Abstract
Type B and non-A, non-B intercalated cells are found within the connecting tubule and the cortical collecting duct. Of these cell types, type B intercalated cells are known to mediate Cl− absorption and HCO3− secretion largely through pendrin-dependent Cl−/HCO3− exchange. This exchange is stimulated by angiotensin II administration and is also stimulated in models of metabolic alkalosis, for instance after aldosterone or NaHCO3 administration. In some rodent models, pendrin-mediated HCO3− secretion modulates acid-base balance. However, the role of pendrin in blood pressure regulation is likely of more physiological or clinical significance. Pendrin regulates blood pressure not only by mediating aldosterone-sensitive Cl− absorption, but also by modulating the aldosterone response for epithelial Na+ channel (ENaC)-mediated Na+ absorption. Pendrin regulates ENaC through changes in open channel of probability, channel surface density, and channels subunit total protein abundance. Thus, aldosterone stimulates ENaC activity through both direct and indirect effects, the latter occurring through its stimulation of pendrin expression and function. Therefore, pendrin contributes to the aldosterone pressor response. Pendrin may also modulate blood pressure in part through its action in the adrenal medulla, where it modulates the release of catecholamines, or through an indirect effect on vascular contractile force. This review describes how aldosterone and angiotensin II-induced signaling regulate pendrin and the contributory role of pendrin in distal nephron function and blood pressure.
Keywords: Blood pressure, Cl−/HCO3− exchange, Epithelial sodium channels, Intercalated cells, Pendrin, Slc26a4
Introduction
Volume contraction increases the release of angiotensin II and aldosterone, which increases renal NaCl absorption, thereby correcting the volume deficit [1]. During volume overload, this response is down regulated, thereby reducing distal nephron NaCl absorption [1]. Most distal NaCl transport occurs within the distal convoluted tubule (DCT), the connecting tubule (CNT), and to a lesser extent, the cortical collecting duct (CCD) [1]. Because the DCT cannot be perfused in vitro, the magnitude of NaCl absorption in this segment cannot be directly quantified. However, studies that directly measured ion flux in renal tubules perfused in vitro demonstrated that net NaCl absorption in the CNT per mm tubule length is approximately twice the absorption in the CCD [2]. Moreover, since the total length of the CNT in vivo is ~6-fold higher than the CCD [1], cumulative NaCl absorption in vivo is greater in the CNT than in the CCD by at least one order of magnitude. While these more proximal segments mediate greater net NaCl absorption than the medullary collecting duct, this latter segment is nevertheless critical in the final regulation of NaCl balance [1].
Experiments using CCDs and CNTs perfused in vitro have also enabled characterization of transport in the CCD and CNT. However, transport in the CCD is much better understood than transport in the CNT since the latter segment is so technically challenging to perfuse in vitro. The CCD is made up of principal and intercalated cells [1]. However, the CNT is composed of CNT and intercalated cells [1,3]. In the CCD, principal cells mediate most Na+ absorption [1,4,5], whereas most Cl− absorption occurs either by intercalated cell-mediated transcellular transport or through paracellular transport [6].
Pendrin is expressed in the apical domains of type B and non-A, non-B intercalated cells, which represent cell types found in the CNT and the CCD [3]. In type B intercalated cells, pendrin mediates Cl− absorption and HCO3− secretion through electroneutral Na+-independent, Cl−/HCO3− exchange [3,7–9]. Apical plasma membrane pendrin protein abundance is greater in the CNT than in the CCD under both basal and stimulated conditions [1,3,10]. This is because the total length of the CNT in vivo is much greater than the CCD and because of the greater pendrin abundance per cell in the CNT than in the CCD.
Models of metabolic alkalosis, such as that seen with aldosterone or NaHCO3 administration [10–14], stimulate pendrin abundance and thereby increase HCO3− secretion and Cl− absorption in the CCD [15,16]. In these treatment models, pendrin knockout mice develop greater alkalosis [10,11], which indicates an impaired ability of these mutant mice to correct the alkalosis. Therefore, the physiological role of type B intercalated cells, and more specifically pendrin, was initially thought to involve restoration of acid-base balance during metabolic alkalosis. However, as will be described in this review, a more important role has been identified in blood pressure regulation and the pressor response to hormones like aldosterone.
The role of intercalated cell subtypes in acid-base regulation
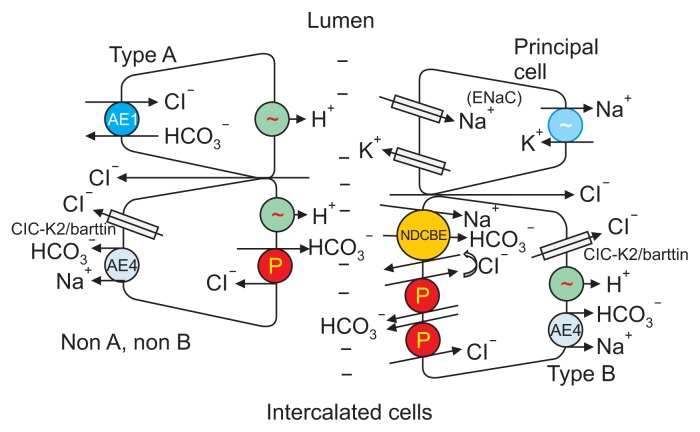
The H+-ATPase is abundantly expressed within intercalated cells. However, H+-ATPase localization within intercalated cells is heterogeneous, since some intercalated cells show apical localization whereas other cells show basolateral or diffuse localization [17,18]. Intercalated cells are therefore classified as type A, type B or non-A, non-B cells, based on their H+-ATPase subcellular distribution and whether or not they express the basolateral Cl−/HCO3− exchanger, AE1 [17,19–21] (Fig. 1 [22]). However, intercalated cell subtypes are also distinguishable by their ultrastructural characteristics [3,23].
Figure 1. Cell types and transporters in the cortical collecting duct (CCD).
Intercalated and principal cell ion transporter distribution within of the CCD is shown. Na+-dependent Cl−/HCO3− exchanger (NDCBE) mediates Na+ and HCO3− absorption, whereas pendrin mediates HCO3− secretion and Cl− absorption. Through the action of these 2 transporters, Cl− and HCO3− are recycled across the apical membrane. Most likely, Cl− is also absorbed through paracellular transport driven by the lumen-negative transepithelial voltage generated by the epithelial Na+ channel (ENaC).
Modified from the article of Wall and Lazo-Fernandez (Annu Rev Physiol 77:363–378, 2015) [22] with original copyright holder’s permission.
The subcellular distribution of the H+-ATPase is thought to determine whether an intercalated cell secretes H+ or HCO3−. Type A intercalated cells secrete H+ through the H+-ATPase (Fig. 1), which is expressed on the apical plasma membrane and is upregulated in models of metabolic acidosis [24,25]. In the type A intercalated cell, this active H+ secretion occurs in parallel with secretion of Cl−, although the mechanism for Cl− secretion is not well understood [26–29].
