE = 4 ms); 2.58% ± 1.89% (ΔTE = 2 ms); and 2.08% ± 1.44% (ΔTE = 1 ms).
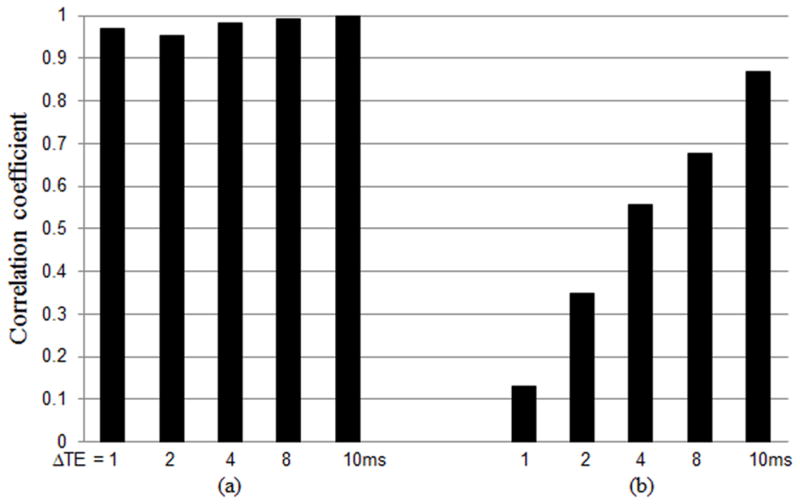
Figure 1 plots the correlation coefficients between the B0 shifts and MTRasym(1 ppm) values measured with the z-spectrum method and the dual gradient echo methods. The data shows that the correlation coefficients gradually increase with longer ΔTEs. The dual gradient echo B0 maps with various ΔTEs are all highly correlated with the z-spectrum B0 map in finding the B0 shifts. However, the correlation coefficients for the MTRasym(1 ppm) values show a large variance among these different dual gradient echo B0 maps, indicating the high sensitivity of gagCEST analysis to the B0 shifts.
Figure 1.
The correlations between the B0 shifts (a) and the MTRasym(1 ppm) values (b) measured with the z-spectrum method and the dual gradient echo methods with ΔTE = 1, 2, 4, 8, and 10 ms from healthy volunteers. The correlation coefficients gradually increase with longer ΔTEs.
Patient Studies
Table 1 lists disease characteristics associated with each patient’s patellofemoral cartilage. Ten patients were diagnosed with OA in the patellofemoral compartments, having symptoms including fibrillation, cartilage defects ranging from thin to full thickness loss. One patient had no patellofemoral cartilage related changes.
Table 1.
Patient information and clinical symptoms associated with the patellofemoral cartilages
| Patient # | SEX | AGE | CLINICAL SYMPTOMS |
|---|---|---|---|
| P01 | F | 34 | tricompartmental OA cartilage fibrillation |
| P02 | F | 39 | tricompartmental OA with full-thickness fissuring involving the patellar apex, as well as probable cartilage defect overlying the medial patellar facet |
| P03 | M | 41 | OA in patellofemoral compartment, with full-thickness cartilage defects |
| P04 | M | 47 | tricompartmental OA, cartilage loss with fissuring is seen involving the medial trochlea and medial patellar facet |
| P05 | M | 24 | tricompartmental OA, increased signal within the patellar superior pole articulating cartilage, representing cartilaginous degeneration |