Type B intercalated cells secrete HCO3− and absorb NaCl through an apical plasma membrane, Na+-independent, electroneutral, Cl−/HCO3− exchanger (pendrin), which is encoded by Slc26a4 [7,9,30,31], and an apical Na+-dependent Cl−/HCO3− exchanger (NDCBE), which is encoded by Slc4a8 [32]. These apical exchangers act serially with net H+ efflux across the basolateral plasma membrane mediated by the H+-ATPase [19–21,31,33,34]. In type B intercalated cells, the basolateral plasma membrane H+-ATPase provides the active step, which generates the driving force for apical anion exchange [35]. In models of metabolic alkalosis, the apical Cl−/HCO3− exchanger and the basolateral membrane H+-ATPase are stimulated, which increases HCO3− secretion by type B intercalated cells [15,36]. From this observation, exchanger-mediated HCO3− secretion is thought to attenuate the alkalosis generated in these treatment models [7,37].
Apical Na+-independent Cl−/HCO3− exchange is also observed in non-A, non-B intercalated cells [38]. Unlike type B intercalated cells, however, the H+-ATPase localizes to the apical, rather than the basolateral, plasma membrane in this cell type [39]. Because this cell type is found primarily in the CNT of the mouse kidney [3], which represents a segment in mouse that has not been successfully perfused in vitro, CNT transport properties, and hence the properties of non-A, non-B intercalated cells, are less well understood than those of type B intercalated cells in the CCD. Nevertheless, given the distribution of Cl− and H+/OH− transporters within non-A, non-B intercalated cells, this cell type most likely absorbs Cl− [40] coupled with the secretion of H+ and HCO3− [38]. Therefore, the luminal fluid of the CNT should ultimately gain CO2 and H2O and lose Cl−. Tsuruoka and Schwartz [38] have shown that CNTs from untreated rabbits secrete HCO3−. If non-A, non-B intercalated cells are the predominant non-type A intercalated cell in this segment, these data suggest that non-A, non-B intercalated cells secrete relatively more HCO3− than H+s under most physiological conditions.
Whether the H+-ATPase of the non-A, non-B intercalated cell provides the active step for apical Cl−/HCO3− exchange, as was observed in the type B intercalated cell [35], is not known. Moreover, in this cell type the relative magnitude of anion exchange-mediated HCO3− secretion and H+-ATPase-mediated H+ secretion is unclear. As such, whether non-A, non-B intercalated cells are acid- or base-secreting cells (or both) is all unclear.
Intercalated cells are thought to regulate renal net acid excretion by secreting H+ or HCO3−. However, as described below, more recent studies have shown that intercalated cells play an important role in fluid and electrolyte balance and blood pressure regulation [9,10].
Pendrin localizes to type B and non-A, non-B intercalated cells in the CCD where it mediates Cl−/HCO3− exchange
In heterologous expression systems, pendrin mediates electroneutral, Na+-independent, Cl−/Cl−, Cl−/HCO3−, and Cl−/I− exchange [41–44]. Pendrin is expressed in the apical regions in a minority of cell types, i.e., type B and non-A, non-B intercalated cells, and colocalizes with NDCBE, which is encoded by Slc4a8 [32]. Because pendrin is an electroneutral Cl−/HCO3− exchanger and because it localizes to the apical region of cells that secrete HCO3− and absorb Cl− through electroneutral Na+-independent, Cl−/HCO3− exchange [7,9,43], we hypothesized that pendrin-mediates this electroneutral anion exchange observed in the rodent CCD. To test this hypothesis, we examined Cl− and total CO2 (HCO3−) transport in CCDs from wild type and pendrin null mice that were administered NaHCO3 and an aldosterone analogue in order to upregulate intercalated cell apical Cl−/HCO3− exchange [7,9,10,15,31]. Because pendrin gene ablation eliminated HCO3− secretion and Cl− absorption observed in CCDs from wild type mice, the apical Cl−/HCO3− exchange observed in the type B intercalated cell is pendrin-dependent [7,9].
Na+ and Cl− exit across the basolateral plasma membrane of type B intercalated cells through Na+-HCO3− cotransporter, AE4 [35], and ClC-K2/barttin Cl− channels [45,46], respectively. H+ exits the cell across the basolateral plasma membrane through the H+-ATPase [34,35].
Cl− absorption in the CCD occurs through 2 separate pathways, which differ in diuretic sensitivity. The first of these is eliminated with the application of epithelial Na+ channel (ENaC) inhibitors, such as amiloride, while the second is blocked with thiazides, which are inhibitors of the NaCl cotransporter (NCC) encoded by Slc12a3 [47]. The magnitude of the amiloride- and the thiazide-sensitive components of NaCl absorption varied between studies and likely depended on the species tested and the treatment model used [32,47]. While thiazides strongly inhibit NCC, NCC cannot mediate thiazide-sensitive Cl− absorption in the CCD because this transporter is not expressed in this segment [32]. However, Leviel et al [32] showed that the thiazide-sensitive component of Cl− absorption observed in the mouse CCD occurs instead through a NDCBE encoded by Slc4a8. The authors also showed that pendrin-mediated Cl−/HCO3− exchange and NDCBE-mediated Na+-dependent Cl−/HCO3− exchange act in parallel to mediate electroneutral NaCl absorption in the CCD. However, in the mouse CCD, the role of NDCBE in Cl− absorption remains controversial [48].
ENaC represents the major mechanism of Na+ absorption by principal cells [4,5]. While ENaC does not directly mediate Cl− transport, ENaC inhibitors markedly reduce Cl− absorption in the CCD [48]. ENaC blockade changes Cl− transport or channel activity either through paracrine signaling or by altering the driving force of Cl− movement. While the mechanism by which ENaC inhibition changes Cl− transport is not known, recent studies have shown that it does not involve pendrin, ClC-5, or CFTR [5,26,48]. Amiloride-sensitive Cl− absorption may involve paracellular Cl− transport or transcellular transport mediated by an electrogenic Cl− exchanger. ENaC-mediated Na+ absorption generates a lumen-negative transepithelial voltage that augments the driving force for electrogenic, transcellular Cl− transport and paracellular Cl− absorption across tight junctions.
Claudin-mediated paracellular Cl− transport is important in both blood pressure regulation and the maintenance of vascular volume. For example, natriuresis, chloriuresis, and reduced blood pressure are observed following claudin 4 gene ablation, particularly when dietary NaCl intake is restricted [49]. Since claudin 4 acts as a Cl− channel and localizes to tight junctions between intercalated cells and principal cells [49,50], ENaC blockade may alter electrogenic Cl− transport in the CCD by eliminating the driving force for claudin 4-mediated paracellular Cl− transport.
The contribution of transcellular Cl− transport relative to paracellular Cl− absorption, and the possible contrasting physiological roles of each, are not well understood. Pei et al [51] had shown that under conditions where energy conservation is needed, the kidney is more dependent on paracellular than on transcellular Cl− absorption. While this study looked primarily at proximal tubule paracellular Cl− absorption, one could speculate that in the CCD, paracellular transport-mediated Cl− absorption is most significant during energy depletion. Since paracellular Cl− absorption is driven by the lumen-negative voltage generated through ENaC-dependent Na+ absorption, ENaC may also be of importance during energy depletion. Conversely, transcellular Cl− transport mediated by type B intercalated cell apical Cl−/HCO3− exchange may be most important when cellular energy is abundant. However, the relative role of each pathway may depend on the need for K+ conservation. For example, in CCDs from mice fed a NaCl-deficient diet, significant thiazide-sensitive NaCl absorption was observed, which reflected NDCBE/pendrin-mediated NaCl absorption [32]. In contrast, in CCDs from mice fed a NaCl-replete diet along with aldosterone, Cl− absorption was unaffected by thiazides, but nearly obliterated with the application of an ENaC inhibitor (benzamil) [48]. Because thiazide-sensitive, NDCBE/pendrin-mediated NaCl absorption is not accompanied by a change in K+ flux [32], whereas ENaC-mediated, amiloride-sensitive NaCl absorption greatly stimulates K+ secretion [52], the thiazide-sensitive, NDCBE/pendrin-mediated component of the mouse Cl− absorption CCD may be most important when K+ conservation is needed.
Pendrin is upregulated with aldosterone
Since aldosterone increases apical anion exchange in the CCD [16,31], other studies have investigated whether aldosterone changes renal pendrin abundance or subcellular distribution. Although the abundance of pendrin at the apical plasma membrane in type B intercalated cells was low under basal conditions [3,10], it increased ~6-fold in response to aldosterone, primarily through sub-cellular redistribution [10]. Moreover, the aldosterone-induced increase in apical anion exchange [15,16,31,53] was eliminated with pendrin gene ablation [7,9]. Therefore, we conclude that both pendrin and type B intercalated cell apical anion exchange are greatly stimulated by aldosterone and that the apical Cl−/HCO3− exchange in type B intercalated cells is mainly pendrin-mediated.
Because pendrin mediates HCO3− secretion in mouse CCD, further experiments have explored the role of pendrin in acid-base balance. As expected, pendrin gene ablation exacerbated the metabolic alkalosis observed with the administration of aldosterone analogues [10]. However, these studies provided surprising evidence that pendrin also modulates blood pressure [10].
Pendrin gene ablation reduces, while pendrin overexpression increases, rodent blood pressure
Since pendrin mediates renal Cl− absorption, we explored its role in NaCl balance [9–11]. Following a NaCl-replete diet, apparent vascular volume was similar in wild type and in pendrin null mice [9,11]. However, following dietary NaCl restriction, pendrin gene ablation increased NaCl excretion, reduced blood pressure, and increased the apparent vascular volume contraction [9,11]. Therefore, the pendrin null kidney has an impaired ability to fully conserve NaCl, which leads to lower blood pressure [9,11,54]. During dietary NaCl restriction, or following an aldosterone infusion, which both upregulate pendrin, the difference in blood pressure between pendrin null and wild type mice was enhanced [9–11,55]. However, since intercalated cells represent only ~1% of total kidney volume [1,56], it is remarkable that this minority cell type had such a significant impact on blood pressure and vascular volume.
Whereas blood pressure was reduced in pendrin null mice, mice that overexpressed pendrin developed hypertension that was very sensitive to dietary Cl− intake [57]. However, whether pendrin-mediated Cl− absorption contributes to NaCl-sensitive hypertension in people has been a topic of great interest.
Pendrin regulates blood pressure and NaCl balance in humans
In 1896, Dr. Vaughan Pendred reported a single family in which congenital deafness and goiter were observed in 2 of the family’s 10 children [58]. This autosomal recessive disorder, called Pendred syndrome, is seen in 7.5 per 100,000 persons [59]. Given the important role it plays in hearing and thyroid function, it was not surprising that the product of the gene responsible for Pendred syndrome (pendrin) is highly expressed in the thyroid and inner ear [60,61]. Early studies, however, had not identified any abnormalities in renal function or blood pressure in people with Pendred syndrome [58]. This view changed, however, after Everett et al [59] had identified and cloned the gene responsible for this syndrome. This discovery facilitated the development of a number of tools, such as anti-pendrin antibodies and pendrin null mice. These tools enabled better study of Pendred syndrome and led to our observations, and the observations of others, showing that pendrin is highly expressed in intercalated cells and that pendrin modulates blood pressure.
After our report showing that Slc26a4 regulates blood pressure in mice [10,55], there was great interest in determining if pendrin also regulates human blood pressure. In the first of these human studies, a retrospective chart review examined the incidence of hypertension in persons with Pendred syndrome and in their unaffected family members [62]. While the study was not adequately powered, it raised the possibility that the absence of pendrin is protective against hypertension. A later study directly investigated blood pressure and NaCl excretion in people with biallelic, inactivating mutations in SLC26a4 [63]. This report demonstrated that pendrin gene ablation reduced blood pressure and induced natriuresis and chloriuresis in humans, similar to what we had shown in our earlier studies in mice.
Pendrin enhances the aldosterone-induced increase in ENaC abundance and function
Although pendrin does not directly mediate Na+ transport, NaCl-restricted pendrin null mice excreted more NaCl than their wild type NaCl-restricted littermates [54]. Therefore, we had asked if pendrin changed the expression of a major renal Na+ transporter [54]. In mice consuming a NaCl-replete diet, where circulating plasma renin and aldosterone were suppressed, pendrin gene ablation did not significantly alter the abundance of renal Na+ transporters such as NHE3, NKCC2, α1Na,K-ATPase, ENaC, and NCC [54]. However, following an aldosterone infusion or dietary NaCl restriction, pendrin gene ablation reduced the abundance of the α, β, and γ ENaC subunits [5,54,55].
Other experiments explored the effect of pendrin gene ablation on ENaC function. Aldosterone administration markedly increased Na+ absorption in the rat and mouse CCD, which was virtually eliminated with the application of ENaC inhibitors [4,5]. Therefore, aldosterone-stimulated Na+ absorption is primarily ENaC-mediated. However, the benzamil-sensitive component of Na+ absorption was reduced by ~60% in CCDs from aldosterone-treated pendrin null mice [5]. These data showed that pendrin gene ablation reduced ENaC abundance and function, which likely contributed to the lower blood pressure observed in these mutant mice.
Aldosterone increases ENaC activity (NPo) by increasing the frequency at which the channel is open (open probability, Po) [64], by increasing the total protein abundance of the ENaC subunits [65], and by increasing the apical plasma membrane ENaC subunit abundance through subcellular redistribution [65]. However, assembly of all 3 ENaC subunits is needed for aldosterone to efficiently deliver ENaC to the apical plasma membrane [66–68].
In other experiments, we performed single channel recordings in principal cells from split open CCDs of aldosterone-treated mice to examine the effect of pendrin gene ablation on channel activity, NPo, channel surface density, N, and channel open probability, Po [5]. Pendrin gene ablation lowered channel activity from the reduced channel surface density due to a decrease in ENaC subunit total protein abundance and ENaC subunit subcellular redistribution [5,54,55]. Pendrin gene ablation also lowered channel activity by reducing channel open probability [5].