| P06 | M | 48 | tricompartmental OA with deep chondral fissuring and a medial chondral defect near the trough of the trochlea and medial patellar facet |
| P07 | M | 32 | OA in patellofemoral compartment, with full-thickness fissuring at the trough of the trochlea and partial thickness fissuring at the apex of the patella |
| P08 | F | 26 | tricompartmental OA with diffuse cartilage thinning and irregularity |
| P09 | F | 28 | OA in the patellofemoral compartment with large osteochondral defect |
| P10 | M | 36 | N/A |
| P11 | F | 46 | tricompartmental OA characterized by cartilage fibrillation and thinning without focal chondral defect |
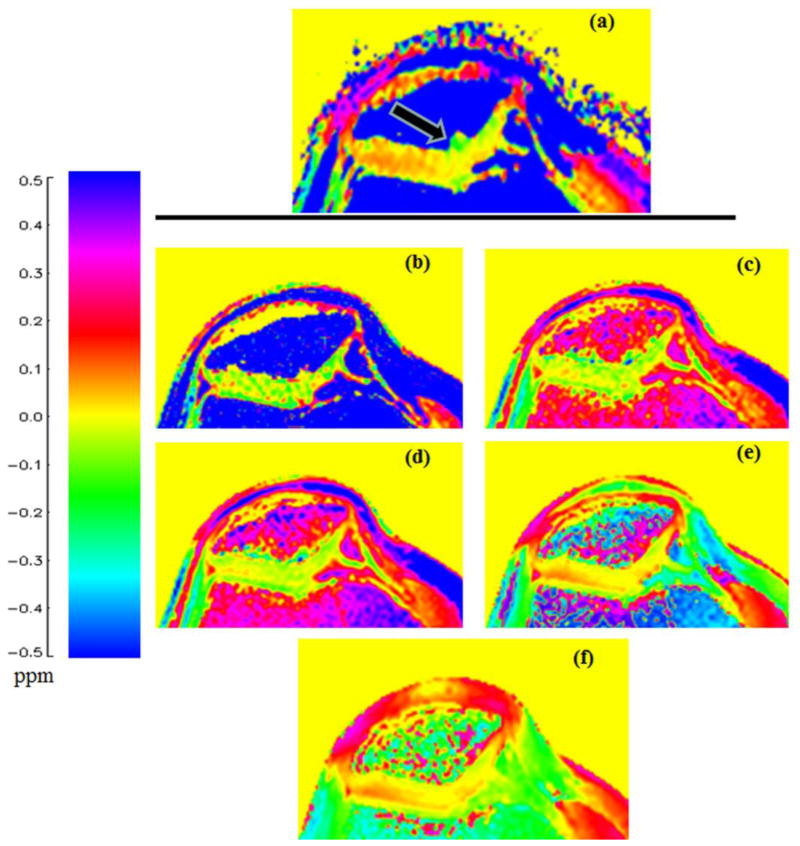
In all patients, B0 frequency shift values obtained from the z-spectrum method and dual gradient echo method ranged from −0.05 to 0.30 ppm and appear free of phase wrapping. Figure 2 is an example of a color coded B0 shift map. The dual gradient echo B0 map with ΔTE = 10 ms shows the most similar pattern in trend and magnitude as the one generated from the z-spectrum method. It appears that there is a larger B0 shift value (around 0.1 ppm) in the synovial fluid area between patellar and trochlear cartilage layers. This effect appears greater with the z-spectrum method. Compared to the B0 map with ΔTE = 10 ms, the B0 map from the z-spectrum method shows more B0 fluctuations at the lateral side and some odd spots (−0.1~−0.2 ppm) at the medial side of the cartilage areas. The B0 map (ΔTE = 10 ms) appears smoother in the cartilage areas and the transition zone is less profound from synovial fluid to cartilages.
Figure 2.
B0 frequency shift maps calculated from the z-spectrum method (a) and the dual gradient echo methods with ΔTE = 1, 2, 4, 8, and 10 ms (b, c, d, e, and f, respectively). The patient was diagnosed with tricompartmental cartilage OA, characterized by fibrillation and an abundance of hematopoietic marrow. For the gagCEST study, only the cartilage area (black arrow) is examined. As ΔTE increases, the gradient echo frequency shifts in the cartilage area exhibits a closer pattern to the z-spectrum B0 frequency shift map (0~0.1 ppm laterally, around 0 ppm medially).