Pendrin modulates ENaC abundance in kidney through paracrine signaling
We and others have explored how pendrin changes ENaC abundance and function [55,69]. Because pendrin and ENaC localize to different cell types [3,7,8,70] (Fig. 1), these proteins cannot directly associate. Moreover, pendrin did not change ENaC by altering the level of hormones that regulate ENaC, such as corticosterone, vasopressin, renin, aldosterone, or thyroid hormone [54,71]. Finally, while pendrin gene ablation downregulated ENaC in kidney, pendrin gene ablation did not change ENaC abundance in the thyroid or colon [54]. Moreover, pendrin gene ablation paradoxically increased ENaC abundance in the inner ear [72]. Therefore, the decrease in ENaC subunit abundance and function observed in pendrin null mice appears restricted to the kidney.
Because ENaC function changes with pH [55], we hypothesized that pendrin-mediated HCO3− secretion increases luminal HCO3− concentration, thereby stimulating ENaC. To test this hypothesis, we employed two separate treatment models, each of which increases luminal HCO3− concentration within the CNT and CCD in vivo. In the first, we gave mice NaHCO3 and aldosterone to stimulate pendrin-mediated HCO3− secretion [15,55], thereby increasing luminal HCO3− concentration in the CNT and CCD. In the second, we added a carbonic anhydrase inhibitor (acetazolamide) to the treatment protocol to increase distal delivery of HCO3− from upstream segments, while down regulating pendrin expression and function [55,73]. When mice were treated with aldosterone and NaHCO3, pendrin gene ablation magnified the metabolic alkalosis and reduced renal ENaC subunit abundance and function [55]. However, adding acetazolamide to the treatment protocol eliminated the differences in renal ENaC subunit abundance and function as well as differences in acid-base balance [55]. One explanation for these findings is that with stimulation of distal HCO3− delivery from upstream segments, pendrin null mice are rescued from the enhanced alkalosis and the subsequent decrease in renal ENaC abundance and function.
In other experiments, mouse principal cells (mpkCCD) were used to determine if changing HCO3− concentration in vitro on the apical side of the cell modulated ENaC abundance and function [55]. When HCO3− concentration on the apical side of the monolayer was increased over a period of 3 to 24 hours, ENaC abundance and function increased, which did not depend on the substituting anion. Therefore, pendrin modulates ENaC, at least in part, either through changes in luminal HCO3− or luminal pH.
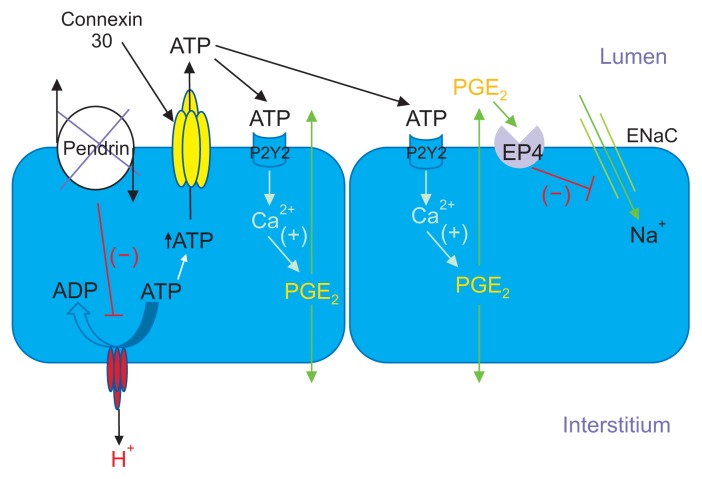
Since H+-ATPase gene ablation in the kidney reduced renal cortical ENaC abundance and function [69], and since pendrin gene ablation significantly reduced H+-ATPase abundance in type B intercalated cells [74], pendrin gene ablation may reduce ENaC function due to reduced type B intercalated cell H+-ATPase function. Because B1-H+-ATPase gene ablation increased urinary excretion of prostaglandin E2 (PGE2), Gueutin et al [69] had hypothesized that B cell H+-ATPase gene ablation reduced ENaC abundance and function through a PGE2-mediated mechanism (Fig. 2 [22]). They observed that a reduction in intercalated cell H+-ATPase abundance, as occurs with pendrin gene ablation, stimulated ATP secretion. Luminal ATP then acted through purinergic receptors on the apical plasma membrane of principal cells in order to stimulate principal cell Ca2+ release, which increased the production of PGE2, and thereby reduced ENaC abundance and function [69] (Fig. 2). These studies are consistent with a large body of work demonstrating that luminal ATP acts through principal cell apical plasma membrane purinergic receptors in order to reduce phosphatidylinositol 4,5-bisphosphate (PIP2) through a phospholipase C-dependent pathway, which lowers ENaC subunit abundance and function [75–77].
Figure 2. Pendrin gene ablation modulates luminal ATP concentration, which changes ENaC abundance and function.
With pendrin gene ablation, H+-ATPase abundance decreases in the type B intercalated cell, thereby increasing intercalated cell ATP content. Luminal ATP concentration then rises through enhanced connexin 30-mediated ATP secretion. Luminal ATP acts on apical membrane purinergic receptors to stimulate calcium release, which increases prostaglandin E2 production (PGE2). PGE2 acts through a receptor-mediated process to reduce ENaC abundance and function.
Modified from the article of Wall and Lazo-Fernandez (Annu Rev Physiol 77:363–378, 2015) [22] with original copyright holder’s permission.
In summary, pendrin gene ablation reduces ENaC abundance and function by changing luminal ATP and HCO3− concentration. However, other pathways may also contribute to this pendrin-ENaC interaction.
Pendrin is upregulated by angiotensin II
Angiotensin II stimulates ENaC-mediated renal Na+ absorption through both short- and long-term mechanisms [78–80]. Therefore, we explored the effect of this hormone on intercalated cell function. In particular, we had asked if angiotensin II application in vitro increased Cl− absorption in the CCD and explored the mechanism by which it occurs [81]. We found that angiotensin II application to the bath induced a 2-fold increase in electroneutral Cl− absorption in CCDs from furosemide-treated wild type mice, but not from pendrin knockout mice [81]. These data indicated that this peptide hormone more likely increased Cl− absorption through transcellular rather than through paracellular transport [81].
Angiotensin II may increase CCD Cl− absorption through a direct effect on apical plasma membrane pendrin abundance or it may stimulate another transporter, thereby increasing the driving force for apical anion exchange. For example, angiotensin II may increase basolateral plasma membrane H+-ATPase abundance in type B intercalated cells, which provides the active transport step for apical anion exchange [35]. To discriminate between these possibilities, we perfused mouse CCDs in vitro in the presence or absence of angiotensin II and then fixed and labeled the tubules for pendrin and the H+-ATPase. Transporter subcellular distribution was quantified by immunogold cytochemistry with morphometric analysis. In type B intercalated cells, we observed no increase in plasma membrane pendrin or H+-ATPase abundance with angiotensin II application in vitro [82] However, angiotensin II induced H+-ATPase subcellular redistribution in type A intercalated cells, which produced a 3-fold increase in its abundance on the apical plasma membrane relative to the cytoplasm [82]. Moreover, this peptide hormone increased HCO3− absorption in the CCD, indicating increased H+ secretion [82], similar to previous observations in the OMCD [83].
In CCDs perfused in vitro, angiotensin II application increased ENaC-mediated Na+ absorption [80] which should have increased the lumen-negative voltage. However, we observed that angiotensin II increased Cl− absorption without changing VT. As such, movement of another ion must shunt the ENaC-mediated current. In the CCD, the lumen-negative VT talls with increased H+ secretion [27], but rises as ENaC-mediated Na+ absorption increases [5]. Since angiotensin II application in vitro increases both H+ secretion and Na+ absorption in the mouse CCD, movement of these 2 ions must shunt the voltage generated by each other.
These data indicate that angiotensin II does not increase pendrin-dependent Cl− absorption in vitro through pendrin subcellular redistribution. Angiotensin II also does not change the driving force for anion exchange by increasing basolateral plasma membrane H+-ATPase abundance. Instead, angiotensin II may increase the driving force for apical anion exchange by lowering intra-cellular Cl− concentration within the type B intercalated cell, such as through activation of a basolateral plasma membrane Cl− channel. However, other mechanisms are possible.
Angiotensin II acts on the angiotensin type 1 receptor to increase apical plasma membrane pendrin abundance through subcellular redistribution, independently of aldosterone [84]. Angiotensin II application in vivo also stimulates the angiotensin type 2 receptor, which acts through nitric oxide to reduce pendrin protein abundance without changing pendrin subcellular distribution [84]. Therefore, the angiotensin type 2 receptor provides a feedback loop, which attenuates the increase in apical plasma membrane pendrin abundance that follows angiotensin type 1a receptor activation by angiotensin II.
Regulation of pendrin by the mineralocorticoid receptor and receptor interaction with aldosterone and angiotensin II
Upon aldosterone binding to the mineralocorticoid receptor (MR), a series of downstream signaling events are triggered, which lead to increased collecting duct transporter or channel activity. In the kidney, the signaling cascade by which aldosterone activates the MR and thereby stimulates ion transport, is best understood for ENaC in principal cells. In addition to aldosterone, cortisol and corticosterone are also MR substrates. However, if a cell expresses 11β-hydroxysteroid dehydrogenase type II, these glucocorticoids are oxidized and inactivated, making aldosterone the preferred receptor ligand [85]. The MR and 11β-hydroxysteroid dehydrogenase type II are clearly expressed in principal cells [85–87]. Convincing MR expression data was also found within type B and non-A, non-B intercalated cells [86,87]. However, because 11β-hydroxysteroid hydrogenase type II expression was either low or absent in intercalated cells [86,88], whether aldosterone is an MR ligand in this cell type is controversial. Aldosterone may activate intercalated cell transporters through the MR, but independently of direct aldosterone binding, such as through oxidative stress or cyclic adenosine monophosphate (cAMP) [89].
Intercalated cell pendrin abundance is reduced with chemical inhibitors of the MR, such as spironolactone [87], either through a direct effect of MR inhibition or through an indirect effect of MR blockade, such as through MR-dependent changes in serum potassium [90]. The reported effect of serum K+ and K+ balance on pendrin abundance has varied between studies, however [12,91–93].
Shibata et al [87] had reported that mouse intercalated cells express a unique MR phosphorylation site. Phosphorylation of the intercalated cell MR at S843 prevented aldosterone binding to the MR, thereby blocking MR activation. However, with increased angiotensin II production, as observed with dietary NaCl restriction, the intercalated cell MR was dephosphorylated at S843 and thereby activated, which augmented aldosterone binding and increased pendrin and H+-ATPase abundance. Because aldosterone-induced S843 MR dephosphorylation was greater with angiotensin II, but reduced with hyperkalemia [87], angiotensin II augments, whereas hyperkalemia attenuates, aldosterone-induced intercalated cell MR activation.
Does pendrin regulate blood pressure through a mechanism that is outside of the kidney?
Blood pressure is regulated not only by the kidney, but also by the central nervous system, by vascular tissue, and through the production of hormones released by the adrenal gland [94]. While studies to date have not demonstrated pendrin expression within the central nervous system, we had observed that pendrin was expressed in both epinephrine- and norepinephrine-producing chromaffin cells within the rat and mouse adrenal medulla where it modulates catecholamine release [95]. While plasma epinephrine and norepinephrine concentrations were similar in wild type and pendrin null mice under basal, unstressed conditions, catecholamine concentrations were higher in pendrin null than in wild type mice after 20 minutes of immobilization stress. Because catecholamine release changes blood pressure, we measured blood pressure before, during, and after immobilization stress, a treatment model associated with increased release of catecholamines. Blood pressure increased in both groups following immobilization stress. However, while blood pressure remained lower in the pendrin null than in the wild type mice before and during the period of stress [95], blood pressure was similar between wild type and pendrin null mice 30 minutes after relief of stress [95]. Therefore, pendrin gene ablation increases catecholamine release, which may limit the drop in blood pressure observed in the absence of renal pendrin-mediated Cl− absorption.
Since blood pressure is the product of systemic vascular resistance and cardiac output, we had explored the effect of pendrin gene ablation on vascular tone [96]. Although we did not observe pendrin expression in vascular tissue, pendrin gene ablation increased thoracic aorta contractile force in response to either α adrenergic agonists (phentolamine) or angiotensin II [96]. This greater contractile force was associated with increased myosin light chain kinase 20 abundance and was eliminated when an angiotensin type 1a receptor inhibitor, i.e. candesartan, was given in vivo [96]. This increased vascular contractility may also limit the hypotension that follows pendrin gene ablation. However, because pendrin expression in vascular tissue is very low, the changes in vascular tone we observed with pendrin gene ablation likely occurred as an indirect effect of pendrin gene ablation, such as through changes in circulating levels of angiotensin II and/or catecholamines [96].
Because previous studies have used global, embryonic pendrin (Slc26a4) gene ablation to study pendrin physiology, some of the phenotype observed in these mutant mice may have occurred due to developmental changes or from an extra-renal effect of pendrin gene ablation. Eliminating these potentially confounding variables will require the development of an inducible, tissue-specific pendrin null mouse.
Conclusions
Angiotensin II and aldosterone increase renal NaCl absorption in part by increasing apical plasma membrane pendrin abundance. While angiotensin II and aldosterone directly stimulate ENaC in principal cells, they also stimulate ENaC activity indirectly, by stimulating pendrin, which then increases ENaC abundance and function. In the kidney, pendrin regulates ENaC abundance and function, at least partly, by changing luminal HCO3− and ATP. However, pendrin is also expressed in tissues outside the kidney that also modulate blood pressure, such as the adrenal medulla. Whether pendrin changes blood pressure, at least in part, through its action outside kidney remains to be determined.