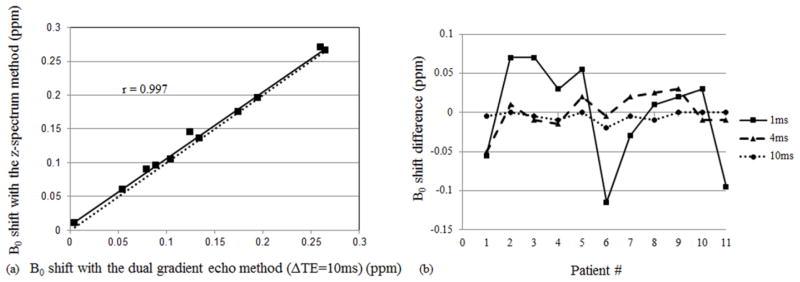
The average B0 shifts in the patellofemoral compartments are: 0.14 ± 0.08 ppm using the z-spectrum method; 0.14 ± 0.08 ppm using the dual gradient echo method (ΔTE = 10 ms); 0.14 ± 0.08 (ΔTE = 8 ms); 0.14 ± 0.09 (ΔTE = 4 ms); 0.13 ± 0.09 (ΔTE = 2 ms); and 0.14 ± 0.10 (ΔTE = 1 ms). The ROI analysis shows that all five B0 maps are strongly correlated with the B0 map from the z-spectrum method (r = 0.997 for ΔTE = 10 ms; r = 0.991 for ΔTE = 8 ms; r = 0.961 for ΔTE = 4 ms; r = 0.974 for ΔTE = 2 ms; r = 0.786 for ΔTE = 1 ms). Figure 3a plots a strong correlation (r = 0.997) between the z-spectrum B0 map and the dual gradient echo B0 map with ΔTE = 10 ms as an example.
Figure 3.
(a) A strong correlation (r = 0.997) between the z-spectrum method and the dual gradient echo method with ΔTE = 10 ms in the B0 inhomogeneity evaluation was shown. The dotted line y=x was plotted as a reference. (b) The B0 shift differences determined with the z-spectrum method and dual gradient echo methods (ΔTE = 1 ms, solid line; ΔTE = 4 ms, dashed line; ΔTE = 10 ms, dotted line). The differences are large with ΔTE = 1 ms and reduced with ΔTE = 10 ms.
The differences of the dual gradient echo based frequency shifts compared to the z-spectrum based frequency shifts were calculated for each subject. The average differences are: −0.005 ± 0.006 ppm (ΔTE = 10 ms); −0.002 ± 0.011 ppm (ΔTE = 8 ms); 0.000 ± 0.023 ppm (ΔTE = 4 ms); −0.008 ± 0.024 ppm (ΔTE = 2 ms); and −0.001 ± 0.064 ppm (ΔTE = 1 ms). Although these average differences did not present an apparent discrepancy, the standard deviations increase gradually from the longer ΔTEs to the shorter ΔTEs. Figure 3b shows the difference of B0 maps determined by the dual gradient echo methods (ΔTE = 1 ms, solid line; ΔTE = 4 ms, dashed line; ΔTE = 10 ms, dotted line), compared to the ones obtained by the z-spectrum method. The frequency differences of the dual gradient echo based B0 map with ΔTE = 1 ms and the z-spectrum based B0 map have a larger variation among those cases.