Acknowledgments
This work was supported by NIH grant DK 104125 (to S. M. Wall). I thank Dr. Peter Kopp for his suggestions.
Footnotes
Conflicts of interest
The author has no conflicts of interest to declare.
References
- 1.Wall SM, Weinstein AM. Cortical distal nephron Cl(−) transport in volume homeostasis and blood pressure regulation. Am J Physiol Renal Physiol. 2013;305:F427–F438. doi: 10.1152/ajprenal.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida AJ, Burg MB. Sodium transport in the rabbit connecting tubule. Am J Physiol. 1982;243:F330–F334. doi: 10.1152/ajprenal.1982.243.4.F330. [DOI] [PubMed] [Google Scholar]
- 3.Wall SM, Hassell KA, Royaux IE, et al. Localization of pendrin in mouse kidney. Am J Physiol Renal Physiol. 2003;284:F229–F241. doi: 10.1152/ajprenal.00147.2002. [DOI] [PubMed] [Google Scholar]
- 4.Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995;6:89–94. doi: 10.1681/ASN.V6189. [DOI] [PubMed] [Google Scholar]
- 5.Pech V, Wall SM, Nanami M, et al. Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol. 2015;309:F154–F163. doi: 10.1152/ajprenal.00564.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlatter E, Greger R, Schafer JA. Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl− transport. Pflugers Arch. 1990;417:317–323. doi: 10.1007/BF00370998. [DOI] [PubMed] [Google Scholar]
- 7.Royaux IE, Wall SM, Karniski LP, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YH, Kwon TH, Frische S, et al. Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol. 2002;283:F744–F754. doi: 10.1152/ajprenal.00037.2002. [DOI] [PubMed] [Google Scholar]
- 9.Wall SM, Kim YH, Stanley L, et al. NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: role in Cl-conservation. Hypertension. 2004;44:982–987. doi: 10.1161/01.HYP.0000145863.96091.89. [DOI] [PubMed] [Google Scholar]
- 10.Verlander JW, Hassell KA, Royaux IE, et al. Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: role of pendrin in mineralocorticoid-induced hypertension. Hypertension. 2003;42:356–362. doi: 10.1161/01.HYP.0000088321.67254.B7. [DOI] [PubMed] [Google Scholar]
- 11.Verlander JW, Kim YH, Shin W, et al. Dietary Cl(−) restriction upregulates pendrin expression within the apical plasma membrane of type B intercalated cells. Am J Physiol Renal Physiol. 2006;291:F833–F839. doi: 10.1152/ajprenal.00474.2005. [DOI] [PubMed] [Google Scholar]
- 12.Mohebbi N, Perna A, van der Wijst J, Becker HM, Capasso G, Wagner CA. Regulation of two renal chloride transporters, AE1 and pendrin, by electrolytes and aldosterone. PLoS One. 2013;8:e55286. doi: 10.1371/journal.pone.0055286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frische S, Kwon TH, Frøkiaer J, Madsen KM, Nielsen S. Regulated expression of pendrin in rat kidney in response to chronic NH4Cl or NaHCO3 loading. Am J Physiol Renal Physiol. 2003;284:F584–F593. doi: 10.1152/ajprenal.00254.2002. [DOI] [PubMed] [Google Scholar]
- 14.Wagner CA, Finberg KE, Stehberger PA, et al. Regulation of the expression of the Cl−/anion exchanger pendrin in mouse kidney by acid-base status. Kidney Int. 2002;62:2109–2117. doi: 10.1046/j.1523-1755.2002.00671.x. [DOI] [PubMed] [Google Scholar]
- 15.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol. 1985;249:F870–F877. doi: 10.1152/ajprenal.1985.249.6.F870. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Austt J, Good DW, Burg MB, Knepper MA. Deoxycorticosterone-stimulated bicarbonate secretion in rabbit cortical collecting ducts: effects of luminal chloride removal and in vivo acid loading. Am J Physiol. 1985;249:F205–F212. doi: 10.1152/ajprenal.1985.249.2.F205. [DOI] [PubMed] [Google Scholar]
- 17.Brown D, Hirsch S, Gluck S. Localization of a proton-pumping ATPase in rat kidney. J Clin Invest. 1988;82:2114–2126. doi: 10.1172/JCI113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown D, Hirsch S, Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell sub-populations. Nature. 1988;331:622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- 19.Alper SL, Natale J, Gluck S, Lodish HF, Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci U S A. 1989;86:5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kim YH, Cha JH, Tisher CC, Madsen KM. Intercalated cell subtypes in connecting tubule and cortical collecting duct of rat and mouse. J Am Soc Nephrol. 1999;10:1–12. doi: 10.1681/ASN.V1011. [DOI] [PubMed] [Google Scholar]
- 21.Teng-umnuay P, Verlander JW, Yuan W, Tisher CC, Madsen KM. Identification of distinct subpopulations of intercalated cells in the mouse collecting duct. J Am Soc Nephrol. 1996;7:260–274. doi: 10.1681/ASN.V72260. [DOI] [PubMed] [Google Scholar]
- 22.Wall SM, Lazo-Fernandez Y. The role of pendrin in renal physiology. Annu Rev Physiol. 2015;77:363–378. doi: 10.1146/annurev-physiol-021014-071854. [DOI] [PubMed] [Google Scholar]
- 23.Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol. 1986;250:F1–F15. doi: 10.1152/ajprenal.1986.250.1.F1. [DOI] [PubMed] [Google Scholar]
- 24.Verlander JW, Madsen KM, Cannon JK, Tisher CC. Activation of acid-secreting intercalated cells in rabbit collecting duct with ammonium chloride loading. Am J Physiol. 1994;266:F633–F645. doi: 10.1152/ajprenal.1994.266.4.F633. [DOI] [PubMed] [Google Scholar]
- 25.Bastani B, Purcell H, Hemken P, Trigg D, Gluck S. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991;88:126–136. doi: 10.1172/JCI115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanami M, Lazo-Fernandez Y, Pech V, et al. ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. I. Stilbene-sensitive Cl− secretion. Am J Physiol Renal Physiol. 2015;309:F251–F258. doi: 10.1152/ajprenal.00471.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanami M, Pech V, Lazo-Fernandez Y, Weinstein AM, Wall SM. ENaC inhibition stimulates HCl secretion in the mouse cortical collecting duct. II. Bafilomycin-sensitive H+ secretion. Am J Physiol Renal Physiol. 2015;309:F259–F268. doi: 10.1152/ajprenal.00120.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wall SM, Fischer MP. Contribution of the Na(+)-K(+)-2Cl(−) cotransporter (NKCC1) to transepithelial transport of H(+), NH(4)(+), K(+), and Na(+) in rat outer medullary collecting duct. J Am Soc Nephrol. 2002;13:827–835. doi: 10.1681/ASN.V134827. [DOI] [PubMed] [Google Scholar]
- 29.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ. Contribution of the Na+-K+-2Cl− cotransporter NKCC1 to Cl− secretion in rat OMCD. Am J Physiol Renal Physiol. 2001;280:F913–F921. doi: 10.1152/ajprenal.2001.280.5.F913. [DOI] [PubMed] [Google Scholar]