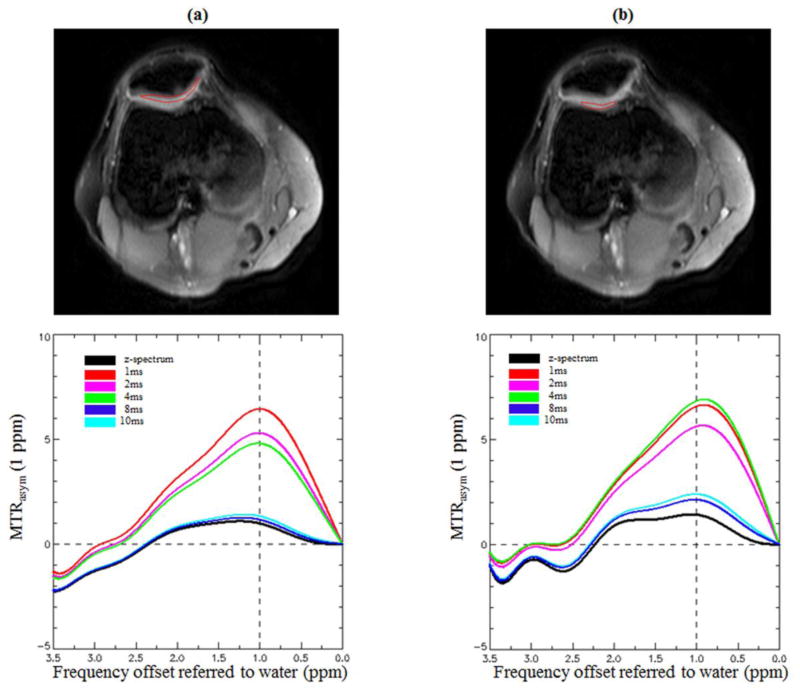
Figure 4 illustrates the MTRasym curves in the patellofemoral compartment with the two methods. The MTRasym curves and MTRasym(1 ppm) values corrected with the z-spectrum method are consistent with those corrected with the high ΔTE (8 and 10 ms) dual gradient echo methods, around 1.5% in the patellar region and around 1.5%~2.0% in the femoral region. However, the MTRasym curves corrected by the low ΔTE B0 maps (1–4 ms) are much further apart from the z-spectrum corrected curve and show abnormally larger MTRasym(1 ppm) values (4%~5%), which may erroneously be interpreted as high GAG content.
Figure 4.
The MTRasym curves from 0 to 3.5 ppm at the patellar (a) and trochlear (b) cartilage regions. The spectra were corrected by both methods. The z-spectrum method correction is shown in black as the reference, and the comparisons with the dual gradient echo B0 mapping corrections with ΔTE = 1, 2, 4, 8, and 10 ms are displayed in different colors. In both patellar and trochlear cartilage, all the MTRasym curves show strong peaks at 1 ppm and visable peaks at 2 ppm. Those two peaks correspond to the two different GAG hydroxyl protons. There is a tendency that the MTRasym curves corrected by higher ΔTE dual gradient echo B0 maps (8 and 10 ms) are closer to the one corrected with the z-spectrum method.
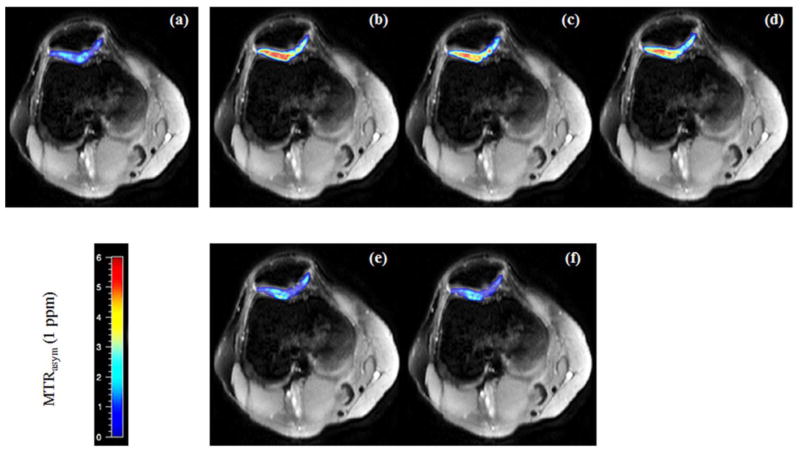
Figure 5 shows examples of typical MTRasym(1 ppm) maps corrected with z-spectrum and dual gradient echo methods for an OA patient. The MTRasym(1 ppm) maps corrected with the low ΔTE dual gradient echo B0 maps (b~d) exhibit abnormally higher signals and larger signal fluctuations (b: 5.6% ± 5.9%, c: 4.8% ± 3.0%, d: 4.5% ± 3.0%) in the pathological cartilage. On the contrary, the MTRasym(1 ppm) maps corrected by the z-spectrum (a) and high ΔTE dual gradient echo B0 maps (e~f) all show low signal characteristics (a: 0.7% ±1.9%, e: 1.1% ±2.0%, f: 1.1% ± 1.8%), which correspond to the GAG loss in the fibrillation stage of the whole arthritis process. When the higher ΔTE dual gradient echo method was used, the gagCEST maps showed smoother transition pattern between medial and lateral compartments.