- 30.Amlal H, Petrovic S, Xu J, et al. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl(−)/HCO3(−) exchanger activity and impairs bicarbonate secretion in kidney collecting duct. Am J Physiol Cell Physiol. 2010;299:C33–C41. doi: 10.1152/ajpcell.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Star RA, Burg MB, Knepper MA. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest. 1985;76:1123–1130. doi: 10.1172/JCI112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leviel F, Hübner CA, Houillier P, et al. The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest. 2010;120:1627–1635. doi: 10.1172/JCI40145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emmons C. New subtypes of rabbit CCD intercalated cells as functionally defined by anion exchange and H+-ATPase activity. J Am Soc Nephrol. 1993;4:838. [Google Scholar]
- 34.Emmons C. H+ extrusion in mouse cortical collecting duct (CCD) intercalated cells. J Am Soc Nephrol. 1997;8:5A. [Google Scholar]
- 35.Chambrey R, Kurth I, Peti-Peterdi J, et al. Renal intercalated cells are rather energized by a proton than a sodium pump. Proc Natl Acad Sci U S A. 2013;110:7928–7933. doi: 10.1073/pnas.1221496110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verlander JW, Madsen KM, Galla JH, Luke RG, Tisher CC. Response of intercalated cells to chloride depletion metabolic alkalosis. Am J Physiol. 1992;262:F309–F319. doi: 10.1152/ajprenal.1992.262.2.F309. [DOI] [PubMed] [Google Scholar]
- 37.Schuster VL. Function and regulation of collecting duct intercalated cells. Annu Rev Physiol. 1993;55:267–288. doi: 10.1146/annurev.ph.55.030193.001411. [DOI] [PubMed] [Google Scholar]
- 38.Tsuruoka S, Schwartz GJ. Mechanisms of HCO(−)(3) secretion in the rabbit connecting segment. Am J Physiol. 1999;277:F567–F574. doi: 10.1152/ajprenal.1999.277.4.F567. [DOI] [PubMed] [Google Scholar]
- 39.Roy A, Al-bataineh MM, Pastor-Soler NM. Collecting duct intercalated cell function and regulation. Clin J Am Soc Nephrol. 2015;10:305–324. doi: 10.2215/CJN.08880914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimizu T, Nakamura M, Imai M. Effect of S-8666 on Cl− transport in the rabbit connecting tubule perfused in vitro. Tohoku J Exp Med. 1991;164:293–298. doi: 10.1620/tjem.164.293. [DOI] [PubMed] [Google Scholar]
- 41.Scott DA, Karniski LP. Human pendrin expressed in Xenopus laevis oocytes mediates chloride/formate exchange. Am J Physiol Cell Physiol. 2000;278:C207–C211. doi: 10.1152/ajpcell.2000.278.1.C207. [DOI] [PubMed] [Google Scholar]
- 42.Scott DA, Wang R, Kreman TM, Sheffield VC, Karniski LP. The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet. 1999;21:440–443. doi: 10.1038/7783. [DOI] [PubMed] [Google Scholar]
- 43.Soleimani M, Greeley T, Petrovic S, et al. Pendrin: an apical Cl−/OH−/HCO3− exchanger in the kidney cortex. Am J Physiol Renal Physiol. 2001;280:F356–F364. doi: 10.1152/ajprenal.2001.280.2.F356. [DOI] [PubMed] [Google Scholar]
- 44.Shcheynikov N, Yang D, Wang Y, et al. The Slc26a4 transporter functions as an electroneutral Cl−/I−/HCO3− exchanger: role of Slc26a4 and Slc26a6 in I− and HCO3− secretion and in regulation of CFTR in the parotid duct. J Physiol. 2008;586:3813–3824. doi: 10.1113/jphysiol.2008.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hennings JC, Andrini O, Picard N, et al. The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol. 2017;28:209–217. doi: 10.1681/ASN.2016010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinelli L, Nissant A, Edwards A, Lourdel S, Teulon J, Paulais M. Dual regulation of the native ClC-K2 chloride channel in the distal nephron by voltage and pH. J Gen Physiol. 2016;148:213–226. doi: 10.1085/jgp.201611623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terada Y, Knepper MA. Thiazide-sensitive NaCl absorption in rat cortical collecting duct. Am J Physiol. 1990;259:F519–F528. doi: 10.1152/ajprenal.1990.259.3.F519. [DOI] [PubMed] [Google Scholar]
- 48.Pech V, Thumova M, Kim YH, et al. ENaC inhibition stimulates Cl− secretion in the mouse cortical collecting duct through an NKCC1-dependent mechanism. Am J Physiol Renal Physiol. 2012;303:F45–F55. doi: 10.1152/ajprenal.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong Y, Yu M, Yang J, et al. The Cap1-claudin-4 regulatory pathway is important for renal chloride reabsorption and blood pressure regulation. Proc Natl Acad Sci U S A. 2014;111:E3766–E3774. doi: 10.1073/pnas.1406741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou J, Renigunta A, Yang J, Waldegger S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc Natl Acad Sci U S A. 2010;107:18010–18015. doi: 10.1073/pnas.1009399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei L, Solis G, Nguyen MT, et al. Paracellular epithelial sodium transport maximizes energy efficiency in the kidney. J Clin Invest. 2016;126:2509–2518. doi: 10.1172/JCI83942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch. 2009;458:157–168. doi: 10.1007/s00424-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanley MJ, Kokko JP. Study of chloride transport across the rabbit cortical collecting tubule. J Clin Invest. 1978;62:39–44. doi: 10.1172/JCI109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YH, Pech V, Spencer KB, et al. Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol. 2007;293:F1314–F1324. doi: 10.1152/ajprenal.00155.2007. [DOI] [PubMed] [Google Scholar]
- 55.Pech V, Pham TD, Hong S, et al. Pendrin modulates ENaC function by changing luminal HCO3−. J Am Soc Nephrol. 2010;21:1928–1941. doi: 10.1681/ASN.2009121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfaller W. Structure Function Correlation on Rat Kidney. New York: Springer-Verlag; 1982. [DOI] [PubMed] [Google Scholar]
- 57.Jacques T, Picard N, Miller RL, et al. Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension. J Am Soc Nephrol. 2013;24:1104–1113. doi: 10.1681/ASN.2012080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pendred V. Deaf-mutism and goitre. Lancet. 1896;2:532. doi: 10.1016/S0140-6736(01)74403-0. [DOI] [Google Scholar]
- 59.Everett LA, Glaser B, Beck JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 60.Wangemann P, Itza EM, Albrecht B, et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Royaux IE, Suzuki K, Mori A, et al. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- 62.Madeo AC, Manichaikul A, Pryor SP, Griffith AJ. Do mutations of the Pendred syndrome gene, SLC26A4, confer resistance to asthma and hypertension? J Med Genet. 2009;46:405–406. doi: 10.1136/jmg.2008.063610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim BG, Yoo TH, Yoo JE, Seo YJ, Jung J, Choi JY. Resistance to hypertension and high Cl− excretion in humans with SLC26A4 mutations. Clin Genet. 2017;91:448–452. doi: 10.1111/cge.12789. [DOI] [PubMed] [Google Scholar]