Figure 5.
The MTRasym(1 ppm) color maps with the z-spectrum B0 mapping correction (a) and dual gradient echo B0 mapping corrections (b, ΔTE = 1 ms; c, ΔTE = 2 ms; d, ΔTE = 4 ms; e, ΔTE = 8 ms; f, ΔTE = 10 ms). The MTRasym(1 ppm) maps with low ΔTE dual gradient echo B0 mapping corrections (b~d) exhibit higher signals in the pathological cartilage than the ones corrected by the z-spectrum method (a) and high ΔTE dual gradient echo methods (e, f).
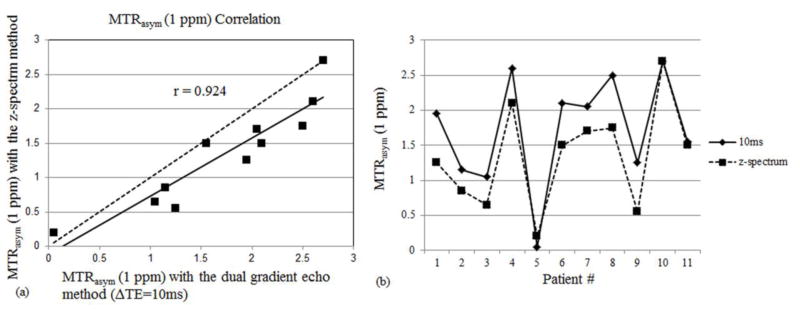
The average MTRasym(1 ppm) value using the z-spectrum method for all patients was measured to be 1.34% ± 0.74% in the patellofemoral region. It is relatively lower compared to the MTRasym(1 ppm) of healthy volunteers (1.93% ± 0.74%). The average MTRasym(1 ppm) values obtained using the dual gradient echo method are 1.72% ± 0.80% (ΔTE = 10 ms), 1.50% ± 0.82% (ΔTE = 8 ms), 1.20% ± 1.93% (ΔTE = 4 ms), 1.95% ± 1.88% (ΔTE = 2 ms), 1.71% ± 4.92% (ΔTE = 1 ms). The z-spectrum method reveals a strong correlation with the dual gradient echo methods using ΔTE = 10 ms (r = 0.924; Figure 6a) and 8 ms (r = 0.712), but a low correlation with the dual gradient echo methods using ΔTE = 4 ms (r = 0.254), 2 ms (r = 0.047) and 1 ms (r = 0.376). Using the dual gradient echo method with ΔTE = 10 ms, the MTRasym(1 ppm) values range from 0.05% ~ 2.70% (Figure 6b), which corresponds to different GAG concentrations in the patients. The tenth patient with no observed patellofemoral cartilage related changes shows the highest MTRasym value and the patients with diseased patellofemoral compartments have various lower values.
Figure 6.
(a) Strong correlation in MTRasym(1 ppm) calculations was observed between the z-spectrum B0 mapping method and the dual gradient echo B0 mapping method with ΔTE = 10 ms. The dotted line y=x was plotted as a reference. (b) MTRasym values from all the eleven patients were listed. The tenth patient with no observed patellofemoral cartilage related changes shows the highest value.
Discussion
The GAG concentration in cartilage plays an important role in diagnosing early stages of OA and tracking the OA progression. The clinical assessment of cartilage is generally based on the local signal changes caused by synovial fluid leaking into fissures, and morphological changes. This assessment tends to neglect cartilage alterations at the molecular level. The current GAG imaging gold standard-dGEMRIC requires contrast agent injection and patient exercise to help the contrast agent diffuse into articular cartilage (42–44), which results in a significantly longer procedure time (2~3 hours). Since patients need to be repositioned, it may require image co-registration at post-processing. These limit its clinical applicability and dGEMRIC may not be feasible on patients with renal impairment. gagCEST imaging has been shown to be able to provide information on the molecular composition of cartilage and to identify the early OA change characteristics, such as the loss of GAG content, which is complementary to current MR morphological imaging. Compared to dGEMRIC, gagCEST has many advantages, such as shorter procedure time, no need for contrast agent injection, and no waiting period with patient exercise.
However, gagCEST is highly sensitive to magnetic field inhomogeneities. In this study, the B0 field inhomogeneity corrections using the dual gradient echo and z-spectrum methods were compared in both healthy volunteers and patients with knee injuries. Our results demonstrated that the dual gradient echo methods with relative longer ΔTEs have a better correlation with the z-spectrum method in both the B0 shift determination and GAG concentration assessment. The longer ΔTE provides a better fit of the phase change within the time interval. The data has shown that B0 shifts of at most 0.30 ppm from the central frequency were observed in the cartilage region. Even within the longest 10 ms ΔTE, the 0.30 ppm frequency shift cause the phase accumulation of 2.41 radians, which is still less than the range of a single π, so the phase wrapping issue could be excluded from the postprocessing consideration. Because the phase is restricted to [−π, +π], ΔTE is suggested to keep within 13 ms in order to prevent the phase wrap. The B0 shift detections with low ΔTEs showed substantial variation compared to the z-spectrum method (Figure 2). Our data showed that using a longer ΔTE, the dual gradient echo B0 mapping correction could also detect gagCEST effects at 3T in vivo in articular cartilage and it had a similar accuracy in finding out the absolute water frequency as in the conventional method (29,30).
The gagCEST imaging protocol, particularly the RF saturation duration and saturation power, should be further optimized in the future. We are currently evaluating a highly sensitive, parallel transmission-based CEST-MRI method (19). Extension to a higher field is still desirable for the gagCEST imaging. Anup et al. has recently reported the better performance of gagCEST imaging at 7T due to less direct saturation effects (22). However, we should keep in mind that 7T imaging is currently not available for clinical diagnosis.
The gagCEST approach in the articular cartilage has a small range of B0 shifts (usually less than 0.3 ppm). Thus, the results from gagCEST studies may not directly be applied to other CEST experiments. Cartilage is a solid-like tissue that originally has a homogeneous composition which helps to maintain B0 field homogeneity. Early OA does not induce much structural alteration; thus, large central frequency changes are not expected. With small frequency shifts, the phase wrapping should not be an issue. Therefore, the dual gradient echo B0 field maps can be obtained more accurately, which enable the detection of frequency shifts in CEST experiments.
One limitation of this study is the relatively small number of subjects. Although the patients showed various levels of GAG concentration, more subjects should be scanned in the future to acquire a robust statistical sample size. Another consideration are implants such as metal screws from surgery as used in ACL reconstruction, which can affect the performance of the shimming box especially at the femoral lateral condyle area even it is away from the cartilage. The dGEMRIC method, which serves as gold standard method in GAG assessment, should also be performed along with gagCEST to validate GAG concentration findings. Comparison with radiological grading like Kellgren-Lawrence system is also desirable. Further, the reason that gagCEST is not a clinical option so far is that it lacks sufficient in vivo validation. True or relative GAG concentration values need to be defined with other methods as references, such as dGEMRIC.
In conclusion, our data indicates that the B0 shift values and MTRasym(1 ppm) values calculated from the commonly used z-spectrum method highly agree with those calculated from the dual gradient echo methods with longer ΔTE values (> 6 ms). The longer ΔTE dual gradient echo B0 mapping method is feasible and can be used as an alternative to correct for the artifacts induced by the B0 inhomogeneity in gagCEST imaging.