- 64.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 65.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999;104:R19–R23. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Valentijn JA, Fyfe GK, Canessa CM. Biosynthesis and processing of epithelial sodium channels in Xenopus oocytes. J Biol Chem. 1998;273:30344–30351. doi: 10.1074/jbc.273.46.30344. [DOI] [PubMed] [Google Scholar]
- 67.Hughey RP, Mueller GM, Bruns JB, et al. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- 68.Mueller GM, Kashlan OB, Bruns JB, et al. Epithelial sodium channel exit from the endoplasmic reticulum is regulated by a signal within the carboxyl cytoplasmic domain of the alpha subunit. J Biol Chem. 2007;282:33475–33483. doi: 10.1074/jbc.M707339200. [DOI] [PubMed] [Google Scholar]
- 69.Gueutin V, Vallet M, Jayat M, et al. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hager H, Kwon TH, Vinnikova AK, et al. Immunocyto-chemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol Renal Physiol. 2001;280:F1093–F1106. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- 71.Kim YH, Pham TD, Zheng W, et al. Role of pendrin in iodide balance: going with the flow. Am J Physiol Renal Physiol. 2009;297:F1069–F1079. doi: 10.1152/ajprenal.90581.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim BG, Kim JY, Kim HN, et al. Developmental changes of ENaC expression and function in the inner ear of pendrin knock-out mice as a perspective on the development of en-dolymphatic hydrops. PLoS One. 2014;9:e95730. doi: 10.1371/journal.pone.0095730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milton AE, Weiner ID. Regulation of B-type intercalated cell apical anion exchange activity by CO2/HCO3−. Am J Physiol. 1998;274:F1086–F1094. doi: 10.1152/ajprenal.1998.274.6.F1086. [DOI] [PubMed] [Google Scholar]
- 74.Kim YH, Verlander JW, Matthews SW, et al. Intercalated cell H+/OH− transporter expression is reduced in Slc26a4 null mice. Am J Physiol Renal Physiol. 2005;289:F1262–F1272. doi: 10.1152/ajprenal.00206.2005. [DOI] [PubMed] [Google Scholar]
- 75.Ma HP, Eaton DC. Acute regulation of epithelial sodium channel by anionic phospholipids. J Am Soc Nephrol. 2005;16:3182–3187. doi: 10.1681/ASN.2005040434. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Listhrop R, Ecelbarger CM, Kishore BK. Renal sodium transporter/channel expression and sodium excretion in P2Y2 receptor knockout mice fed a high-NaCl diet with/without aldosterone infusion. Am J Physiol Renal Physiol. 2011;300:F657–F668. doi: 10.1152/ajprenal.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pochynyuk O, Bugaj V, Rieg T, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beutler KT, Masilamani S, Turban S, et al. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 79.Brooks HL, Allred AJ, Beutler KT, Coffman TM, Knepper MA. Targeted proteomic profiling of renal Na(+) transporter and channel abundances in angiotensin II type 1a receptor knockout mice. Hypertension. 2002;39:470–473. doi: 10.1161/hy02t2.102959. [DOI] [PubMed] [Google Scholar]
- 80.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.ASN.0000013292.78621.FD. [DOI] [PubMed] [Google Scholar]
- 81.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM. Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol. 2007;292:F914–F920. doi: 10.1152/ajprenal.00361.2006. [DOI] [PubMed] [Google Scholar]
- 82.Pech V, Zheng W, Pham TD, Verlander JW, Wall SM. Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol. 2008;19:84–91. doi: 10.1681/ASN.2007030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rothenberger F, Velic A, Stehberger PA, Kovacikova J, Wagner CA. Angiotensin II stimulates vacuolar H+ -ATPase activity in renal acid-secretory intercalated cells from the outer medullary collecting duct. J Am Soc Nephrol. 2007;18:2085–2093. doi: 10.1681/ASN.2006070753. [DOI] [PubMed] [Google Scholar]
- 84.Verlander JW, Hong S, Pech V, et al. Angiotensin II acts through the angiotensin 1a receptor to upregulate pendrin. Am J Physiol Renal Physiol. 2011;301:F1314–F1325. doi: 10.1152/ajprenal.00114.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kyossev Z, Walker PD, Reeves WB. Immunolocalization of NAD-dependent 11 beta-hydroxysteroid dehydrogenase in human kidney and colon. Kidney Int. 1996;49:271–281. doi: 10.1038/ki.1996.39. [DOI] [PubMed] [Google Scholar]
- 86.Ackermann D, Gresko N, Carrel M, et al. In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol. 2010;299:F1473–F1485. doi: 10.1152/ajprenal.00437.2010. [DOI] [PubMed] [Google Scholar]
- 87.Shibata S, Rinehart J, Zhang J, et al. Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab. 2013;18:660–671. doi: 10.1016/j.cmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, Lee JW, Chou CL, et al. Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A. 2017;114:E9989–E9998. doi: 10.1073/pnas.1710964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayuzawa N, Fujita T. Activation of mineralocorticoid receptor in salt-sensitive hypertension. Curr Hypertens Rep. 2015;17:552. doi: 10.1007/s11906-015-0552-2. [DOI] [PubMed] [Google Scholar]
- 90.Terker AS, Yarbrough B, Ferdaus MZ, et al. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol. 2016;27:2436–2445. doi: 10.1681/ASN.2015070815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Procino G, Milano S, Tamma G, et al. Co-regulated pendrin and aquaporin 5 expression and trafficking in Type-B intercalated cells under potassium depletion. Cell Physiol Biochem. 2013;32:184–199. doi: 10.1159/000356638. [DOI] [PubMed] [Google Scholar]
- 92.Quentin F, Chambrey R, Trinh-Trang-Tan MM, et al. The Cl−/HCO3− exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol. 2004;287:F1179–F1188. doi: 10.1152/ajprenal.00211.2004. [DOI] [PubMed] [Google Scholar]
- 93.Xu N, Hirohama D, Ishizawa K, et al. Hypokalemia and pendrin induction by aldosterone. Hypertension. 2017;69:855–862. doi: 10.1161/HYPERTENSIONAHA.116.08519. [DOI] [PubMed] [Google Scholar]
- 94.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 95.Lazo-Fernandez Y, Aguilera G, Pham TD, et al. Pendrin localizes to the adrenal medulla and modulates catecholamine release. Am J Physiol Endocrinol Metab. 2015;309:E534–E545. doi: 10.1152/ajpendo.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sutliff RL, Walp ER, Kim YH, et al. Contractile force is enhanced in Aortas from pendrin null mice due to stimulation of angiotensin II-dependent signaling. PLoS One. 2014;9:e105101. doi: 10.1371/journal.pone.0105101. [DOI] [PMC free article] [PubMed] [Google Scholar]