Acknowledgments
This study was supported in part by Wright Center of Innovation in Biomedical Imaging and OSU medical center imaging signature program and grants from the National Institutes of Health (R21CA156945, R01EB009731).
Abbreviations used
- OA
osteoarthritis
- MRI
magnetic resonance imaging
- CEST
chemical exchange saturation transfer
- gagCEST
glycosaminoglycan chemical exchange saturation transfer
- APT
amide proton transfer
- GAG
glycosaminoglycan
- SNR
signal to noise ratio
- IRB
Institutional Review Board
- TSE
turbo spin echo
- FOV
field of view
- NSA
number of signal average
- SENSE
sensitivity encoding
- ROI
region of interest
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of disabilities and associated health conditions among adults—United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:120–125. [PubMed] [Google Scholar]
- 2.Watt I. Osteoarthritis revisited---again! Skeletal Radiol. 2009;38:419–423. doi: 10.1007/s00256-008-0637-y. [DOI] [PubMed] [Google Scholar]
- 3.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 4.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expeditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–1407. doi: 10.1002/art.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan WP, Lang P, Stevens MP, Sack K, Majumdar S, Stoller DW, Basch C, Genant HK. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157:799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 8.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Beaudoin G, Choquette D, Haraoui B, Tannenbaum H, Meyer JM, Beary JF, Cline GA, Pelletier JP. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rational for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emad Y, Ragab Y, Shaarawy A, Abou-zeid A, Saad A, Fawzy M, Jokhdar H, Rasker JJ. Can magnetic resonance imaging differentiate undifferentiated arthritis based on knee imaging? J Rheumatol. 2009;36:1963–1979. doi: 10.3899/jrheum.081320. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein F, Mosher T, Hunter D. Imaging of knee osteoarthritis: data beyond the beauty. Curr Opin Rheumatol. 2007;19:435–443. doi: 10.1097/BOR.0b013e328248b4be. [DOI] [PubMed] [Google Scholar]
- 12.Subburaj K, Kumar D, Souza RB, Alizai H, Li X, Link TM, Majumdar S. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40:2134–2141. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menezes NM, Gray ML, Hartke JR, Burstein D. T2 and T1rho MRI in articular cartilage systems. Magn Reson Med. 2004;51:503–509. doi: 10.1002/mrm.10710. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23Na MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47(2):284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madelin G, Lee JS, Inati S, Jerschow A, Regatte RR. Sodium inversion recovery MRI of the knee joint in vivo at 7T. J Magn Reson. 2010;207(1):42–52. doi: 10.1016/j.jmr.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143:79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 17.Ling W, Eliav U, Navon G, Jerschow A. Chemical exchange saturation transfer by intermolecular double-quantum coherence. J Magn Reson. 2008;194:29–32. doi: 10.1016/j.jmr.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104:4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keupp J, Baltes C, Harvey PR, van den Brink J. Parallel RF Transmission based MRI Technique for Highly Sensitive Detection of Amide Proton Transfer in the Human Brain at 3T. Proceddings of the 19th Annual meeting of ISMRM; Montreal, Quebec, Canada. 2011; p. #1826. [Google Scholar]
- 20.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, van Zijl PCM. Chemical exchange saturation transfer imaging and spectroscopy. Prog NMR Spectrosc. 2006;48:109–136. [Google Scholar]
- 22.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, Hariharan H, Reddy R. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3T and 7T. Magn Reson Med. 2012;68:588–594. doi: 10.1002/mrm.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Pro Natl Acad Sci U S A. 2008;105:2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt B, Zbýn S, Stelzeneder D, Jellus V, Paul D, Lauer L, Bachert P, Trattnig S. Cartilage quality assessment by using glycosaminoglycan chemical exchange saturation transfer and (23)Na MR imaging at 7T. Radiology. 2011;260:257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]
- 25.Saar G, Zhang B, Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration changes in the intervertebral disc via chemical exchange saturation transfer. NMR Biomed. 2012;25:255–261. doi: 10.1002/nbm.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Chan Q, Anthony MP, Cheung KM, Samartzis D, Khong PL. Assessment of glycosaminoglycan concentration changes in the intervertebral disc via chemical exchange saturation transfer. NMR Biomed. 2011;24:1137–1144. doi: 10.1002/nbm.1671. [DOI] [PubMed] [Google Scholar]
- 27.Malemud CJ. Changes in proteoglycans in osteoarthritis: biochemistry, ultrastructure and biosynthetic processing. J Rheumatol Suppl. 1991;21:60–62. [PubMed] [Google Scholar]
- 28.Juras V, Bittsansky M, Majdisova Z, Szomolanyi P, Sulzbacher I, Gabler S, Stampfl J, Schuller G, Trattnig S. In vitro determination of biomechanical properties of human articular cartilage in osteoarthritis using multi-parametric MRI. J Magn Reson. 2009;197:40–47. doi: 10.1016/j.jmr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61:1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Blakeley JO, Hua J, Kim M, Laterra J, Pomper MG, van Zijl PC. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magn Reson Med. 2008;60:842–849. doi: 10.1002/mrm.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Payen J, Wilson DA, Traystman RJ, van Zijl PCM. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nature Med. 2003;9:1085–90. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 32.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PCM. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27:1129–36. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 33.Jokivarsi KT, Hiltunen Y, Tuunanen PI, Kauppinen RA, Grohn OHJ. Correlating tissue outcome with quantitative multiparametric MRI of acute cerebral ischemia in rats. J Cereb Blood Metab. 2010;30:415–27. doi: 10.1038/jcbfm.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Lai B, Wilson DA, Laterra J, van Zijl PC. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med. 2003;50:1120–1126. doi: 10.1002/mrm.10651. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu D-X, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PCM. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature Med. 2011;17:130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mougin OE, Coxon RC, Pitiot A, Gowland PA. Magnetization transfer phenomenon in the human brain at 7T. Neroimage. 2010;49:272–281. doi: 10.1016/j.neuroimage.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, Shah ZK, Patel M, Sammet S, Wei L, Bahnson RR, Knopp MV. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging. 2011;33:647–654. doi: 10.1002/jmri.22480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mindischberge C, Robinson S, Rauscher A, Barth M, Moser E. Robust field map generation using a triple-echo acquisition. J Magn Reson Imaing. 2004;20:730–734. doi: 10.1002/jmri.20158. [DOI] [PubMed] [Google Scholar]
- 39.Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by B0 and B1 field inhomogeneities in pH-sensitive chemical exchange saturation transfer (CEST) imaging. Magn Reson Med. 2007;58:1207–1215. doi: 10.1002/mrm.21398. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Wen Z, Zhang G, Huang F, Lu S, Wang X, Hu S, Chen M, Zhou J. Three-Dimensional Turbo-Spin-Echo Amide Proton Transfer MR Imaging at 3-Tesla and Its Application to High-Grade Human Brain Tumors. Mol Imaging Biol. 2012 doi: 10.1007/s11307-012-0563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dula AN, Asche EM, Landman BA, Welch EB, Pawate S, Sriram S, Gore JC, Smith SA. Development of chemical exchange saturation transfer at 7 T. Magn Reson Med. 2011;66:831–838. doi: 10.1002/mrm.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bashir A, Gray ML, Burstein D. Gd-DTPA2− as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 43.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd(DTPA)2− enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 44.Stubendorff JJ, Lammentausta E, Struglics A, Lindberg L, Heinegard D, Dahlberg LE. Is cartilage sGAG content related to early changes in cartilage disease? Implications for interpretation of dGEMRIC. Osteoarthritis Cartilage. 2012;20:396–404. doi: 10.1016/j.joca.2012.01.015. [DOI] [PubMed] [Google Scholar]